Abstract
It is well known that oxidative stresses induce the production of amyloid β (Aβ) in the brain, lens, and retina, leading to age-related diseases. In the present study, we investigated the effects of ferulic acid on the Aβ levels in H2O2-stimulated human lens epithelial (HLE) SRA 01/04 cells. Three types of Aβ peptides (Aβ1-40, Aβ1-42, and Aβ1-43) were measured by ELISA, and the levels of mRNA for the expressed proteins related to Aβ production (APP, BACE1, and PS proteins) and degradation (ADAM10, NEP, and ECE1 proteins) were determined by quantitative real-time RT-PCR. H2O2 stimulation augmented gene expression of the proteins related to Aβ production, resulting in the production of three types of Aβ peptides. Treatment with 0.1 μM ferulic acid attenuated the augmentations of gene expression and production of the proteins related to the secretion of three types of Aβ peptides in the H2O2-stimulated HLE cells. These results provided evidence of antioxidative functions of ferulic acid for lens epithelial cells.
1. Introduction
A sequential proteolytic processing of amyloid precursor protein (APP) cleaved by β-secretase (β site APP cleaving enzyme, BACE1) [1] and γ-secretase [a presenilin complex (PS)] [2] leads to the production of amyloid β (Aβ) peptides. The cleavage of APP at different positions by γ-secretase mainly produces three Aβ peptides, Aβ1–40, Aβ1–42, and Aβ1–43 peptides [3–7]. The most abundant variant of Aβ is Aβ1–40, but Aβ1–42 and Aβ1–43, the longer forms, are more neurotoxic than Aβ1–40. In addition, the neurotoxicity of Aβ1–43 is higher than that of Aβ1–42, although the accumulation of Aβ1–43 is lower than that of Aβ1–42 [7]. However, the APP cleaved by α-secretase (a disintegrin and metalloprotease domain protease 10, ADAM10) generates nonamyloidogenic soluble APPα [8–10]. Neprilysin (NEP) [11] and endothelin converting enzyme (ECE) [12] degrade the Aβ peptide. The production of Aβ is a normal process; however, the overproduction of Aβ, or an increased proportion of Aβ1–42 and Aβ1–43, appears to cause an early onset of cataract and Alzheimer's disease [13, 14].
Cataract represents a disease of increasing lens opacity, and it has been reported that Aβ accumulation in the human lens causes lens opacification [15–18]. In addition, there have been several reports that oxidative stress can increase the accumulation and toxicity of Aβ peptides in the lens, retina, and brain and that enhanced Aβ accumulation leads to oxidative stress [14, 19–24]. Therefore, it is expected that drugs that inhibit the effect of Aβ peptides and lower oxidative stress can be used as anticataract eye drops.
Ferulic acid (4-hydroxy-3-methoxycinnamic acid) is a widely distributed constituent of plants and is used as an antioxidant to prevent the oxidation of substrates such as lipids, proteins, DNA, and carbohydrates [25, 26]. Ferulic acid has been shown to possess some scavenging activity toward hydroxyl radicals and peroxynitrite [27, 28]. Recently, several studies have shown that ferulic acid has beneficial effects against Alzheimer's disease [29–31], diabetes [32], and cancer [33]. Alzheimer's disease is a neurodegenerative disorder, and it had been shown that one of the main pathophysiological features of Alzheimer's disease was the presence of extracellular senile plaques consisting essentially of Aβ, a peptide thought to be a leading cause of neurotoxicity [34]. It was reported that ferulic acid could be a suitable molecule to specifically bind to Aβ and inhibit fibril formation [35]. Furthermore, its compact structure could also be used for specific interactions with Aβ mature fibrils, thereby possibly promoting their destabilization [35]. Ferulic acid inhibits Aβ aggregation, destabilizes preformed Aβ fibrils in vitro [36], and protects cultured neuronal cells against Aβ-induced cytotoxicity [29]. Moreover, long-term administration of ferulic acid protects mice against Aβ-induced learning and memory deficits in vivo [30, 31]. Therefore, ferulic acid may be a useful molecule for the inhibition of Aβ production and accumulation.
In this study, we investigated the changes in Aβ production and accumulation in human lens epithelial (HLE) SRA 01/04 cells stimulated with hydrogen peroxide (H2O2). In addition, we demonstrated the preventive effects of ferulic acid on Aβ production and accumulation in HLE cells.
2. Materials and Methods
2.1. Cell Culture and Treatment
The HLE cell line SRA 01/04 was incubated in Dulbecco's Modified Eagle's Medium (DMEM) containing 10 μg/L gentamicin and 10% (v/v) fetal bovine serum (FBS) under humidified air containing 5% CO2, at 37°C. Each treatment was usually carried out on the third day after seeding of 0.4 × 104 cells/cm2 (at approximately 80% confluency), and the culture medium was changed to non-FBS medium 1 hour before each experiment. The experiment was initiated by changing the medium to fresh medium containing 0–100 μM of H2O2 and the HLE cells were cultured for 24 hours to produce Aβ1–40, Aβ1–42, and Aβ1–43 (H2O2-stimulated HLE cells). Ten μM of H2O2 and 0–30 μM of ferulic acid included in the medium were used for 24-hour cultures to observe the effect of ferulic acid on Aβ production (H2O2-stimulated HLE cells in the presence of ferulic acid).
2.2. Quantitative Real-Time RT-PCR
The experiment was performed as described previously [14, 37]. Briefly, extraction and purification of the total RNA from HLE cells were performed using an RNase-Free DNase kit and an RNeasy Mini kit (Qiagen, Tokyo, Japan), and an RNA PCR kit (AMV Ver. 3.0, Takara Bio Inc., Shiga, Japan) was used for the reaction according to the manufacturer's instructions. Total RNA (1 μg, OD260/OD280 values > 1.8) was used for the reverse transcription (RT) reaction, and the reaction was performed at 42°C for 15 min, followed by 5 min at 95°C. The PCR reactions were performed using LightCycler® FastStart DNA Master SYBR Green I according to the manufacturer's instructions (Roche Diagnostics Applied Science, Mannheim, Germany), and the quantities of the PCR products were measured fluorometrically in a real-time manner using a LightCycler® DX 400 (Roche Diagnostics Applied Science). In this study, the specific primers (10 pmol) for APP, BACE1, PS1, PS2, ADAM10, NEP, ECE1, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: 5′-TGGTGGGCGGTGTTGTCATA-3′ (forward; FOR) and 5′-TGGATTTTCGTAGCCGTTCTGC-3′ (reverse; REV) for APP; 5′-GCAAGGAGTACAACTATGAC-3′ (FOR) and 5′-AGCTTCAAACACTTTCTTGG-3′ (REV) for BACE1; 5′-ATCATCTGCATAGTCCTCTC-3′ (FOR) and 5′-AGACAGCTTTGATGTTCAAG-3′ (REV) for PS1; 5′-TACAAGTACCGCTGCTACAAGTTC-3′ (FOR) and 5′-GCACTTCCCCAAGGTAGATATAGG-3′ (REV) for PS2; 5′-CACATGATTCTGGAACAGAG-3′ (FOR) and 5′-GTTGTTAAGTTTGTCCCCAG-3′ (REV) for ADAM10; 5′-CTGATATCAACACTCCAAAGC-3′ (FOR) and 5′-TCATCGTAGGTTGCATAGAG-3′ (REV) for NEP; 5′-AGAATGAGATTGTGTTTCCG-3′ (FOR) and 5′-CTATGCCACCAAAGTTTAAGG-3′ (REV) for ECE1; and 5′-TGCACCACCAACTGCTTAGC-3′ (FOR) and 5′-GGCATGGACTGTGGTCATGAG-3′ (REV) for GAPDH. The conditions for PCR were 95°C for 10 min (hot start), 60 cycles of 95°C for 10 s (denaturing), 63°C for 10 s (annealing), and 72°C for 5 s (extension). The differences in the threshold cycles for target groups (APP, BACE1, PS1, PS2 ADAM10, NEP, and ECE1) and GAPDH were used to calculate the levels of mRNA expression in HLE cells.
2.3. Measurement of Aβ
The measurements of Aβ1–40, Aβ1–42, and Aβ1–43 levels were performed as previously described [14, 38]. Approximately 2.5 × 106 HLE cells were collected with a cell scraper (Asahi Glass, Tokyo, Japan), homogenized in 250 μL of 70% formic acid, and then centrifuged at 9,100 ×g for 15 min at 4°C. The supernatants were added to 4.75 mL of 1 M Tris base, and the mixtures were used for the measurements of the Aβ1–40, Aβ1–42, and Aβ1–43 peptides. The Aβ1–40, Aβ1–42, and Aβ1–43 levels were measured using the human β amyloid (40) ELISA kit (dynamic range, 1–100 pM, Wako, Osaka, Japan), the human β amyloid (42) ELISA kit (dynamic range, 0.1–20 pM, Wako), and the human amyloid β (1–43) (FL) ELISA kit (dynamic range, 0.51–32.5 pM, IBL, Gunma, Japan) according to the manufacturer's instructions, respectively. The Aβ levels were expressed as pmol/g of protein according to our previous reports. The protein levels in the samples used to determine Aβ1–40, Aβ1–42, and Aβ1–43 were assessed using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA).
2.4. Measurement of Cell Viability
HLE cells with or without added H2O2 and ferulic acid treatments were incubated in 96-well microplates (Iwaki, Chiba, Japan). The viability of the HLE cells was calculated by the Cell Count Reagent SF kit (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's instructions. The absorbance at 450 nm was measured, and the cell viability (%) was represented as the percentage of the absorbance measured for unstimulated HLE cells for each point (normal cells).
2.5. Statistical Analyses
All values were presented as mean ± standard error (SE), and statistical differences were evaluated by one-way analysis of variance followed by Dunnett's multiple comparisons. P values of less than 0.05 were considered significant.
3. Results
3.1. The Effect of H2O2 Stimulation on Aβ Production and Accumulation in HLE Cells
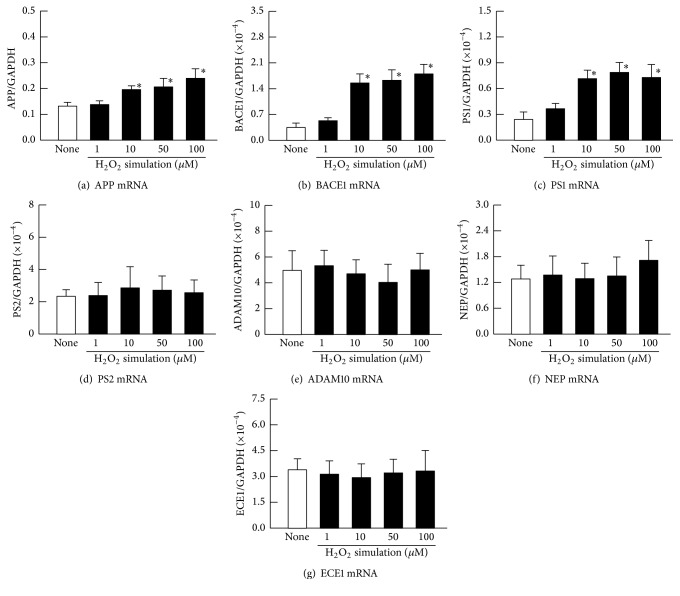
Figures 1(a)–1(c) shows the levels of Aβ accumulation in the H2O2-stimulated HLE cells. The Aβ1–40 and Aβ1–42 levels were enhanced with increasing H2O2 concentration. Although the Aβ1–43 was not detected in the unstimulated HLE cells, an accumulation of Aβ1–43 was observed with stimulation by 10–100 μM H2O2. Figure 1(d) shows the cell viability in the H2O2-stimulated HLE cells. The cell viability in the 1 μM and 10 μM H2O2-stimulated HLE cells was similar to that of the unstimulated HLE cells (None). However, the HLE cell viabilities in the presence of 50 μM and 100 μM H2O2 were decreased, and the cell viability at 100 μM H2O2 was significantly lower than that of the untreated cells (None). Figure 2 shows the mRNA levels for proteins related to Aβ production (APP, BACE1, PS1, and PS2) and to degradation (ADAM10, NEP, and ECE1) in the H2O2-stimulated HLE cells. APP, BACE1, and PS1 mRNAs were increased with increasing concentrations of H2O2, while PS2 mRNA levels were not altered. The H2O2 stimulation did not change the mRNA levels of proteins related to Aβ degradation (ADAM10, NEP, and ECE1).
Figure 1.
Effect of H2O2 stimulation on Aβ1–40 (a), Aβ1–42 (b), Aβ1–43 (c), and cell viability (d) in HLE cells. The HLE cells were stimulated by 1–100 μM H2O2 for 24 h. Open columns (none) = unstimulated HLE cells. Closed columns = H2O2-stimulated HLE cells. N.D. = not detectable. The data are presented as the means ± SE of 5–8 experiments. ∗P < 0.05, versus none for each category.
Figure 2.
Effect of H2O2 stimulation on mRNA levels of APP (a), BACE1 (b), PS1 (c), PS2 (d), ADAM10 (e), NEP (f), and ECE1 (g) in HLE cells. The HLE cells were stimulated with 1–100 μM H2O2 for 24 h. Open columns (none) = unstimulated HLE cells. Closed columns = H2O2-stimulated HLE cells. The data are presented as the means ± SE of 5–8 experiments. ∗P < 0.05, versus none for each category.
3.2. The Preventive Effects of Ferulic Acid on Enhanced Aβ Production and Accumulation in H2O2-Stimulated HLE Cells
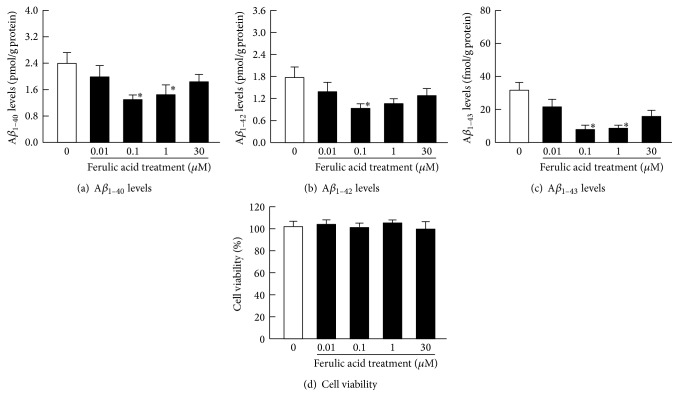
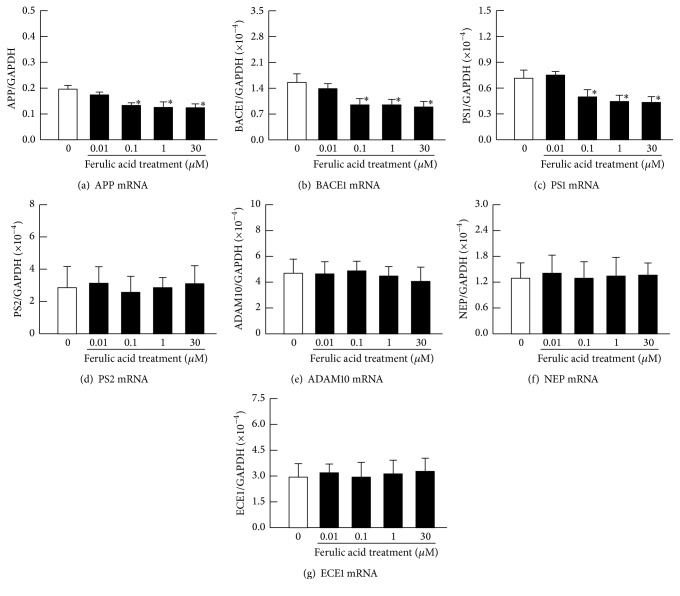
Figures 3(a)–3(c) show the effects of ferulic acid on the specific Aβ levels in the 10 μM H2O2-stimulated HLE cells. At 0.01–0.1 μM of ferulic acid, Aβ levels were relatively decreased in the H2O2-stimulated HLE cells. However, at 30 μM of ferulic acid, the Aβ levels were higher than those at 0.1 μM of ferulic acid; thus, the preventive effects of the Aβ level increase peaked at 0.1 μM of ferulic acid. Figure 3(d) shows the effect of ferulic acid on cell viability in the H2O2-stimulated HLE cells. A decrease in cell viability was not observed at 0.01–30 μM of ferulic acid. Figure 4 shows the effects of ferulic acid on the mRNA levels for proteins related to Aβ production (APP, BACE1, PS1, and PS2) and degradation (ADAM10, NEP, and ECE1) in the H2O2-stimulated HLE cells. Ferulic acid decreased the mRNA levels of APP, BACE1, and PS1. However, the mRNA levels of PS2 and the mRNA levels for proteins related to Aβ degradation (ADAM10, NEP, and ECE1) in HLE cells treated with ferulic acid were similar to the HLE cells without ferulic acid treatment.
Figure 3.
The effect of ferulic acid on Aβ1–40 (a), Aβ1–42 (b), Aβ1–43 (c), and cell viability (d) in 10 μM H2O2-stimulated HLE cells. The 10 μM H2O2 and 0–30 μM ferulic acid were added simultaneously to the medium, and the HLE cells were incubated for 24 h. Open columns = H2O2-stimulated HLE cells. Closed columns = H2O2-stimulated HLE cells with ferulic acid treatment. The data are presented as the means ± SE of 5–8 experiments. ∗P < 0.05, versus H2O2-stimulated HLE cells in the absence of ferulic acid (open columns) for each category.
Figure 4.
The effect of ferulic acid on mRNA levels of APP (a), BACE1 (b), PS1 (c), PS2 (d), ADAM10 (e), NEP (f), and ECE1 (g) in H2O2-stimulated HLE cells. The 10 μM H2O2 and 0–30 μM ferulic acid were added simultaneously to the medium, and the HLE cells were incubated for 24 h. Open columns = H2O2-stimulated HLE cells. Closed columns = H2O2-stimulated HLE cells with ferulic acid treatment. The data are presented as the means ± SE of 5–8 experiments. ∗P < 0.05, versus H2O2-stimulated HLE cells in the absence of ferulic acid (open columns) for each category.
3.3. The Effects of Ferulic Acid on Aβ Production and Accumulation in HLE Cells
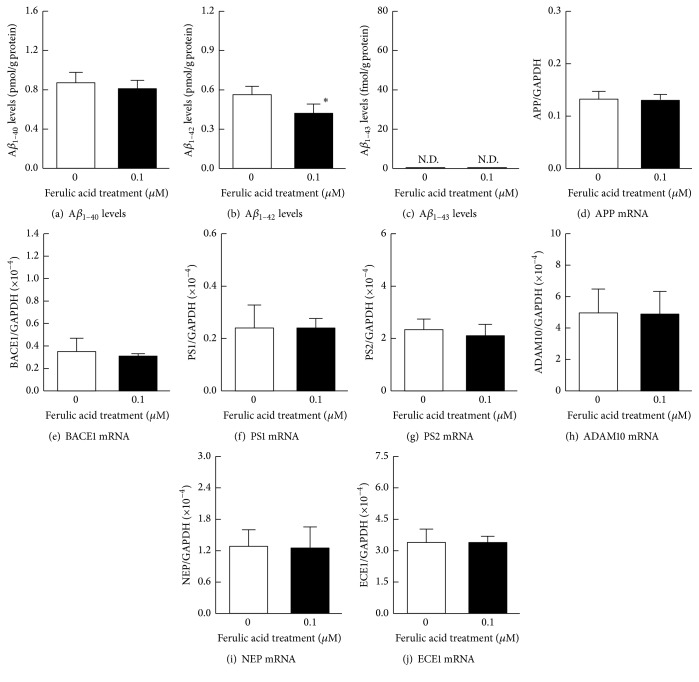
Because 0.1 μM of ferulic acid showed the greatest suppression of the production of Aβ, we further determined the impact of 0.1 μM of ferulic acid on the HLE cells without H2O2 stimulation (Figure 5). As shown in Figure 5, the Aβ1–43 level was not detected by the HLE cells treated with or without 0.1 μM ferulic acid, and Aβ1–40 levels and the mRNA levels of proteins related to Aβ production (APP, BACE1, PS1, and PS2) and degradation (ADAM10, NEP, and ECE1) were similar to those of the naïve HLE cells. On the other hand, the Aβ1–42 levels in HLE cells, treated with 0.1 μM ferulic acid, were significantly lower than that without ferulic acid.
Figure 5.
The effect of 0.1 μM of ferulic acid on the production of Aβ1–40 (a), Aβ1–42 (b), Aβ1–43 (c) levels, and mRNA levels of APP (d), BACE1 (e), PS1 (f), PS2 (g), ADAM10 (h), NEP (i), and ECE1 (j) in HLE cells. The 0.1 μM of ferulic acid was added simultaneously to the medium, and the HLE cells were incubated for 24 h. Open columns = the naïve HLE cells. Closed columns = HLE cells in the presence of 0.1 μM ferulic acid. N.D. = not detectable. The data are presented as the means ± SE of five experiments. ∗P < 0.05, versus naïve HLE cells (open columns) for each category.
4. Discussion
In the cataractous lens, an accumulation of Aβ peptide was observed, and lens opacification occurred via oxidative stress [14, 15]. Therefore, the prevention of Aβ accumulation in the lens is important for cataract therapy. In this study, we investigated whether treatments with ferulic acid prevented the Aβ production and accumulation in the human lens and showed that ferulic acid attenuated the increase in the mRNA levels of proteins related to Aβ production (APP, BACE1, and PS1) and prevented the accumulation of Aβ1–40, Aβ1–42, and Aβ1–43 in H2O2-stimulated HLE cells.
Previous reports showed that the Aβ levels in the retina and brain were enhanced by oxidative stress [19–24]. In addition, we also reported that H2O2 in the rat lens induced lipid peroxidation and led to the accumulation of Aβ1–42 in the lens epithelium [14]. Therefore, in our current study, the HLE cells were exposed to H2O2 to enhance the Aβ production. The results clearly suggested that 10 μM of H2O2 induced Aβ production, whereas 0.01–0.1 μM of ferulic acid significantly decreased the mRNAs (APP, BACE1, and PS1) related to Aβ production (Figure 4) and the Aβ production in H2O2-stimulated HLE cells (Figure 3). It was previously reported that oxidative stress increased the accumulation of Aβ in the lens, retina, and brain [14, 19–24]. In addition, ferulic acid has been shown to possess some scavenging activity for hydroxyl radicals and peroxynitrite [27, 28] and to inhibit the oxidation of substrates. Taken together, our results suggested that 0.1 μM and 1 μM of ferulic acid may prevent H2O2 effects, resulting in a decrease of specific mRNAs and in Aβ accumulation. However, the mRNA levels of proteins related to Aβ production (APP, BACE1, and PS) in HLE cells incubated with 30 μM of ferulic acid were similar to that of the H2O2-stimulated HLE cells incubated with 0.1 μM of ferulic acid (Figure 4). However, the Aβ1–40, Aβ1–42, and Aβ1–43 levels in the HLE cells stimulated with 30 μM of ferulic acid were elevated when compared with those of the H2O2-stimulated HLE cells incubated with 0.1 μM of ferulic acid (Figure 3). It was reported that ferulic acid undergoes specific interactions with Aβ mature fibrils, possibly promoting their destabilization [35]. Moreover, ferulic acid inhibited Aβ aggregation and destabilized preformed Aβ fibrils [36]. From these findings, the destabilization of Aβ fibrils by ferulic acid may be related to the difference in the levels of Aβ in the HLE cells treated with 0.1 μM and 30 μM of ferulic acid. In this study, the Aβ monomer in/on the cells would be measured, since the HLE cells were collected, and all protein fractions were precipitated with 70% formic acid. We tried to measure the Aβ levels in medium; however the Aβ levels were not detected by using ELISA method. We hypothesize that both the fibrils and monomeric Aβ are present in the medium but were below the detection level of the ELISA assay. For the certification of this hypothesis, there is a need to measure the levels of Aβ in the medium by using high resolution mass spectrometry in future studies.
On the other hand, 0.1 μM of ferulic acid shows different effects between Aβ1–40 and Aβ1–42 levels (Figure 5). It was known that the Aβ1–42 was easy to aggregate in comparison with Aβ1–40; however, the amount of Aβ1–42 production was lower than that of Aβ1–40 [7]. Therefore, the destabilization of Aβ aggregation by ferulic acid may strongly affect the Aβ1–42 in comparison with Aβ1–40. Further studies are needed to elucidate the precise mechanisms for the prevention of Aβ production and the accumulation by ferulic acid in the lens. In addition, it is important to measure the ratio of Aβ fibrils and Aβ monomers to clarify the molecular mechanism involved in the downregulation of APP, BACE1, and PS1 mRNAs in ferulic acid treated cells. Therefore, we are now investigating the protein expression and aggregation of Aβ in H2O2-stimulated HLE cells incubated with 0.1 μM and 30 μM of ferulic acid by using western blotting and immunostaining methods. Moreover, we are developing a drug delivery system for ferulic acid to the lens. In the future, we will determine the in vivo effects of a ferulic acid ophthalmic formulation on lens opacification and Aβ accumulation in the UPL rat model.
5. Conclusions
We have shown that stimulation with H2O2 leads to increased mRNA levels of proteins related to Aβ production (APP, BACE1, and PS) and to an enhanced accumulation of Aβ1–40, Aβ1–42, and Aβ1–43 in HLE cells. In addition, we showed that treatments with ferulic acid attenuated the increases in the mRNA levels of proteins related to Aβ production and prevented Aβ1–40, Aβ1–42, and Aβ1–43 accumulation in H2O2-stimulated HLE cells. An ophthalmic eye drop formulation containing ferulic acid could prevent both oxidative stress and Aβ accumulation in HLE cells, resulting in the suppression of lens opacification during cataract. This study provides significant information that can therefore be used to design further studies aimed at developing anticataract drugs.
Acknowledgments
This work was supported in part by Grant 25288075, from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflicts of Interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Vassar R., Bennett B. D., Babu-Khan S., et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 2.Takasugi N., Tomita T., Hayashi I., et al. The role of presenilin cofactors in the γ-secratase complex. Nature. 2003;422(6930):438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 3.Fraser P.-E., Nguyen J.-T., Inouye H., et al. Fibril formation by primate, rodent, and dutch-hemorrhagic analogues of Alzheimer amyloid β-protein. Biochemistry. 1992;31(44):10716–10723. doi: 10.1021/bi00159a011. [DOI] [PubMed] [Google Scholar]
- 4.Bush A. I., Pettingell W. H., Multhaup G., et al. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzo A., Yankner B. A. β-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(25):12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J., Yee A., Brewer H. B., Jr., Das S., Potter H. Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature. 1994;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 7.Saito T., Suemoto T., Brouwers N., et al. Potent amyloidogenicity and pathogenicity of Aβ243. Nature Neuroscience. 2011;14(8):1023–1032. doi: 10.1038/nn.2858. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H., Koo E. H. The amyloid precursor protein: beyond amyloid. Molecular Neurodegeneration. 2006;1(1, article 5) doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kukar T.-L., Ladd T.-B., Bann M.-A., et al. Substrate-targeting γ-secretase modulators. Nature. 2008;453(7197):925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong P. C. Translational control of BACE1 may go awry in Alzheimer's disease. Neuron. 2008;60(6):941–943. doi: 10.1016/j.neuron.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Iwata N., Takaki Y., Fukami S., Tsubuki S., Saido T. C. Region-specific reduction of Aβ-degrading endopeptidase, neprilysin, in mouse hippocampus upon aging. Journal of Neuroscience Research. 2002;70(3):493–500. doi: 10.1002/jnr.10390. [DOI] [PubMed] [Google Scholar]
- 12.Eckman E. A., Reed D. K., Eckman C. B. Degradation of the Alzheimer's amyloid β peptide by endothelin-converting enzyme. The Journal of Biological Chemistry. 2001;276(27):24540–24548. doi: 10.1074/jbc.m007579200. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J., Selkoe D.-J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 14.Nagai N., Ito Y. Excessive hydrogen peroxide enhances the attachment of amyloid β1-42 in the lens epithelium of UPL rats, a hereditary model for cataracts. Toxicology. 2014;315(1):55–64. doi: 10.1016/j.tox.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein L. E., Muffat J. A., Cherny R. A., et al. Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet. 2003;361(9365):1258–1265. doi: 10.1016/S0140-6736(03)12981-9. [DOI] [PubMed] [Google Scholar]
- 16.Meehan S., Berry Y., Luisi B., Dobson C. M., Carver J. A., MacPhee C. E. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. Journal of Biological Chemistry. 2004;279(5):3413–3419. doi: 10.1074/jbc.m308203200. [DOI] [PubMed] [Google Scholar]
- 17.Moncaster J. A., Pineda R., Moir R. D., et al. Alzheimer's disease amyloid-β links lens and brain pathology in down syndrome. PLOS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010659.e10659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun G., Moncaster J. A., Koutras C., et al. δ-catenin is genetically and biologically associated with cortical cataract and future alzheimer-related structural and functional brain changes. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0043728.e43728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamagno E., Bardini P., Obbili A., et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiology of Disease. 2002;10(3):279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 20.Tamagno E., Guglielmotto M., Aragno M., et al. Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the β-amyloid precursor protein. Journal of Neurochemistry. 2008;104(3):683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melov S., Wolf N., Strozyk D., Doctrow S. R., Bush A. I. Mice transgenic for Alzheimer disease β-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radical Biology & Medicine. 2005;38(2):258–261. doi: 10.1016/j.freeradbiomed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Tong Y., Zhou W., Fung V., et al. Oxidative stress potentiates BACE1 gene expression and Aβ generation. Journal of Neural Transmission. 2005;112(3):455–469. doi: 10.1007/s00702-004-0255-3. [DOI] [PubMed] [Google Scholar]
- 23.Shen C., Chen Y., Liu H., et al. Hydrogen peroxide promotes Aβ production through JNK-dependent activation of γ-secretase. Journal of Biological Chemistry. 2008;283(25):17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda A., Tamaoka A., Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. Journal of Neuroscience Research. 2010;88(5):1137–1145. doi: 10.1002/jnr.22271. [DOI] [PubMed] [Google Scholar]
- 25.Rice-Evans C. A., Miller N. J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 26.Kroon P. A., Williamson G. Hydroxycinnamates in plants and food: current and future perspectives. Journal of the Science of Food and Agriculture. 1999;79(3):355–361. [Google Scholar]
- 27.Pannala A.-S., Razaq R., Halliwell B., Singh S., Rice-Evans C. A. Inhibition of peroxynitrite dependent tyrosine nitration by hydroxycinnamates: nitration or electron donation? Free Radical Biology and Medicine. 1998;24(4):594–606. doi: 10.1016/s0891-5849(97)00321-3. [DOI] [PubMed] [Google Scholar]
- 28.Kanski J., Aksenova M., Stoyanova A., Butterfield D. A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. Journal of Nutritional Biochemistry. 2002;13(5):273–281. doi: 10.1016/s0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 29.Sultana R., Ravagna A., Mohmmad-Abdul H., Calabrese V., Butterfield D. A. Ferulic acid ethyl ester protects neurons against amyloid β-peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. Journal of Neurochemistry. 2005;92(4):749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 30.Yan J.-J., Cho J.-Y., Kim H.-S., et al. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. British Journal of Pharmacology. 2001;133(1):89–96. doi: 10.1038/sj.bjp.0704047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H.-S., Cho J.-Y., Kim D.-H., et al. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of β-amyloid peptide (1 - 42) in mice. Biological and Pharmaceutical Bulletin. 2004;27(1):120–121. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- 32.Jung E. H., Kim S. R., Hwang I. K., Ha T. Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. Journal of Agricultural and Food Chemistry. 2007;55(24):9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 33.Hudson E. A., Dinh P. A., Kokubun T., Simmonds M. S. J., Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiology Biomarkers & Prevention. 2000;9(11):1163–1170. [PubMed] [Google Scholar]
- 34.Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sgarbossa A., Giacomazza D., di Carlo M. Ferulic acid: a hope for Alzheimer's disease therapy from plants. Nutrients. 2015;7(7):5764–5782. doi: 10.3390/nu7075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono K., Hirohata M., Yamada M. Ferulic acid destabilizes preformed β-amyloid fibrils in vitro. Biochemical and Biophysical Research Communications. 2005;336(2):444–449. doi: 10.1016/j.bbrc.2005.08.148. [DOI] [PubMed] [Google Scholar]
- 37.Nagai N., Ito Y. Dysfunction in cytochrome c oxidase caused by excessive nitric oxide in human lens epithelial cells stimulated with interferon-γ and lipopolysaccharide. Current Eye Research. 2012;37(10):889–897. doi: 10.3109/02713683.2012.689070. [DOI] [PubMed] [Google Scholar]
- 38.Nagai N., Ito Y., Sasaki H. Hyperglycemia enhances the production of amyloid β1–42 in the lenses of Otsuka long-evans Tokushima Fatty rats, a model of human type 2 diabetes. Investigative Ophthalmology and Visual Science. 2016;57(3):1408–1417. doi: 10.1167/iovs.15-19026. [DOI] [PubMed] [Google Scholar]