Abstract
The genome of the highly infectious bacterium Burkholderia pseudomallei harbors an atp operon that encodes an N‐type rotary ATPase, in addition to an operon for a regular F‐type rotary ATPase. The molecular architecture of N‐type ATPases is unknown and their biochemical properties and cellular functions are largely unexplored. We studied the B. pseudomallei N1No‐type ATPase and investigated the structure and ion specificity of its membrane‐embedded c‐ring rotor by single‐particle electron cryo‐microscopy. Of several amphiphilic compounds tested for solubilizing the complex, the choice of the low‐density, low‐CMC detergent LDAO was optimal in terms of map quality and resolution. The cryoEM map of the c‐ring at 6.1 Å resolution reveals a heptadecameric oligomer with a molecular mass of ~141 kDa. Biochemical measurements indicate that the c17 ring is H+ specific, demonstrating that the ATPase is proton‐coupled. The c17 ring stoichiometry results in a very high ion‐to‐ATP ratio of 5.7. We propose that this N‐ATPase is a highly efficient proton pump that helps these melioidosis‐causing bacteria to survive in the hostile, acidic environment of phagosomes.
Keywords: Burkholderia pseudomallei, c‐ring stoichiometry, cryoEM, N‐type rotary ATPase, proton homeostasis
Subject Categories: Microbiology, Virology & Host Pathogen Interaction; Structural Biology
Introduction
Rotary ATPases are dynamic membrane protein complexes, which play a crucial role in cellular bioenergetics, as they produce adenosine triphosphate (ATP) by exploiting the transmembrane electrochemical ion gradient. Working in reverse, they can pump ions against the gradient by using energy released by hydrolysis of ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi). Rotary ATPases share a common multiprotein subunit architecture forming two functionally tightly coupled parts, which act as two reversible motors 1, 2. The water‐soluble subcomplex harbors three nucleotide conversion sites in the three catalytic β‐subunits 3, 4. The membrane‐embedded subcomplex consists of rotor and stator proteins, which use the proton‐ or sodium‐motive force (pmf, smf) to translocate ions and drive ATP synthesis, or to pump ions in the opposite direction. Torque is transmitted from the membrane to the soluble part by the central stalk. Rotary ATPases can have up to three peripheral stalks, which are static and stabilize the complex during operation. F‐type ATP synthases have a single outer stalk, while the A‐ and V‐type ATPases have two or three 5. Vacuolar (V‐type) ATPases in eukaryotic cells work exclusively as proton pumps 6, while F‐ and A‐type ATPases of bacteria or archaea can operate both in ATP synthesis and hydrolysis direction, depending on the physiological state of the cell. Recent advances in structural biology have provided remarkable new insights into previously unknown parts of F‐ and V‐type rotary ATPases, especially the motor complex in the membrane 7, 8, 9, 10, 11.
The membrane rotors of rotary ATPases known to date consist of 8–15 copies of c‐subunits in the c‐ring 12, 13. The ring stoichiometry is determined by the amino acid sequence of the c‐subunit 14, 15, 16. The usual c‐subunit found in most F‐type ATP synthases is a simple α‐helix hairpin. The c‐subunit sequence does not only determine the stoichiometry but also the type of ion (H+ or Na+), translocated by the ATPase/synthase for energy conversion. It is the sole determinant in all ATP‐dependent bioenergetics processes that decides whether ATP production in a given organism is powered by the pmf or the smf. The C‐terminal α‐helix of each c‐subunit contains a conserved and functionally essential carboxylate (Glu or Asp), which lies at the outer surface of the c‐ring in the membrane center. Ions bind to or are released from these sites through two conserved half channels from the cytoplasmic (matrix) side and from the cell exterior or lumenal side 17. The c‐ring stoichiometry defines the number of ions that are transported along this pathway and hence the ion‐to‐ATP ratio in rotary ATPases/synthases of any given species 13, 14, 18.
An in silico study of the distribution of rotary ATPase operons identified a novel atp operon in several phylogenetically independent groups of bacteria and archaea 19. The operon was always found in addition to other F‐/A‐type atp operons in the same genome. The protein complexes encoded by these so‐called “novel” or “next to” atp operons are referred to as N‐type ATPases and were predicted to be “predominantly Na + ‐selective” 19. A later study of the halotolerant alkaliphilic cyanobacterium Aphanothece halophytica showed that, besides the conventional (F‐type) H+‐ATP synthase, this bacterium indeed expresses a Na+‐coupled rotary ATPase of the N‐type 20, suggesting a role in counteracting salt stress. Apart from this report, no structural or biochemical data are available of any N‐type ATPase. In particular, the stoichiometry and structure of their bioenergetically decisive c‐rings are unknown.
We undertook to investigate the structure of the N‐type ATPase rotor ring from the Gram‐negative, pathogenic β‐proteobacterium Burkholderia pseudomallei, the causative agent of pneumonia‐causing melioidosis/glanders 21, 22, 23. Using single‐particle cryoEM, we obtained a map at 6.1 Å resolution that reveals the basic architecture of an N‐type rotor ring composed of 17 identical c‐subunits. Biochemical evidence indicates that the c‐subunits of this rotor predominantly bind H+, indicating that physiologically, the B. pseudomallei N‐type ATPase is proton‐coupled.
Results and Discussion
Expression of the Burkholderia pseudomallei N‐type ATPase and purification of the c‐ring
The N‐type atp operon of B. pseudomallei has nine open reading frames (Fig 1A). The order of appearance of the genes is similar to conventional bacterial F‐type atp operons 24, except that atpD (β‐subunit) and atpC (ε‐subunit) are shifted to the front of the operon. The translated amino acid sequences indicate a close relationship to F‐ATP synthase subunits, with the exception of atpQ, which encodes putative a membrane protein of yet unknown function. The gene encoding the 8.3 kDa c‐subunit (atpE) is located in the central region of the N‐ATPase operon. Based on the sequence alignment with known F‐type ATP synthase c‐subunits, it is predicted to form a hairpin of two transmembrane helices that contains two carboxylates at the conserved ion‐binding site (Fig 1B), similar to other Na+‐ and H+‐binding c‐rings 25, 26.
Figure 1. N‐atp operon and c‐subunit sequence of Burkholderia pseudomallei aligned with other known c‐subunits.
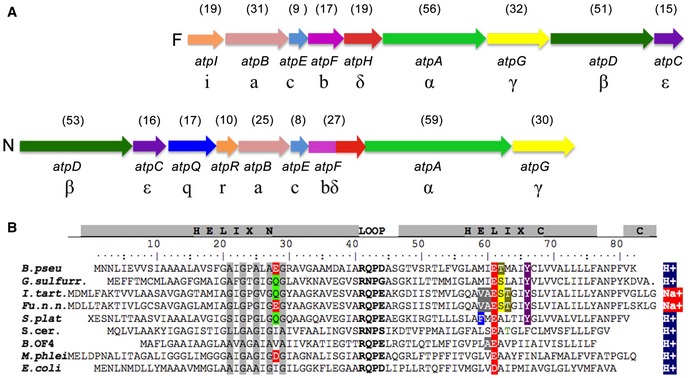
- Gene order of the F‐type atp operon (top) compared to the N‐type atp operon (bottom). In the N‐atp operon, the atpD and atpC precede atpB and are followed by atpQ and atpR, which are absent in the F‐atp operon. Arrows indicate coding sequences. The theoretical molecular masses of the F‐ATP and the N‐ATPase gene products are given in kDa (brackets) above each gene. In B. pseudomallei, the F‐type operon is located on the genomic chromosome 1 and the N‐type operon is located on genomic chromosome 2, flanked by virulence‐specific genes 22.
- Amino acid alignment of selected H+‐ and Na+‐specific F‐type c‐subunits with the N‐type c‐subunit of B. pseudomallei. The conserved loop region is bold and conserved residues involved in ion binding are colored. The conserved glycine motif as discussed in the text is highlighted in gray. Species are as follows: Burkholderia pseudomallei (numbering), Geobacter sulfurreducens, Ilyobacter tartaricus, Fusobacterium nucleatum subsp. nucleatum, Spirulina platensis, Saccharomyces cerevisiae, Bacillus pseudofirmus OF4, Mycobacterium phlei, and Escherichia coli.
To show that the genes of the N‐type ATPase encode a real protein complex and to produce the complete N‐type ATPase of B. pseudomallei, we cloned the entire N‐atp operon into a pHERD28T Burkholderia expression vector system 27. The vector pHERD_BPN1No carrying the complete set of N‐ATPase subunits was expressed in the non‐pathogenic host Burkholderia thailandensis. Western blot analysis of B. thailandensis membranes with a c‐subunit‐specific antibody gave a strong signal for a c‐ring oligomer of the same molecular mass as the c‐ring complex that was isolated from Escherichia coli cells expressing only the B. pseudomallei c‐subunit. This indicates that the B. pseudomallei c‐oligomer does not alter its stoichiometry when it is expressed either by itself or together with all other N‐type ATPase subunits in an expression host (Fig 2A and B). This observation is in line with our earlier work on the heterologous expression of c‐rings from Ilyobacter tartaricus or Caldalkalibacillus thermarum TA2, indicating that the c‐ring stoichiometry is conserved in host cells and does not depend on external factors such as lipid composition, expression medium, or growth temperature 28, 29, 30.
Figure 2. Oligomeric size of the Burkholderia pseudomallei N‐type ATPase rotor ring.
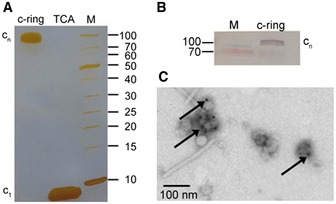
- SDS–PAGE of the isolated B. pseudomallei c‐ring before and after TCA treatment, which dissociates the complex into monomers.
- Western blot of the B. pseudomallei N‐ATPase in Burkholderia membranes with a specific anti‐c‐subunit antibody. The rotor ring shows the same oligomeric size as the isolated complex in (A).
- Negative‐stain electron microscopy of the B. pseudomallei N‐ATPase β‐subunit with immunogold‐labeled His6‐tag in inverted membrane vesicles.
Negative‐stain electron microscopy (EM) was performed with inverted membrane vesicles of B. thailandensis cells expressing the B. pseudomallei N‐type ATPase, taking advantage of an anti‐His6‐tag on the β‐subunit (atpD) of the soluble N1 headgroup (the equivalent of the F1‐α3β3 head in F‐type ATP synthase) to attach an immunogold label for visualization. Gold beads were found on the outside of the vesicles only after N‐type ATPase induction (Fig 2C), showing that the B. pseudomallei N‐type rotary ATPase β‐subunit was successfully expressed and the N1No ATPase was assembled and incorporated into the membrane of B. thailandensis host cells.
Following our previously established strategy for expressing c‐subunits and their assembly into c‐rings, we used a heterologous E. coli expression system 28, 30. Burkholderia pseudomallei atpE was cloned into a T7 expression vector and the c‐subunit was expressed in the E. coli strain Lemo21. The c‐subunit did not have an affinity tag, and we used a purification method that bases on ammonium sulfate precipitation 31. SDS‐gel electrophoresis indicated a band at ~90–100 kDa. The SDS‐stable oligomeric c‐ring complex dissociated into monomeric subunits upon acid treatment (Fig 2A). The subunit mass was determined by MALDI‐MS as 8,349.6 kDa for the unmodified protein and 8,378.3 kDa for the formylated protein, in good agreement with predictions (Fig EV1). The monomer mass together with the slow migration of the oligomer on the SDS–polyacrylamide gel suggested an unusually high c‐ring stoichiometry for the B. pseudomallei N‐type c‐ring.
Figure EV1. MALDI mass spectrum of purified Burkholderia pseudomallei c‐subunit.
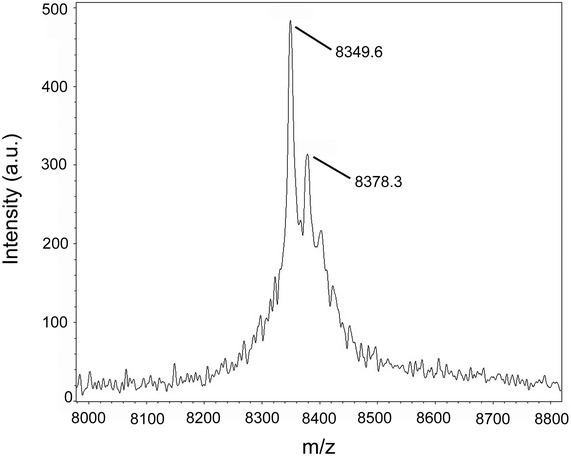
Peaks were identified at 8,349.6 m/z and 8,378.3 m/z, corresponding to the molecular mass of unformylated (predicted 8,349.96 Da) or formylated (predicted 8,377.96 Da) B. pseudomallei c‐subunit.
Ion specificity
To address the ion specificity of the isolated B. pseudomallei c‐ring oligomer, the detergent‐solubilized complex was exposed to N‐cyclohexyl‐N′‐(4‐(dimethylamino)‐α‐naphtyl)‐carbodiimide (NCD‐4), a fluorescent analogue of the ATP synthase inhibitor N,N′‐dicyclohexylcarbodiimide (DCCD) (Figs 3 and EV2). NCD‐4 reacts covalently with the protonated, ion‐binding carboxylates of c‐rings. Therefore, it can be used to directly characterize the type of ion that is bound in a given ATP synthase by measuring a continuous increase of fluorescence upon addition of the fluorophore. For example, in the case of the Na+ binding c‐ring from I. tartaricus, the reaction can be specifically and immediately stopped by adding 15 mM NaCl resulting in 1% remaining labeling activity 31. Similarly, in a H+ binding c‐ring, the increase of fluorescence is completely stopped (no measurable remaining activity) by deprotonation of the ion‐binding carboxylates, caused by the shift of pH to 9.0 in the cuvette 32. For testing our B. pseudomallei c‐oligomer, we chose to initiate the reaction at pH 6.0 at which the ion‐binding c‐oligomer carboxylates are still protonated and is able to react rapidly with NCD‐4, while all water‐exposed carboxylates (e.g., loop region, Fig 1B) are deprotonated and are not involved in the reaction. A continuous increase of fluorescence was monitored for several minutes and then quantified. The reaction starts with a linear increase of fluorescence (Fig 3A, blue arrow). We then tested the effect of Na+ (Fig 3A, red arrow) and depletion of H+ (pH 9.0) on the reaction efficiency (Fig 3B, red arrow). Li+ and Cs+ ions were used as controls to test the effect of other, non‐physiological monovalent cations on the labeling reaction (Fig EV2). The addition of 15 mM Na+ reduced NCD‐4 labeling from the initial rate (defined as 100%) to 40% remaining labeling activity. The effect of 15 mM Li+ (55% remaining) and Cs+ (44% remaining) was similar, while a pH increase to 9.0 (= complete deprotonation of c‐ring glutamates) again resulted in an immediate and complete stop of the reaction with only 1% of rest labeling. A further increase of the Na+, Li+, and Cs+ concentration (150 mM) to a value similar to a physiological concentration of blood serum decreased the total NCD‐4 labeling efficiency to 27, 17, and 24%, respectively (Figs 3A and EV2). A quantification of the labeling efficiencies is given in Table EV1. Note that the rather high increase of cationic strength in the measurement causes effects on fluorescence quenching of the fluorescent probe, as it has been described previously 33. In addition, the combined effects of salt on the availability of water and the critical micelle concentration of the detergent need to be taken into consideration. Importantly however, the observation contrasts with the strong and immediate effect of Na+ on NCD‐4 labeling of Na+ binding c‐rings from I. tartaricus 31, while it matches that observed with a c‐ring isolated from a H+ ATP synthase remarkably well 32. In contrast to what had been predicted from the polypeptide sequence 19, these biochemical data suggest that the B. pseudomallei N‐type ATPase c‐ring is predominantly H+ selective, indicating it is coupled to protons and hence contributes to or utilizes the proton gradient, not the sodium gradient, across the bacterial membrane.
Figure 3. Effect of Na+ and high pH on the kinetics of the NCD‐4 modification of the Burkholderia pseudomallei N‐type ATPase rotor ring.
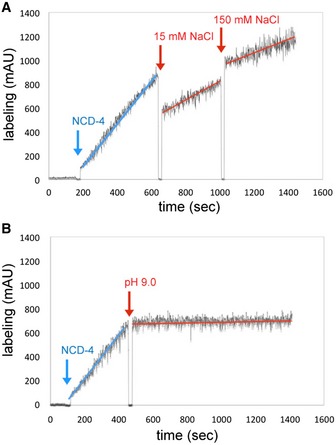
- Addition of 15 and 150 mM NaCl (red arrows) decelerated the reaction due to unspecific salt effects.
- Increasing the pH to 9.0 by adding 2.5 M Tris base stock solution (red arrow) abolished NCD‐4 binding to Glu61. Results of typical experiments are shown. The experiments were repeated three times each.
Figure EV2. Effect of Li+ and Cs+ on the kinetics of the NCD‐4 modification of the Burkholderia pseudomallei N‐type ATPase c‐ring.
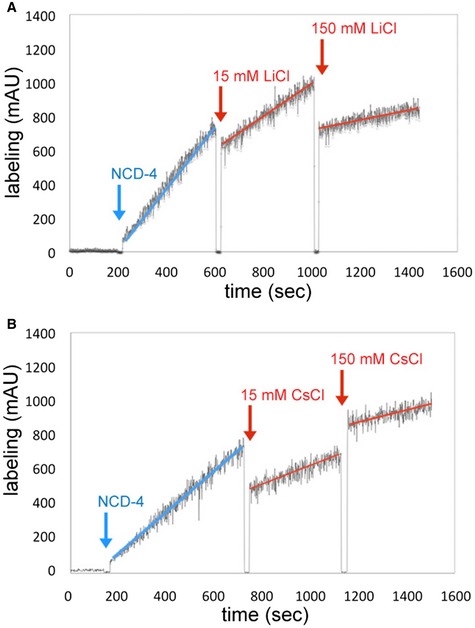
- Addition of 15 or 150 mM LiCl resulted in a drop in labeling efficiency to 55 or 17%.
- Addition of 15 or 150 mM CsCl reduced the labeling efficiency to 44 or 24%, illustrating the effect of salt in the buffer 33 and the overall dilution of the reaction volume. Results of typical experiments are shown. Each experiment was repeated three times.
Single‐particle cryoEM
We proceeded to determine the structure of the rotor ring by single‐particle cryoEM. Like some other rotor rings 14, the B. pseudomallei c‐ring complex proved to be stable in many solubilizing agents tested. We investigated the c‐ring in the three detergents lauryl dimethyl amineoxide (LDAO), dodecyl maltoside (DDM), octaethylene glycol monododecyl ether (C12E8), and the amphipathic polymer surfactant amphipol A8‐35 34. The choice of surfactant proved to be a decisive factor for the success of the analysis and the map resolution obtained. There was a clear correlation between the density of the detergent or amphipol, micelle size, and the resolution achieved (Table EV2).
Notwithstanding the protein complex mass of 141 kDa, which is low for cryoEM, the c‐ring complex was visible in thin layers of amorphous ice (Fig EV3A). A total of 213,000 particles in LDAO, 76,000 in DDM, 115,000 in C12E8, and 226,000 particles in amphipol A8‐35 were picked semi‐automatically. In 2D class averages, top views of rings in LDAO or DDM showed 17 density characteristics of transmembrane α‐helices in projection (Fig 4A and B). In LDAO, two concentric rings of 17 staggered α‐helix densities were resolved clearly (Fig 4B). In C12E8, the 17 α‐helix densities were poorly resolved (Fig 4C), and in amphipol A8‐35, they were not resolved at all (Fig 4D), even though the largest number of particles contributed to the average. Transmembrane helices also stood out in side views of c‐ring complexes solubilized in DDM and especially LDAO, whereas this was not the case in C12E8 or amphipol A8‐35.
Figure EV3. Electron cryo‐microscopy of the Burkholderia pseudomallei N‐type ATPase rotor ring.
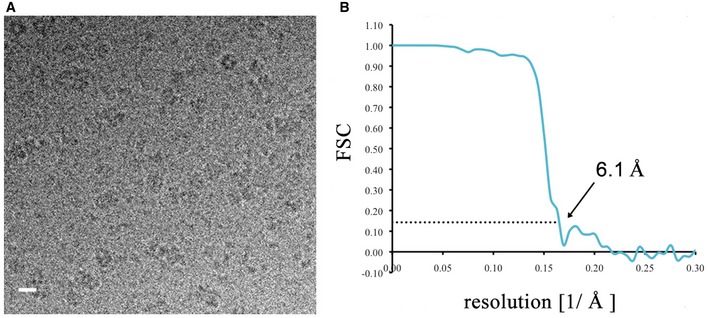
- Representative electron micrograph of rotor rings in vitreous buffer (scale bar, 10 nm).
- Gold‐standard FSC curve 59 for the density map of the rotor ring in LDAO. The resolution at 0.143 FSC was 6.1 Å (arrow).
Figure 4. Electron cryo‐microscopy of the Burkholderia pseudomallei N‐type ATPase rotor ring.
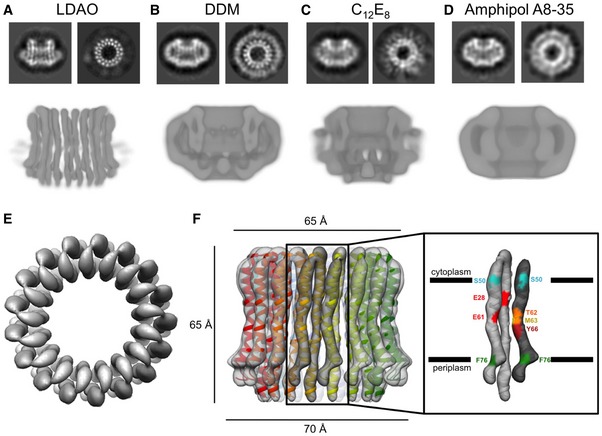
-
A–DRepresentative 2D class averages of the rotor ring showing the ring from the side and top as well as slices through 3D maps after refinement for (A) LDAO, (B) DDM, (C) C12E8, and (D) amphipol A8‐35.
-
ETop view of the rotor ring in LDAO with 17 identical hairpin‐like c‐subunits fitted.
-
FSide view of the c‐ring in LDAO with the refined model fitted. The helices in the outer ring are slightly shorter than the inner helices. Two adjacent c‐subunits form a functional ion‐binding unit. The positions of residues involved in ion binding or defining the membrane boundary are shown in different colors.
The 2D class averages of the top and side views explain why LDAO works best. Due to the small diameter of its micelle, LDAO covers only about half the length of the transmembrane ring helices. The large difference in density between protein and amorphous ice (Table EV2) ensures high contrast of the solvent‐exposed part of the protein, which enables more accurate particle alignment during image processing and hence, higher resolution. Moreover, the density of LDAO is closer to that of amorphous ice than the other surfactants (Table EV2). As a result, contrast matching between solvent and protein 35 in LDAO is minimal and protein contrast is highest, which again helps the alignment accuracy.
3D refinement and particle polishing 36 with the best 2D class averages of LDAO‐solubilized complexes resulted in a 6.1 Å map of the rotor ring (Figs EV3B and 4E and F). The individual α‐helices, which form the 17 identical c‐subunits were well‐resolved. The c‐ring of the B. pseudomallei N‐type ATPase is a cylinder with a height of ~65 Å. Its outer diameter is ~70 Å at the periplasmic side, ~58 Å in the center of the lipid bilayer, and ~65 Å at the cytoplasmic side, giving the rotor a slight hourglass shape that is characteristic of many c‐ring rotors of F‐ and V‐type ATPases 37, 38 (Movie EV1). Its hydrophobic central cavity with a diameter of ~30 Å is presumably filled with lipid 39.
Model building and structure analysis
Seventeen copies of a helix hairpin with the B. pseudomallei polypeptide sequence modeled on the Fusobacterium nucleatum c‐ring structure 26 were fitted to the EM map and refined against the cryoEM density using real‐space‐refinement in Phenix (Fig 4F). The fit of the Cα chain was consistent with the conserved RQPD sequence in the loop connecting the two helices and the conserved pattern of glycine/alanine residues in the N‐terminal helix at the interface to the next c‐subunit in the ring (Fig 1B). It has been shown that if some of the conserved glycine residues are changed to alanine, the helical packing changes, which can result in higher c‐ring stoichiometries 16, 32, 40, 41. The A×G×A×A×G sequence in the N‐terminal helix of the B. pseudomallei c‐ring thus contributes to helix packing and to the unusually high c17 stoichiometry.
Ring architecture
Overall dimensions of the B. pseudomallei c‐ring closely resemble those of the 15‐subunit Spirulina platensis c‐ring 42, even though the former has two more subunits. The inner N‐terminal α‐helices are mostly straight, bending only very slightly toward the central hydrophobic cavity. The outer, C‐terminal helices are S‐shaped, with a slight kink near the ion‐binding site (Glu61) and a sharper kink at Phe76 on the periplasmic membrane surface. On the cytoplasmic side, Ser50 marks the boundary of the hydrophobic, membrane‐embedded region, indicating a lipid bilayer thickness of 35 Å (Fig 4F).
Ion‐binding site
The ion‐binding Glu61 is located near the center of the lipid bilayer. In our B. pseudomallei model, its carboxylate sidechain points outward, as in the X‐ray structures of several F‐type ATPase rotor rings 14. This acidic residue, which is crucial for activity in all rotary ATPases, is surrounded by sidechains involved in Na+ or H+ coordination 32, 37, 42. A second glutamate (Glu30) in the N‐terminal helix points toward the ion‐binding site, reminiscent of c‐ring structures with a two‐carboxylate ion‐binding motif 25, 26. This site has no apparent influence on the NCD‐4 modification reaction (Fig 3). Therefore, as in other c‐rings with the two‐carboxylate motif, this second glutamate is protonated at all times and serves as a hydrogen‐bond donor. Finally, Thr62, Met63, and Tyr66 in the C‐terminal helix are likely to contribute to the hydrogen‐bonding network around the proton‐binding site. Even though, at 6.1 Å, the map does not resolve sidechains, it is safe to state that the c‐rings of N‐type and F‐type ATPases are very similar with respect to overall architecture, including the layout of their ion‐binding sites, allowing us to conclude that both types work essentially in the same way.
Analysis of No subunits in contact with the c‐ring based on sequence comparison
We compared the polypeptide sequences of other subunits in the No subcomplex that are homologous to subunits γ, ε, and a (Fig 1A), which, in F‐type ATPases, are close to or in direct contact with the c‐ring. As expected, critical residues, in particular the functionally essential Arg164 in subunit a 43 and the c‐ring contacting His40 in ε 44 are conserved, as is the overall pattern of hydrophobic and polar sidechains (Fig EV4). Notable exceptions are the residues at the interface between subunit γ and the c‐ring, in particular the conserved motif at Tyr207/Glu208 (Fig EV5). In B. pseudomallei, this residue pair is exchanged against Val/His, and this is likely to affect the c‐ring/γ interaction. In general though, the larger diameter of the c‐ring and higher c‐subunit stoichiometry of B. pseudomallei are not reflected in the primary subunit sequences. We conclude that not only the amino acid sequences but also the structures of the neighboring subunits in the No subcomplex are overall conserved and similar to those of the conventional F‐type ATPases. This includes in particular the lengths of helices 5 and 6 of the a‐subunit that wrap around the c‐ring 7, 8, 9, 10, 11, which can thus adapt to the larger diameter of the B. pseudomallei c17 ring.
Figure EV4. Subunits adjacent to the c‐ring of N‐type ATPase.

Alignment of F‐type and N‐type atpG, atpC, and atpB, comparing sequences of the predicted γ‐, ε‐, and a‐subunits of Burkholderia pseudomallei (BC), Spirulina platensis (SP), Bacillus pseudofirmus OF4 (OF), Ilyobacter tartaricus (IT), and Escherichia coli (EC) having a c17, c15, c13, c11, and c10 ring. Differences were observed in the γ‐subunit of B. pseudomallei.
Figure EV5. Cartoon model of the Escherichia coli γ‐subunit.
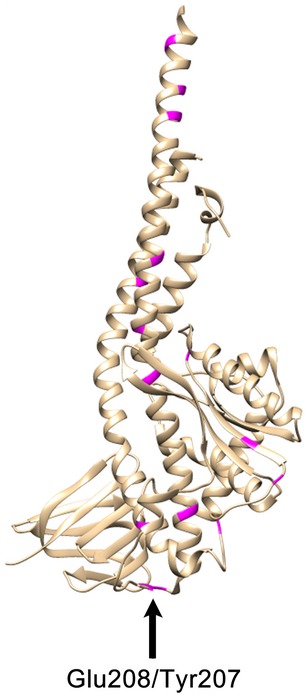
The structure from Cingolani & Duncan 60 was used. The positions of different residues marked in magenta in Fig EV4 are highlighted in the E. coli structure. Glu208 and Tyr207, which are exchanged to Val and His in Burkholderia pseudomallei interact with the c‐ring 61.
Conclusions
We determined the cryoEM structure of a c‐ring of an N‐type ATPase from the bacterium B. pseudomallei. The c‐ring has an unexpected heptadecameric c17 subunit stoichiometry. This finding expands the range of known c‐ring stoichiometries that so far extended from 8 13 to 15 12, and refutes an earlier speculation that N‐type c‐rings would turn out to be on the small side of the range 19.
Each c‐subunit in the B. pseudomallei c17 ring is a hairpin of two α‐helices with an ion‐binding site at the interface between adjacent c‐subunits, as in the F‐type ATPases. The c‐ and K‐rings of the V‐type ATPases from Saccharomyces cerevisiae and Enterococcus hirae, respectively, are even larger, equivalent to 20 single‐hairpin c‐subunits 10, 38. The K‐ring from E. hirae only contains 10 ion‐binding sites, owing to the fact that each K‐subunit is a double‐helix hairpin with only one single acidic ion‐binding glutamate 38. The high degree of sequence similarity between N‐type and F‐type operons supports the notion that an N1No‐type ATPase shares the same structural features and functionalities of an F1Fo‐ATPase. However, given that the B. pseudomallei N‐type ATPase can translocate 17 ions per complete rotation, this results in an unusually high ion‐to‐ATP ratio of 5.7, making it a highly efficient proton pump.
It has been reported that during the early stages of infection that causes melioidosis, B. pseudomallei can exist in acidic, membrane‐bound compartments such as polyphagosomes 45, 46. As the B. pseudomallei genome also encodes a conventional F‐type ATP synthase, we propose that the N‐type rotary ATPase, as a highly efficient ATP‐driven proton pump, enables the bacterium to cope with acid stress and maintain pH homeostasis to survive in this hostile environment, while the F‐type ATP synthase generates ATP by oxidative phosphorylation (Fig 5). In support of this notion is also the fact that the N‐type operon is flanked by genes on chromosome 2 that are involved in the bacterium's virulence, while the F‐type operon resides on chromosome 1 of the B. pseudomallei genome (Fig 1) 22. Furthermore, we also speculate that the c17 ring in the N‐ATPase may also play a role in H+ homeostasis of the bacterial cell during infection, enabling the cell to express a secretion apparatus, which is essential for its phagosome escape 23 (Fig 5, stage 4). Future work is required to address the role of N‐type ATPases in cell homeostasis and pathogenicity in more detail.
Figure 5. Proposed role of the Burkholderia pseudomallei N‐type ATPase in H+ homeostasis and phagosome escape during the early state of infection.
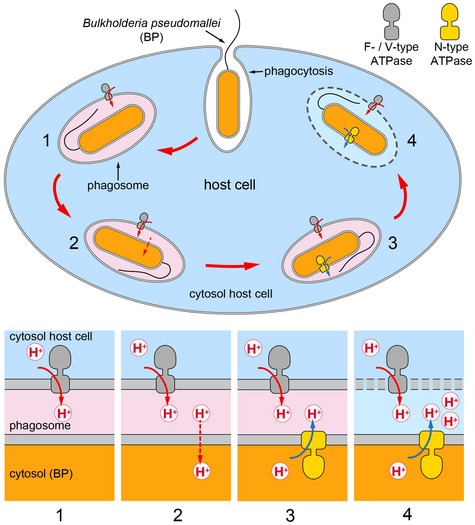
Burkholderia pseudomallei enters macrophage phagosomes that are acidified by a host cell‐specific V‐type ATPases (1). Due to the high pH gradient, protons leak through the cell membrane of B. pseudomallei and acidify the B. pseudomallei cytosol (2). The proposed role of the N‐type ATPase is to redress the internal pH by pumping protons out of the bacterial cell and to maintain protein homeostasis (3). B. pseudomallei expresses secretion apparatus (bsa) system, which is essential for phagosome escape (4). Drawing adapted from Wiersinga et al 23.
Materials and Methods
Cloning of pHERD28T_atpBP
The N‐atp operon was amplified from genomic DNA of B. pseudomallei by four different PCRs. Due to the high GC content (70%), Extender Polymerase (5 Prime) and a combinatorial enhancer solution (CES) were used 47. Four fragments were created containing the restriction sites BglII and NdeI (fragment 1: 2,035 bp), NdeI and ApaI (fragment 2: 1,755 bp), ApaI and SspI (fragment 3: 1,548 bp), and SspI and HindIII (fragment 4: 2,106 bp). The fragments were blunt‐end‐cloned into a pJET1.2 kit for amplification (Fermentas), restricted, and fragments 1 and 2 were assembled with fragments 3 and 4 by simultaneous cloning into a pET24a vector, which was digested with BglII and ApaI or ApaI and HindIII, respectively. By restriction of pET24a_1+2 and pET14a_3+4, two new larger fragments (3,738 bp and 3,596 bp, respectively) were obtained and simultaneously cloned into a pBAD_HisB vector digested with BglII and HindIII. Subsequently, the entire N‐atp operon (7,441 bp) was digested from the pBAD_HisB vector by NcoI and HindIII and ligated in a digested pHERD28T vector resulting in the pHERD_BPN1No vector for expression in B. thailandensis cells under control of the pBAD promotor.
Expression and purification of the Burkholderia pseudomallei N‐type ATPase c‐ring
The B. pseudomallei N‐type ATPase c‐ring was heterologously expressed in E. coli Lemo21 using a pt7‐7 expression vector 48. Protein expression in 2 l LB medium was induced with 0.5 mM IPTG at OD600 of 0.8 and cells were harvested after 4 h of incubation at 37°C under continuous shaking at 120 rpm. Cells were harvested by centrifugation at 5,000 g and 4°C for 30 min and resuspended in 10 ml/l cell culture of 50 mM potassium phosphate buffer (KPB) pH 8.0. Cells were disrupted by several passages through a microfluidizer at 4°C and 1,200 bar in the presence of 1 mM Pefablock (Sigma‐Aldrich, D), 1 mM dithiothreitol (DTT), and traces of deoxyribonuclease A (DNase A). Cell debris was removed by centrifugation for 35 min at 24,000 g and 4°C. Membranes were collected at 230,000 g for 60 min at 4°C and resuspended in 50 mM KPB pH 8.0, 100 mM NaCl, and 10% (v/v) glycerol. The c‐ring was extracted from E. coli membranes with 2% (w/v) N‐lauroylsarcosine for 12 min at 66°C. Contaminating proteins solubilized with the c‐ring were salted out with 70% saturated (NH4)2SO4 in 50 mM Tris–HCl pH 8.0 buffer. The c‐ring was dialyzed against 20 mM Tris/HCl with a pH adjusted to 8.0 at 22°C until the protein precipitated. The c‐ring precipitate was collected by centrifugation and resuspended in 3% (w/v) n‐dodecyl‐β‐D‐maltoside (DDM) and 20 mM Tris/HCl pH 8.0. The protein was further purified by size‐exclusion chromatography (Äkta Purifier) in 20 mM Tris/HCl pH 8.0, 150 mM NaCl, 0.05% (w/v) DDM on a Superose 6 column (GE Healthcare, Munich, D). The eluted protein was concentrated by centrifugation, and the final protein concentration was determined by the BCA assay (Pierce).
NCD‐4 modification reaction of the Burkholderia pseudomallei c‐ring
NCD‐4 modification was performed with a 60 μl sample containing 0.45 mg/ml c‐ring in 20 mM Tris/HCl pH 8.0 and 1.5% (w/v) DDM adjusted to pH 6.0 by the addition of 0.5 M 2‐(N‐morpholino)ethanesulfonic acid (MES)/HCl, pH 5.0. The reaction was started with 100 μM NCD‐4 (Invitrogen) prepared from a stock solution in 10% (w/v) DDM. The continuous increase of fluorescence was monitored in an F‐450 Hitachi Fluorescence Spectrophotometer (λex = 324 nm, λem = 440 nm) at 25°C.
Expression, Western blot analysis, and immunogold labeling of the Burkholderia pseudomallei N‐type ATPase
Vector pHERD_BPN1No was transformed in B. thailandensis E264 for quasi‐homologous expression of the N‐type ATPase. Protein expression was induced with 0.2% arabinose at OD600 of 0.6 and cells were harvested after shaking at 120 rpm for 4 h at 37°C. Cells were harvested at 5,000 g and 4°C for 30 min and resuspended in 10 ml/l culture of 20 mM Tris/HCl buffer pH 8.0. Membrane vesicles were prepared by several passages through a French press at 4°C and 1,000 bar in the presence of 1 mM Pefablock, 1 mM DTT and traces of deoxyribonuclease A. Cell debris was removed by centrifugation at 24,000 g and 4°C for 35 min. Membrane vesicles were harvested at 230,000 g and 4°C for 60 min and resuspended in 20 mM Tris/HCl buffer pH 8.0, 100 mM NaCl, 5% (v/v) glycerol, 5 mM MgCl2, and 0.1 mM Pefablock.
Western blot analysis of the expression was performed with a specific antibody against the ATPase c‐subunit (ATP synthase subunit c, AS09591 Agrisera Antibodies) and an alkaline phosphatase‐conjugated second antibody (anti‐mouse IgG, A4312 Sigma‐Aldrich). Membrane vesicles were labeled with a specific antibody (monoclonal anti‐polyhistidine antibody produced in mouse, H1029 Sigma‐Aldrich) against the His‐tag on the β‐subunit of the N‐type ATPase and a secondary antibody carrying a 8‐nm gold particle. The labeled vesicles were deposited on a carbon‐coated grid, negatively stained with 1% (w/v) uranyl acetate and examined in a CM120 electron microscope (Phillips).
Electron cryo‐microscopy
For electron cryo‐microscopy, 3 μl aliquots of purified c‐ring in amphipol A8‐35, DDM, C12E8, or LDAO at a concentration of 2.5 mg/ml were loaded on glow‐discharged holey carbon grids (Quantifoil R2/2). Grids were plunge‐frozen in a Vitrobot (FEI) at 75% humidity and 10°C with blotting times ranging from 7 to 11 s. Micrographs were collected at liquid nitrogen temperature on a JEOL 3200 FSC electron microscope, operating at 300 kV with an in‐column energy filter and equipped with a K2 Summit direct electron detector camera (Gatan). The slit width was adjusted to 20 eV. Images were recorded in counting mode at a nominal magnification of 30,000×, corresponding to a pixel size of 1.14 Å at the specimen. The total exposure time was 5 s (amphipol A8‐35, DDM and C12E8) or 7 s (LDAO) leading to an accumulated dose of 40–70 electrons per Å2. Each image was fractionated into 25–35 subframes with an accumulation time of 0.2 s. Defocus values in the final datasets range from 1 to 4 μm.
Image processing
Global beam‐induced motion of images was corrected with MotionCorr 49, and the contrast transfer function for each image was determined with CTFFIND3 50. RELION‐1.3 51 was used for automated particle selection and manual deletion of false‐positive and addition of false‐negative particles. c‐ring symmetry was determined by 2D classifications using Relion‐1.3, XMIPP 52 and EMAN 53 with phase‐flipped CTF correction. For 3D reconstruction, a starting model was generated in EMAN 53. Unsymmetrized 2D and 3D classifications were used in Relion‐1.3 to discard false‐positive particles. A subsequent 3D classification was performed with one class, applying 17‐fold symmetry at an angular sampling of 3.75°. The output of the 3D classification of particles in LDAO was used as a starting model for the final 3D autorefinement. Particle‐based beam‐induced movement correction and particle polishing were performed in Relion‐1.3 36, 54 using a running average of seven movie frames and a standard deviation of one pixel for the translational alignments.
Model building and refinement
The WHATIF homology modeling server 55 was used to generate a model of the B. pseudomallei N‐type ATPase rotor ring based on the F. nucleatum subsp nucleatum c‐subunit 26. The two c‐subunit sequences are 46% identical and 76% of all residues are conserved; 17 copies of the homology model were fitted to the EM map in COOT 56 and Chimera 57. The resulting structure was refined in PHENIX 58. The EM data were deposited in the Electron Microscopy Data Bank (EMDB) with accession code EMD‐3546.
Author contributions
SS performed cloning, expression, biochemical experiments, and negative‐stain EM. MW performed electron cryo‐microscopy and data processing. DJM supported the cryoEM work and devised the data collection strategy. TM initiated and supervised the project. SS, MW, WK, and TM interpreted the data and wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Movie EV1
Review Process File
Acknowledgements
We thank Herbert Schweizer and Linnell Randall from Colorado State University for providing the pHERD28T expression vector, Julian Langer (Max Planck Institute of Biophysics, Frankfurt) for MALDI‐MS of the B. pseudomallei c‐ring, and Friederike Joos for immunogold labeling of the N‐type ATPase. This work was funded by the Max Planck Society, the Collaborative Research Centre (SFB) 807 of the German Research Foundation (DFG), the Cluster of Excellence Frankfurt “Macromolecular Complexes” (DFG Project EXC 115), and by the Wellcome Trust (WT110068/Z/15/Z).
EMBO Reports (2017) 18: 526–535
References
- 1. Junge W, Nelson N (2015) ATP synthase. Annu Rev Biochem 84: 631–657 [DOI] [PubMed] [Google Scholar]
- 2. Okuno D, Iino R, Noji H (2011) Rotation and structure of F0F1‐ATP synthase. J Biochem 149: 655–664 [DOI] [PubMed] [Google Scholar]
- 3. Boyer PD (1993) The binding change mechanism for ATP synthase–some probabilities and possibilities. Biochim Biophys Acta 1140: 215–250 [DOI] [PubMed] [Google Scholar]
- 4. Walker JE (2013) The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans 41: 1–16 [DOI] [PubMed] [Google Scholar]
- 5. Stewart AG, Sobti M, Harvey RP, Stock D (2013) Rotary ATPases: models, machine elements and technical specifications. Bioarchitecture 3: 2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cotter K, Stransky L, McGuire C, Forgac M (2015) Recent insights into the structure, regulation, and function of the V‐ATPases. Trends Biochem Sci 40: 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allegretti M, Klusch N, Mills DJ, Vonck J, Kühlbrandt W, Davies KM (2015) Horizontal membrane‐intrinsic alpha‐helices in the stator a‐subunit of an F‐type ATP synthase. Nature 521: 237–240 [DOI] [PubMed] [Google Scholar]
- 8. Hahn A, Parey K, Bublitz M, Mills DJ, Zickermann V, Vonck J, Kühlbrandt W, Meier T (2016) Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol Cell 63: 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morales‐Rios E, Montgomery MG, Leslie AGW, Walker JE (2015) Structure of ATP synthase from Paracoccus denitrificans determined by X‐ray crystallography at 4.0 Å resolution. Proc Natl Acad Sci USA 112: 13231–13236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao JH, Benlekbir S, Rubinstein JL (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V‐ATPase. Nature 521: 241–245 [DOI] [PubMed] [Google Scholar]
- 11. Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, Grigorieff N, Rubinstein JL (2015) Structure and conformational states of the bovine mitochondrial ATP synthase by cryo‐EM. Elife 4: e10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pogoryelov D, Yu J, Meier T, Vonck J, Dimroth P, Müller DJ (2005) The c15 ring of the Spirulina platensis F‐ATP synthase: F1/F0 symmetry mismatch is not obligatory. EMBO Rep 6: 1040–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watt IN, Montgomery MG, Runswick MJ, Leslie AGW, Walker JE (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci USA 107: 16823–16827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meier T, Faraldo‐Gómez JD, Börsch M (2011) ATP synthase—a paradigmatic molecular machine In Molecular machines in biology, Frank J. (ed.), pp 208–238. New York, NY: Cambridge University Press; [Google Scholar]
- 15. Müller DJ, Dencher NA, Meier T, Dimroth P, Suda K, Stahlberg H, Engel A, Seelert H, Matthey U (2001) ATP synthase: constrained stoichiometry of the transmembrane rotor. FEBS Lett 504: 219–222 [DOI] [PubMed] [Google Scholar]
- 16. Pogoryelov D, Klyszejko AL, Krasnoselska GO, Heller EM, Leone V, Langer JD, Vonck J, Muller DJ, Faraldo‐Gómez JD, Meier T (2012) Engineering rotor ring stoichiometries in the ATP synthase. Proc Natl Acad Sci USA 109: E1599–E1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kühlbrandt W, Davies KM (2016) Rotary ATPases: a new twist to an ancient machine. Trends Biochem Sci 41: 106–116 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson SJ (2010) ATP synthase: from sequence to ring size to the P/O ratio. Proc Natl Acad Sci USA 107: 16755–16756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dibrova DV, Galperin MY, Mulkidjanian AY (2010) Characterization of the N‐ATPase, a distinct, laterally transferred Na+‐translocating form of the bacterial F‐type membrane ATPase. Bioinformatics 26: 1473–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soontharapirakkul K, Promden W, Yamada N, Kageyama H, Incharoensakdi A, Iwamoto‐Kihara A, Takabe T (2011) Halotolerant cyanobacterium Aphanothece halophytica contains an Na+‐dependent F1F0‐ATP synthase with a potential role in salt‐stress tolerance. J Biol Chem 286: 10169–10176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabbar Z, Currie BJ (2013) Melioidosis and the kidney. Nephrology (Carlton) 18: 169–175 [DOI] [PubMed] [Google Scholar]
- 22. Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, Ulrich RL, Ronning CM, Brinkac LM, Daugherty SC et al (2004) Structural flexibility in the Burkholderia mallei genome. Proc Natl Acad Sci USA 101: 14246–14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ (2006) Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei . Nat Rev Microbiol 4: 272–282 [DOI] [PubMed] [Google Scholar]
- 24. Walker JE, Saraste M, Gay NJ (1984) The unc operon. Nucleotide sequence, regulation and structure of ATP‐synthase. Biochim Biophys Acta 768: 164–200 [DOI] [PubMed] [Google Scholar]
- 25. Preiss L, Langer JD, Yildiz Ö, Eckhardt‐Strelau L, Guillemont JEG, Koul A, Meier T (2015) Structure of the mycobacterial ATP synthase F0 rotor ring in complex with the anti‐TB drug bedaquiline. Sci Adv 1: e1500106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schulz S, Iglesias‐Cans M, Krah A, Yildiz Ö, Leone V, Matthies D, Cook GM, Faraldo‐Gómez JD, Meier T (2013) A new type of Na+‐driven ATP synthase membrane rotor with a two‐carboxylate ion‐coupling motif. PLoS Biol 11: e1001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu D, Damron FH, Mima T, Schweizer HP, Yu HD (2008) PBAD‐based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Appl Environ Microbiol 74: 7422–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meier T, Dimroth P (2002) Intersubunit bridging by for the unusual stability Na+ ions as a rationale of the c‐rings of Na+‐translocating F1F0 ATP synthases. EMBO Rep 3: 1094–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meier T, Morgner N, Matthies D, Pogoryelov D, Keis S, Cook GM, Dimroth P, Brutschy B (2007) A tridecameric c ring of the adenosine triphosphate (ATP) synthase from the thermoalkaliphilic Bacillus sp. strain TA2.A1 facilitates ATP synthesis at low electrochemical proton potential. Mol Microbiol 65: 1181–1192 [DOI] [PubMed] [Google Scholar]
- 30. Meier T, Yu J, Raschle T, Henzen F, Dimroth P, Müller DJ (2005b) Structural evidence for a constant c11 ring stoichiometry in the sodium F‐ATP synthase. FEBS J 272: 5474–5483 [DOI] [PubMed] [Google Scholar]
- 31. Meier T, Matthey U, von Ballmoos C, Vonck J, Krug von Nidda T, Kühlbrandt W, Dimroth P (2003) Evidence for structural integrity in the undecameric c‐rings isolated from sodium ATP synthases. J Mol Biol 325: 389–397 [DOI] [PubMed] [Google Scholar]
- 32. Preiss L, Yildiz Ö, Hicks DB, Krulwich TA, Meier T (2010) A new type of proton coordination in an F1F0‐ATP synthase rotor ring. PLoS Biol 8: e1000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adenier A, Aaron JJ (2002) A spectroscopic study of the fluorescence quenching interactions between biomedically important salts and the fluorescent probe merocyanine 540. Spectrochim Acta A Mol Biomol Spectrosc 58: 543–551 [DOI] [PubMed] [Google Scholar]
- 34. Zoonens M, Popot JL (2014) Amphipols for each season. J Membr Biol 247: 759–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kühlbrandt W (1982) Discrimination of protein and nucleic acids by electron microscopy using contrast variation. Ultramicroscopy 7: 221–232 [DOI] [PubMed] [Google Scholar]
- 36. Scheres SH (2014) Beam‐induced motion correction for sub‐megadalton cryo‐EM particles. Elife 3: e03665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meier T, Polzer P, Diederichs K, Welte W, Dimroth P (2005a) Structure of the rotor ring of F‐type Na+‐ATPase from Ilyobacter tartaricus . Science 308: 659–662 [DOI] [PubMed] [Google Scholar]
- 38. Murata T, Yamato I, Kakinuma Y, Leslie AG, Walker JE (2005) Structure of the rotor of the V‐type Na+‐ATPase from Enterococcus hirae . Science 308: 654–659 [DOI] [PubMed] [Google Scholar]
- 39. Meier T, Matthey U, Henzen F, Dimroth P, Müller DJ (2001) The central plug in the reconstituted undecameric c cylinder of a bacterial ATP synthase consists of phospholipids. FEBS Lett 505: 353–356 [DOI] [PubMed] [Google Scholar]
- 40. Matthies D, Preiss L, Klyszejko AL, Muller DJ, Cook GM, Vonck J, Meier T (2009) The c13 ring from a thermoalkaliphilic ATP synthase reveals an extended diameter due to a special structural region. J Mol Biol 388: 611–618 [DOI] [PubMed] [Google Scholar]
- 41. Preiss L, Klyszejko AL, Hicks DB, Liu J, Fackelmayer OJ, Yildiz Ö, Krulwich TA, Meier T (2013) The c‐ring stoichiometry of ATP synthase is adapted to cell physiological requirements of alkaliphilic Bacillus pseudofirmus OF4. Proc Natl Acad Sci USA 110: 7874–7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pogoryelov D, Yildiz Ö, Faraldo‐Gómez JD, Meier T (2009) High‐resolution structure of the rotor ring of a proton‐dependent ATP synthase. Nat Struct Mol Biol 16: 1068–1073 [DOI] [PubMed] [Google Scholar]
- 43. Lightowlers RN, Howitt SM, Hatch L, Gibson F, Cox GB (1987) The proton pore in the Escherichia coli F0F1‐ATPase: a requirement for arginine at position 210 of the a‐subunit. Biochim Biophys Acta 894: 399–406 [DOI] [PubMed] [Google Scholar]
- 44. Hermolin J, Dmitriev OY, Zhang Y, Fillingame RH (1999) Defining the domain of binding of F1 subunit e with the polar loop of F0 subunit c in the Escherichia coli ATP synthase. J Biol Chem 274: 17011–17016 [DOI] [PubMed] [Google Scholar]
- 45. Dejsirilert S, Kondo E, Chiewsilp D, Kanai K (1991) Growth and survival of Pseudomonas pseudomallei in acidic environments. Jpn J Med Sci Biol 44: 63–74 [DOI] [PubMed] [Google Scholar]
- 46. Egan AM, Gordon DL (1996) Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect Immun 64: 4952–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ralser M, Querfurth R, Warnatz HJ, Lehrach H, Yaspo ML, Krobitsch S (2006) An efficient and economic enhancer mix for PCR. Biochem Biophys Res Commun 347: 747–751 [DOI] [PubMed] [Google Scholar]
- 48. Tabor S, Richardson CC (1985) A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82: 1074–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li X, Mooney P, Zheng S, Booth CR, Braunfeld MB, Gubbens S, Agard DA, Cheng Y (2013) Electron counting and beam‐induced motion correction enable near‐atomic‐resolution single‐particle cryo‐EM. Nat Methods 10: 584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mindell JA, Grigorieff N (2003) Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol 142: 334–347 [DOI] [PubMed] [Google Scholar]
- 51. Scheres SH (2012) RELION: implementation of a Bayesian approach to cryo‐EM structure determination. J Struct Biol 180: 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marabini R, Masegosa IM, San Martin MC, Marco S, Fernandez JJ, de la Fraga LG, Vaquerizo C, Carazo JM (1996) Xmipp: an image processing package for electron microscopy. J Struct Biol 116: 237–240 [DOI] [PubMed] [Google Scholar]
- 53. Ludtke SJ, Baldwin PR, Chiu W (1999) EMAN: semiautomated software for high‐resolution single‐particle reconstructions. J Struct Biol 128: 82–97 [DOI] [PubMed] [Google Scholar]
- 54. Bai XC, Fernandez IS, McMullan G, Scheres SH (2013) Ribosome structures to near‐atomic resolution from thirty thousand cryo‐EM particles. Elife 2: e00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vriend G (1990) What If—a molecular modeling and drug design program. J Mol Graph 8: 52–56 [DOI] [PubMed] [Google Scholar]
- 56. Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- 58. Zwart PH, Afonine PV, Grosse‐Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, McKee E, Moriarty NW, Read RJ, Sacchettini JC et al (2008) Automated structure solution with the PHENIX suite. Methods Mol Biol 426: 419–435 [DOI] [PubMed] [Google Scholar]
- 59. Henderson R, Sali A, Baker ML, Carragher B, Devkota B, Downing KH, Egelman EH, Feng Z, Frank J, Grigorieff N et al (2012) Outcome of the first electron microscopy validation task force meeting. Structure 20: 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cingolani G, Duncan TM (2011) Structure of the ATP synthase catalytic complex F1 from Escherichia coli in an autoinhibited conformation. Nat Struct Mol Biol 18: 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pogoryelov D, Nikolaev Y, Schlattner U, Pervushin K, Dimroth P, Meier T (2008) Probing the rotor subunit interface of the ATP synthase from Ilyobacter tartaricus . FEBS J 275: 4850–4862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Table EV1
Table EV2
Movie EV1
Review Process File


