Abstract
Background
There is limited information on the presentation and characteristics of psychotic illness experienced by people with autism spectrum disorder (ASD).
Aims
To describe autistic and psychotic phenomenology in a group of individuals with comorbid ASD and psychosis (ASD–P) and compare this group with populations affected by either, alone.
Method
We studied 116 individuals with ASD–P. We compared features of their ASD with people with ASD and no comorbid psychosis (ASD–NP), and clinical characteristics of psychosis in ASD–P with people with psychosis only.
Results
Individuals with ASD–P had more diagnoses of atypical psychosis and fewer of schizophrenia compared with individuals with psychosis only. People with ASD–P had fewer stereotyped interests/behaviours compared with those with ASD–NP.
Conclusions
Our data show there may be a specific subtype of ASD linked to comorbid psychosis. The results support findings that psychosis in people with ASD is often atypical, particularly regarding affective disturbance.
The relationship between psychotic illnesses (particularly schizophrenia) and autism spectrum disorder (ASD) is complex, with suggestions that there is substantial overlap between the two conditions.1 However, they differ considerably in age at onset, with the former usually first becoming apparent in adolescence and early adulthood and the latter in early childhood. People with ASD may experience comorbid psychotic illnesses such as schizophrenia and bipolar disorder (we have termed this comorbidity ‘ASD–P’), and evidence is accumulating that individuals with ASD are at greater risk of developing psychotic illnesses than those in the general population. One study indicated rates as high as 28%.2 Selten et al concluded from a recent population-based study of people with an ASD that their odds of having a comorbid psychosis were between 5.6 and 5.8, depending on the type of psychosis.3 Another recent study has also reported epidemiological evidence for a connection between developmental disorders manifest in childhood and psychotic experiences in adolescence.4 Descriptions of the phenomenology of psychotic illness in ASD come from case studies and small case series (for example Clarke et al5), but these have been unsystematic, and it remains uncertain how psychotic illness presents in the ASD population. One possibility is that there may be a subtype of ASD that carries a higher risk of psychotic illness driven by common genetic variants (probably copy number variants rather than single nucleotide polymorphisms) (see King & Lord1 for a summary). If this were the case, people with ASD who develop psychosis might differ significantly in the presentation of their ASD from those without psychosis, and the psychotic illness would have similar characteristics across people with ASD. A second possibility is that features of ASD are misdiagnosed as psychotic symptoms, with difficulties in reading others' intentions resembling paranoia, difficulties in expressive communication resembling thought disorder, and ‘melt downs’ resembling catatonia.6 Third, these psychopathological alternatives might simply be epiphenomena of different constructs and language from separate literatures applied to the same mental phenomena. It could be that people with ASD who develop psychosis have experiences that can fit into both understandings, and so could simultaneously be considered part of their ASD and part of their psychosis. To better understand the relationship between ASD and psychotic illness, we report below on a cross-sectional study of people with ASD–P. For genetic reasons or because of diagnostic confusion, we hypothesise that in individuals with ASD–P both conditions would take a recognisably distinct form, different from the manifestations of either ASD or psychotic illness as it manifests in singly affected populations.
Method
Written informed consent was obtained from all participants prior to study procedures taking place. Individuals aged 16 or older were considered eligible to give consent for themselves. Where participants were found to lack capacity to consent to participate in research, advice from an informant who knew them well, such as a family member, was obtained in accordance with UK law. Recruitment and data collection for the ASD–P group took place between January 2010 and June 2013. The Cambridgeshire 3 Research Ethics Committee, UK, approved the project.
Participants and measures
The total number of participants with ASD and comorbid psychosis (the ASD–P group) was 116 (men n = 89, women n = 27). They were initially identified and referred to the study by clinicians in services across the UK, through charities and by self-referral.
The sample size in this study represents the maximum number of eligible individuals recruited within a constrained time period and was not determined by formal sample-size calculation. Recruitment was undertaken by a number of different agencies, and it was not possible to know the number of individuals invited to take part but who did not respond. None of those who consented to participate withdrew.
Eligibility criteria for ASD–P group
Participants were required to: (a) have a clinical diagnosis of ASD at the time of referral to the study, or (b) meet criteria on the Autism Diagnostic Observation Schedule (ADOS)7 at the time of involvement in the study, or (c) meet criteria on the Autism Diagnostic Interview-Revised (ADI-R)8 for a lifetime diagnosis. A maximum of one point below threshold on any one of three ADI-R diagnostic scales was accepted as indicative of ASD for individuals with no clinical diagnosis of ASD, as in all cases of referral ASD was suspected by the person's clinical team. Details of the participants' age at administration of the ADI-R was obtained; in all but one individual the ADI-R was conducted on enrolment in the study.
History of a comorbid psychotic illness was determined in two stages. Participants were initially included if they had a prior clinical diagnosis of psychotic illness or gave an account of an episode that was clearly psychotic. Their participation was subsequently confirmed if psychotic symptoms were elicited by F.V.L. using the Diagnostic Interview for Psychosis (DIP)9 or the Psychiatric Assessment Schedule for Adults with Developmental Disabilities (Mini PAS-ADD).10 Both instruments can be used to generate diagnoses using the Operational Criteria Checklist (OPCRIT).11 The Mini PAS-ADD was used when individuals had an intellectual disability and were unable to fully self-report their experiences. In two cases, neither the participant nor an informant was available. For these participants, the OPCRIT was completed using the participant's notes, either by F.V.L or by a trained member of a research network. Diagnoses were generated using ICD-10,12 DSM-IV-TR13 and Research Diagnostic Criteria (RDC)14 algorithms in the OPCRIT. Participants were considered to have research-significant psychosis if they met criteria for a disorder with features of psychosis (ICD-10 F20-F39 diagnoses that include psychosis in the description) on any one of the three algorithms.
In practice, there was little confusion between symptoms of ASD and of psychosis in our participants. Psychosis was always associated with a change from previous functioning and rarely involved a person's special interests or repetitive behaviours. Additionally, the psychotic illnesses described had all been treated by experienced mental health professionals.
Verbal IQ was also collected for participants using the Wechsler Abbreviated Scales of Intelligence (WASI).15
ASD comparison group
ADI-R data were also available from a group of individuals with both clinically and ADI-R-, ADOS- or Adult Asperger Assessment (AAA)16-confirmed ASD and without a known history of psychotic illnesses, substance use disorders, epilepsy or genetic disorders associated with autism (n = 69; men n = 32, women n = 37). They were recruited via the Cambridge arm of the Medical Research Council Autism Imaging Multicentre Study (MRC AIMS) project,17 and are termed the ASD–no psychosis (ASD–NP) group. Verbal IQ scores, collected via the WASI, were also available for this group.
Comparison group with psychosis
The comparison group with psychosis only came from the ÆSOP study, a three-centre, prospective survey of first-onset psychoses carried out in the UK between 1997 and 1999. It comprised 568 individuals with clinically relevant psychosis. Full details of the cohort are available elsewhere.18
Missing data
Of the 116 participants, 13 were missing verbal IQ data, 32 were missing ADI-R data, and 3 were missing OPCRIT data. A subset of 75 participants had full data available and were used for comparison of ASD traits (men n = 63, women n = 12). This subset did not differ significantly from the initial sample in terms of gender, diagnosis or verbal IQ. However, they were significantly younger than those with missing data (t(42) = 4.9, P<0.001), normally because developmental information from parents was often unavailable in older participants because of death, estrangement or non-participation.
Procedures
The relative proportions of different types of psychosis in the study population (DSM-IV-TR diagnoses) were compared with the relative proportions of different categories of psychoses reported in the ÆSOP study,18 excluding those with substance-induced psychosis. These categories were defined as follows:
affective psychosis – DSM-IV 296.x4, 296.4, 296.89;
schizophrenia (including schizoaffective and schizophreniform disorders) – DSM-IV 295.xx;
other psychosis (called non-affective psychosis in the ÆSOP study) – DSM-IV 297.xx, 298.8, 298.9.
Substance-induced psychosis is not a diagnosis generated by the OPCRIT and therefore none of the participants with ASD–P could be given this diagnosis. However, although substance use did occur in some individuals in the ASD–P group around the time of first onset of illness, there were no individuals where there was a clear role of substance use in aetiology. Data were also reported regarding the characteristics and course of the psychopathology specifically experienced by individuals in the ASD–P group together with details of medication prescription. A more detailed description of the types of psychotic experiences of participants is given in online supplement DS1.
Statistical analysis
Variables were compared between groups using Fisher's exact or t-tests as appropriate. Multivariate analysis of covariance (MANCOVA) was used to test for differences between ASD–P and ASD–NP groups on the ADI-R diagnostic algorithm domain scores for qualitative abnormalities in reciprocal social interaction (scale A), qualitative abnormalities in communication (scale B (verbal)) and restricted, repetitive and stereotyped patterns of behaviour (scale C). To give more insight into the relationships between the scales and the covariates, we then fitted separate regression models between each of the scales A–C and the covariates. Variables included as covariates were gender, age at which the ADI-R was conducted and verbal IQ. A Bonferroni correction to compensate for these multiple analyses was applied, with P-values of 0.017 (0.05/3) or smaller considered significant.
Results
Characteristics of the psychotic illness in the ASD–P group (n = 116)
Age at onset of psychotic illness varied considerably, with some individuals reporting onset in early childhood or in middle age (range: 6–55 years, 2 over 45, 6 younger than 12). The rate of onset of illness was also variable, with the greatest number (42%) reporting an insidious (greater than 6 months) decline from previous functioning. Impairment during illness was also variable, but 71% of participants reported severe impairment (complete lack of normal functioning for at least 2–3 days, or any admission). The majority (53%) had had at least one episode of illness lasting more than 2 years, although much shorter episodes were also reported.
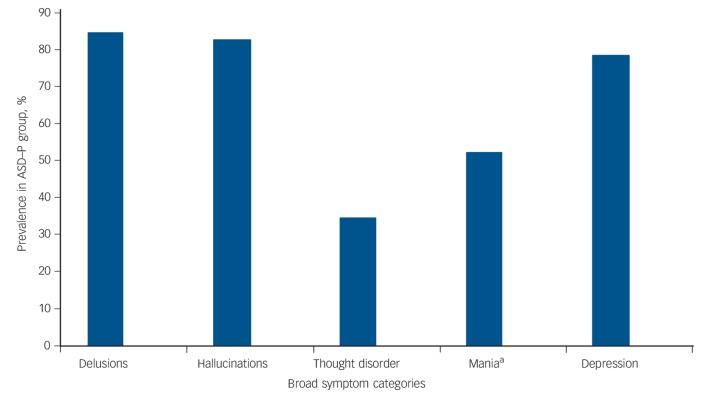
Mood and psychotic symptoms were grouped into broad categories. For mood symptoms, the DSM-IV defining criteria for major depressive or manic episode were used (presence of low mood and/or loss of pleasure for depression, elevated mood and/or irritability for mania). Figure 1 shows the rates of each symptom category in the ASD–P group. In total, 99 (85%) participants had a lifetime-ever experience of core affective symptoms (either depression and/or mania) and 74 (64%) people reported that affective symptoms occurred concurrently with psychotic symptoms, although this was not associated with high rates of DSM-IV-TR affective psychotic illness (see below). There was agreement between two or more diagnostic systems for 79% of participants. The most frequent concordant diagnosis was psychosis not otherwise specified (psychosis-NOS), in 32% of individuals. The second most frequent concordant diagnosis was schizophrenia (21%). All other concordant diagnoses occurred at a frequency of less than 10%.
Fig. 1.
Broad symptom categories and their prevalence in the autism spectrum disorder and psychosis (ASD–P) group.
a. Mania includes both hypomanic and manic symptoms (defined by duration).
Diagnoses of psychosis (ASD–P n = 71, ÆSOP n = 538)
In total, 71 participants in the ASD–P group had a DSM-IV-TR diagnosis of psychotic illness, and these diagnoses were compared with those in the ÆSOP study. There were significant differences between the relative proportions of different diagnoses between the ÆSOP study and the current study (Fisher's exact test, P<0.01) (Fig. 2). Affective psychoses occurred with similar frequency in the two samples (Fisher's exact test, P = 0.38). Schizophrenia was less frequent in the ASD–P group (Fisher's exact test, P<0.01), and other psychoses were proportionately much more common in the ASD–P group (Fisher's exact test, P<0.01). This difference seems to have been caused by the large number of participants in the ASD–P group with a diagnosis of psychosis-NOS (n = 37, 52%). Rates of psychosis-NOS were not available for the ÆSOP study sample, so direct statistical comparison is not possible.
Fig. 2.
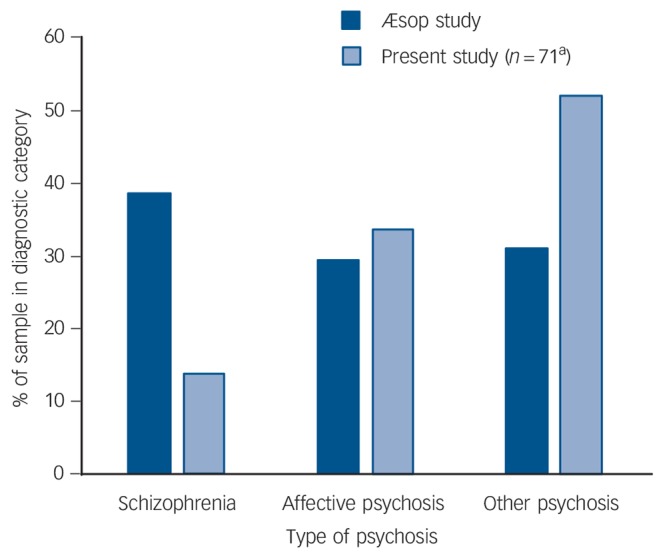
Prevalence of DSM-IV-TR diagnosis by group.
This figure compares the prevalence of DSM-IV-TR-diagnosed psychosis between a general psychiatric sample (ÆSOP study – psychosis)18 and the ASD and comorbid psychosis (ASD–P) group. ‘Schizophrenia’ here includes schizophrenia, schizophreniform and schizoaffective disorders. Affective psychosis includes those with major depressive episode with psychotic features, manic episode with psychotic features and bipolar disorder with psychosis. ‘Other psychosis’ includes psychotic disorder not otherwise specified, which was present in 52% of the ASD–P sample. a. Of the full ASD–P data-set (n = 75), four had a DSM-IV-TR diagnosis of major depressive episode without psychotic features and were thus excluded from this comparison.
Medication prescription (ASD–P n = 92)
Information about regular psychotropic medication use at the time of the study was available for 92 participants. Only four of these participants were not taking psychotropic medication: one had stopped, and three had never been prescribed them. Of the remainder, 71 (77% of those for whom medication data were available) were taking at least one antipsychotic medication, with 8% (6/71) being prescribed clozapine. In total, 46 (50%) were taking a single medication, 32 (35%) were taking two medications and 10 (11%) were taking three. (See online Fig. DS1 for details of the medications taken.) Among those people taking two or more medications, the most common combination was an antidepressant/mood stabiliser and one or two antipsychotics. Three participants were also prescribed one or more benzodiazepines in addition to an antipsychotic. Two were prescribed procyclidine. Three participants volunteered that they had either idiosyncratic reactions or were unusually sensitive to psychotropics. Data regarding other medications beyond those reported here were not available.
Autistic features (ASD–P n = 75)
Differences between the ASD–P group with full data (n = 75) and the ASD–NP (n = 69) groups are reported in Table 1. Compared with participants in the ASD–NP group, the ASD–P group had significantly fewer women, were significantly older when the ADI-R was completed and had a significantly lower verbal IQ. Gender, age at administration of the ADI-R and verbal IQ were included in the analysis as covariates.
Table 1.
Demographic profile of ASD–Psychosis (ASD–P) and ASD-no psychosis (ASD–NP) groups used for comparison of Autism Diagnostic Interview-Revised responses
| Group differences |
||||
|---|---|---|---|---|
| ASD–P (n = 75) | ASD–NP (n = 69) | Difference/OR (95% CI) | P | |
| Age, mean (range) s.d. | 27.7 (17–55) 7.6 | 27.8 (18–49) 7.6 | Difference = −0.1 (−2.5 to 2.4) | 0.967 |
| Gender, male: n (%) | 63 (84) | 32 (46) | OR = 0.2 (0.07 to 0.38) | <0.001 |
| Verbal IQ, mean (range) s.d. | 95.5 (55–133) 23.1 | 113.3 (67–137) 14.7 | Difference = −17.5 (−24 to −11) | <0.001 |
OR, odds ratio.
Raw, unadjusted differences between groups on the ADI-R scales were all less than one scale point and not significantly different (P>0.1). The results of the MANCOVA are shown in Table 2 and indicate a significant effect of group membership on scale C score. In the subsequent univariate analyses by scale, group membership only had a significant effect (P = 0.004) on scale C. The largest effect of group membership (as judged by partial eta-squared (η2)) was seen on scale C; within the univariate regression on scale C, group membership had the largest effect (η2 = 0.06, between a small and medium effect size – see Cohen19). Focusing on scale C, neither verbal IQ nor age significantly affected scores at the uncorrected or Bonferroni-corrected significance level. The ASD–P group scored 1.4 points less on average, a moderate effect size in terms of Cohen's d (scale C s.d. = 2.5, d = 1.4/2.5≈0.6). When another regression model was fitted that included all second-order interaction terms, no interactions approached significance (all P-values >0.1).20
Table 2.
Results of the Multivariate analysis of covariance (MANCOVA) applied to scales A, B and C of the Autism Diagnostic Interview-Revised (ADI-R) and the three univariate regressions separately relating each of the ADI-R scales to the covariatesa
| Multivariate analysis |
Univariate analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Term | Pillai statistic |
F(3,137) (P) | η2 | ADI-R, scale A |
ADI-R, scale B |
ADI-R, scale C |
|||
| F(1,139) (P) | η2 | F(1,139) (P) | η2 | F(1,139) (P) | η2 | ||||
| Gender | 0.07 | 3.4 (0.019) | 0.07 | 5.7 (0.018) | 0.04 | 9.0 (0.003) | 0.06 | 4.9 (0.029) | 0.03 |
| Age | 0.05 | 2.2 (0.090) | 0.05 | 5.2 (0.024) | 0.04 | 1.3 (0.253) | 0.01 | 2.4 (0.126) | 0.02 |
| Verbal IQ | 0.10 | 4.9 (0.003) | 0.11 | 6.8 (0.01) | 0.05 | 15.0 (<0.001) | 0.10 | 2.1 (0.150) | 0.01 |
| Group | 0.07 | 3.2 (0.025) | 0.06 | 3.3 (0.070) | 0.02 | 4.0 (0.046) | 0.03 | 8.7 (0.004) 0.06 | |
Univariate analysis are all based on type III sums of squares. Partial eta-squared (η2) is a measure of effect size (small: 0.01; medium: 0.09; large: 0.25; based on the square of the Pearson correlation effect sizes from Cohen19). Multivariate η2 = 1 – Λ1/s where Λ = Wilks' lambda and s = minimum of the number of levels of the factor minus 1, or the number of dependent variables (here, s = 1 for continuous variables).20
Discussion
Summary of results
Our results indicate that individuals with ASD who have also developed a comorbid psychotic illness differ significantly in their autistic phenotypic profile from individuals with ASD alone. The ASD–P group showed significantly fewer lifetime stereotyped, repetitive or restrictive interests/behaviours. In addition, the diagnosis profile in the ASD–P group differed from that of individuals with psychosis only. Individuals with ASD–P had lower rates of schizophrenia and higher rates of psychosis-NOS. Our description of the psychosis seen in the ASD–P group came from the largest sample of its kind known to us.
Meaning of the results
Difficulties in diagnosing psychotic illness in people with ASD have been highlighted.6 Others have commented on the misdiagnosis of ASD as schizophrenia.21 Therefore, careful steps were taken in the current study to ensure that only individuals with clear diagnoses of both disorders were included. This took place in two stages – first, by recruitment from clinical services experienced in the diagnosis and treatment of psychosis, and second, by confirming both ASD characteristics and mental health history using standardised and well-validated instruments.
The results indicate that the spectrum of psychotic illness experienced by adults with ASD may be different from that experienced by the population without a diagnosis of ASD. This is evident from the different distribution of DSM-IV-TR diagnoses and the high number of people with psychosis-NOS (Fig. 2). The duration of illness reported by individuals with ASD in this study generally did not meet the duration criteria for DSM-IV-TR schizophrenia (minimum of 6 months' disturbance with 1 month of active symptoms), indicating a more acute, transient course than that seen in the general population (similar to that reported elsewhere22). This atypical diagnostic distribution for psychotic illness is supported by observations from studies of mental health problems in people with ASD previously reported.23 It is possible that this difference is explained by individuals with ASD responding differently to the questions used to derive psychotic diagnoses since the measures used were not standardised for use with people who have ASDs. However, the descriptions of symptoms obtained from participants, and referrals from clinical services suggest there is validity in these results.
The prominence of affective symptoms in people with concurrent ASD and psychosis is informative in light of family medical histories. For example, there are higher rates of bipolar disorder in the families of people with Asperger syndrome,24 indicating plausible shared genetic risks of affective disturbance among some people on the autism spectrum. This is supported by recent research showing the increased risk of ASD in families with a history of schizophrenia and/or bipolar disorder.25 A family history of depression or anxiety is also more common in people with ASD than in the general population.26 Thus, it seems likely that major mood disorders and psychosis share risk pathways with ASD, as potentially diverse as having a common genetic mechanism or because the long-term experience and stress of having an ASD increases the risks of developing mood disorders in adolescence and adulthood. Both of these elements are consistent with a stress–vulnerability model of affective disorders and/or psychotic illness.27 In addition, the considerable clinical heterogeneity of the psychotic illness we report in the ASD–P group also argues against there being a unique form of psychotic illness linked to ASD and consequent upon a major effect of a single genetic variant predisposing to both conditions.
We propose that our observations support the existence of an underlying neurodevelopmental vulnerability to developing psychosis in some people with ASD. We suggest that this is driven by an, as-yet, poorly understood genetic mechanism, compounded by elevated rates of depression and anxiety, perhaps linked to the stress of sensory differences, social difficulties, unemployment, bullying or other problems faced by many people with ASD. There is currently little standardised stressful life-event data from an ASD population, and this is an important area of future research.
Beyond potentially atypical presentations of psychosis, some support for there being a biological vulnerability to psychotic illness comes from our observations that individuals with this comorbidity tend to have a different pattern of autistic characteristics. The finding that they have, on average, significantly fewer restricted, repetitive and stereotyped behaviours and interests than those with ASD alone is informative on a number of levels. It may represent the genetic fractionation of the parts of the autism triad,28 and could suggest that the genetic risk factors for social–communication difficulties share a greater association with genetic risk factors for psychosis (for example, FOXP2)29 than restricted and repetitive behaviours.
Diagnostic systems
Although a detailed discussion of the differences between diagnostic systems is beyond the scope of this paper, it is worth highlighting that individuals with ASD, who may experience psychosis differently from the general population, do not appear to fit easily into existing diagnostic categories. It has been well-described that there is to some extent a lack of agreement between diagnostic systems (for example Cheniaux et al30), which is why the current study chose a concordance approach to the inclusion of participants, rather than relying on any one diagnostic system. In particular, the lack of an exclusion criterion for affective symptoms in ICD-10-defined schizophrenia highlighted by Cheniaux et al30 may be relevant to people with ASD and psychosis, as it appears that affective symptoms are prominent in the individuals reported here. It also suggests that diagnosing psychosis in people with ASD may require particular care on the part of clinicians and a critical appraisal of the diagnostic systems used in their practice. Unfortunately, this may have implications for treatment that is based on diagnosis, as individuals with ASD may be inadequately served if only a single diagnostic system is relied upon to determine eligibility for treatment, particularly where a decision is made to offer or exclude treatment based on a diagnosis of psychosis using a single diagnostic system.
Models of the relationship between ASD and psychosis
In light of this sample, it may be important to reconsider models that have been proposed to describe the relationships between ASD and psychosis. In our view, a possible model would emphasise the dimensional nature of mood symptoms, psychotic symptoms and autistic characteristics. Our results suggest that the overlap between ASD and schizophrenia (as opposed to psychosis more broadly) may be limited. Indeed, some have questioned whether schizophrenia might be considered part of the autism spectrum.1 If it is, it most likely represents a somewhat different part of the spectrum from the affective psychosis that predominated in this current sample.
Generalisability and limitations
Our sample may not be representative of all people who have ASD and psychotic illness. Sampling was conducted as widely as possible throughout England, with a small number of participants from other parts of the UK, but was not systematic. Population-based studies have identified autistic-like features in the developmental histories of people who develop schizophrenia31 and, in retrospective studies of people with schizophrenia, ASD is diagnosed frequently.32 There may be lower numbers of people meeting criteria for schizophrenia in this study because of self-selection bias. Factors such as negative symptoms, poorer outcome or lower IQ,33 may have disproportionately discouraged certain individuals from participating. A population-based epidemiological study with the resolution to examine properly both psychotic and autistic features in adulthood would be costly and difficult to conduct, given difficulties with diagnostic uncertainty when two spectrums overlap, but might settle the issue. Currently, some of the best evidence with a developmental perspective is a population-based study that found an association between childhood neurodevelopmental diagnoses, including ASD, and psychotic experiences in adolescence, an association not mediated by lower IQ.3 There is also a birth cohort study showing very similar results.34 These are methodologically strong studies with robust results. However, more research will be needed to further describe psychosis in people with ASD and to understand its relationship to psychosis seen in people without ASD.
A limitation of this study is the lack of comparison samples collected for the purpose of directly comparing them with the ASD–P group. Relying on previously published data and samples meant that, at times, methods used for analysis were less robust than they would have been with a well-matched sample. The difference in verbal IQ between the ASD–P and ASD–NP groups, for example, was most likely because of methodological differences in recruitment (for example the ASD–NP participants were recruited for a study that required them to complete questionnaires, which would have excluded individuals with lower verbal IQ). Because verbal IQ is possibly related to ADI-R scores, the use of covariance in the analysis of group variance in the ADI-R scores raises questions regarding the validity of the results.35 However, existing research suggests that the effect of non-verbal IQ on the domain of interest (restricted, repetitive and stereotyped behaviours) is mixed, with circumscribed interests being positively correlated with higher non-verbal IQ and repetitive use of objects being negatively correlated.36 Thus, it could be argued that the same drive for repetition is simply being expressed differently depending on non-verbal IQ, whereas the overall level of these types of behaviour does not vary because of non-verbal IQ. Additionally, even if verbal IQ is not used as a covariate, the results reported for scale C of the ADI-R remain significant, indicating to us that this is a real and interesting difference between those with ASD who experience psychosis and those who do not. It is also important to acknowledge that the phenomenological data in this study were rated only once so, despite the use of standardised measures and diagnostic algorithms, rater bias may have affected the results.
Final remarks and suggestions for future research
What is clear from this research is that individuals who experience concurrent ASD and psychotic illness exist and are treated in mental health services. Sometimes, the ASD has not been recognised prior to the first onset of psychiatric illness, and thus clinicians on the front line are often left treating not only the psychosis but are also facing the challenges of identifying an underlying ASD.6 Mental health services in the UK are yet to be fully equipped to support people with both psychotic illness and ASD. Thus, for many, their psychotic illness will be treated but their quality of life will suffer because there may be no aftercare or support services tailored to the needs of those with ASD. Although not captured in this study, this type of variability emerged in our discussions with participants and should be an area of urgent action at a procedural and policy level. In addition, more research is needed into aetiological and phenotypic overlaps between ASD and psychosis, utilising a dual-diagnosis cohort compared with two control groups, each singly diagnosed with ASD or psychosis. The development of a multidimensional model for understanding the relationship between these two conditions would require cohorts to be described not solely by diagnosis, but using dimensional measures. Peralta & Cuesta37 have summarised the complexities inherent in creating a dimensional measure of psychosis, but a promising effort to develop an effective system was presented by Läge et al.38 Their approach involved categorical and dimensional aspects, encompassing affective and psychotic features. It is not difficult to picture how integrating features of ASD into such a model would be possible, given the known dimensions within ASD and the well-validated measures already in use with this population.
Acknowledgments
We particularly thank all the participants and their families who took part, the clinicians and others who kindly approached them on our behalf, and Dr Isabel Clare for training and guidance with the IQ assessments.
The MRC AIMS Consortium is a UK collaboration between the Institute of Psychiatry (IoP) at King's College, London, the Autism Research Centre, University of Cambridge and the Autism Research Group, University of Oxford. The Consortium members (in alphabetical order) are: Anthony J. Bailey (Oxford), Simon Baron-Cohen (Cambridge), Patrick F. Bolton (IoP), Edward T. Bullmore (Cambridge), Sarah Carrington (Oxford), Marco Catani (IoP), Bhismadev Chakrabarti (Cambridge), Michael C. Craig (IoP), Eileen M. Daly (IoP), Sean C. L. Deoni (IoP), Christine Ecker (IoP), Francesca Happé (IoP), Julian Henty (Cambridge), Peter Jezzard (Oxford), Patrick Johnston (IoP), Derek K. Jones (IoP), Meng-Chuan Lai (Cambridge), Michael V. Lombardo (Cambridge), Anya Madden (IoP), Diane Mullins (IoP), Clodagh M. Murphy (IoP), Declan G. M. Murphy (IoP), Greg Pasco (Cambridge), Amber N. V. Ruigrok (Cambridge), Susan A. Sadek (Cambridge), Debbie Spain (IoP), Rose Stewart (Oxford), John Suckling (Cambridge), Sally J. Wheelwright (Cambridge), Steven C. Williams (IoP), and C. Ellie Wilson (IoP).
See editorial, pp. 241–242, this issue.
Footnotes
Declaration of interest
None.
Funding
F.V.L. was funded by a Medical Research Council (UK) PhD studentship and additional funding was provided by the Baily Thomas Charitable Trust, and we are grateful to them both for their support. A.J.H. is supported by the Health Foundation. M.-C.L. was supported by the William Binks Autism Neuroscience Fellowship at the University of Cambridge and the O'Brien Scholars Program within the Child and Youth Mental Health Collaborative at the Centre for Addiction and Mental Health and The Hospital for Sick Children, Toronto. S.B.-C. was supported by the Autism Research Trust. A.P.W., P.B.J. and A.J.H.'s contributions were supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East of England at the Cambridgeshire & Peterborough NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the MRC, the NHS, the NIHR or the Department of Health. This study also received support from the UK Medical Research Council Autism Imaging Multicentre Study (MRC AIMS) Consortium.
References
- 1. King BH, Lord C. Is schizophrenia on the autism spectrum? Brain Res 2011; 1380: 34–41. [DOI] [PubMed] [Google Scholar]
- 2. Mouridsen SE, Rich B, Isager T. Psychiatric disorders in adults diagnosed as children with atypical autism. A case control study. J Neural Transm 2008; 115: 135–8. [DOI] [PubMed] [Google Scholar]
- 3. Selten J-P, Lundberg M, Rai D, Magnusson C. Risks for non-affective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: a population-based study. JAMA Psychiatry 2015; 72: 483–9. [DOI] [PubMed] [Google Scholar]
- 4. Khandaker GM, Stochl J, Zammit S, Lewis G, Jones PB. A population-based longitudinal study of childhood neurodevelopmental disorders, IQ and subsequent risk of psychotic experiences in adolescence. Psychol Med 2014; 44: 3229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarke D, Baxter M, Perry D, Prasher V. The diagnosis of affective and psychotic disorders in adults with autism: seven case reports. Autism 1999; 3: 149–64. [Google Scholar]
- 6. Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2: 1013–27. [DOI] [PubMed] [Google Scholar]
- 7. Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Austism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 1989; 19: 185–212. [DOI] [PubMed] [Google Scholar]
- 8. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24: 659–85. [DOI] [PubMed] [Google Scholar]
- 9. Castle DJ, Jablensky A, McGrath JJ, Carr V, Morgan V, Waterreus A, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med 2006; 36: 69–80. [DOI] [PubMed] [Google Scholar]
- 10. Prosser H, Moss S, Costello H, Simpson N, Patel P, Rowe S. Reliability and validity of the Mini PAS-ADD for assessing psychiatric disorders in adults with intellectual disabilities. J Intellect Disabil Res 1998; 42: 264–72. [DOI] [PubMed] [Google Scholar]
- 11. McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48: 764. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organisation International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). WHO, 2007. [Google Scholar]
- 13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM-IV). APA, 2000. [Google Scholar]
- 14. Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35: 773. [DOI] [PubMed] [Google Scholar]
- 15. Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, 1999. [Google Scholar]
- 16. Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger Assessment (AAA): a diagnostic method. J Autism Dev Disord 2005; 35: 807–19. [DOI] [PubMed] [Google Scholar]
- 17. Lai M-C, Lombardo M V, Ruigrok AN V, Chakrabarti B, Wheelwright SJ, Auyeung B, et al. Cognition in males and females with autism: similarities and differences. PLoS One 2012; 7: e47198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirkbride JB, Fearon P, Morgan C, Dazzan P, Morgan K, Tarrant J, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AESOP study. Arch Gen Psychiatry 2006; 63: 250–8. [DOI] [PubMed] [Google Scholar]
- 19. Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd edn). Erlbaum, 1988. [Google Scholar]
- 20. Green SB, Salkind NJ, Akey T. Using SPSS for Windows: Analyzing and Understanding Data. Prentice-Hall, 1997. [Google Scholar]
- 21. Tantam D. Lifelong eccentricity and social isolation. I. Psychiatric, social, and forensic aspects. Br J Psychiatry 1988; 153: 777–82. [DOI] [PubMed] [Google Scholar]
- 22. Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32: 1910–7. [DOI] [PubMed] [Google Scholar]
- 23. Tantam D. Autism Spectrum Disorders through the Life Span. Jessica Kingsley, 2012. [Google Scholar]
- 24. DeLong R. Autism and familial major mood disorder: are they related? J Neuropsychiatry Clin Neurosci 2004; 16: 199–213. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan PF, Magnusson C, Reichenberg A, Boman M, Dalman C, Davidson M, et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry 2012; 69: 1099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smalley SL, McCracken J, Tanguay P. Autism, affective disorders, and social phobia. Am J Med Genet 1995; 60: 19–26. [DOI] [PubMed] [Google Scholar]
- 27. Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull 1984; 10: 300–12. [DOI] [PubMed] [Google Scholar]
- 28. Happé F, Ronald A. The “fractionable autism triad”: a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev 2008; 18: 287–304. [DOI] [PubMed] [Google Scholar]
- 29. Tolosa A, Sanjuán J, Dagnall AM, Moltó MD, Herrero N, de Frutos R. FOXP2 gene and language impairment in schizophrenia: association and epigenetic studies. BMC Med Genet 2010; 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheniaux E, Landeira-Fernandez J, Versiani M. The diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder and unipolar depression: interrater reliability and congruence between DSM-IV and ICD-10. Psychopathology 2009; 42: 293–8. [DOI] [PubMed] [Google Scholar]
- 31. Jones P, Rodgers B, Murray R, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344: 1398–402. [DOI] [PubMed] [Google Scholar]
- 32. Hallerback MU, Lugnegard T, Gillberg C. Is autism spectrum disorder common in schizophrenia? Psychiatry Res 2012; 198: 12–7. [DOI] [PubMed] [Google Scholar]
- 33. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry 2008; 165: 579–87. [DOI] [PubMed] [Google Scholar]
- 34. Sullivan S, Rai D, Golding J, Zammit S, Steer C. The association between autism spectrum disorder and psychotic experiences in the Avon longitudinal study of parents and children (ALSPAC) birth cohort. J Am Acad Child Adolesc Psychiatry 2013; 52: 806–14.e2. [DOI] [PubMed] [Google Scholar]
- 35. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol 2001; 110: 40–8. [DOI] [PubMed] [Google Scholar]
- 36. Holtmann M, Bölte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Dev Med Child Neurol 2007; 49: 361–6. [DOI] [PubMed] [Google Scholar]
- 37. Peralta V, Cuesta MJ. A dimensional and categorical architecture for the classification of psychotic disorders. World Psychiatry 2007; 6: 100–1. [PMC free article] [PubMed] [Google Scholar]
- 38. Läge D, Egli S, Riedel M, Strauss A, Möller H-J. Combining the categorical and the dimensional perspective in a diagnostic map of psychotic disorders. Eur Arch Psychiatry Clin Neurosci 2011; 261: 3–10. [DOI] [PubMed] [Google Scholar]