Abstract
Objectives
Bisphosphonates are widely used as first-line treatment for primary and secondary prevention of fragility fractures. Whilst they have proved effective in this role, there is growing concern over their long-term use, with much evidence linking bisphosphonate-related suppression of bone remodelling to an increased risk of atypical subtrochanteric fractures of the femur (AFFs). The objective of this article is to review this evidence, while presenting the current available strategies for the management of AFFs.
Methods
We present an evaluation of current literature relating to the pathogenesis and treatment of AFFs in the context of bisphosphonate use.
Results
Six broad themes relating to the pathogenesis and management of bisphosphonate-related AFFs are presented. The key themes in fracture pathogenesis are: bone microdamage accumulation; altered bone mineralisation and altered collagen formation. The key themes in fracture management are: medical therapy and surgical therapy. In addition, primary prevention strategies for AFFs are discussed.
Conclusions
This article presents current knowledge about the relationship between bisphosphonates and the development of AFFs, and highlights key areas for future research. In particular, studies aimed at identifying at-risk subpopulations and organising surveillance for those on long-term therapy will be crucial in both increasing our understanding of the condition, and improving population outcomes.
Cite this article: N. Kharwadkar, B. Mayne, J. E. Lawrence, V. Khanduja. Bisphosphonates and atypical subtrochanteric fractures of the femur. Bone Joint Res 2017;6:144–153. DOI: 10.1302/2046-3758.63.BJR-2016-0125.R1.
Keywords: Bisphosphonates, Atypical fractures of the femur, Femoral geometry, Bone remodelling
Article focus
Guidelines from The National Institute for Health and Care Excellence recommend bisphosphonates for all patients over 50 at risk of, and those under 50 with a history of, fragility fracture. However, its use has been linked to atypical fractures of the femur (AFFs). This article aims to provide a summary of the current evidence linking bisphosphonate use to AFFs, while presenting current strategies for their treatment and prevention.
Key messages
Bisphosphonates have been shown to increase microdamage accumulation and alter both bone mineralisation and collagen formation.
It is thought that these mechanisms contribute to AFFs.
Little is known about risk factors for bisphosphonate-related AFFs, and future studies should focus on identifying at-risk subpopulations.
Strengths and limitations
Our MEDLINE search is readily reproducible, using clear and robust search terms.
Our article comprehensively addresses all aspects of pathogenesis and management of AFFs related to the use of bisphosphonates.
The limitation of electronic databases means that this may not include all available literature.
Introduction
Osteoporosis, a condition associated with significant morbidity and mortality, results in patients having an increased risk of fractures. Bisphosphonates are a class of drug commonly prescribed to mitigate this risk. Approximately 50% of women over the age of 50 sustain an osteoporosis-related fracture or a fragility fracture during their lifetime, and one in five patients die within 12 months of the fracture.1-4 Clinical trials of bisphosphonates have demonstrated a significant decrease in the incidence of spinal and hip fractures and a concomitant reduction in associated costs and healthcare utilisation.5-12 Subsequent systematic reviews have supported the use of bisphosphonates as first-line treatment for primary and secondary prevention of fragility fractures in women, as well as the prevention and treatment of steroid-induced osteoporosis.13-16 However, despite their success, there has been growing concern over the long-term use of bisphosphonates. This is predominantly due to reports of increased risk of atypical subtrochanteric fractures of the femur (AFFs), which are thought to be secondary to bisphosphonate-related suppression of bone remodelling.17 The first such case series was published in 2005 and described AFFs and other non-vertebral fractures in nine adults following three to eight years of alendronate therapy.18 The fractures were atraumatic, and occurred earlier in the treatment period when alendronate was co-administered with either glucocorticoids or oestrogen. Since then, there has been a surge in published reports describing similar cases and investigating the association on a large scale, with one nationwide cohort study in Sweden finding the age-adjusted relative risk of AFFs with any use of bisphosphonates to be 47.3.19-26
In 2009, in response to the reported association between bisphosphonates and AFFs, the leadership of the American Society for Bone and Mineral Research (ASBMR) appointed a task force to address key questions relating to this problem.17 A multidisciplinary expert group reviewed pertinent published reports concerning AFFs, as well as preclinical studies that could provide insight into their pathogenesis. The task force defined major and minor features for complete and incomplete AFFS, which were updated in 2013 (Table I).27 The report recommended that in order to designate a fracture of the femur as atypical, at least four of five major features must be present. Conversely, minor features, despite being commonly associated with atypical fractures, are not required for the diagnosis. The ASBMR task force examined 310 case reports, of which 291 (94%) identified bisphosphonate use as a comorbidity, with a mean treatment duration of seven years. Despite this work, much uncertainty continues to surround the pathogenesis of these fractures, and their relative infrequency precludes widespread knowledge of the optimum management. The objective of this article is to review this evidence, whilst presenting the current available strategies for the management of bisphosphonate-related AFFs.
Table I.
Atypical fracture of the femur: major and minor features27
| Major features |
|---|
| - The fracture is associated with minimal or no trauma, as in a fall from a standing height or lower |
| - The fracture line originates at the lateral cortex and is substantially transverse in its orientation, although it may become oblique as it progresses medially across the femur |
| - Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex |
| - The fracture is noncomminuted or minimally comminuted |
| - Localised periosteal or endosteal thickening of the lateral cortex is present at the fracture site (“beaking” or “flaring”) |
| Minor features |
| - Generalised increase in cortical thickness of the femoral diaphysis |
| - Unilateral or bilateral prodromal symptoms such as dull or aching pain in the groin or thigh |
| - Bilateral incomplete or complete femoral diaphyseal fractures |
| - Delayed fracture healing |
| - Comorbid conditions (e.g. vitamin D deficiency, rheumatoid arthritis, hypophosphataemia) |
| - Use of pharmaceutical agents (e.g. bisphosphonates, glucocorticoids, proton pump inhibitors) |
Materials and Methods
We conducted a literature search of journal articles using the MEDLINE, Embase, Cochrane Library and Google Scholar databases in June 2016. No date restrictions were placed on the search. We used the key words ‘bisphosphonates atypical fracture’, ‘bisphosphonates subtrochanteric fracture’, ‘bisphosphonate mechanism’, ‘bisphosphonate bone healing’, ‘bisphosphonate microfracture’, ‘bisphosphonate collagen’, ‘bisphosphonate complications’, ‘subtrochanteric femoral fracture’ and ‘subtrochanteric fracture treatment’.
From the search results, articles with irrelevant titles were discounted, with the remaining abstracts examined for relevance. Reference listings of the remaining articles were also searched and scrutinised for relevance. The reference list generated was modified during the peer-review process in accordance with comments from the reviewers.
Mechanism of action of bisphosphonates
Bisphosphonates contain a backbone of two phosphonate groups covalently bonded to a central carbon atom, which in turn has two side chains (R1 and R2) that determine the chemical properties of the compound. They are carbon-substituted analogues of pyrophosphate, a ubiquitous molecule used in biochemical reactions throughout the body. Like pyrophosphate, bisphosphonates bind to hydroxyapatite and are thus absorbed by bone. Investigation of this binding mechanism led to the initial theory, proposed by Fleisch et al in 1969, that bisphosphonates achieve inhibition of bone resorption by reducing hydroxyapatite dissolution.28 However, subsequent structure-activity relation studies showed no close correlation between the levels of hydroxyapatite binding and inhibition of resorption, giving rise to the theory that bisphosphonates act directly on bone cells (Fig. 1).29 Proposed mechanisms of action include:
Fig. 1.
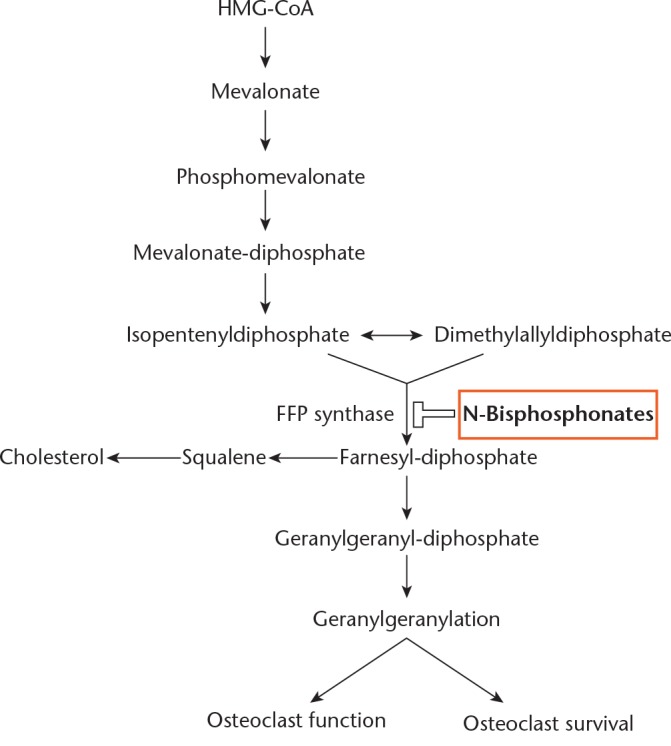
Schematic illustration of pathway target of nitrogen-containing bisphosphonates (N-bisphosphonates) in the inhibition of osteoclast function and survival; N-bisphosphonates target and inhibit farnesyl diphosphate (FPP) synthase (data used to create figure taken from Fleisch29)
- inhibition of osteoclast attachment to bone;32
- inhibition of osteoclast differentiation or recruitment;33-37
- interference with osteoclast structural features (cytoskeleton), necessary for bone resorption.38-40
Although all bisphosphonates concentrate in, and therefore selectively act on, bone, their mechanism of action may differ according to their side chains.29 Sato et al35 examined the association of alendronate (4-amino-1-hydroxybutylidene-1, 1-biphosphonic acid) with bone particles and its localisation in rat bone at one and six days after injection. They subsequently examined its effects on: osteoclast ultrastructure in situ; rat and chicken osteoclast resorption in vitro; and osteoclast intracellular calcium and cyclic adenosine monophosphate levels. Their findings suggested that alendronate binds to resorption surfaces and is locally released during subsequent osteoclastic acidification, preventing bone resorption and membrane ruffling without destroying the osteoclasts.
Masarachia et al41 noted the predilection of alendronate for osteoclasts over osteoblasts at a ratio of 8:1. In addition, they demonstrated the presence of alendronate deep within the mineralised matrix, where it lies dormant, activated only during osteoclastic acidification to prevent resorption.
Further structural refinement led to the creation of two further subclasses of bisphosphonates: nitrogen containing (NBP); and non-nitrogen containing (NNBP) bisphosphonates. This prompted further investigation into the mechanism of action of bisphosphonates at the molecular level. It was found that these two subclasses utilise two different pathways to produce their effects. The NNBP subclass (etidronate, tiludronate) affects bone resorption through its metabolites, which form toxic ATP analogues that induce osteoclast apoptosis. The NBP subclass (alendronate, ibandronate, pamidronate, risedronate, and zoledronate) inhibits the mevalonate pathway, a fundamental metabolic pathway in eukaryotes and bacteria that plays a key role in osteoclast formation and function, through their inhibition of farnesyl diphosphate synthase (a tyrosine phosphatase).42-51
Pathogenesis of bisphosphonate-related AFFs
Despite the beneficial effects of bone turnover suppression on bone strength, severe suppression of bone turnover by bisphosphonates is thought to play a major role in the development of AFFs. Current estimates place the age-adjusted relative risk of an AFF with any use of bisphosphonates at 47.3, with risk of fracture increasing over time from ten times the baseline after two years of therapy to 50 times the baseline thereafter. Current estimates of the number needed to harm are 2000 per year use, with 78% of AFFs associated with bisphosphonate use.19
The accumulation of bisphosphonates at sites of high osteogenic activity, such as areas of microfracture repair, can result in a local amplification of their effects, leading to potentially pathogenic changes in bone mineralisation, collagen cross-linking and the mineral and organic matrix.51 The following is a list of possible pathogenic mechanisms for AFFs, as suggested by Shane et al:17
“- Microdamage accumulation
- Changes to bone mineralisation, including increased mineralisation and reduced heterogeneity of mineralisation
- Alterations to the normal pattern of collagen cross-linking, either due to changes to the maturity of cross-links formed by enzymatic processes or advanced glycation end-product accumulation
- Variations in rates of bone turnover
- Reduced vascularity
- Anti-angiogenic effects.”
The effect of bisphosphonates on microdamage accumulation
In normal bone, microdamage caused by cyclic loading is a natural physiologic event that initiates bone remodelling. It accumulates in states of reduced bone turnover, as is found physiologically in advanced age.52 Bone treated with bisphosphonate has an exaggerated reduction in bone turnover, and has been shown to gather microdamage exponentially over time.53,54 It is notable that a relatively small reduction in bone turnover is required to trigger this exponential increase in microdamage, with one study of risedronate showing a 40% slowing in turnover causing a three-fold increase in the volume of microdamage.55,56
Brennan et al57 used a sheep model of osteoporosis to examine the effects of bisphosphonates on osteocyte apoptosis and microdamage accumulation. They reported an increased number of micro cracks with bisphosphonate therapy in skeletally mature ewes. Despite this, there was no associated increase in the risk of fracture. Indeed, a direct link between bisphosphonate-associated microdamage accumulation and atypical fractures is not yet established in humans. Furthermore, studies of iliac crest biopsies have provided conflicting data about whether the microdamage accumulates with bisphosphonate treatment in humans. One study evaluated women treated for an average of five years with alendronate, showing a significant increase in crack density in the treatment group when compared with a treatment-naïve group.58 However, these results were contradicted by a second study of a comparable population which showed no association between bisphosphonate treatment and microdamage accumulation in the iliac crest.59 Neither of these studies evaluated samples from the femoral cortex and, because the accumulation of microdamage is site-specific, the effect on microdamage in the femoral diaphysis is currently unknown.
The effect of bisphosphonates on bone mineralisation
The suppression of bone turnover alters bone mineral and matrix properties. Bisphosphonates prolong the life of existing bone remodelling units and reduce the formation of new ones. This prolongation allows for a greater percentage of bone remodelling units to become older and fully mineralised, leading to an increase in the homogeneity of mineralisation. Although increased mineralisation results in increased strength and stiffness of the bone, it also results in more brittle bone which is consequently more susceptible to fracture.60 Boskey et al61 used infrared spectroscopic imaging to investigate the material properties of iliac crest biopsies taken from seven alendronate-treated women and ten age-matched controls. The group showed that whilst alendronate treatment increases bone mass, it decreases tissue heterogeneity, which could impair tissue mechanical properties.
Healthy, heterogeneous bone contains areas of varying compliance and stiffness, which can halt the propagation of microfractures. In bisphosphonate-exposed bone, increased homogeneity in the organic matrix, combined with the increased homogeneity of mineralisation, may allow further propagation of micro cracks, leading to a higher fracture risk.
The effect of bisphosphonates on collagen
Collagen, which constitutes 90% of the organic matrix of bone, is cross-linked both enzymatically and non-enzymatically. The enzymatic process is mediated by lysyl and prolyl hydroxylases, resulting in the trivalent collagen cross-links. These cross-links yield a more stable collagen matrix and seem to be positively associated with stiffness and strength in bone.62,63 Saito et al64 used a canine model to show that the total number of enzymatic cross-links is unaffected by three years of bisphosphonate treatment. Non-enzymatic cross-linking, on the other hand, occurs when reducing sugars interact with free amino groups in collagen, resulting in advanced glycation end products (AGEs). Increased AGE concentration in bone has been shown to significantly increase the brittleness of bone.62 Bisphosphonates suppress bone turnover, increasing mean tissue age. As the concentration of AGEs within bone increases over time, it is thought that AGE concentration is increased within bisphosphonate-treated bone. Multiple canine studies have shown that one to three years of treatment with bisphosphonate results in increased AGE concentration in bone.63,64 There is currently no human data on the accumulation of AGEs in patients who are undergoing long-term bisphosphonate therapy, thus the extent to which they accumulate in patients remains unclear.
Epidemiology of AFFs
Many epidemiological studies have sought to clarify the relationship between bisphosphonate use and AFFS, with varying results. Such studies broadly fall into two categories: smaller studies utilising radiographs to categorise fractures; and larger studies using registry data complete with fracture classification (either subtrochanteric (ST) or femoral shaft (FS)).27 Most studies using registry data have found no change in the rates of subtrochanteric or fractures of the femoral shaft since the use of bisphosphonates for osteoporosis prevention and treatment was approved.65-71 However, these studies are limited firstly by the fact that not all ST and FS fractures are atypical, and secondly by the poor sensitivity and specificity of registry data.72,73 Conversely, the majority of studies utilising radiographic data have shown a strong association between bisphosphonates and AFFs.19,22-25,26,74-82 These studies remain limited by their size and ability to accurately ascertain drug exposure.82 The 2013 ASBMR task force reported that although a causal relationship was yet to be proven, the studies reporting a relationship between bisphosphonates and AFFs are consistent and robust.27
Risk factors for AFFS
There has been much recent focus on identifying risk factors for developing AFFs in patients receiving bisphosphonate therapy. A 2013 study by Franceschetti et al suggested that metabolic characteristics may play a key role in fracture development.83 The group identified an inadequate response of parathyroid hormone to hypocalcaemia as the primary risk factor in these patients, and suggest obesity, early menopause and younger age (< 70 years) as other potential risk factors. Mechanical factors have also been identified as potential risk factors for development of an AFF. Taormina et al84 analysed pre-fracture radiographs of 53 bisphosphonate users who went on to develop AFFs, and compared them with control radiographs and pre-fracture radiographs from patients who went on to sustain intertrochanteric fractures. The study revealed associations between neck-shaft angle and centre-edge angle with development of AFF. Although no current guidelines exist, the presence of these risk factors in patients receiving long-term bisphosphonate therapy should prompt consideration of surveillance for development of AFF.
Preventing bisphosphonate-related AFFs
There is considerable controversy over the optimum duration of bisphosphonate therapy. Concern surrounding AFFs, as well as osteonecrosis of the jaw, prompted the Food and Drug Administration (FDA) to re-evaluate the efficacy of continuing bisphosphonate therapy beyond three to five years.85,86 Their review focused on three long-term extension trials — the Fosamax Fracture Intervention Trial Long-Term (FLEX) extension; the Reclast Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly–Pivotal Fracture Trial (HORIZONPFT) extension; and the Actonel Vertebral Efficacy with Risedronate Therapy–Multinational Trial (VERT-MN) extension, in which the duration of treatment ranged from six to ten years.86 The FLEX trial randomly assigned 1099 postmenopausal women, who had previously received an average of five years of daily alendronate therapy, to continued alendronate treatment or placebo for an additional five years.87 The HORIZONPFT extension trial used a similar design with a shorter treatment period (three years of treatment followed by three years of placebo or active extension).88 Both studies used changes in bone mineral density as their primary end points and reported fractures as exploratory end points. Both showed significant reduction in the risk of vertebral fracture with continuation of bisphosphonate treatment.
Pooled data from the three extension trials specific to those patients who were treated with bisphosphonates for six years or more (2496 patients) showed vertebral and non-vertebral osteoporotic fracture rates of between 9.3% and 10.6%. By comparison, patients who were switched to the placebo for the extension period (starting at four years for alendronate and three years for risedronate and zolendronate) showed lower fracture rates of between 8.0% and 8.8%.85 This raises the question of whether continued bisphosphonate therapy beyond three to five years imparts additional fracture-prevention benefit. This question is further complicated by the observation that bone loss on cessation of bisphosphonate therapy is modest when alendronate and zolendronate are the agents of choice, whereas a much greater bone loss occurs upon the discontinuation of risedronate. No data are currently available post cessation of ibandronate therapy.89
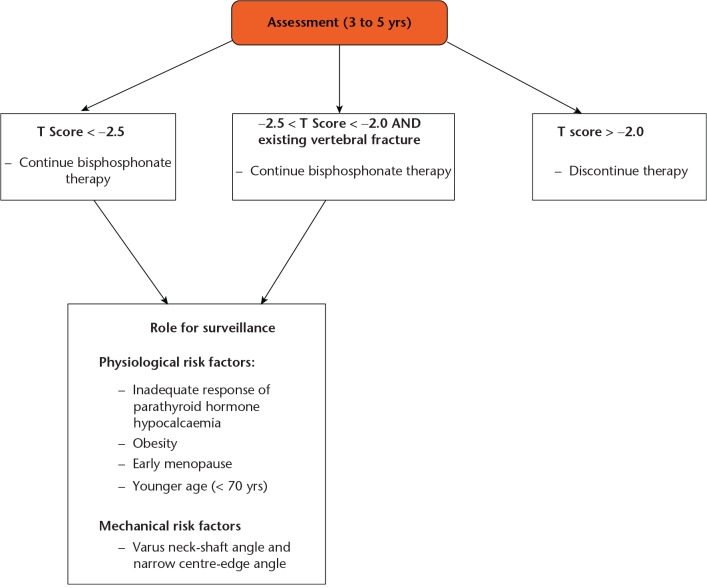
Overall, the evidence regarding this increased risk of fracture with continuation of bisphosphonate therapy beyond three to five years remains limited, with data from randomised controlled trials suggesting that an overall reduction in the risk of vertebral fractures persists. There does, however, remain a lack of consistent evidence that prolonged bisphosphonate therapy is associated with a significant reduction in non-vertebral fractures. This matter was addressed by Black et al in 2012,89 who made several recommendations. Firstly, they recommend that patients with low bone mineral density at the femoral neck (T score below −2.5) after three to five years of treatment are at the highest risk for vertebral fractures and therefore appear to benefit most from continuation of bisphosphonates. Secondly, patients with an existing vertebral fracture who have a somewhat higher (although not higher than −2.0) T score for bone mineral density may also benefit from continued therapy. Finally, patients with a femoral neck T score above −2.0 have a low risk of vertebral fracture and are unlikely to benefit from continued treatment. Unfortunately, a large proportion of patients are asymptomatic prior to developing a fracture, and the current literature has failed to establish clear surveillance guidelines for those on long-term bisphosphonate therapy. Interval femoral radiograph monitoring may be used to identify early stress reactions/fractures, though this relies on experienced radiologists.84 Further investigation into the benefits and risks of long-term therapy, as well as surveillance of fracture risk after discontinuation of bisphosphonates, is needed to optimise outcomes for patients (Fig. 2).
Fig. 2.
Flow chart showing possible pathway for prevention of bisphosphonate-related atypical fractures of the femur (data used to create figure taken from Shane et al27).
Surgical management of bisphosphonate-related AFFs
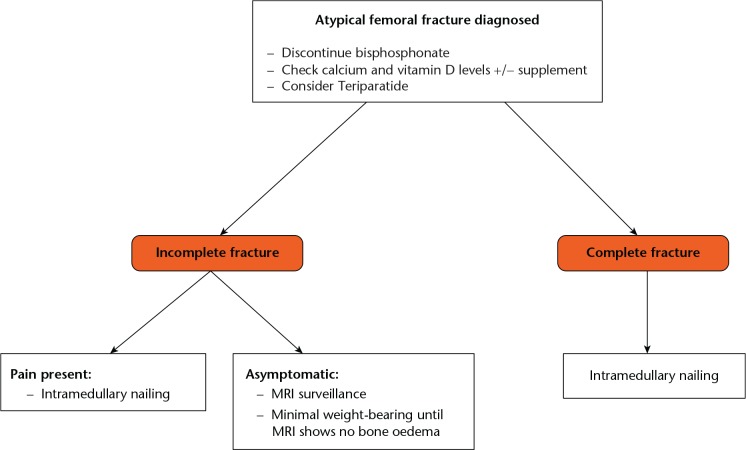
The ASBMR task force recognised that there are no controlled studies evaluating surgical treatment strategies for AFFs.27 Their recommendations, therefore, were opinion-based and represented the consensus of the orthopaedic surgeons who served on the task force. They developed a hierarchical approach to management depending on whether the fracture is complete or incomplete (Fig. 3).27,90
Fig. 3.
Flow chart showing treatment pathway for bisphosphonate-related atypical fractures of the femur (data used to create figure taken from Shane et al27).
History of thigh or groin pain in a patient on bisphosphonate therapy
It is essential to rule out an impending fracture of the femur in these patients with anteroposterior and lateral plain radiographs of the hip, including the full diaphysis of the femur. If the radiographs are normal but clinical suspicion remains high, a technetium bone scan or MRI of the femur should be performed. Bone marrow oedema is indicative of an active stress fracture, which should be managed conservatively with partial weight-bearing, cessation of bisphosphonate therapy, calcium and vitamin D supplementation and the commencement of teriparatide.27 Close follow-up with MRI is needed to monitor the resolution of bone marrow oedema and prevent progression to complete fracture.27,51
Complete subtrochanteric/diaphyseal fracture of the femur
Although no studies have examined different surgical options for atypical fractures, intramedullary nails are preferred over other fixation devices as they offer biological and biomechanical advantages. Biologically, fractures treated by intramedullary nail heal by endochondral ossification.91 Although bisphosphonates do not impair the initial phase of fracture healing or the development of a proliferative callus, they do inhibit osteoclastic remodelling. This results in a longer remodelling phase and delays the transformation of the calcified cartilage callus to mature bone. However, the alternative surgical strategy of using metal plates requires intramembranous bone healing, which is inhibited by bisphosphonates. From a biomechanical point of view, plates are inherently inferior to nails because of their more lateral position (longer lever arm on the proximal fixation) and their non–load sharing characteristics. Therefore overall, plate-screw constructs are not recommended for AFFs.16 A full-length intramedullary nail should be used, and the medullary canal should be over-reamed to facilitate insertion of the reconstruction nail and to prevent fracture of the remaining shaft. Irrespective of symptoms, the contralateral femur must be evaluated radiographically for the presence of a fracture.22
Outcomes for complete AFFs are generally poor, with some studies showing 53% requiring revision after the initial intramedullary nailing.92 Saleh et al52 proposed that pre-emptive autologous bone marrow grafting can be considered although, at present, no studies have examined the efficacy of this procedure in AFFs.
Incomplete subtrochanteric/femoral shaft fractures
The management of incomplete atypical fractures depends on several factors, including symptoms, plain radiographs, and MRI. For incomplete fractures accompanied by pain, prophylactic intramedullary nail fixation is recommended.19-22,88,89 Femoral radiographs with cortical thickening and stress reaction should be examined for the presence of a radiolucent fracture line across the lateral cortex, which is a poor prognostic indicator warranting prophylactic fixation with an intramedullary nail.80 For patients with incomplete fractures and no pain, limited weight bearing and avoidance of vigorous activity is advised until a surveillance MRI shows no bone oedema.
Medical management of bisphosphonate-related AFFs
Discontinuing bisphosphonates
A large observational study, from a health maintenance organisation in California, examined the occurrence of a contralateral AFF following an index atypical fracture in patients who either continued or discontinued the use of bisphosphonates.93 Dell et al75 showed the incidence of bilateral AFFs to be 41% in patients who continued bisphosphonates for three or more years after the index AFF, compared with 19% in patients who discontinued the drug. Similarly, Schilcher et al19 demonstrated that following atypical fracture, discontinuing bisphosphonate therapy achieved a 70% per-year reduction in the relative risk of developing contralateral atypical fracture. As a result, the International Osteoporosis Foundation Fracture Working Group has recently recommended that bisphosphonate therapy be ceased following an AFF.94
Calcium and vitamin D supplementation
Dietary calcium and vitamin D status should be assessed, and adequate supplementation prescribed if necessary, as this reduces the risk of all fractures by 12% to 26%.95-97 Recommendations for optimal treatment should include daily intakes of 1000 mg/day to 1200 mg/day of calcium and a minimum of 1000 IU to 2000 IU of vitamin D3, along with regular monitoring of serum 25-hydroxyvitamin D and parathyroid hormone (PTH) levels.98,99
Teriparatide (1–34 PTH)
Teriparatide, recombinant PTH 1–34 (1–34 PTH), should be considered in patients with AFFs.51 It improves bone turnover and microarchitecture in patients on long-term alendronate treatment and enhances fracture healing by increasing callus formation and mechanical strength.100-105 Two clinical trials also showed that teriparatide shortened the healing time in patients with osteoporotic fractures.106 Although no randomised studies exist to examine the effect of teriparatide in atypical fractures, the above studies provide a robust argument for its use in these patients.
In conclusion, although there is evidence of a relationship between long-term use of bisphosphonates and a specific type of subtrochanteric and fracture of the femoral shaft, this association does not prove causation. These atypical fractures are characterised by unique clinical features (prodromal pain and bilaterality) and unique radiographic features (transverse or short oblique orientation, absence of comminution, cortical thickening, stress fracture or stress reaction and delayed healing). It must be emphasised that bisphosphonates are vital drugs for the prevention of common osteoporotic fractures, and atypical fractures are rare. The decision to terminate bisphosphonate therapy after five years is still a controversial one, though patients with a low risk of fractures seem unlikely to benefit from treatment beyond this period.
Once an atypical fracture occurs, bisphosphonates must be stopped, and patients should receive daily calcium and vitamin D supplementation. Fixation should be performed using intramedullary nailing, with post-operative teriparatide commenced to augment healing. Large-scale data gathering should aid the identification of any subpopulations at particular risk of atypical fractures, and guide treatment and surveillance strategies. Physicians and patients should be made aware of the possibility of AFFs and of the potential for bilaterality. Future research should facilitate increased surveillance, help to establish the true incidence of, and risk factors for, these fractures, and finally include studies to address their surgical and medical management.
Footnotes
Author Contribution: N. Kharwadkar: Study Design, Literature search, Preparation of paper.
B. Mayne: Literature search, Preparation of paper.
J. E. Lawrence: Literature search, Preparation of paper.
V. Khanduja: Conception of study, Study design, Preparation and finalisation of paper.
ICMJE Conflict of Interest: None declared.
Funding Statement
V. Khanduja reports consultancy and grant fees received from several commercial entities, none of which is related to this article.
References
- 1. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 2009;181:265-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papaioannou A, Kennedy CC, Ioannidis G, et al. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int 2009;20:703-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010;152:380-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haleem S, Lutchman L, Mayahi R, Grice JE, Parker MJ. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury 2008;39:1157-1163. [DOI] [PubMed] [Google Scholar]
- 5. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-1541. [DOI] [PubMed] [Google Scholar]
- 6. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-1822. [DOI] [PubMed] [Google Scholar]
- 7. Chrischilles EA, Dasbach EJ, Rubenstein LM, et al. The effect of alendronate on fracture-related healthcare utilization and costs: the fracture intervention trial. Osteoporos Int 2001;12:654-660. [DOI] [PubMed] [Google Scholar]
- 8. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-2082. [DOI] [PubMed] [Google Scholar]
- 9. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 2002;112:281-289. [DOI] [PubMed] [Google Scholar]
- 10. Eastell R, Lang T, Boonen S, et al. Effect of once-yearly zoledronic acid on the spine and hip as measured by quantitative computed tomography: results of the HORIZON Pivotal Fracture Trial. Osteoporos Int 2010;21:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quandt SA, Thompson DE, Schneider DL, et al. Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of-1.6 to -2.5 at the femoral neck: the Fracture Intervention Trial. Mayo Clin Proc 2005;80:343-349. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz AV, Bauer DC, Cummings SR, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res 2010;25:976-982. [DOI] [PubMed] [Google Scholar]
- 13. Wells G, Cranney A, Peterson J, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008;1:CD004523. [DOI] [PubMed] [Google Scholar]
- 14. Homik J, Cranney A, Shea B, et al. Bisphosphonates for steroid induced osteoporosis. Cochrane Database Syst Rev 2000;2:CD001347. [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008;1:CD001155. [DOI] [PubMed] [Google Scholar]
- 16. Khosla S. Increasing options for the treatment of osteoporosis. N Engl J Med 2009;361:818-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010;25:2267-2294. [DOI] [PubMed] [Google Scholar]
- 18. Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 2005;90:1294-1301. [DOI] [PubMed] [Google Scholar]
- 19. Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011;364:1728-1737. [DOI] [PubMed] [Google Scholar]
- 20. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg [Br] 2007;89-B:349-353. [DOI] [PubMed] [Google Scholar]
- 21. Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury 2008;39:224-231. [DOI] [PubMed] [Google Scholar]
- 22. Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 2008;358:1304-1306. [DOI] [PubMed] [Google Scholar]
- 23. Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg [Am] 2009;91-A:2556-2561. [DOI] [PubMed] [Google Scholar]
- 24. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012;27:2544-2550. [DOI] [PubMed] [Google Scholar]
- 25. Murphy CG, O’Flanagan S, Keogh P, Kenny P. Subtrochanteric stress fractures in patients on oral bisphosphonate therapy: an emerging problem. Acta Orthop Belg 2011;77:632-637. [PubMed] [Google Scholar]
- 26. Meier RPH, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012;172:930-936. [DOI] [PubMed] [Google Scholar]
- 27. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014;29:1-23. [DOI] [PubMed] [Google Scholar]
- 28. Fleisch H, Russell RG, Francis MD. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 1969;165:1262-1264. [DOI] [PubMed] [Google Scholar]
- 29. Fleisch H. Bisphosphonates–history and experimental basis. Bone 1987;8(Suppl 1):S23-S28. [PubMed] [Google Scholar]
- 30. Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, Thesing CW, Bijvoet OL. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner 1986;1:27-39. [PubMed] [Google Scholar]
- 31. Flanagan AM, Chambers TJ. Dichloromethylenebisphosphonate (Cl2MBP) inhibits bone resorption through injury to osteoclasts that resorb Cl2MBP-coated bone. Bone Miner 1989;6:33-43. [DOI] [PubMed] [Google Scholar]
- 32. Carano A, Teitelbaum SL, Konsek JD, Schlesinger PH, Blair HC. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest 1990;85:456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Löwik CW, Van der Pluijm G, Van der Wee-Pals LJ, van Treslong-De Groot HB, Bijvoet OL. Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts. J Bone Miner Res 1988;3:185-192. [DOI] [PubMed] [Google Scholar]
- 34. Cecchini MG, Felix R, Fleisch H, Cooper PH. Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. J Bone Miner Res 1987;2:135-142. [DOI] [PubMed] [Google Scholar]
- 35. Hughes DE, MacDonald BR, Russell RGG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest 1989;83:1930-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papapoulos SE, Hoekman K, Löwik CWGM, Vermeij P, Bijvoet OL. Application of an in vitro model and a clinical protocol in the assessment of the potency of a new bisphosphonate. J Bone Miner Res 1989;4:775-781. [DOI] [PubMed] [Google Scholar]
- 37. Cecchini MG, Fleisch H. Bisphosphonates in vitro specifically inhibit, among the hematopoietic series, the development of the mouse mononuclear phagocyte lineage. J Bone Miner Res 1990;5:1019-1027. [DOI] [PubMed] [Google Scholar]
- 38. Miller SC, Jee WSS. The effect of dichloromethylene diphosphonate, a pyrophosphate analog, on bone and bone cell structure in the growing rat. Anat Rec 1979;193:439-462. [DOI] [PubMed] [Google Scholar]
- 39. Plasmans CMT, Jap PHK, Kuijpers W, Slooff TJ. Influence of a diphosphonate on the cellular aspect of young bone tissue. Calcif Tissue Int 1980;32:247-266. [DOI] [PubMed] [Google Scholar]
- 40. Sato M, Grasser W. Effects of bisphosphonates on isolated rat osteoclasts as examined by reflected light microscopy. J Bone Miner Res 1990;5:31-40. [DOI] [PubMed] [Google Scholar]
- 41. Masarachia P, Weinreb M, Balena R, Rodan GA. Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone 1996;19:281-290. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt A, Rutledge SJ, Endo N, et al. Protein-tyrosine phosphatase activity regulates osteoclast formation and function: inhibition by alendronate. Proc Natl Acad Sci U S A 1996;93:3068-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A 1999;96:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 2001;296:235-242. [PubMed] [Google Scholar]
- 45. Suri S, Mönkkönen J, Taskinen M, et al. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone 2001;29:336-343. [DOI] [PubMed] [Google Scholar]
- 46. Fisher JE, Rodan GA, Reszka AA. In vivo effects of bisphosphonates on the osteoclast mevalonate pathway. Endocrinology 2000;141:4793-4796. [DOI] [PubMed] [Google Scholar]
- 47. Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys 2000;373:231-241. [DOI] [PubMed] [Google Scholar]
- 48. Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep 2003;5:65-74. [DOI] [PubMed] [Google Scholar]
- 49. Reszka AA, Rodan GA. Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem 2004;4:711-719. [PubMed] [Google Scholar]
- 50. Frith JC, Mönkkönen J, Auriola S, Mönkkönen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 2001;44:2201-2210. [DOI] [PubMed] [Google Scholar]
- 51. Lehenkari PP, Kellinsalmi M, Näpänkangas JP, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol 2002;61:1255-1262. [DOI] [PubMed] [Google Scholar]
- 52. Saleh A, Hegde VV, Potty AG, Lane JM. Bisphosphonate therapy and atypical fractures. Orthop Clin North Am 2013;44:137-151. [DOI] [PubMed] [Google Scholar]
- 53. Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res 2007;22:1759-1765. [DOI] [PubMed] [Google Scholar]
- 54. Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 2006;39:872-879. [DOI] [PubMed] [Google Scholar]
- 55. Allen MR, Iwata K, Sato M, Burr DB. Raloxifene enhances vertebral mechanical properties independent of bone density. Bone 2006;39:1130-1135. [DOI] [PubMed] [Google Scholar]
- 56. Komatsubara S, Mori S, Mashiba T, et al. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res 2004;19:999-1005. [DOI] [PubMed] [Google Scholar]
- 57. Brennan O, Kennedy OD, Lee TC, Rackard SM, O’Brien FJ. Effects of estrogen deficiency and bisphosphonate therapy on osteocyte viability and microdamage accumulation in an ovine model of osteoporosis. J Orthop Res 2011;29:419-424. [DOI] [PubMed] [Google Scholar]
- 58. Stepan JJ, Burr DB, Pavo I, et al. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone 2007;41:378-385. [DOI] [PubMed] [Google Scholar]
- 59. Chapurlat RD, Arlot M, Burt-Pichat B, et al. Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 2007;22:1502-1509. [DOI] [PubMed] [Google Scholar]
- 60. Donnelly E, Meredith DS, Nguyen JT, et al. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res 2012;27:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int 2009;20:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vashishth D, Gibson GJ, Khoury JI, et al. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 2001;28:195-201. [DOI] [PubMed] [Google Scholar]
- 63. Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 2009;20:887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporos Int 2008;19:1343-1354. [DOI] [PubMed] [Google Scholar]
- 65. Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res 2009;24:1095-1102. [DOI] [PubMed] [Google Scholar]
- 66. Abrahamsen B, Eiken P, Eastell R. Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab 2010;95:5258-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abrahamsen B. Older women who use bisphosphonate for longer than 5 years may have increased odds of a subtrochanteric or femoral shaft fracture, but absolute risk is low. Evid Based Med 2011;16:168-169. [DOI] [PubMed] [Google Scholar]
- 68. Hsiao FY, Huang WF, Chen YM, et al. Hip and subtrochanteric or diaphyseal femoral fractures in alendronate users: a 10-year, nationwide retrospective cohort study in Taiwanese women. Clin Ther 2011;33:1659-1667. [DOI] [PubMed] [Google Scholar]
- 69. Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res 2011;26:993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nieves JW, Bilezikian JP, Lane JM, et al. Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int 2010;21:399-408. [DOI] [PubMed] [Google Scholar]
- 71. Vestergaard P, Schwartz F, Rejnmark L, Mosekilde L. Risk of femoral shaft and subtrochanteric fractures among users of bisphosphonates and raloxifene. Osteoporos Int 2011;22:993-1001. [DOI] [PubMed] [Google Scholar]
- 72. Spangler L, Ott SM, Scholes D. Utility of automated data in identifying femoral shaft and subtrochanteric (diaphyseal) fractures. Osteoporos Int 2011;22:2523-2527. [DOI] [PubMed] [Google Scholar]
- 73. Narongroeknawin P, Patkar NM, Shakoory B, et al. Validation of diagnostic codes for subtrochanteric, diaphyseal, and atypical femoral fractures using administrative claims data. J Clin Densitom 2012;15:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: A systematic review of case/case series studies. Bone 2010;47:169-180. [DOI] [PubMed] [Google Scholar]
- 75. Dell R, Greene D, Tran D. Stopping bisphosphonate treatment decreases the risk of having a second atypical femur fracture. [abstract] Read at the annual meeting of the American Academy of Orthopaedic Surgeons San Francisco;2012. [Google Scholar]
- 76. Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use. N Engl J Med 2010;362:1848-1849. [DOI] [PubMed] [Google Scholar]
- 77. Giusti A, Hamdy NA, Dekkers OM, et al. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011;48:966-971. [DOI] [PubMed] [Google Scholar]
- 78. Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int 2009;20:1353-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012;27:977-986. [DOI] [PubMed] [Google Scholar]
- 80. Lo JC, Huang SY, Lee GA, et al. Clinical correlates of atypical femoral fracture. Bone 2012;51:181-184. [DOI] [PubMed] [Google Scholar]
- 81. Thompson RN, Phillips JR, McCauley SH, Elliott JR, Moran CG. Atypical femoral fractures and bisphosphonate treatment: experience in two large United Kingdom teaching hospitals. J Bone Joint Surg [Br] 2012;94-B:385-390. [DOI] [PubMed] [Google Scholar]
- 82. Abrahamsen B. Atypical femur fractures: refining the clinical picture. J Bone Miner Res 2012;27:975-976. [DOI] [PubMed] [Google Scholar]
- 83. Franceschetti P, Bondanelli M, Caruso G, et al. Risk factors for development of atypical femoral fractures in patients on long-term oral bisphosphonate therapy. Bone 2013;56:426-431. [DOI] [PubMed] [Google Scholar]
- 84. Taormina DP, Marcano AI, Karia R, Egol KA, Tejwani NC. Symptomatic atypical femoral fractures are related to underlying hip geometry. Bone 2014;63:1-6. [DOI] [PubMed] [Google Scholar]
- 85. Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis–where do we go from here? N Engl J Med 2012;366:2048-2051. [DOI] [PubMed] [Google Scholar]
- 86.No authors listed. Food and Drug Administration. Background Document for Meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. September 9, 2011. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM270958.pdf (date last accessed 29 September 2016).
- 87. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006;296:2927-2938. [DOI] [PubMed] [Google Scholar]
- 88. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27:243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis–for whom and for how long? N Engl J Med 2012;366:2051-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Teo BJ, Koh JS, Goh SK, et al. Post-operative outcomes of atypical femoral subtrochanteric fracture in patients on bisphosphonate therapy. Bone Joint J 2014;96-B:658-64. [DOI] [PubMed] [Google Scholar]
- 91. Das De S, Setiobudi T, Shen L, Das De S. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg [Br] 2010;92-B:679-686. [DOI] [PubMed] [Google Scholar]
- 92. Cao Y, Mori S, Mashiba T, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 2002;17:2237-2246. [DOI] [PubMed] [Google Scholar]
- 93. Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma 2010;24:75-81. [DOI] [PubMed] [Google Scholar]
- 94. Silverman SL, Kupperman ES, Bukata SV, Members of IOF Fracture Working Group. Fracture healing: a consensus report from the International Osteoporosis Foundation Fracture Working Group. Osteoporos Int 2016;27:2197-2206. [DOI] [PubMed] [Google Scholar]
- 95. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257-2264. [DOI] [PubMed] [Google Scholar]
- 96. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007;370:657-666. [DOI] [PubMed] [Google Scholar]
- 97. Holick MF. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging 2007;24:1017-1029. [DOI] [PubMed] [Google Scholar]
- 98. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003;77:204-210. [DOI] [PubMed] [Google Scholar]
- 99. Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004;19:745-751. [DOI] [PubMed] [Google Scholar]
- 100. Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab 2011;96:1627-1632. [DOI] [PubMed] [Google Scholar]
- 101. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 1999;14:960-968. [DOI] [PubMed] [Google Scholar]
- 102. Andreassen TT, Fledelius C, Ejersted C, Oxlund H. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand 2001;72:304-307. [DOI] [PubMed] [Google Scholar]
- 103. Skripitz R, Andreassen TT, Aspenberg P. Parathyroid hormone (1-34) increases the density of rat cancellous bone in a bone chamber. A dose-response study. J Bone Joint Surg [Br] 2000;82-B:138-141. [DOI] [PubMed] [Google Scholar]
- 104. Zanchetta JR, Bogado CE, Ferretti JL, et al. Effects of teriparatide (recombinant human parathyroid hormone (1-34)) on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 2003;18:539-543. [DOI] [PubMed] [Google Scholar]
- 105. Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010;25:404-414. [DOI] [PubMed] [Google Scholar]
- 106. Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg [Am] 2011;93-A:1583-1587. [DOI] [PubMed] [Google Scholar]