Abstract
Typical centrioles are made of microtubules organized in ninefold symmetry. Most animal somatic cells have two centrioles for normal cell division and function. These centrioles originate from the zygote, but because the oocyte does not provide any centrioles, it is surprising that the zygotes of many animals are thought to inherit only one centriole from the sperm. Recently, in the sperm of Drosophila melanogaster, we discovered a second centriolar structure, the proximal centriole-like structure (PCL), which functions in the zygote. Whether the sperm of other insects has a second centriolar structure is unknown. Here, we characterized spermiogenesis in the red flour beetle, Tribolium castaneum. Electron microscopy suggests that Tribolium has one microtubule-based centriole at the tip of the axoneme and a structure similar to the PCL, which lacks microtubules and lies in a cytoplasmic invagination of the nucleus. Immunostaining against the orthologue of the centriole/PCL protein, Ana1, also recognizes two centrioles near the nucleus during spermiogenesis: one that is microtubule-based at the tip of the axoneme, suggesting it is the centriole; and another that is more proximal and appears during early spermiogenesis, suggesting it is the PCL. Together, these findings suggest that Tribolium sperm has one microtubule-based centriole and one microtubule-lacking centriole.
Keywords: centriole, Tribolium, sperm, Ana1, atypical centrioles, PCL
1. Introduction
The centriole is a cellular organelle that is essential for fertilization and embryo development (reviewed in [1]). Most somatic cells have a pair of centrioles, each consisting of microtubules arranged in ninefold radial symmetry. The older centriole is known as the mother centriole, and it forms the axoneme (a microtubule-based subcellular skeletal structure) of the cilium (aka flagellum) during interphase. The younger centriole, known as the daughter centriole, matures into a mother centriole in preparation for cell division. Before mitosis, the centrioles duplicate (each centriole serves as a platform for the formation of a single new centriole nearby) to have four centrioles, and two are inherited by each daughter cell [2–4]. Since two centrioles are required for normal cell division, a fundamental question in reproductive biology is from where the first two centrioles in a zygote arise [5].
In most sexually reproducing animals, the female gamete does not provide any functional centrioles to the zygote; rather, the male gamete (spermatozoon) provides the centrioles [5,6]. Because the zygote requires two centrioles, the sperm of many animals, like fish, amphibians and nematodes, have two centrioles. These two centrioles are the daughter centriole that is called the proximal centriole, which is found near the nucleus, and the mother centriole that is called the distal centriole, which is found at the base of the sperm tail's axoneme [7–9]. These two sperm centrioles function after fertilization in the zygote and serve as a platform for the formation of new zygotic centrioles. These first two pairs of centrioles are inherited by daughter cells and duplicated to generate the centrioles of the animal's somatic cells. This pattern of centriole inheritance ensures that all somatic cells inherit exactly two centrioles, as abnormalities in number can cause devastating developmental defects and cancer [10]. Since precise centriole number is critical, it is surprising that the insect and mammalian spermatozoa appear to have only one centriole, and the origin of the second centriole is unknown (reviewed in [11]).
In insects, only the centriole that forms the axoneme is present (the homologue of the mammalian distal centriole) [12]. This centriole has been found with a variable number of microtubules, but most commonly have either nine triplet or doublet microtubules [13], as has been shown in Drosophila melanogaster (triplets) [14] and Mastotermes darwiniensis (doublets) [15]. Alternative microtubule arrangements have been observed in species like Sciara (60–90 singlet microtubules) [16] and Caenorhabditis elegans (nine singlet microtubules) [8]. Since all these centrioles have microtubules, the structural definition of a centriole is a cylindrical subcellular structure made of microtubules usually in ninefold or radial symmetry [17–19].
Recently, an atypical centriole was discovered in Drosophila sperm: the proximal centriole-like structure (PCL), which forms during early spermiogenesis (haploid sperm development). The initiation of PCL formation requires the same set of proteins as are required to initiate centriole formation (i.e. Asl, Plk4, Sas-6), and the PCL is composed of centriole-specific proteins (e.g. Sas-4, Ana1 and Bld10) [20]. The PCL is formed at the proximal end of the centriole, later shifts into the centriolar adjunct (a specialized form of PCM found in insect sperm [21]) and finally resides at the base of the nucleus, after the centriolar adjunct is eliminated from the spermatozoon in a process known as centrosome reduction [20,22]. The PCL lacks microtubules and has a novel structure, consisting of a 100 nm wide electron dense wall with a 20 nm wide central tubule and translucent interstitial material between them [22,23]. After fertilization in the zygote, the inherited PCL forms a centrosome that emanates a microtubule aster. The PCL is essential to form a new daughter centriole, and to form one of the spindle poles during cell division [22,24]. Aspects of the PCL's formation mechanism, composition, and function overlap with those of a centriole, although the PCL structure is distinct from that of a centriole. These findings raise the hypothesis that the PCL is a new type of centriole: an atypical centriole, which is structurally defined as a microtubule-lacking cylindrical subcellular structure, made of centriole-specific proteins. Therefore, Drosophila sperm has two centriolar structures, one typical and one atypical, which resolves the origin of the second centriole in this species.
In order to test if the PCL is a Drosophila ‘anomaly’ or represents a new type of centriole found in insects, we examined the centrioles of the red flour beetle (Tribolium) during spermatogenesis (sperm development). Tribolium castaneum (red flour beetle) is a representative species of the largest, most diverse eukaryotic order (Coleoptera), including over 300 000 species [25]. Tribolium and Drosophila shared a common insect ancestor about 300 million years ago, and if the PCL was present in this ancestor, Tribolium would be expected to have a PCL. Therefore, studying Tribolium will provide insight into the diversity and generality of centriolar structures in insects.
Our findings suggest that Tribolium sperm has two centriolar structures: the first is an axoneme-attached centriole with nine doublet microtubules that was previously described in many insect studies, and the second is a new type of centriole that lacks microtubules and has a unique structure that is similar, but not identical, to the Drosophila PCL. Together these results suggest that insects have a spectrum of centriolar structures ranging from the typical centriole with radial triplet, doublet or singlet microtubules to the structurally distinct atypical centriole without microtubules. It also suggests that, while the structure of sperm centrioles can be diverse, the number is conserved.
2. Results and discussion
2.1. Tribolium spermiogenesis
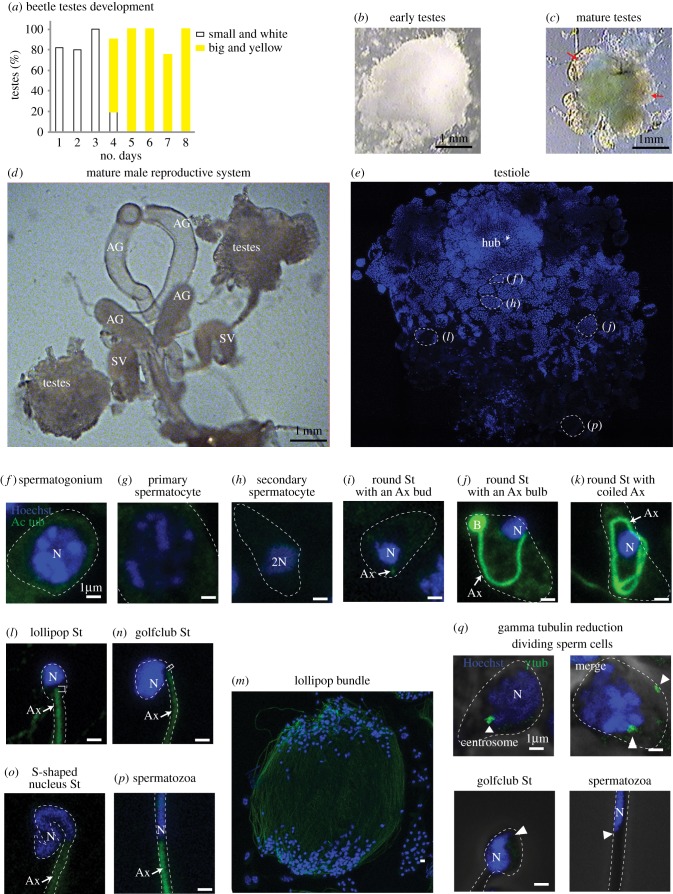
Tribolium's spermatogenesis was previously described briefly, but no immunofluorescent systematic study of spermiogenesis has been published [26,27]. Therefore, to study the sperm centrioles, we first characterized Tribolium's male reproductive organ (testis) and later spermiogenesis using immunofluorescence. Tribolium testes size grows dramatically after eclosion from pupae. Initially, on day 2, it is small, delicate and encased in white fat (figure 1a,b). On day 4, the testes become yellow and large, and have several (at most 6) lobes (testioles) that are arranged around the vas deferens, which carries the spermatozoa to the seminal vesicle [28] (figure 1c,d). In each testiole, spermatogenesis is organized along an axis that begins with stem cells at the ‘hub’ and ends with spermatozoa at the vas deferens (figure 1e).
Figure 1.
Tribolium spermiogenesis. (a–c) As the beetles eclose from the pupa, their testes change (graph in a). They start as small white testes, surrounded by a big white cloud of fat (b). Around day 4, they change to large and yellow with no more than six clear lobes (testioles marked with red arrows) and very little fat (c). Though the early testes (b) appears equal in size to day 4 testes (c), in reality, the early testes fat fills the volume difference between the smaller testes and the larger ones on day 4. (d) The complete male reproductive system. (e) A single testiole stained with Hoechst (blue) to recognize the nuclei. Specific areas are presented in panels (f)–(p) as labelled. The Hoechst staining becomes lighter in later-stage sperm. (f–p) Sperm cells at distinct stages are stained with Hoechst (blue) and anti-acetylated-tubulin (green). (q) Anti-γ-tubulin (green) staining is observed in dividing-stage sperm but is undetectable in late-stage sperm (golfclub spermatid and spermatozoa) at the expected site of the centriole (arrowhead). Abbreviations: AG, accessory gland; SV, seminal vesicle; Ax, axoneme; N, nucleus; B, bulb; St, spermatid. Scale bars, 1 µm.
Next, we characterized Tribolium spermiogenesis. The early sperm cells (spermatogonia) are small, round and have round nuclei with uniform Hoechst DNA staining. During cell division, the condensed DNA is stained more intensely (figure 1f). The post-mitotic spermatocytes are big, round and have big nuclei that contain patchy Hoechst staining (figure 1g). They are organized in a cyst, but meiosis occurs asynchronously [27]. The secondary spermatocytes, which are found between meiosis I and II, are smaller than primary spermatocytes and have more condensed DNA (figure 1h). Early round spermatids that are formed after meiosis have an axoneme bud labelled by acetylated tubulin (figure 1i). A small gap between the acetylated tubulin-labelled axoneme and the nucleus is maintained throughout spermatogenesis. Later, the axoneme elongates and curves within the elliptical spermatid, while the tip of the axoneme forms a bulb of intensely stained acetylated tubulin (figure 1j). Next, the axoneme elongates further and coils around the nucleus (figure 1k). After elongation, the spermatids, with round nuclei and long axonemes that appear as lollipops (‘lollipop spermatids’), are organized antiparallel to each other [26] (figure 1l,m). Then, spermatids transition to ellipsis-shaped nuclei with a severely kinked neck region (‘golfclub spermatids’) (figure 1n), and as the spermatids mature, the nuclei narrow and twist into an S-shape (‘S-spermatid’) (figure 1o). Lastly, the nuclei straighten in late spermatids, and spermatozoa have a needle shaped nuclei (figure 1p).
The early sperm centrosome is labelled by the universal centrosomal marker γ-tubulin, and the marker is later eliminated during spermiogenesis in a process known as centrosome reduction [5]. Anti γ-tubulin immunostaining shows two dots in dividing sperm cells, but not in differentiating spermatids or spermatozoa (figure 1q), suggesting that, like in other animal sperm, the Tribolium sperm centrosome is reduced during spermiogenesis. Note that in Drosophila, proteins are eliminated during centrosome reduction, but the sperm centriole and PCL are maintained with some structural changes in spermatozoa [22].
2.2. The Tribolium axoneme-attached centriole has doublet microtubules
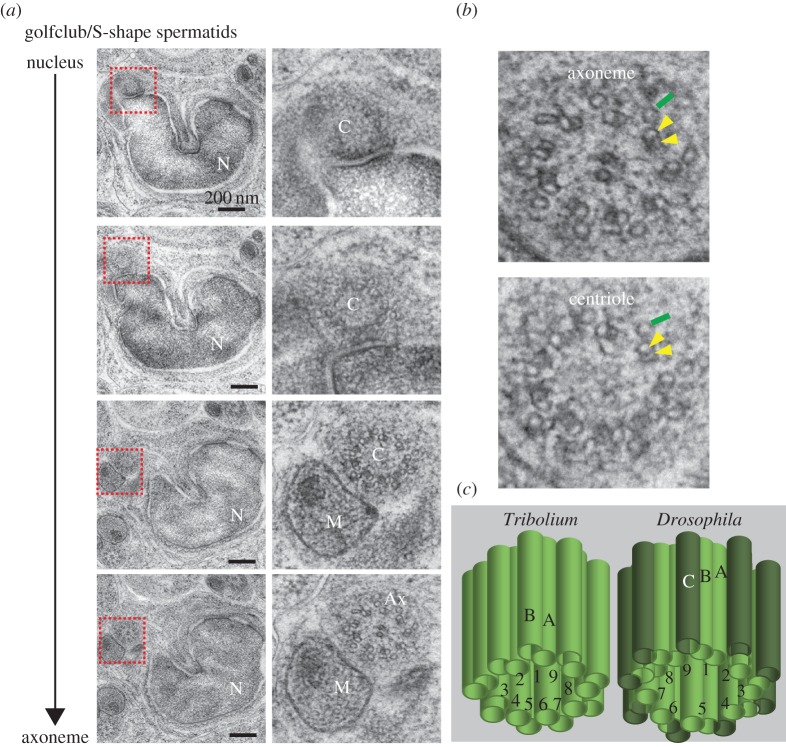
The centrioles of insect sperm have structural diversity, but with the exception of the PCL, they all have microtubules [12]. To investigate the number, structure and microtubules of centrioles in Tribolium sperm, we performed high-pressure freezing–freeze substitution (HPF-FS) and transmission electron microscopy (TEM) serial section analysis at the junction between the nucleus and flagellum. This technique was integral in determining the PCL structure in Drosophila, as traditional chemical fixation tends to disrupt its delicate ultrastructure [22,23].
To study the centriolar structures in Tribolium sperm, we selected the intermediate spermatid stage (golfclub/S-shape) because Drosophila's PCL is observed most robustly in this stage. We found that in the sperm flagella, the axoneme in the spermatid is composed of nine accessory tubules, with nine doublets (microtubules A and B) and two central microtubules, as previously described [27]. Traditionally, this organization is referred to as a 9 + 9 + 2 axoneme and is found in many insect groups including other beetles [27,29,30] (figure 2). The base of the axoneme is made of nine accessory tubules, with nine doublets and no central microtubules (9 + 9 + 0), suggesting this is the centriole. This doublet microtubule-based centriole arises from the previously described pair of centrioles with triplet microtubules in spermatocytes [27]. This suggests that the axoneme-attached centriole is modified throughout spermatogenesis as part of centrosome reduction, which has been described in other animals [22,31]. Since centrioles can have nine doublets [32,33], we hypothesize that the axoneme-attached structure is the centriole in Tribolium.
Figure 2.
The centriole of Tribolium spermatids has doublet microtubules. (a) High-pressure freezing (HPF), freeze substitution (FS) and serial section TEM analysis of Tribolium golfclub/S-shape spermatid showing the transition from a centriole lacking central microtubules to an axoneme with central microtubules. Abbreviations: Ax, axoneme; C, centriole; M, mitochondria; N, nucleus. (b) Magnification of axoneme and centriole cross-sections. Yellow arrowheads: the microtubules in the doublet microtubules; green line: accessory microtubule. (c) Model of the axoneme-attached centriole. The centriole is made of doublet microtubules labelled A and B in ninefold symmetry, which is different than Drosophila's sperm's axoneme-attached centriole's triplets, labelled A, B and C.
2.3. Tribolium sperm has a proximal centriole-like structure
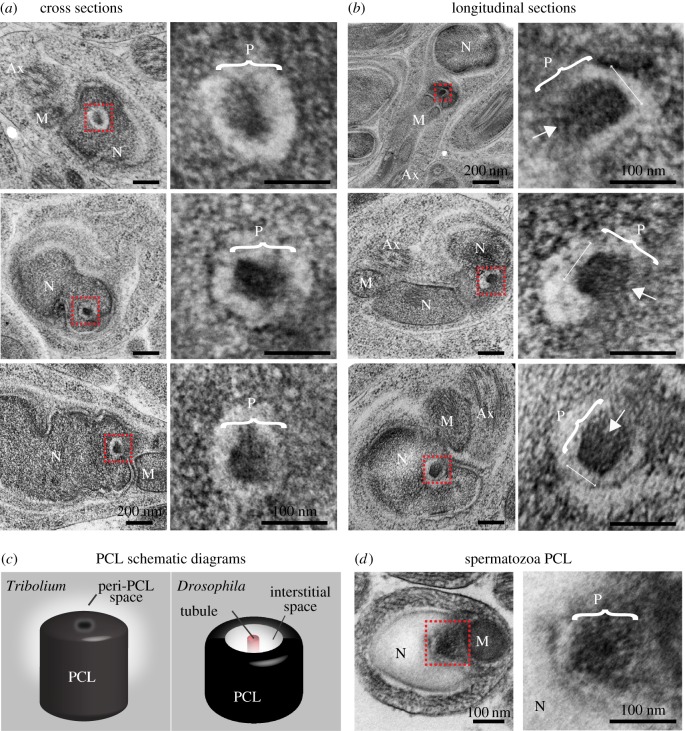
To investigate the presence of a second centriole in the sperm, we analysed the nucleus and axoneme junction. We found a distinct structure between the axoneme-attached centriole and the nucleus (figure 3a,b). This structure is located in a cytoplasmic invagination of the nucleus and has no microtubules. It is composed of an electron-dense structure with a diameter of 84 ± 6 nm (n = 13), and is surrounded by translucent material. The overall shape and the dimensions of this structure are similar to the Drosophila PCL (figure 3c) [22]. In cross-section, the structure does not possess the central tubule found in the Drosophila PCL (figure 3a), but a barely detectable electron-dense central core can be observed in longitudinal and semi-longitudinal sections (figure 3b). Also, the position differs from that of the Drosophila PCL, which is found adjacent to the axoneme-attached centriole. However, the electron-dense structure's position in Tribolium is similar to the position of the human proximal centriole: between the base of the nucleus and the axoneme-attached distal centriole [34,35]. This structure is observed in spermatozoa, suggesting it is maintained throughout spermiogenesis (figure 3d). Therefore, we hypothesize that this second structure is the homologue of the Drosophila PCL. This Tribolium PCL lacks microtubules and therefore would traditionally not be defined as a centriole; however, we propose that, like the Drosophila PCL, the Tribolium PCL is an atypical centriole.
Figure 3.
The 2nd centriole of Tribolium spermatids lack microtubules. (a,b) Representative HPF-FS TEM semi cross-section (a) and semi-longitudinal (b) analysis of Tribolium golfclub spermatids. Images on the right are insets of images on the left, signified by the red box. Brackets indicate the axis representing the PCL diameter. Arrows indicate the location of the barely detectable electron-dense central core. (c) A model of the second centriolar structure in spermatids. The structure of the PCL (P) is slightly different than Drosophila's PCL. (d) Representative TEM sections of the second centriolar structure in Tribolium spermatozoa. Abbreviations: Ax, axoneme; M, mitochondria; N, nucleus. Scale bars, 1 µm.
2.4. Tribolium Ana1 labels two spermatid centrioles
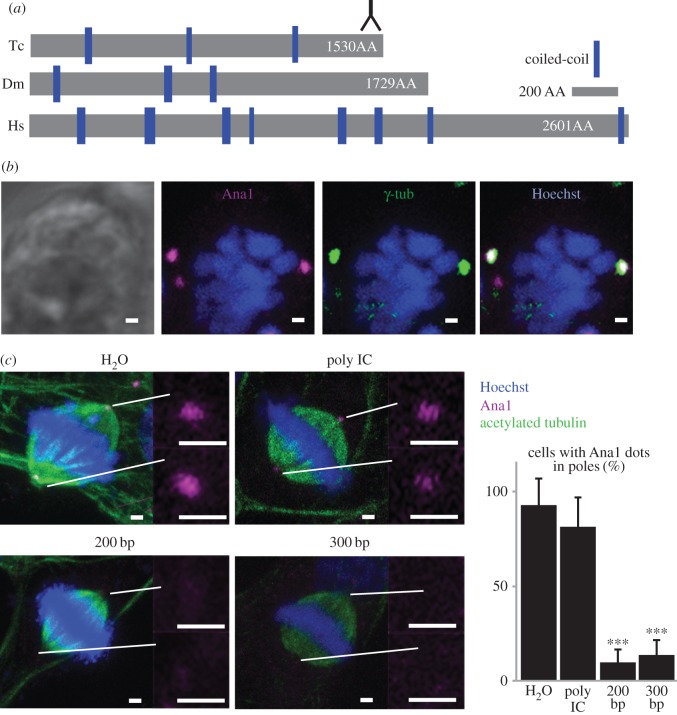
To test the hypothesis that the Tribolium PCL is the second centriolar structure, we tested if it contains the PCL marker and conserved centriolar protein Ana1/CEP295 [20,36,37]. Reciprocal BLASTp between Drosophila and Tribolium identified a large (172 KD) protein that is 28% identical and has three predicted short coiled-coil domains (figure 4a) (hypothetical protein TcasGA2_TC014427) [38]. We generated an antibody against the C-terminus of Tribolium Ana1 (amino acids 1506–1530). As expected from a centriolar marker, this antibody recognizes two dots (which often appear as one large, fused dot) that colocalize with γ-tubulin in the spindle poles of dividing Tribolium sperm cells and BCIRL-TcA-CLG1 cells (figure 4b). RNAi treatment of the BCIRL-TcA-CLG1 cells significantly reduced the Ana1 staining (figure 4c), indicating that the antibody is specific to Ana1.
Figure 4.
Tribolium Ana1. (a) Schematic comparison of Drosophila (Dm) Ana1, Tribolium (Tc) Ana1 and human CEP295 (Hs). Inversed ‘Y’ symbol indicates the region recognized by the antibody. (b) Colocalization of γ-tubulin and Ana1 in dividing TcA cells. (c) Ana1 labelling is reduced in TcA cells when treated with two different lengths of RNAi fragments against Ana1 (200 and 300 bp), but is not diminished significantly when treated with water (H2O) or a non-specific double-stranded RNAi (Poly IC). ***p < 0.001 by t-test. n = 3. Scale bars, 1 µm.
2.5. Anti-Ana1 recognizes two centriolar structures in spermatids
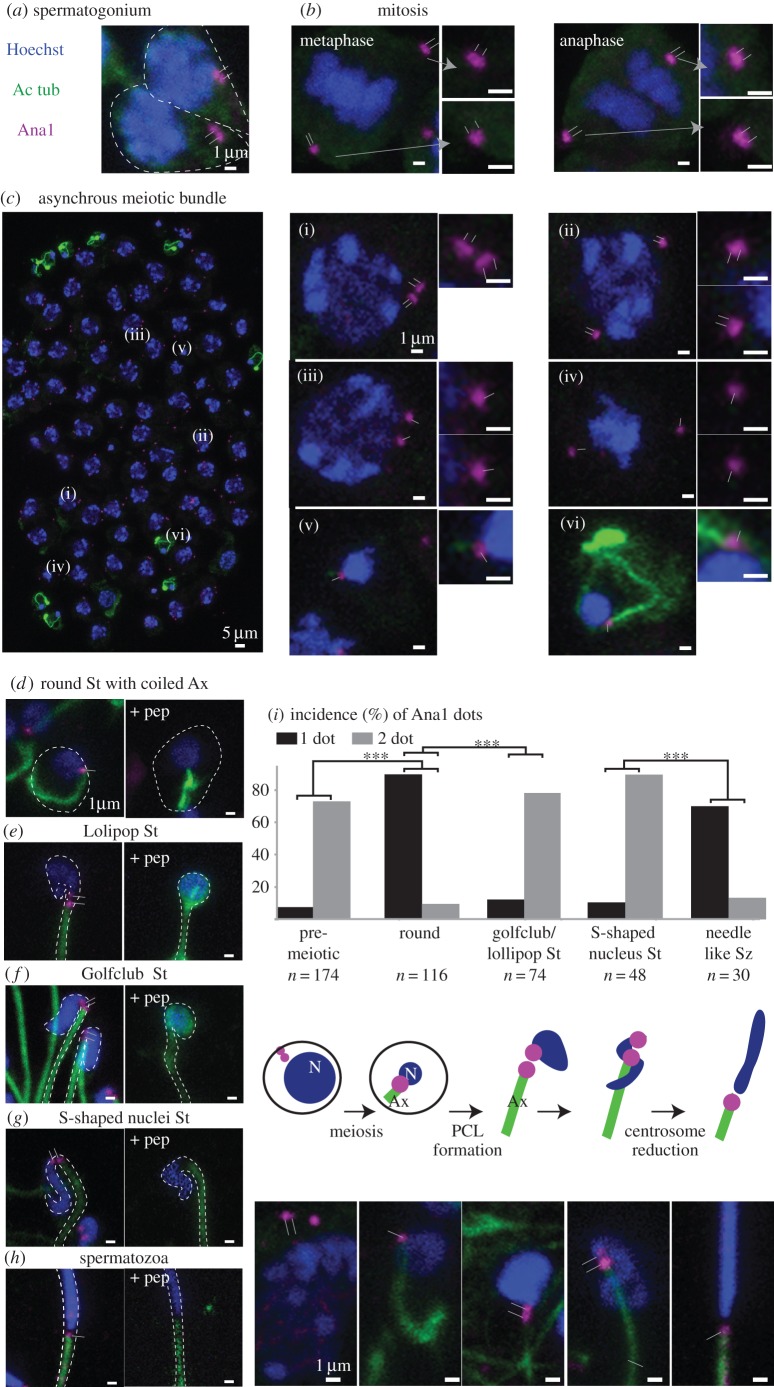
To characterize the centrioles during spermatogenesis, we stained testes with the anti-Ana1 antibody. Interphase spermatogonia have two cytoplasmic Ana1-labelled centrioles that are adjacent to one another (figure 5a). Mitotic spermatogonia have two centrioles that localize to each spindle pole (figure 5b). Primary spermatocytes have two pairs of cytoplasmic Ana1-labelled centrioles; the centrioles are often very close together so that they appear as one elongated dot (figure 5c(i)). During meiosis I, each pair of centrioles migrates to opposite poles (figure 5c(ii)). At the end of meiosis I, the secondary spermatocyte has two centrioles (figure 5c(iii)). Dividing secondary spermatocytes (figure 5c(iv)) produce two round spermatids, with one Ana1-labelled centriole in each cell. This Ana1-labelled centriole is located between the nucleus and the axoneme, and the axoneme is initially shorter and later is elongated (figure 5c(v,vi)). This location suggests that the Ana1-labelled axoneme-attached centriole found in round spermatids is homologous to the Drosophila centriole and the human distal centriole. Interestingly, in Tribolium, the Ana1-labelled axoneme-attached centriole is observed in spermatozoa; this is different from Drosophila, where Ana1 cannot be detected from both centrioles due to centrosome reduction [39].
Figure 5.
A 2nd centriole forms during Tribolium spermatogenesis. (a–h) Anti-Ana1 labelling throughout Tribolium spermatogenesis. (c) As expected, an asynchronous meiotic bundle has spermatocytes, each with two centrosomes with a total of four centrioles (i–iii). Each secondary spermatocyte has one centriole at opposite poles (iv), and early spermatid stages have one centriole (v–vi). (d–h) Anti-Ana1 dots are found at the base of the nucleus throughout spermiogenesis and this staining is abolished in the presence of a competitive peptide. (i) Quantification of Ana1 dots in different stages of sperm: one dot (black); two dots (grey). A schematic diagram showing the emergence of the second centriolar structure. p < 0.001 by chi-square test. Abbreviations: Ax, axoneme; St, spermatid; Sz, spermatozoa; N, nucleus. Thin lines indicate position of centriole and PCL. Scale bars, 1 µm.
During the transition from a round spermatid to a lollipop spermatid, a second Ana1-labelled dot appears between the axoneme-attached centriole and the nucleus (figure 5d,e). Both this proximal dot and the axoneme-attached centriole persist in golfclub spermatids and S-shaped spermatids (figure 5f,g). At the S-shaped spermatid stage, the proximal Ana1 dot is in the expected position for the PCL (near the centriole), but its precise position is distinct. The emergence of the Ana1-labelled PCL post-meiosis is consistent with the timing of the emergence of Drosophila PCL. After the S-shaped spermatid stage (figure 5h), the Ana1 dot is not detected at the Tribolium PCL, which is consistent with the reduction of Drosophila PCL. Finally, the Ana1 staining is abolished in the presence of a competitive peptide (figure 5e–h). Quantification of these data shows significant changes between one and two dots, which correlates with the known timing of meiosis, centrosome reduction and the PCL's emergence in Drosophila (figure 5i). Together, this immunostaining study indicates presence of a second centriolar structure during Tribolium spermiogenesis, which is found between the axoneme-attached centriole and the nucleus.
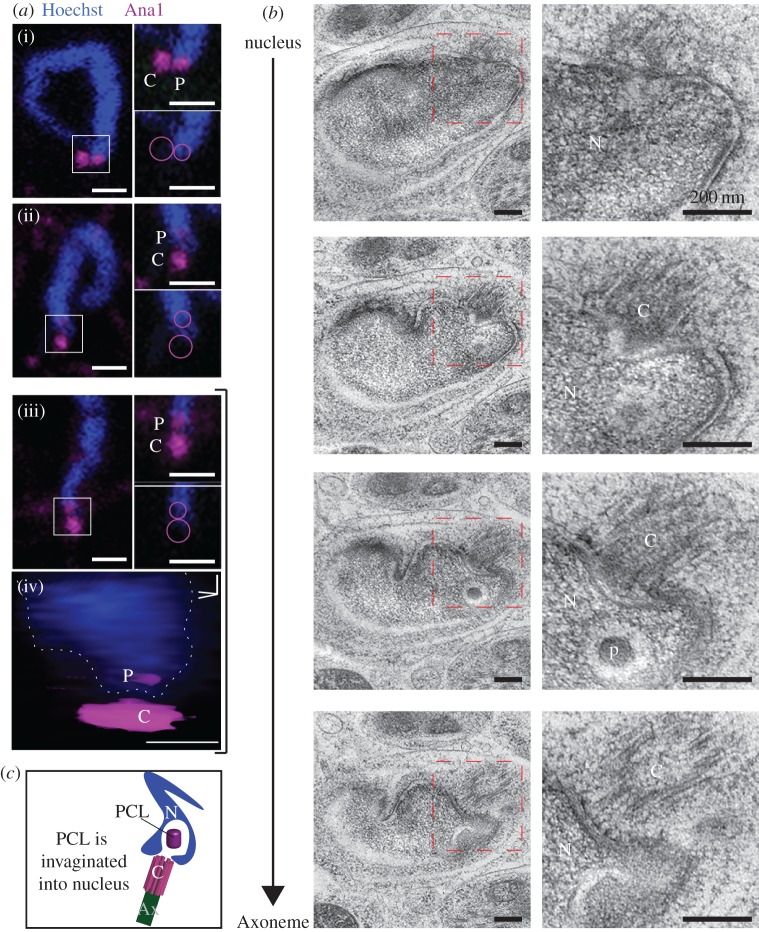
Finally, to investigate if the electron-dense structure we observed using EM is the same as the proximal Ana1 dot, we examined the proximal Ana1 dot and the nucleus base using single frames (figure 6a(i–iii)) and three-dimensional reconstitution (figure 6a(iv)), and found that the proximal Ana1 dot is located inside the nucleus boundary. Since serial-section electron microscopy finds that the PCL is the only distinct cytosolic structure invaginated to the nucleus base (figure 6b,c), these data strongly suggest that the proximal Ana1 dot is the Tribolium PCL.
Figure 6.
The 2nd Ana-1 labelled centriole is the PCL. (a) Single 0.3 µm thick confocal sections showing that the proximal Ana1 dot is located inside the nucleus boundary in golfclub/S-shaped spermatid stage (i–iii). Circles mark the location of the centriole and PCL in the inset. Three-dimensional volume model of the PCL in an S-shaped spermatid iii (iv). Scale bars, 1 µm. (b) Serial section TEM analysis showing that only the PCL is found in the cytosolic invagination within the nucleus at the golfclub/S-shape spermatid stage. Scale bars, 200 nm. (c) A schematic diagram showing the location of the PCL in a cytosolic invagination within the nucleus. Abbreviations: Ax, axoneme; C, centriole; N, nucleus; P, PCL.
3. Concluding remarks
Here, we examined the centrioles of Tribolium during spermatogenesis and found that Tribolium sperm has two centrioles: (i) as described previously, a centriole made of doublet microtubules instead of triplet microtubules, and (ii) a previously undescribed atypical centriole, the Tribolium PCL, that is made of a 100 nm electron-dense core surrounded by electron-translucent material. Like Drosophila's PCL, the Tribolium PCL localizes near the centriole that forms the axoneme, but its relative position and ultrastructure are distinct. This suggests that insect sperm centrioles can have a range of structures.
There are several potential explanations why the PCL was not detected in the many electron microscopy studies of insect and beetle sperm:
(1) The PCL is small; it is about the size of a single electron microscopy ultrathin section (approx. 70 nm), making it easy to miss.
(2) The PCL is found in different locations relative to the centriole in different species preventing location-based identification.
(3) The PCL has a different structure depending on the species, making it hard to identify based on structural criteria.
(4) The PCL is masked by the electron-dense centriolar adjunct that surrounds it. Electron microscopy studies in a variety of beetles have reported the presence of centriolar adjunct that could be masking the PCL [40–42]. Observing the internal structure of the PCL, which distinguishes it from the centriolar adjunct, requires non-standard electron microscopy techniques, such as high pressure freezing with freeze substitution. Alternatively, immunostaining can be used to distinguish the PCL, which is made of centriolar proteins, from the centriolar adjunct, which is made of PCM proteins.
Centrioles can be defined in many ways based on their structure, protein composition and function [11,17]. Traditionally, centrioles are defined based on their structure and composition; centrioles are defined as cylindrical subcellular structures containing microtubules [17–19]. Our studies suggest that the sperm's atypical centrioles are small (approx. 100 nm) electron-dense subcellular structures composed of centriole-specific proteins, which lacks microtubules.
Recognizing centriolar structures solely based on microtubules may be causing a systemic bias against structurally atypical centrioles. Perhaps atypical sperm centrioles are more common than is currently acknowledged. The diversity of centriole structures may be of particular importance when considering mammalian sperm. While human and primate spermatids begin with two centrioles, spermatozoa is thought to possess only one intact centriole because the other centriole degenerates [43]. However, since the structure of atypical centrioles is small, delicate and variable, maybe mammalian sperm possess a currently undetected centriole. Therefore, our findings argue for directed research on whether other animal sperm (i.e. that of insects and mammals) has an atypical centriole in addition to their typical centriole.
In light of broader knowledge on sperm and embryo centrioles, our findings argue for a conserved model for centriole inheritance during sexual reproduction. In this model, the spermatozoon inherits exactly two centrioles with diverse structures. Altogether, these findings expand the definition of centriolar structures to include those that do not have microtubules, but have centriole-specific proteins.
4. Methods
4.1. Beetle cultivation and dissection
Vermillion White and Georgia-1 red flour beetles were grown in a 30°C incubator in mason jars with filter paper lids. Beetles were given whole-wheat flour, supplemented with 5% brewers yeast, to eat. Testes were dissected from males by lightly squeezing using forceps at the thorax until their genitalia were exposed. A second pair of forceps was used to hold and pull the genitalia while maintaining firm pressure on the beetle's thorax. Upon removal, testes were selected, isolated from the remainder of the reproductive system and placed in 1X PBS.
4.2. Antibodies
Antibodies were generated in rabbits against the C-terminus of Tribolium castaneum Ana1 (Cys-RAKDVEQRFFELQDHSGKKE) and affinity purified by Pacific Immunology. This antibody was used at 1 : 800 for immunofluorescence and did not work well for westerns. GTU88 anti-γ-tubulin and acetylated tubulin (Sigma T6557 and T7451 respectively) were used at 1 : 100.
4.3. Immunofluorescence
Slides with beetle testes were prepared using a modified version of Basiri et al. [44]. Dissected beetle testes were placed in 20 µl of freshly prepared 3.7% formaldehyde (Sigma, 252549) for five minutes on a charged slide (Azer Scientific, EMS200A+). Formaldehyde was then wicked away and replaced with 10 µl of 1X PBS. The testes were then teased apart, and a sigmacote (Sigma, SL2) coverslip was pressed onto the sample. Then, the slide was placed in liquid nitrogen for at least 10 min. The slide was removed quickly from nitrogen, then the coverslip was removed using forceps, and, lastly, the slide was placed in a pre-chilled coplin jar of ice-cold methanol for 2 min. Next, the slide was placed in 1X PBS for 1 min, then placed for 10 min in fresh 1X PBS with 3% Triton X-1000 at room temperature. PBST-B was prepared by adding 1% BSA to PBST, and slides were then placed in PBST-B for 20 min. Antibodies diluted in PBST-B with 1% RNase A were added to the slides and they were covered in parafilm. Next, the slides were placed in a humidity chamber for at least 1 h incubation at room temperature, or overnight incubation at 4°C. Slides were washed three times in PBST for 5 min each. The secondary antibody mixture was prepared by diluting the antibody in PBST with 1% RNase A. Secondary antibodies used were: 1 : 800 Donkey anti-Mouse conjugated to Alexa Fluor 488 (Jackson ImmunoResearch 715-545-150), 1 : 600 Donkey anti-Rabbit conjugated to a Cy5 fluor (Jackson ImmunoResearch 711-165-152), or 1 : 800 Goat anti-Rabbit conjugated to Alexa Fluor 650 (Fisher 84546 respectively), and 2 µg ml−1 Hoechst 33258. The secondary antibody mixture was added to slides, and they were covered in parafilm. Next, they were incubated for at least 1 h at room temperature. Lastly, slides were then washed three times with PBST for 5 min each, followed by three times with 1× PBS for 5 min each. Immunostained slides were sealed and imaged using a Leica confocal microscope Sp8.
Sperm images were taken using magnification of 640X and zoom of 6× with 512 × 512 pixel density. Figures 1, 4 and 5 show a projection using Leica LAS X software. Figure 6 shows a single Z section and three-dimensional rendering using Leica LAS X software. Using Photoshop, images were cropped to 200 pixels by 100 pixels, or 100 pixels by 100 pixels (10 × 10 or 25 × 25 for insets), the intensity was modified to allow easy visualization, and the panels were resized to 300 dpi for publication.
To perform peptide inhibition, a mixture of primary antibody and 10 µg ml−1 of peptide was incubated at 4°C for 1 h prior to staining.
4.4. High-pressure freezing–freeze substitution and transmission electron microscopy
For TEM analysis of Tribolium, the testes and seminal vesicles of Tribolium were dissected and immediately processed using the high-pressure freezer system (Leica EM HPM100) in 20% BSA. The frozen samples were dehydrated and en bloc stained using a freeze substitution preprocessor (Leica EM AFS2) in 96% acetone with 1.5% OsO4 (osmium crystals were dissolved in acetone and 4% water). The freeze substitution started with −90°C for 6 h, then warmed up from −90°C to −10°C over 15 h (5.3° slope). Then, the samples were warmed to −3°C over 1 h (7° slope) while being washed with 96% acetone (at −7°C). Then, they were warmed from −3°C to 4°C over 1 h (7° slope) while being washed twice with 100% acetone. Lastly, the dehydrated samples were infiltrated and embedded in EMBed 812 resin while warming up to room temperature. The ultrathin sectioning (70 nm) was performed using an ultramicrotome (Leica EM UC6), and sections were post-stained with 6% uranyl acetate (in 1 : 1 70% ethanol and 100% methanol), and Reynolds lead citrate (3–4%) (in pre-boiled ddH2O). Then, the sections were imaged using TEM (JEOL 1400-plus) operating at 80 kV.
4.5. Cell culture
TcA cells were acquired from Dr Cindy Goodman at the USDA [45]. They were grown in a 29°C incubator without CO2 and with Ex-Cell 420 media (Sigma, 14420), supplemented with 10% FBS and 1% streptomycin.
4.6. RNAi
Tribolium genomic DNA was purified using chloroform/phenol extractions. Then, we amplified two fragments of Ana1 (200 bp and 300 bp) using Ana1 primers (see below) with a T7 promoter sequence (underlined) added on in both forward and reverse directions. Next, we generated dsRNAi using the T7 flash transcription kit from Epicentre. We added the RNAi to the cell culture media at a concentration of 100 ng ml−1. RNAi was incubated for 96 h, and then cells were fixed in ice-cold methanol for 5 min and stained for immunofluorescence.
Ana 1 primers:
| T7-F200 T7-F300 T7-R200 T7-R300 |
TAATACGACTCACTATAGGGAAACCACAACGGTCAAGACGAAAC TAATACGACTCACTATAGGGACACTTCCGCGAAAAAATGACGATC TAATACGACTCACTATAGGGTTTCTTGGAGCATTTGCACGAATC TAATACGACTCACTATAGGGCCTCTTCAGGGATCACATCGCTGG |
4.7. Statistical methods
Experiments were repeated at least three times (n > 3), and statistical analyses (average ± standard deviation) were done with excel. A two-tailed, unpaired Student's t-test was used to determine p-value (p). Chi-squared tests were done to compare two categorical variables (one focus versus two foci) to acquire a p-value. p-value designations are: *p < 0.05, **p < 0.01, ***p < 0.001.
Acknowledgements
We would like to thank Dotty Sorenson, Sasha Meshinchi, Jeff Harrison and Chris Edwards for assistance at the Microscopy & Image Analysis Laboratory (MIL) in the University of Michigan Medical School; the EPIC facility of Northwestern University's NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1121262) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN, and Charlene Wilke at the Biological Imaging Facility in the Northwestern University. We would like to thank Yoshi Tomoyasu, Courtney Clark-Hatchel and Ralph Rübsam for advice, Andrew Zelhof and Sue Brown for beetles, and Cindy Goodman and the USDA ARS for the TcA cell line.
Authors' contributions
T.A.-R. supervised the project. E.L.F. led the light microscopy part of the project. K.J. preformed EM. A.H. performed westerns. R.R. helped with figure 5. A.Z. assisted with cell culture. M.K. assisted with the production of RNAi.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grant no. 1121176 (MCB) from the National Science Foundation and R01GM098394 from the National Institute of General Medical Sciences.
References
- 1.Bornens M. 2012. The centrosome in cells and organisms. Science 335, 422–426. (doi:10.1126/science.1209037) [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. 2007. Revisiting the role of the mother centriole in centriole biogenesis. Science 316, 1046–1050. (doi:10.1126/science.1142950) [DOI] [PubMed] [Google Scholar]
- 3.Avidor-Reiss T, Gopalakrishnan J, Blachon S, Polyanovsky A. 2012. Centriole duplication and inheritance in Drosophila melanogaster. In The centrosome: cell and molecular mechanisms of functions and dysfunctions in disease (ed. Schatten H.), pp. 3–31. New York, NY: Humana Press. [Google Scholar]
- 4.Fu J, Hagan IM, Glover DM. 2015. The centrosome and its duplication cycle. Cold Spring Harb. Perspect. Biol. 7, a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schatten G. 1994. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 165, 299–335. (doi:10.1006/dbio.1994.1256) [DOI] [PubMed] [Google Scholar]
- 6.Borrego-Pinto J, Somogyi K, Karreman MA, Konig J, Muller-Reichert T, Bettencourt-Dias M, Gonczy P, Schwab Y, Lenart P. 2016. Distinct mechanisms eliminate mother and daughter centrioles in meiosis of starfish oocytes. J. Cell Biol. 212, 815–827. (doi:10.1083/jcb.201510083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schatten H, Schatten G, Mazia D, Balczon R, Simerly C. 1986. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc. Natl Acad. Sci. USA 83, 105–109. (doi:10.1073/pnas.83.1.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, Li Y, White JG. 2001. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 105, 547–558. (doi:10.1016/S0092-8674(01)00338-5) [DOI] [PubMed] [Google Scholar]
- 9.Yabe T, Ge X, Pelegri F. 2007. The zebrafish maternal-effect gene cellular atoll encodes the centriolar component sas-6 and defects in its paternal function promote whole genome duplication. Dev. Biol. 312, 44–60. (doi:10.1016/j.ydbio.2007.08.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, Godinho SA. 2011. Centrosomes and cilia in human disease. Trends Genet. 27, 307–315. (doi:10.1016/j.tig.2011.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avidor-Reiss T, Khire A, Fishman EL, Jo KH. 2015. Atypical centrioles during sexual reproduction. Front. Cell Dev. Biol. 3, 21 (doi:10.3389/fcell.2015.00021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riparbelli MG, Dallai R, Callaini G. 2010. The insect centriole: a land of discovery. Tissue Cell 42, 69–80. (doi:10.1016/j.tice.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 13.Dallai R, Gottardo M, Beutel RG. 2016. Structure and evolution of insect sperm: new interpretations in the age of phylogenomics. Annu. Rev. Entomol. 61, 1–23. (doi:10.1146/annurev-ento-010715-023555) [DOI] [PubMed] [Google Scholar]
- 14.Tates AD. 1971. Cytodifferentiation during spermatogenesis in Drosophila melanogaster: an electron microscope study. Leiden, Netherlands: Rijksuniversiteit de Leiden. [Google Scholar]
- 15.Baccetti B, Dallai R. 1978. The spermatozoon of arthropoda. XXX. The multiflagellate spermatozoon in the termite Mastotermes darwiniensis. J. Cell Biol. 76, 569–576. (doi:10.1083/jcb.76.3.569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips DM. 1967. Giant centriole formation in Sciara. J. Cell Biol. 33, 73–92. (doi:10.1083/jcb.33.1.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheatley DN. 1982. The centriole: a central enigma of cell biology, pp. 21–49. Amsterdam, The Netherlands: Elsevier Biomedical Press; Sole. [Google Scholar]
- 18.Chapman MJ, Dolan MF, Margulis L. 2000. Centrioles and kinetosomes: form, function, and evolution. Q. Rev. Biol. 75, 409–429. (doi:10.1086/393621) [DOI] [PubMed] [Google Scholar]
- 19.Winey M, O'Toole E. 2014. Centriole structure. Phil. Trans. R. Soc. B 369, 20130457 (doi:10.1098/rstb.2013.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blachon S, Cai X, Roberts KA, Yang K, Polyanovsky A, Church A, Avidor-Reiss T. 2009. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 182, 133–144. (doi:10.1534/genetics.109.101709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallai R, Paoli F, Mercati D, Lupetti P. 2016. The centriole adjunct of insects: need to update the definition. Tissue Cell 48, 104–113. (doi:10.1016/j.tice.2016.02.001) [DOI] [PubMed] [Google Scholar]
- 22.Khire A, et al. 2016. Centriole remodeling during spermiogenesis in Drosophila. Curr. Biol. 26, 3183–3189. (doi:10.1016/j.cub.2016.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottardo M, Callaini G, Riparbelli MG. 2015. Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton (Hoboken) 72, 576–584. (doi:10.1002/cm.21260) [DOI] [PubMed] [Google Scholar]
- 24.Blachon S, Khire A, Avidor-Reiss T. 2014. The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics 197, 199–205. (doi:10.1534/genetics.113.160523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maderspacher F. 2008. Genomics: an inordinate fondness for beetles. Curr. Biol. 18, R466–R468. (doi:10.1016/j.cub.2008.04.068) [DOI] [PubMed] [Google Scholar]
- 26.Dias G, Yotoko KS, Gomes LF, Lino-Neto J. 2012. Uncommon formation of two antiparallel sperm bundles per cyst in tenebrionid beetles (Coleoptera). Naturwissenschaften 99, 773–777. (doi:10.1007/s00114-012-0949-6) [DOI] [PubMed] [Google Scholar]
- 27.Dias G, Lino-Neto J, Mercati D, Dallai R. 2015. The sperm ultrastructure and spermiogenesis of Tribolium castaneum (Coleoptera: Tenebrionidae) with evidence of cyst degeneration. Micron 73, 21–27. (doi:10.1016/j.micron.2015.03.003) [DOI] [PubMed] [Google Scholar]
- 28.Sokoloff A. 1974. The biology of Tribolium, with special emphasis on genetic aspects. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Dallai R. 2014. Overview on spermatogenesis and sperm structure of Hexapoda. Arthropod Struct. Dev. 43, 257–290. (doi:10.1016/j.asd.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 30.Dallai R, Afzelius BA. 1993. Axonemal structure and insect phylogeny. Bolletino di zoologia 60, 423–429. (doi:10.1080/11250009309355851) [Google Scholar]
- 31.Manandhar G, Simerly C, Schatten G. 2000. Centrosome reduction during mammalian spermiogenesis. Curr. Top. Dev. Biol. 49, 343–363. (doi:10.1016/S0070-2153(99)49017-9) [DOI] [PubMed] [Google Scholar]
- 32.O'Toole ET, McDonald KL, Mantler J, McIntosh JR, Hyman AA, Muller-Reichert T. 2003. Morphologically distinct microtubule ends in the mitotic centrosome of Caenorhabditis elegans. J. Cell Biol. 163, 451–456. (doi:10.1083/jcb.200304035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottardo M, Callaini G, Riparbelli MG. 2015. The Drosophila centriole—conversion of doublets into triplets within the stem cell niche. J. Cell Sci. 128, 2437–2442. (doi:10.1242/jcs.172627) [DOI] [PubMed] [Google Scholar]
- 34.Sathananthan AH, Ratnam SS, Ng SC, Tarin JJ, Gianaroli L, Trounson A. 1996. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Hum. Reprod. 11, 345–356. (doi:10.1093/HUMREP/11.2.345) [DOI] [PubMed] [Google Scholar]
- 35.Miller SW, Avidor-Reiss T, Polyanovsky A, Posakony JW. 2009. Complex interplay of three transcription factors in controlling the tormogen differentiation program of Drosophila mechanoreceptors. Dev. Biol. 329, 386–399. (doi:10.1016/j.ydbio.2009.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knorz VJ, Spalluto C, Lessard M, Purvis TL, Adigun FF, Collin GB, Hanley NA, Wilson DI, Hearn T. 2010. Centriolar association of ALMS1 and likely centrosomal functions of the ALMS motif-containing proteins C10orf90 and KIAA1731. Mol. Biol. Cell 21, 3617–3629. (doi:10.1091/mbc.E10-03-0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izquierdo D, Wang WJ, Uryu K, Tsou MF. 2014. Stabilization of cartwheel-less centrioles for duplication requires CEP295-mediated centriole-to-centrosome conversion. Cell Rep. 8, 957–965. (doi:10.1016/j.celrep.2014.07.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya Y, Yoshiba S, Gupta A, Watanabe K, Kitagawa D. 2016. Cep295 is a conserved scaffold protein required for generation of a bona fide mother centriole. Nat. Commun. 7, 12567 (doi:10.1038/ncomms12567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khire A, Vizuet AA, Davila E, Avidor-Reiss T. 2015. Asterless reduction during spermiogenesis is regulated by Plk4 and is essential for zygote development in Drosophila. Curr. Biol. 25, 2956–2963. (doi:10.1016/j.cub.2015.09.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackie JB, Walker MH. 1974. A study of the conjugate sperm of the dytiscid water beetles Dytiscus marginalis and Colymbetes fuscus. Cell Tissue Res. 148, 505–519. (doi:10.1007/BF00221935) [DOI] [PubMed] [Google Scholar]
- 41.Gassner G, Childress D, Klemetson DJ. 1975. Spermiogenesis in boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 4, 115–125. (doi:10.1016/0020-7322(75)90010-0) [Google Scholar]
- 42.Werner M, Zissler D, Peschke K. 1999. Structure and energy pathways of spermatozoa of the rove beetle Aleochara bilineata (Coleoptera, Staphylinidae). Tissue Cell 31, 413–420. (doi:10.1054/tice.1999.0052) [DOI] [PubMed] [Google Scholar]
- 43.Manandhar G, Simerly C, Schatten G. 2000. Highly degenerated distal centrioles in rhesus and human spermatozoa. Hum. Reprod. 15, 256–263. (doi:10.1093/humrep/15.2.256) [DOI] [PubMed] [Google Scholar]
- 44.Basiri ML, Blachon S, Chim YC, Avidor-Reiss T. 2013. Imaging centrosomes in fly testes. J. Visualized Experiments e50938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman CL, Stanley D, Ringbauer JA Jr, Beeman RW, Silver K, Park Y. 2012. A cell line derived from the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). In Vitro Cell. Dev. Biol. Anim. 48, 426–433. (doi:10.1007/s11626-012-9524-x) [DOI] [PubMed] [Google Scholar]