Abstract
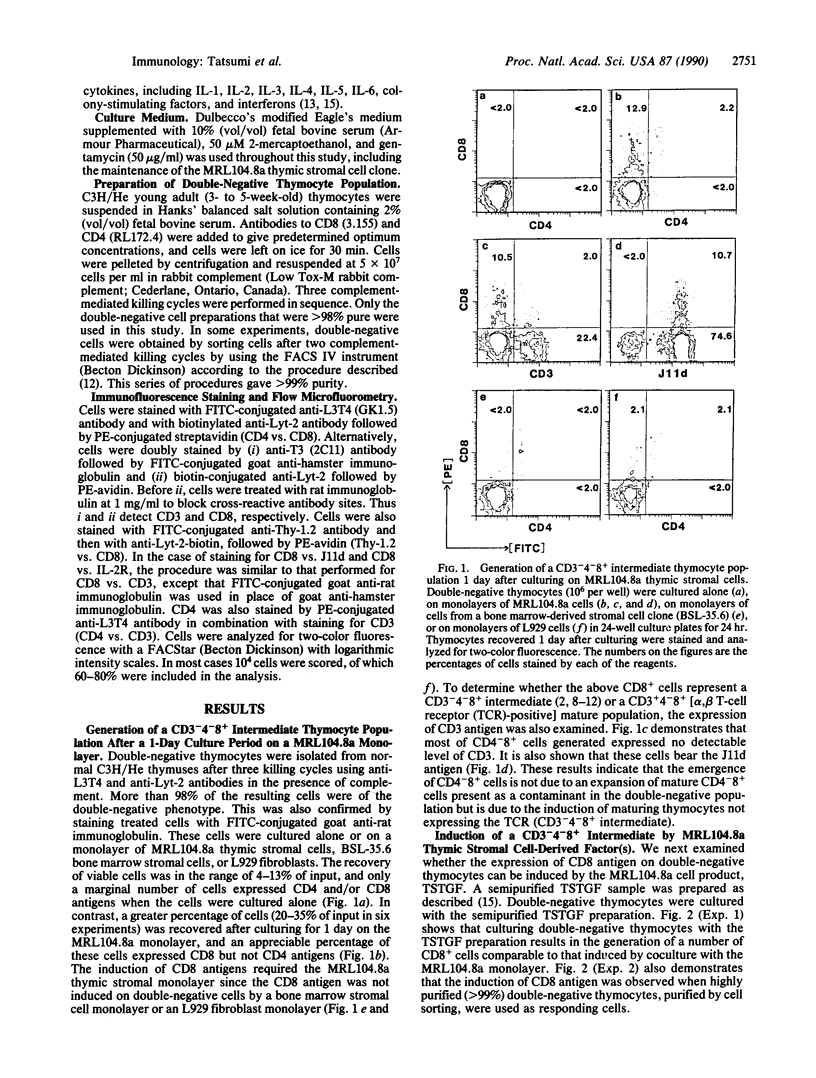
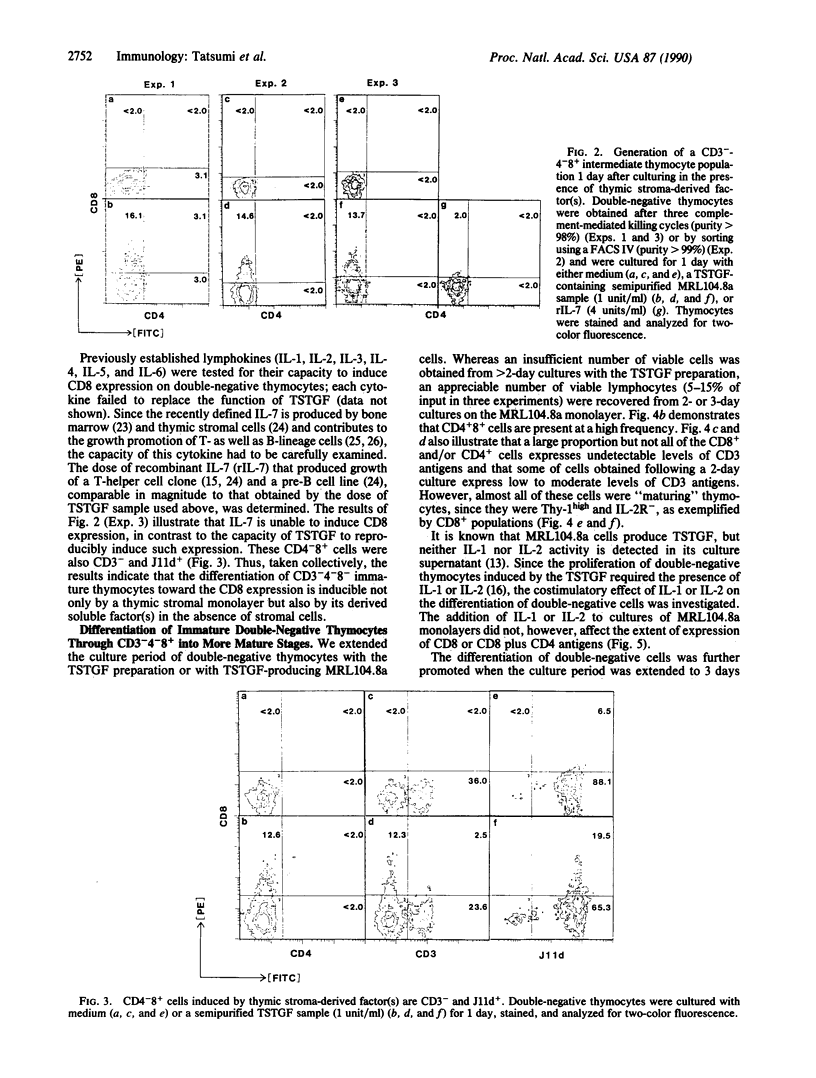
We have investigated the capacity of our established thymic stromal cell clone (MRL104.8a) or its derived factor(s) to induce the differentiation of immature thymocytes. Culture of purified adult murine double-negative (CD4-CD8-, indicated here as CD4-8-) thymocytes on the MRL104.8a thymic stromal cell monolayer for 1 day resulted in the induction of an appreciable percentage of CD4-8+ thymocytes. A bone marrow-derived stromal cell monolayer or a L929 fibroblast monolayer failed to generate CD4-8+ cells. This differentiation could also be induced by a semipurified sample of the MRL104.8a culture supernatant, which contained a thymic stroma-derived T-cell growth factor capable of contributing to the growth of double-negative immature thymocytes. CD4-8+ thymocytes generated 1 day after coculture with the MRL104.8a cells or the sample containing thymic stroma-derived T-cell growth factor were found to be CD3- and J11d+, excluding the possibility of expansion of mature (CD3+4-8+) thymocytes present in the thymus. More importantly, when the culture period was extended to 2 or 3 days, an appreciable number of CD4+8+ and single-positive (CD4+) cells were generated on the MRL104.8a monolayer. Thus, these results provide the direct demonstration that CD3-4-8- immature thymocytes are promoted to differentiate through a rapidly cycling intermediate (CD3-4-8+) into double- and single-positive cells by a specialized thymic stromal component.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceredig R., Glasebrook A. L., MacDonald H. R. Phenotypic and functional properties of murine thymocytes. I. Precursors of cytolytic T lymphocytes and interleukin 2-producing cells are all contained within a subpopulation of "mature" thymocytes as analyzed by monoclonal antibodies and flow microfluorometry. J Exp Med. 1982 Feb 1;155(2):358–379. doi: 10.1084/jem.155.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Sekaly R. P., MacDonald H. R. Differentiation in vitro of Lyt 2+ thymocytes from embryonic Lyt 2- precursors. Nature. 1983 May 19;303(5914):248–250. doi: 10.1038/303248a0. [DOI] [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Edison L., Mathieson B. J., Chused T. M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985 Sep 1;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Ogata M., Hikita I., Maruo S., Sugihara S., Matsubara H., Takai Y., Hamaoka T., Fujiwara H. Model for clonal elimination in the thymus. Proc Natl Acad Sci U S A. 1989 May;86(10):3773–3777. doi: 10.1073/pnas.86.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Budd R. C., Howe R. C. A CD3- subset of CD4-8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur J Immunol. 1988 Apr;18(4):519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Howe R. C., Pedrazzini T., Lees R. K., Budd R. C., Schneider R., Liao N. S., Zinkernagel R. M., Louis J. A., Raulet D. H. T-cell lineages, repertoire selection and tolerance induction. Immunol Rev. 1988 Aug;104:157–182. doi: 10.1111/j.1600-065x.1988.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Y., Saitoh M., Ogata M., Kosaka H., Tatsumi Y., Kiyotaki C., Hamaoka T., Fujiwara H. Thymic stroma-derived T cell growth factor (TSTGF). IV. Capacity of TSTGF to promote the growth of L3T4- Lyt-2- thymocytes by synergy with phorbol myristate acetate or various IL. J Immunol. 1989 Feb 15;142(4):1195–1202. [PubMed] [Google Scholar]
- Morrissey P. J., Goodwin R. G., Nordan R. P., Anderson D., Grabstein K. H., Cosman D., Sims J., Lupton S., Acres B., Reed S. G. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989 Mar 1;169(3):707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Nikolić-Zugić J., Bevan M. J. Thymocytes expressing CD8 differentiate into CD4+ cells following intrathymic injection. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8633–8637. doi: 10.1073/pnas.85.22.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Matsubara H., Takai Y., Kosaka H., Katagiri T., Sano H., Ishimura K., Fujita H., Hamaoka T., Fujiwara H. Capacities of a newly established thymic stromal cell clone to express Ia antigens and to produce interleukin-6, colony-stimulating factor, and thymic stroma-derived T-cell growth factor. J Leukoc Biol. 1989 Jan;45(1):69–78. doi: 10.1002/jlb.45.1.69. [DOI] [PubMed] [Google Scholar]
- Ogata M., Sato S., Sano H., Hamaoka T., Doi H., Nakanishi K., Asano Y., Itoh T., Fujiwara H. Thymic stroma-derived T cell growth factor (TSTGF). I. Functional distinction of TSTGF from interleukins 2 and 4 and its preferential growth-promoting effect on helper T cell clones. J Immunol. 1987 Oct 15;139(8):2675–2682. [PubMed] [Google Scholar]
- Palacios R., Pelkonen J. Prethymic and intrathymic mouse T-cell progenitors. Growth requirements and analysis of the expression of genes encoding TCR/T3 components and other T-cell-specific molecules. Immunol Rev. 1988 Aug;104:5–27. doi: 10.1111/j.1600-065x.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Williams A. F. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987 Nov 1;166(5):1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Ogata M., Sano H., Mizushima Y., Muramatsu M., Doi H., Itoh T., Hamaoka T., Fujiwara H. Thymic stroma-derived T-cell growth factor (TSTGF): II. Biochemical and functional characterization. J Leukoc Biol. 1988 Sep;44(3):149–157. doi: 10.1002/jlb.44.3.149. [DOI] [PubMed] [Google Scholar]
- Scollay R., Wilson A., D'Amico A., Kelly K., Egerton M., Pearse M., Wu L., Shortman K. Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol Rev. 1988 Aug;104:81–120. doi: 10.1111/j.1600-065x.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Shortman K., Wilson A., Egerton M., Pearse M., Scollay R. Immature CD4- CD8+ murine thymocytes. Cell Immunol. 1988 May;113(2):462–479. doi: 10.1016/0008-8749(88)90042-1. [DOI] [PubMed] [Google Scholar]
- Sprent J., Lo D., Gao E. K., Ron Y. T cell selection in the thymus. Immunol Rev. 1988 Jan;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Takeda S., Gillis S., Palacios R. In vitro effects of recombinant interleukin 7 on growth and differentiation of bone marrow pro-B- and pro-T-lymphocyte clones and fetal thymocyte clones. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1634–1638. doi: 10.1073/pnas.86.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Day L. M., Scollay R., Shortman K. Subpopulations of mature murine thymocytes: properties of CD4-CD8+ and CD4+CD8- thymocytes lacking the heat-stable antigen. Cell Immunol. 1988 Dec;117(2):312–326. doi: 10.1016/0008-8749(88)90121-9. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Sato S., Ogata M., Sakamoto K., Sano H., Shima J., Yamamoto H., Fujiwara H., Hamaoka T. Mediation of in vivo tumor-neutralizing activity by Lyt-2+ as well as L3T4+ T cell subsets. Jpn J Cancer Res. 1988 Jan;79(1):91–98. doi: 10.1111/j.1349-7006.1988.tb00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]