Abstract
Target of rapamycin (TOR) promotes reinitiation at upstream ORFs (uORFs) in genes that play important roles in stem cell regulation and organogenesis in plants. Here, we report that the small GTPase ROP2, if activated by the phytohormone auxin, promotes activation of TOR, and thus translation reinitiation of uORF‐containing mRNAs. Plants with high levels of active ROP2, including those expressing constitutively active ROP2 (CA‐ROP2), contain high levels of active TOR. ROP2 physically interacts with and, when GTP‐bound, activates TOR in vitro. TOR activation in response to auxin is abolished in ROP‐deficient rop2 rop6 ROP4 RNAi plants. GFP‐TOR can associate with endosome‐like structures in ROP2‐overexpressing plants, indicating that endosomes mediate ROP2 effects on TOR activation. CA‐ROP2 is efficient in loading uORF‐containing mRNAs onto polysomes and stimulates translation in protoplasts, and both processes are sensitive to TOR inhibitor AZD‐8055. TOR inactivation abolishes ROP2 regulation of translation reinitiation, but not its effects on cytoskeleton or intracellular trafficking. These findings imply a mode of translation control whereby, as an upstream effector of TOR, ROP2 coordinates TOR function in translation reinitiation pathways in response to auxin.
Keywords: endosomes, phosphorylation, phytohormone auxin, S6K1, signal transduction
Subject Categories: Plant Biology, Protein Biosynthesis & Quality Control, Signal Transduction
Introduction
Target of rapamycin (TOR) is a main sensor of cell growth in response to nutrients, energy status, and growth factors and is conserved from humans to yeasts and plants. Mammalian TOR (mTOR) occurs in two structurally and functionally distinct complexes: TOR complex 1 (TORC1) and TOR complex 2 (TORC2) (Zoncu et al, 2011; Shimobayashi & Hall, 2014). mTORC1—comprising mTOR, raptor, and mLST8—is sensitive to the immunosuppressant drug rapamycin and regulates cell growth by activating ribosome biogenesis, transcription, and protein synthesis (Hara et al, 2002; Loewith et al, 2002). mTORC2 mediates cell metabolism and cytoskeletal organization (Cybulski & Hall, 2009). The mTORC1 pathway promotes 5′‐cap‐dependent translation via phosphorylation of ribosomal protein S6 kinases (mS6Ks) and eIF4E‐binding proteins (m4E‐BPs) (Ma & Blenis, 2009; Sonenberg & Hinnebusch, 2009). The key eukaryotic translation initiation factor 3 (eIF3) (Hinnebusch, 2006) serves as a scaffold for mS6K phosphorylation by mTOR (Ma & Blenis, 2009). When activated, TOR binds and phosphorylates eIF3‐bound S6K1, triggering its dissociation from eIF3 and further activation (Holz et al, 2005). The pathways leading to mTOR activation seem to depend on a group of small GTPases, including Rheb (Ras homologue enriched in brain; Long et al, 2005), Rac1 (Saci et al, 2011), and Rag (Betz & Hall, 2013), that play a variety of roles within cells (Tee & Blenis, 2005; Sancak et al, 2008). The ribosome is an upstream mTORC2 effector in yeast and mammals, and thus can trigger its activation (Zinzalla et al, 2011).
Plant TOR has multifaceted roles in plant growth and homeostasis. The Arabidopsis genome contains a single essential TOR gene, down‐regulation of which correlates with decreased plant size, resistance to stress (Menand et al, 2002; Deprost et al, 2007), and elevated life span (Ren et al, 2012). Arabidopsis RAPTOR and LST8 are structural and functional components of the TORC1 (Mahfouz et al, 2006; Dobrenel et al, 2011; Moreau et al, 2012). The best‐characterized substrate of TORC1 in plant translation is S6K1 (Schepetilnikov et al, 2011; Xiong & Sheen, 2012); indeed, Arabidopsis plants silenced for TOR expression display significantly reduced polysome abundance (Deprost et al, 2007), suggesting a role for TOR in plant translational control.
We have previously characterized a novel regulatory TOR function in plant translation (Schepetilnikov et al, 2013). TOR is critically required for translation reinitiation of mRNAs that harbor upstream open reading frames within their leader regions (uORF‐mRNAs). Such mRNAs encode many potent proteins such as transcription factors, protein kinases, cytokines, and growth factors (Schepetilnikov et al, 2013); defects in translation of uORF‐mRNAs result in severe developmental anomalies (Zhou et al, 2010). Reinitiation is usually less efficient than initiation at the first ORF and occurs mainly after translation of short uORFs (Kozak, 2001); thus, the latter can be used to down‐modulate the production of critical effector proteins. Mutants of subunit h of the reinitiation factor eIF3 (eIF3h) compromise translation reinitiation on uORF‐mRNAs without affecting initiation events (Kim et al, 2004), and eIF3h functions in reinitiation under the control of TOR (Schepetilnikov et al, 2013). To promote reinitiation events, active TOR binds preinitiation complexes and polyribosomes to maintain the phosphorylation status of eIF3h (Schepetilnikov et al, 2013). Recently, it was demonstrated that translational control at uORFs plays a key role in Arabidopsis stem cell regulation and organogenesis (Zhou et al, 2014). Plant TOR is activated in response to glucose (Xiong et al, 2013), the plant hormone auxin (Schepetilnikov et al, 2013), and the pathogenicity factor, cauliflower mosaic virus (CaMV) protein TAV (Schepetilnikov et al, 2011), via as yet uncharacterized signal transduction pathways. Auxin is an important regulator of plant developmental processes that can act via activation of members of a multigenic family of 11 small ROP (Rho‐like GTPases from plants) GTPases (Winge et al, 1997; Vanneste & Friml, 2009). ROPs function in cellular signaling by regulating, among other things, cell shape and auxin responses (Xu et al, 2010). Given that auxin has been suggested as an upstream signal that can trigger ribosomal protein S6 (rpS6) kinase phosphorylation (Beltrán‐Peña et al, 2002; Turck et al, 2004), and was directly implicated in TOR phosphorylation and activation of the TOR pathway toward translation (Schepetilnikov et al, 2013), we focus on the relationships and possible links between small ROP GTPases and TOR.
Here, we report that ROP2 and TOR interact physically in vitro and that ROP2 GTPase, if active, triggers TOR phosphorylation, activating the TOR signaling pathway and translation of a highly controlled class of mRNAs harboring regulatory uORFs. Our results uncover a novel mechanism of translation reinitiation control involving small GTPase ROP2 via TOR.
Results
TOR associates with ROP2 via direct binding
Given that auxin activates both TOR protein kinase (Schepetilnikov et al, 2013) and plasma membrane‐associated ROPs—particularly ROPs 2 and 6 in Arabidopsis (Xu et al, 2010; Fig 1A)—we asked whether TOR and ROPs interact. In Arabidopsis, RAC/ROPs are encoded by 11 genes that comprise a closely related, multigenic family; ROPs 2, 4, and 6 form a distinct subgroup in a phylogenetic tree based on 11 Arabidopsis ROP sequences (Fig 1B). First, using the yeast two‐hybrid system, we found specific interactions of TOR with ROPs 2, 4, and 6 (Fig 1C). We selected ROP2—the most abundant of the ROP GTPases according to the Genevestigator database (Fig EV1A)—to further examine its association with TOR. Strikingly, GST‐ROP2 binds recombinant TOR physically in a GST pull‐down assay, but interacts neither with Arabidopsis GTPase Sar1b, which is unrelated to Rho GTPases and functions in ER‐Golgi trafficking (Bar‐Peled & Raikhel, 1997; Jones et al, 2003), nor with GST alone, and only weakly with human GTPase Rheb (Fig 1D), indicating plant specificity in TOR binding. Although ROPs shuttle between a GTP‐bound active form and a GDP‐bound inactive form, our GST pull‐down approach suggested that Arabidopsis TOR can interact with GTP, the non‐hydrolyzable analogue guanylyl‐imidodiphosphate (GMP‐PNP), and GDP‐bound GST‐ROP2 (Appendix Fig S1A). Third, we determined that TOR and ROP2 co‐immunoprecipitate; endogenous ROPs co‐immunoprecipitated with green fluorescent protein‐tagged TOR (GFP‐TOR) in 35S:GFP‐TOR expressing Arabidopsis, but not with GFP (35S:GFP line; Fig 1E; production of complete TOR in the 35S:GFP‐TOR transgenic line was confirmed by sequence coverage identified from MS/MS data; Appendix Fig S1B). In planta, endogenous ROPs co‐immunoprecipitated with endogenous TOR using anti‐TOR, but not control rabbit serum (NRS; Fig 1F). Thus, ROP2 was identified as a direct TOR interactor in vitro that associates with TOR‐containing complexes in Arabidopsis.
Figure 1. Identification of ROP2 as a binding partner of TOR .

- Schematic representation of Arabidopsis TOR (S2424 phosphorylation site indicated) and ROP functional domains (G domains, the positions of Q64 and D121, and C‐terminal basic K/R‐CaaL motifs are indicated).
- Phylogenetic tree of 11 Rop family member proteins. ROPs 2, 4, and 6 are classified in a subgroup (red).
- ROP2, ROP4, and ROP6 identified as putative TOR interactors by the yeast two‐hybrid (Y2H) system. BD‐ROPs 1–6 were assayed for interaction with AD‐TOR. Equal OD600 units and 1/10 and 1/100 dilutions were spotted from left to right.
- GST pull‐down assay: ROP2‐, Rheb‐, Sar1b‐tagged GST, and GST alone were assayed for interaction with recombinant TOR as indicated on the left panel. GST‐fusion protein bound (B) and unbound (U) fractions were stained by Coomassie blue.
- Immunoprecipitation (IP) experiments with anti‐GFP‐Trap magnetic beads on crude extracts of GFP‐TOR and GFP transgenic plants; for Western blots, 10% of the input and 100% of IP fractions were analyzed with anti‐GFP, anti‐TOR, and anti‐ROP antibodies (Abs).
- Endogenous TOR was immunoprecipitated from Arabidopsis extract with anti‐TOR Abs (IP) and assayed for association with ROPs by immunoblotting. 10% of the input and 100% of IP or normal rabbit serum (NRS) were analyzed by anti‐ROP antibodies.
- Y2H: TOR and its N‐terminal domain (NTOR) interact with ROP2 and dominant‐negative ROP2 (DN‐ROP2). AD‐TOR, ‐NTOR, and ‐CTOR were assayed for interaction with BD‐ROP2 or ‐ROP2 mutants ‐CA‐ROP2 and ‐DN‐ROP2 as indicated.
- Left panel: GST pull‐down assay: ROP2‐, DN‐ROP2‐, CA‐ROP2‐tagged GST, and GST alone were assayed for interaction with recombinant TOR. Fractions were stained by Coomassie blue. Right panel: Quantification of TOR binding to GST‐fusion proteins. The value for TOR binding to GST‐ROP2 was set as 100%. Statistical analysis is based on one‐way ANOVA test. Data are presented as mean ± SEM (P < 0.05, n = 3).
Source data are available online for this figure.
Figure EV1. Characterization of ROPs 1–6 from Arabidopsis .

- ROPs 1–6 transcription profiles were taken from the Genevestigator database (http://www.genevestigator.ethz.ch).
- Alignment of the C‐terminal tail patterns of ROPs 1–6 from Arabidopsis and human RAC1. Two motifs are indicated: basic lysine residues (motif I), and a CxxL (x = aliphatic amino acid) geranylgeranylation motif (motif II). Two deletion variants used are indicated by solid lines. Alignment done in agreement with BLOSUM 62 and Jonson amino acid substitution matrices (similar residues are printed in reverse type).
- GST pull‐down assay: ROP2‐, ROP2ΔII‐, ROP2Δ(I+II)‐tagged GST, and GST alone were assayed for interaction with recombinant TOR. Fractions were stained by Coomassie blue. Right panel: Quantification of TOR binding to GST‐fusion proteins. Results shown represent the means obtained in three independent experiments, and error bars indicate SD. The value for TOR binding to GST‐ROP2 was set as 1.
Source data are available online for this figure.
We next delineated the region of TOR responsible for binding ROP2: The N‐terminal half of TOR (NTOR), but not the TOR C‐terminus, interacts with ROP2 in the yeast two‐hybrid system (Fig 1G). To determine whether GTP charging is critical for ROP2 binding to TOR, we assayed NTOR interactions with both constitutively active GTP‐bound ROP2 (CA‐ROP2) and the dominant‐negative nucleotide‐free ROP2 (DN‐ROP2). CA‐ROP2 carries a ROP2‐Q64L mutation that abolishes GTP hydrolysis, thus keeping ROP2 in the GTP‐bound active state, while a mutation in the consensus aspartate in the G4 motif (ROP2‐D121N) results in lowered nucleotide affinity (Wu et al, 2001; Berken & Wittinghofer, 2008) (Fig 1A). Our results indicate that nucleotide‐free DN‐ROP2 binds to both TOR and NTOR in the yeast two‐hybrid system (Fig 1G). Moreover, CA‐ROP2 binds TOR and NTOR only weakly or not at all in our conditions. Accordingly, TOR interacts reproducibly more strongly with nucleotide‐free ROP2 than with ROP2 or CA‐ROP2 GST fusions in GST pull‐down assays (Fig 1H). This is similar to the human GTPase Rheb, whose binding affinity to TOR is reduced by GTP charging to enable the TOR complex to adopt a configuration that is catalytically active, when GTP‐bound Rheb activates mTOR (Long et al, 2005). Rac1, another member of the Rho family of GTPases, which also binds directly to mTOR in a GTP‐bound state‐independent manner through the C‐terminal RKR stretch of amino acids (Saci et al, 2011), facilitates mTOR localization to cellular membranes. A similar motif involving the four basic lysine residues (motif I) is found at the C‐terminus of ROPs 1–6 (Fig EV1B) upstream of a CxxL (x = aliphatic amino acid) geranylgeranylation motif (motif II) required for plasma membrane targeting (Fu et al, 2005, 2009; Sorek et al, 2011; Xu et al, 2014). Deletion of motif II alone did not affect binding of ROP2 to TOR (Fig EV1C), while deleting a longer fragment involving both motifs I and II impaired this interaction, suggesting that ROP2 binds TOR through the C‐terminal polylysine stretch of amino acids.
TOR is up‐regulated in plants with elevated levels of GTP‐bound ROP2
To study whether ROP2 and TOR can functionally interact, we examined the effect of ROP2 on TOR phosphorylation status. To test TOR activation, we measured levels of TOR phosphorylated at S2424 using anti‐(mTOR‐S2448‐P) antibodies [mTOR S2448 epitope can be aligned with the S2424 epitope in Arabidopsis TOR (Schepetilnikov et al, 2013)]. Anti‐(mTOR‐S2448‐P) antibodies specifically recognize both wild‐type Arabidopsis TOR and its phosphorylation mimic TOR‐S2424D transiently expressed in Arabidopsis suspension culture protoplasts (Appendix Fig S2A). In contrast, a TOR‐specific phosphorylation site knockout (S2424A) diminished TOR recognition to endogenous levels. Accordingly, phosphorylation of S6K1 overexpressed in protoplasts, at the TOR‐specific hydrophobic motif residue T449 [anti‐(mS6K1‐T389‐P) antibodies] (Schepetilnikov et al, 2011; Xiong & Sheen, 2012), increased strongly upon overexpression of TOR or a TOR phosphorylation mimic. Unlike TOR‐S2424D, overexpression of TOR‐S2424A did not promote S6K1 phosphorylation at T449, suggesting that phosphorylation of S2424 contributes to Arabidopsis TOR activation. These results demonstrate that mTOR‐S2448‐P antiserum is specific for TOR phosphorylated at S2424.
We demonstrated earlier that external auxin treatment of Arabidopsis seedlings promotes TOR activation (Schepetilnikov et al, 2013). As expected, incubation of 7 days after germination (dag) seedlings with auxin analogue 1‐naphthylacetic acid (NAA) promoted TOR phosphorylation that was abolished by a second generation TOR inhibitor, AZD‐8055, regardless of NAA treatment (Fig EV2A). AZD‐8055 binds to the TOR kinase domain within the ATP‐binding pocket and inactivates TOR (Chresta et al, 2010; Montane & Menand, 2013). Next, we examined yuc1D (renamed from yucca; Zhao et al, 2001) and curlyfolia1D (cuf1D; Cui et al, 2013) plants characterized by high spatial auxin accumulation, which both exhibited pointed and slightly curled downward leaves (Fig EV2B). Importantly, significantly elevated TOR phosphorylation levels were observed in extracts from yuc1D and cuf1D as compared with that of WT plants (Fig EV2C). To examine levels of active GTP‐bound ROPs in yuc1D and cuf1D plants, we used a pull‐down assay with a ROP‐interactive CRIB motif‐containing protein 1 (Ric1) that specifically targets activated forms of RAC/ROPs and compared our results to those obtained with CA‐ROP2 (Wu et al, 2000; Miyawaki & Yang, 2014). Indeed, Ric1 fused to GST (GST‐Ric1), but not GST alone, interacted preferentially with recombinant ROP2 charged with the non‐hydrolyzable GTP analogue GMP‐PNP, but not with ROP2 preincubated with GDP (Appendix Fig S2B). As shown in Fig EV2C (right panels), this approach revealed elevated levels of ROPs‐GTP in extracts from both yuc1D and cuf1D as compared with that of WT plants, where a statistically more significant increase of active ROP2 levels was demonstrated in cuf1D. Thus, to further establish functional interaction between ROP2 and TOR in planta, we employed Arabidopsis mutants cuf1D and CA‐ROP2, the latter being transgenic for constitutively active GTP‐bound ROP2 (CA‐ROP2). Note that the phenotypes of CA‐ROP2 and cuf1D are similar (Fig 2A). We correlated endogenous GTP‐bound ROP levels pulled down by GST‐Ric1 from cuf1D and WT, or CA‐ROP2 and WT extracts (Fig 2B). As expected, CA‐ROP2 displayed strongly elevated GTP‐bound ROP2 levels due to ROP2 mutant overexpression. GTP‐bound ROP2 levels in the cuf1D mutant were elevated as compared to WT plants. There were no obvious differences in the levels of mRNA encoding ROP and TORC1 components between cuf1D and WT plants (Fig 2C), suggesting that GTP‐bound ROP levels are elevated through a posttranscriptional mechanism.
Figure EV2. TOR phosphorylation at S2424 is elevated in response to auxin and in plants with high endogenous auxin levels.

- WT seedlings were treated with either NAA or AZD‐8055, or TOR was inactivated by AZD‐8055 in seedlings treated with NAA for 8 h. TOR levels and its phosphorylation status were analyzed by immunoblot with anti‐AtTOR Abs (anti‐TOR) and anti‐(mTOR‐S2448‐P) Abs, respectively. Loading control was stained with Coomassie blue. Right: Quantification of ratio between TOR‐P and TOR. The value for TOR‐P/TOR in WT was set as 1.
- Rosettes representative of WT, yuc1D, and cuf1D plants.
- Analysis of active TOR and ROPs‐GTP levels in WT, yuc1D, and cuf1D 7‐dag seedlings. Input: Total ROPs, TOR total, and its phosphorylation levels were analyzed by Western blot. GST‐Ric1 and loading control were stained with Coomassie blue. Bottom left: Quantification of ratio between TOR‐P and TOR (n = 3). The value for TOR‐P/TOR in WT was set as 1. Right panels: Analysis of active ROPs by GST‐Ric1 IP in seedling extracts. GST‐Ric1‐bound ROPs were detected using anti‐ROP antibodies. Quantification of ratio between ROPs‐GTP and GST‐Ric1. The value for ROPs‐GTP/GST‐Ric1 in WT was set as 1.
Figure 2. TOR signaling is up‐regulated in Arabidopsis with elevated active ROP2 levels.

- Rosettes representative of WT, cuf1D, and CA‐ROP2 plants.
- GST‐Ric1 (or GST) pull‐down IP assays targeting active GTP‐bound ROPs in WT, cuf1D, and CA‐ROP2 seedlings. Active ROPs and total ROPs were detected with an anti‐ROP Abs. GST‐Ric1 and a loading control (LC) were stained with Coomassie blue.
- The level of endogenous mRNAs, including actin (ACT), glycerol‐3‐phosphate dehydrogenase C2 (GAPC2), expressed protein (EXP), and others indicated below the bar graphs in WT and cuf1D, was examined by qRT–PCR. The RNA value in WT extracts was set as 100%.
- TOR and S6K1 levels and their phosphorylation status in either cuf1D and WT, or CA‐ROP2 and WT were analyzed by immunoblot with anti‐AtTOR Abs (anti‐TOR), anti‐(mTOR‐S2448‐P) and anti‐mS6K1, and anti‐(mS6K1‐T389‐P) Abs, respectively. The density of bands on Western blots was quantified, and WT values were set as 100%.
- Left panel: Analysis of active ROPs by GST‐Ric1 IP in WT and CA‐ROP2 extracts from 7‐dag seedlings grown with or without 1 μM AZD‐8055. Total ROPs and GST‐Ric1 bound ROPs were detected using anti‐ROP antibodies. GST‐Ric1 and LC were stained with Coomassie blue. Input: Total ROPs, TOR total, and its phosphorylation levels were analyzed by Western blot. Right panel: Quantification of ratio between ROPs‐GTP and GST‐Ric1. The value for ROPs‐GTP in WT and CA‐ROP2 was set as 1.
Next, both mutants characterized by high levels of GTP‐bound ROPs were used to assess the phosphorylation status of TOR and its downstream target S6K1. We confirmed that phosphorylation of TOR at S2424 in CA‐ROP2 as well as in cuf1D extracts prepared from 7‐dag seedlings was greatly elevated as compared with WT extracts (Fig 2D). We also observed a significant increase in S6K1 phosphorylation at the TOR‐responsive motif residue T449 in cuf1D and CA‐ROP2 as compared with WT plants. This further confirms that TOR signaling is up‐regulated in extracts with high GTP‐bound ROP2 levels. There was no significant difference in TOR protein levels between mutants and the corresponding WT extracts. Accordingly, TOR phosphorylation at S2424 was abolished by AZD‐8055 in WT and CA‐ROP2 seedlings (Fig 2E). Given that AZD‐8055 treatment of WT and CA‐ROP2 plants only slightly altered GTP‐bound total ROP levels (Fig 2E, right panel), TOR could be considered dispensable for ROP activation.
To show directly that ROP2 induces TOR signaling activation in response to auxin treatment, we depleted ROPs 2, 4, and 6 (Fig 3A and B; Ren et al, 2016). First, we found that knockout of only ROP2 substantially reduces the level of ROPs immunoprecipitated by TOR to levels similar to that observed in rop2 rop6 and rop2 rop6 ROP4 RNAi plants, strongly suggesting that ROP2 plays a pivotal role in TOR association (Fig 3C). Time‐course analysis revealed that the levels of phosphorylated TOR in WT plants increased eightfold in response to auxin (Fig 3D). Importantly, induction of TOR by auxin was abolished in rop2 rop6 ROP4 RNAi, although the initial level of phosphorylated TOR in ROP‐deficient extract was somewhat higher than in WT plants (Fig 3D), possibly due to induction of other TOR upstream effectors. Taken together, these results demonstrate that ROP2 largely mediates the activation of TOR in response to auxin.
Figure 3. ROP2 mediates auxin signaling toward TOR .
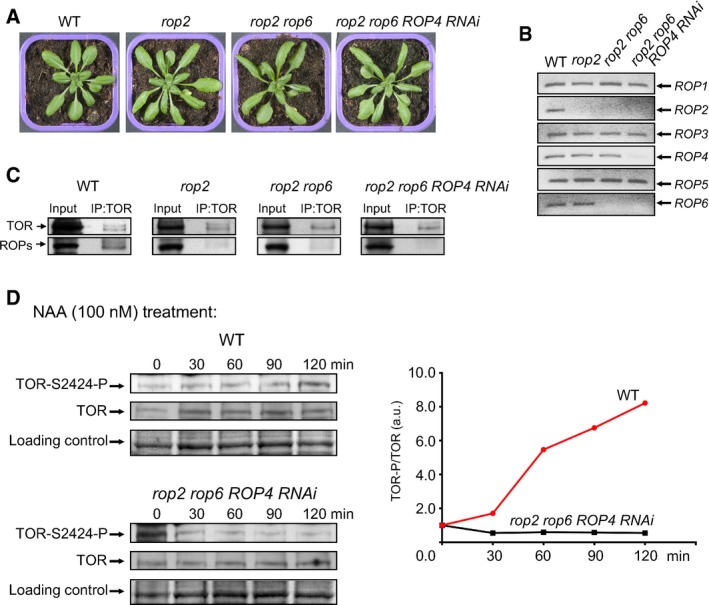
- Rosettes representative of WT, rop2, rop2 rop6, and rop2 rop6 ROP4 RNAi plants.
- The level of endogenous ROP mRNAs in different ROP‐deficient plants was examined by a semiquantitative RT–PCR.
- Endogenous TOR was immunoprecipitated from WT and ROP‐deficient Arabidopsis extracts by immunoblotting with anti‐TOR Abs (IP) and assayed for association with ROPs. 10% of the input and 100% of IP were analyzed by anti‐ROP antibodies.
- Time‐course of TOR and TOR‐P accumulation in extracts from 7‐dag seedlings before (0 min) and after transfer to medium with NAA analyzed by immunoblot with anti‐TOR and anti‐(mTOR‐S2448‐P) Abs. The value of TOR‐P/TOR at 0 min for each line was set as 1.
Source data are available online for this figure.
We also crossed CA‐ROP2 with the GFP‐TOR line (Fig 4) to test how GFP‐TOR phosphorylation is affected by high CA‐ROP2 levels in planta. Although the phenotype of GFP‐TOR/CA‐ROP2 resembles that of CA‐ROP2 (Fig 4A), there was no obvious difference in TORC1 component mRNA levels other than the expected increase in ROP2 mRNA levels (Fig 4B). Note that we used specific primers to discriminate between GFP‐TOR and endogenous TOR mRNAs (Appendix Fig S3). However, the TOR phosphorylation level in CA‐ROP2/GFP‐TOR was elevated by about ninefold above that in GFP‐TOR (Fig 4C), again showing that GTP‐bound ROP2 boosts TOR phosphorylation.
Figure 4. GFP‐TOR in CA‐ROP2 background is highly phosphorylated and functionally and developmentally active.

- Rosettes representative of WT, GFP‐TOR, CA‐ROP2, and GFP‐TOR/CA‐ROP2 plants.
- The level of endogenous mRNAs including endogenous TOR (TOR end) and both GFP‐TOR and TOR end (TOR mix) and others indicated below the bar graphs in GFP‐TOR and GFP‐TOR/CA‐ROP2 was examined by qRT–PCR. The RNA value in GFP‐TOR extracts was set as 100%. Values, expressed in arbitrary units, are averages of three technical replicates, and error bars indicate SD.
- Total and active ROP and TOR levels, and TOR phosphorylation status in either GFP‐TOR or GFP‐TOR/CA‐ROP2 were analyzed as described in Fig 2B and D, respectively.
- In vitro phosphorylation kinetics of recombinant S6K1 (S6K1) using GFP‐TOR immunoprecipitated from GFP‐TOR or GFP‐TOR/CA‐ROP2. S6K1 total and phosphorylation levels were followed by Western blot using anti‐S6K1‐T389‐P or anti‐S6K1 Abs. Total S6K1 was stained by Coomassie blue.
- Representative images of pavement cell (PC) morphology in the second true leaf of 21‐day‐old WT plants and the GFP‐TOR, CA‐ROP2, and GFP‐TOR/CA‐ROP2 mutant lines. Scale bars are 30 μm. Bottom panel: Quantitative analysis of cell circularity (left) and cell sizes (right) is shown as box and whisker plots with median, lower Q1 and upper Q3 quartiles, error bars indicating minimum to maximum range, and presented using unpaired t‐test. *P < 0.05; **P < 0.001; ns, non‐significant (n = 46).
Source data are available online for this figure.
To assay kinase activity of TOR immunoprecipitated from either GFP‐TOR/CA‐ROP2 or GFP‐TOR extracts (TOR IP), we compared recombinant S6K1 phosphorylation at TOR‐responsive T449 in vitro using equal amounts of total TOR IP. Consistently, a higher kinase activity of GFP‐TOR was found in GFP‐TOR/CA‐ROP2 (Fig 4D). Taken together, these results suggest that GTP‐bound ROP2 is a putative candidate to impact TOR signaling activation.
When active, ROP2, together with its binding partner RIC4, interacts with the actin cytoskeleton and promotes the lobing of epidermal pavement cells in Arabidopsis leaves, increasing their circularity (Fu et al, 2002; Fig 4E cf. CA‐ROP2 versus WT). In GFP‐TOR, the size of the epidermal pavement cells is reproducibly increased as expected for a TOR overexpressor (Menand et al, 2002), while their shape remains unaffected (Fig 4E cf. WT versus GFP‐TOR). Accordingly, CA‐ROP2/GFP‐TOR cells are reproducibly bigger, but their circularity is similar to that in CA‐ROP2. Moreover, GFP‐TOR overexpression, as well as its activation by CA‐ROP2, did not influence further the lobe‐promoting ROP2 function in cytoskeleton rearrangements, but rather promoted cell growth.
ROP2 promotes TOR accumulation close to the cell periphery
ROPs associate closely with plasma membrane due to a prenylation motif II at the C‐terminus (Sorek et al, 2011). Results showing that ROP2 interacts physically and functionally with TOR suggest that ROP2 may function in regulating relocation of TOR to the plasma membrane. Microscopic observation showed that transiently expressed GFP‐TOR was distributed diffusely in the cytoplasm in Nicotiana benthamiana cells, mainly at the cell periphery, but appeared as multiple dots upon co‐expression with myc‐ROP2, and especially with myc‐CA‐ROP2 (Fig 5A). Moreover, the number and size of GFP‐TOR dots increased upon co‐expression of myc‐DN‐ROP2 (Fig 5A, bottom panels). To locate GFP‐TOR dots between the cell periphery and the perinuclear region, we realized a series of confocal cross sections, 0.95 μM in depth, from the top to the central section of cells overexpressing both GFP‐TOR and myc‐DN‐ROP2 (Appendix Fig S4A). GFP‐TOR dots close to the cell periphery/plasma membrane disappeared from view and reappeared progressively toward the central section, strongly suggesting GFP‐TOR localization proximal to the plasma membrane. Note the levels of myc‐tagged ROP2, DN‐ROP2, and CA‐ROP2 production in N. benthamiana (Fig 5B).
Figure 5. ROP2 determines TOR appearance as multiple dots close to the cell periphery in an ROP2 C‐terminus‐dependent fashion.

-
ANicotiana benthamiana epidermal cells transiently expressing GFP‐TOR (left panel) and co‐transformed (from left to right) with myc‐ROP2, or myc‐CA‐ROP2, or myc‐DN‐ROP2. Quantitative analysis of GFP‐TOR aggregate number (box and whiskers plot, left; P < 0.05, n = 6) and sizes (scatter plot, right; P < 0.0001, n = 6). The box and whisker plot is with median, lower Q1 and upper Q3 quartiles, error bars indicating minimum to maximum range. Statistical analysis is based on one‐way ANOVA test.
-
BImmunoblot analysis with anti‐myc or anti‐GFP of transiently co‐expressed GFP‐TOR without or with myc‐ROP2, myc‐CA‐ROP2, or myc‐DN‐ROP2 in N. benthamiana cells.
-
C–FFluorescence micrographs showing N. benthamiana cells transiently expressing: (C) GFP‐TOR; (D) GFP‐TOR (upper left), RFP‐ROP2 (upper center), GFP‐TOR (middle left), RFP‐CA‐ROP2 (middle center), GFP‐TOR (bottom left), and RFP‐DN‐ROP2 (bottom center); (E) RFP‐ROP2 (left), RFP‐ROP2∆II (CAFL) (center), and RFP‐ROP2Δ(I+II) (right); (F) GFP‐TOR (upper left), RFP‐ROP2 (upper center), GFP‐TOR (middle left), RFP‐ROP2ΔII (middle center), GFP‐TOR (bottom left), and RFP‐ROP2Δ(I+II) (bottom center). Merged images are shown on the right.
-
GImaging fluorescence assays showing root cells of Arabidopsis 7‐dag GFP‐TOR and GFP‐TOR/CA‐ROP2 cells.
-
HIntracellular distribution of TOR and active TOR. Western blot analysis of various fractions following microsome isolation from GFP‐TOR and GFP‐TOR/CA‐ROP2. The total homogenate (total), nuclear fraction pellet (P10), pellet (P30), pellet (P100), supernatant (S100) were analyzed by Western blot with corresponding antibodies.
Next, we investigated the subcellular co‐localization of GFP‐TOR and red fluorescent protein‐tagged ROP2 (RFP‐ROP2) expressed transiently in N. benthamiana cells. GFP‐TOR was distributed diffusely, mainly at the cell periphery (Fig 5C). In contrast, when GFP‐TOR was co‐expressed together with RFP‐ROP2 or RFP‐CA‐ROP2, GFP‐TOR appeared as small dots on the periphery of epidermal cells, close to the plasma membrane (Fig 5D). Although ROP2 overexpression induced GFP‐TOR association with subcellular structures, neither RFP‐ROP2 nor RFP‐CA‐ROP2 was found co‐localized with GFP‐TOR. In contrast, the dominant‐negative ROP2 mutant (RFP‐DN‐ROP2), while promoting formation of GFP‐TOR‐containing particles, associated within these subcellular structures (Fig 5D). Moreover, nucleotide‐free ROP2, DN‐ROP2, exhibits tight TOR binding activity, and it appears that TOR is trapped by DN‐ROP2, suggesting that nucleotide charging is required for dissociation of ROP2 from the TOR complex. In control experiments, GFP or RFP fused to different ROP2 variants, either alone or in different combinations, did not reveal similar structures in epidermal cells (Appendix Fig S4B and C). In addition, neither Rheb, CA‐ and DN‐Rheb (Appendix Fig S4D), nor Sar1b, CA‐ and DN‐Sar1b derivatives were able to induce GFP‐TOR association with subcellular structures (Appendix Fig S4E).
To elucidate the role of C‐terminal motifs I and II, we investigated the subcellular co‐localization of RFP‐ROP2ΔII and RFP‐ROP2Δ(I+II) with GFP‐TOR. The ROP2 deletion mutant lacking the C‐terminal CAFL (motif II) that interacted strongly with TOR in vitro (Fig EV1C), failed to promote formation of GFP‐TOR‐containing particles when co‐expressed as an RFP‐fusion together with GFP‐TOR (Fig 5F). With a ROP2 construct lacking motif I responsible for TOR interaction (Fig EV1C), no GFP‐TOR association with subcellular structures was seen. In addition, microscopic observation showed that both the polybasic domain and prenylation motif of ROP2 are responsible for ROP2 attachment to the plasma membrane. Indeed, co‐localization with the plasma membrane (PM) was somewhat disturbed upon transient expression of a C‐terminal RFP‐ROP2 deletion mutant (Figs 5E and EV3A). Thus, ROP2 motif I is involved in TOR binding, while motif II is required for targeting of TOR into subcellular structures.
Figure EV3. Appearance of GFP‐TOR as punctate dots in response to NAA treatment correlates with an increase of active TOR in microsomes.

- Fluorescence micrographs showing Nicotiana benthamiana cells transiently expressing plasma membrane (PM) marker GFP‐BD‐CVIL (left panels), RFP‐ROP2 (upper center), RFP‐ROP2ΔII (middle center), and RFP‐ROP2Δ(I+II) (bottom center). Merged images are shown on the right. PM marker consists of green fluorescent protein (GFP) fused to C‐terminal polybasic domain (BD) and isoprenylation motif (CVIL).
- Imaging fluorescence assays showing root cells of Arabidopsis 7‐dag GFP and GFP‐TOR seedlings before and after treatment with 100 nM NAA.
- Intracellular distribution of TOR and active TOR in WT seedlings before (left panel) and after treatment by 100 nM NAA for 8 h (right panel). Western blot analysis of various fractions: the total homogenate (total), nuclear fraction pellet (P10), 30,000 g pellet (P30), 100,000 g pellet (P100), 100,000 g supernatant (S100).
- Nicotiana benthamiana cells transiently co‐expressing GFP‐TOR, RFP‐Golgi, or both with either myc‐DN‐Sar1b or myc‐DN‐ROP2.
- Immunoblot analysis with anti‐myc Abs of cells.
In planta, punctate dots have been observed by fluorescence microscopy in the root cells of WT seedlings either treated by external auxin (Fig EV3B) or GTP‐bound ROP2 expressing GFP‐TOR/CA‐ROP2 seedlings (Fig 5G). Analysis of intracellular distribution of TOR suggested the presence of TOR mainly in supernatant (S100) and partially in microsomal (P100) fractions (Fig EV3C). Although NAA treatment of Arabidopsis seedlings had no significant effect on TOR intracellular distribution, active TOR‐P levels in microsomes were drastically increased. Likewise, the level of active TOR‐P in microsomes isolated from GFP‐TOR/CA‐ROP2 was increased significantly as compared with GFP‐TOR (Fig 5H). Taken together, our results suggest that the appearance of punctate dots correlates with elevated levels of active TOR in microsomes.
We also tracked whether the dominant‐negative DN‐Sar1b mutant that prevents COPII vesicle formation and blocks protein exit from the ER to the Golgi apparatus (Andreeva et al, 2000) can also trigger formation of GFP‐TOR punctate dots. Overexpression of myc‐DN‐Sar1b in N. benthamiana cells inhibits vesicle trafficking and leads to redistribution of Golgi markers to a polygonal network resembling ER structures (Fig EV3D). However, myc‐DN‐Sar1b failed to replace myc‐DN‐ROP2 in GFP‐TOR punctate dot induction, and, vice versa, myc‐DN‐ROP2 overexpression provokes formation of GFP‐TOR aggregates that are not co‐localized with RFP‐Golgi marker, but failed to affect Golgi transport (Fig EV3D and E). Thus, we concluded that ROP2 is highly specific for TOR association.
GFP‐TOR co‐localizes to endosomes in response to ROP2 overexpression
To establish the nature of the mobile intercellular particles to which TOR relocates upon ROP2 overexpression, several RFP‐fused marker constructs were overexpressed transiently together with GFP‐TOR and FLAG‐CA‐ROP2 in N. benthamiana leaves (Fig EV4). Only one out of seven different markers—a transiently expressed RFP‐RabC1 that specifically labels endosomes (Rutherford & Moore, 2002)—co‐localized with GFP‐TOR upon co‐expression of either FLAG‐ROP2, or FLAG‐CA‐ROP2, or FLAG‐DN‐ROP2, indicating that TOR can associate with endosomes (Fig 6A).
Figure EV4. GFP‐TOR‐specific co‐localization with RFP‐RabC1 that labels endosomes.

-
A–GImaging fluorescence assays showing Nicotiana benthamiana cells transiently co‐expressing FLAG‐CA‐ROP2 with GFP‐TOR (left panels), and intracellular markers (central panels) that specifically label early endosomes (A, RFP‐RabC1), endosomes (B, RFP‐RabE1d), autophagosomes (C, RFP‐ATG8a), peroxisomes (mCherry‐peroxi), mitochondria (E, mCherry‐mito), late endosomes (F, RFP‐ARA7), and Golgi (G, GmMan1‐tdTomato); merged images (right). Scale bars are 5 μm.
Figure 6. TOR localizes to endosomal structures in a ROP2‐sensitive fashion.

- Co‐localization analysis of GFP‐TOR and RFP‐RabC1 in Nicotiana benthamiana epidermal cells expressing FLAG‐ROP2 (upper panels), FLAG‐DN‐ROP2 (central panels), and FLAG‐CA‐ROP2 (bottom panels).
- 35S:RFP‐RabC1 Arabidopsis line transiently expressing GFP‐TOR and FLAG‐CA‐ROP2. GFP‐TOR (left), RFP‐Rab1C (center), merged image (right).
- 35S:GFP‐TOR Arabidopsis line transiently expressing RFP‐RabC1 and FLAG‐CA‐ROP2. GFP‐TOR (left), RFP‐RabC1 (center), merged image (right).
- Microscopy images of cells stained with FM4‐64 treated with brefeldin A (BFA) in the root elongation zone of GFP‐TOR 7‐dag seedlings. GFP‐TOR and FM4‐64 were detected in the core of the BFA compartment.
To confirm these results in Arabidopsis, we used 35S:GFP‐TOR and 35S:RFP‐RabC1 lines stably producing GFP‐TOR and RFP‐RabC1, respectively. Consistent with results in N. benthamiana, GFP‐TOR co‐localized mostly with the endosome‐like structures revealed by RFP‐RabC1 when GFP‐TOR and FLAG‐CA‐ROP2 were transiently co‐expressed in 35S:RFP‐RabC1 (Fig 6B), confirming GFP‐TOR association with endosomes. The reciprocal combination, for example, RFP‐Rab1C and FLAG‐CA‐ROP2 expressed transiently in an Arabidopsis line transgenic for GFP‐TOR, displayed RFP‐ and GFP‐labeled particles that were mostly co‐localized (Fig 6C). We conclude that TOR is targeted by ROP2 to endosome‐like structures that quickly dissociate during or after TOR association with endosomes. GFP‐TOR can be visualized on endosomes with the DN‐ROP2 mutant.
We next determined the effect of brefeldin A (BFA) on the distribution of FM4‐64 fluorescent endocytosis marker and GFP‐TOR. BFA inhibits the formation of exocytic vesicles but does not block plasma membrane (PM) internalization through endocytosis (Richter et al, 2007). If GFP‐TOR associates with endocytic compartments, it would be integrated into aggregates of endomembranes together with FM4‐64 in the presence of BFA. Here, in BFA‐treated cells that retained accumulation of GFP‐TOR, selected internalized FM4‐64 aggregates were found co‐localized with GFP‐TOR (Fig 6D). These results further support our hypothesis that GFP‐TOR is relocated to endosomes in response to ROP2 activation.
uORF‐mRNA loading on polysomes is under the control of CA‐ROP2, which functions through TOR
Translation of a special class of uORF‐mRNAs via reinitiation requires TOR activation in response to the phytohormone auxin (Schepetilnikov et al, 2013). To establish the role of active ROP2 in the control of translation reinitiation, we performed comparative polysome profile analysis in WT and CA‐ROP2 seedling‐derived extracts. CA‐ROP2 plants are characterized by a slightly increased ratio of polysomes to fraction of monosomes and ribosomal subunits as compared with WT and AZD‐8055‐treated CA‐ROP2 plants (Fig 7A). To monitor polysomal loading of uORF‐mRNAs in different ROP2 activation conditions, we selected several endogenous uORF‐mRNAs, such as ARF3, ARF5, and bZIP11, translation of which includes one or more reinitiation events depending on the uORF configuration within their leader regions, as well as uORF‐less mRNAs encoding actin and glycerol‐3‐phosphate dehydrogenase C2 (GAPC2; Fig 7B). To avoid translation repression of bZIP11 by sucrose, seedlings were grown on agar medium containing 30 mM sucrose (Wiese et al, 2004).
Figure 7. GTP‐ROP2 mounts up abundance of uORF‐mRNA in polysomes in an AZD‐8055‐sensitive manner.
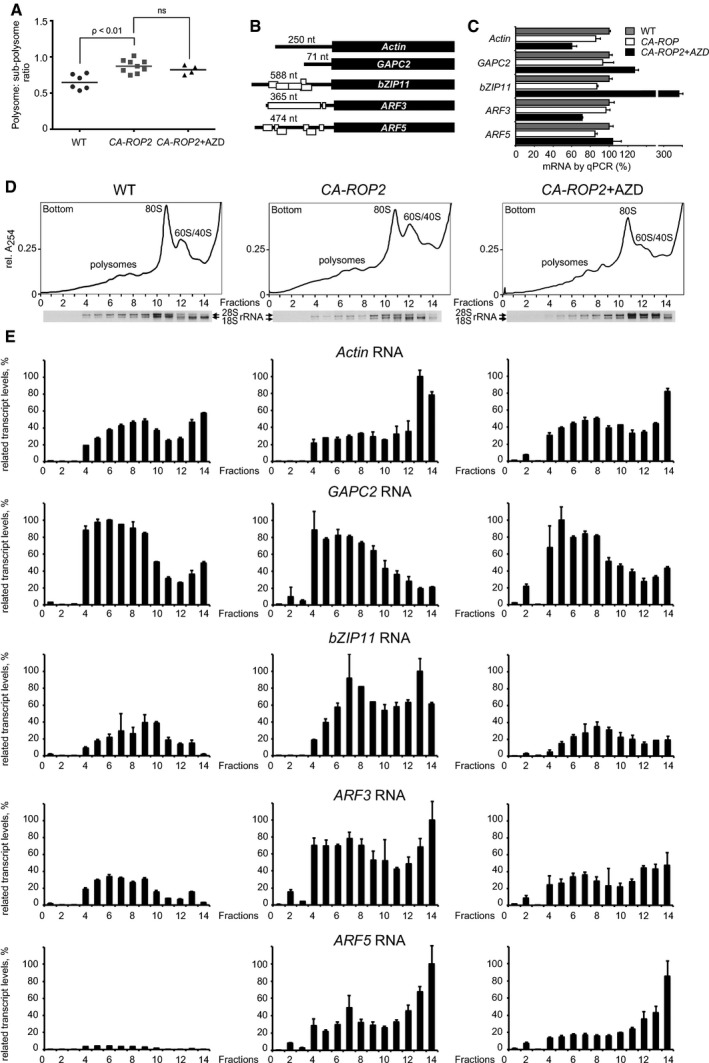
- Statistical analyses of ratio between polysomal and non‐polysomal fractions obtained by sucrose gradient fractionation of extracts isolated from WT seedlings and CA‐ROP2 seedlings grown without or with 0.5 μM AZD‐8055.
- uORF (open rectangles) configuration within selected mRNAs.
- qRT–PCR of each mRNA in total extracts. The RNA value in WT extracts was set as 100%.
- Ribosome sedimentation profiling from extracts prepared from WT (left panel) and CA‐ROP2 7‐dag seedlings treated (right panel) or not (central panel) with 0.5 μM AZD‐8055. Positions of ribosomal subunits (60S/40S), monosomes (80S), and polysomes are indicated. 18S and 28S rRNA distribution was monitored by agarose gel electrophoresis.
- mRNA association with polyribosomes, 80S, and 60S/40S ribosomal subunits was monitored by qRT–PCR in sucrose gradient fractions and presented as graph bars. The highest value of each selected polysome‐bound mRNA among WT, CA‐ROP2, and CA‐ROP2 + AZD was set as 100%.
mRNA association with polysomes, 80S ribosomes, and ribosomal subunits in extracts of WT seedlings and CA‐ROP2 seedlings grown without or with AZD‐8055 was monitored in parallel experiments by quantitative RT–PCR (qRT–PCR) for each indicated endogenous mRNA. Note that distribution among ribosomal fractions of the housekeeping gene EXP was stably maintained under all conditions tested (Appendix Fig S6). There was also no drastic difference in total transcript levels either between CA‐ROP2 and WT extracts, or upon their growth on AZD‐8055, except that levels of bZIP11 mRNA were surprisingly high upon AZD‐8055 treatment (Figs 7C and EV5A). As shown in Fig 7D and E, efficient GAPC2 mRNA loading on polysomes was barely affected by high active ROP2 levels or by TOR inactivation. In contrast, upon loading of actin mRNA into polysomes, a somewhat toxic effect of CA‐ROP2 was apparent. Translation/reinitiation events within bZIP11, ARF3, and ARF5 5′‐UTRs impede or block ribosomal movement toward the main ORF, causing inefficient translation of uORF‐mRNAs under WT conditions (Zhou et al, 2010). The increased abundance of initiating/reinitiating 40S, and likely uORF‐translating 80S, within their long leaders shifts these mRNAs toward 80S or even light polysomal fractions (Fig 7E, WT). Polysomal accumulation of bZIP11, ARF3, and ARF5 mRNAs was reproducibly elevated in CA‐ROP2‐derived extracts as compared with WT extracts (Fig 7E, CA‐ROP2). The abundant appearance of uORF‐mRNAs in both polysomal and subpolysomal fractions in CA‐ROP2 likely reflects increased translation of the main ORF due to improved reinitiation at uORFs, while a few 40S can still be captured by uORFs within the leader region in planta. For example, ARF5 mRNA loading, which is nearly negligible in WT seedlings (six uORFs inhibit ARF5 mRNA translation by 16‐fold; Zhou et al, 2010), was improved significantly upon TOR activation in CA‐ROP2. We observed that only a small fraction of total uORF‐mRNAs loaded on sucrose gradients associates with ribosomes in WT, and a much larger fraction of uORF‐mRNA binds ribosomes in CA‐ROP2 conditions.
Figure EV5. Ribosome profiling of uORF‐containing mRNA in WT Arabidopsis without or with TOR inhibitor.

- qRT–PCR of each mRNA in total extracts. The RNA value in WT extracts was set as 100%.
- The level of heavy polysomes is reduced in WT Arabidopsis treated by AZD‐8055 (AZD). Extracts prepared from 7‐dag seedlings growing without (WT) and with 0.5 μM AZD‐8055 on agar plates (WT + AZD) were subjected to velocity sedimentation through sucrose density gradients. Gradients were fractionated while scanning at 254 nm, and the resulting absorbance profiles are shown (WT and WT + AZD). Positions of ribosomal subunits (RS), monosomes (80S), and polysomes are indicated. rRNA distribution was monitored by agarose gel electrophoresis.
- AZD‐8055 treatment down‐regulates, albeit not significantly, the abundance of bZIP11 and the already low polysomal levels of ARF5 mRNA in WT Arabidopsis. Distribution of mRNAs (actin, GAPC2, bZIP11, ARF3, and ARF5) in fractions were analyzed by qRT–PCR. Note that the highest value of each polysome‐bound mRNA from WT, CA‐ROP2, CA‐ROP2 + AZD (Fig 7) was used as 100% for both WT and WT + AZD.
- TOR and TOR phosphorylation status were analyzed in polysomes prepared from WT, CA‐ROP2 seedlings and CA‐ROP2 line treated by AZD‐8055. Three samples from polysomes and two from 80S and ribosomal subunits were taken to monitor TOR by immunoblotting with anti‐TOR (lower panels) and phospho‐TOR with anti‐(mTOR‐S2448‐P) (central panels). Data shown are representative of two independent blots.
We next investigated mRNA abundance across sucrose gradients in WT versus CA‐ROP2 (Appendix Fig S5). WT and CA‐ROP2 extracts were separated by centrifugation and roughly collected as eight large fractions—polysomes, subpolysomes (80S/60S and 40S), and the non‐ribosomal (NR) top gradient fraction. Indeed, a higher proportion of uORF‐mRNA (about 64% of bZIP11, 69% of ARF3, and 76% of ARF5 mRNAs) sedimented to the NR top fraction of the gradient with WT, while only 20–25% of uORF‐mRNA remained in the NR fraction in CA‐ROP2 conditions, regardless of the fact that total transcript levels did not differ between WT and CA‐ROP2 extracts. Note that roughly 10–15% of uORF‐less mRNAs were present in NR in both WT and CA‐ROP2.
As expected, TOR inactivation by AZD‐8055 abolished cell reinitiation ability. AZD‐8055 treatment diminished further loading of bZIP11 and ARF3 mRNAs on WT polysomes, while ARF5 loading remained low under our WT + AZD‐8055 conditions (Fig EV5C). Translation reinitiation efficiency depends on retention of active TOR in polyribosomes after the preceding initiation event (Schepetilnikov et al, 2013). Here, the phosphorylation level of TOR found in WT extract 80S and ribosomal subunit fractions was below the limit of detection of our antibodies (Fig EV5D). In contrast, in CA‐ROP2 plants, TOR is phosphorylated and associates not only with 80S and ribosomal subunit fractions but also with light polysomes, which contain two or three translating ribosomes on average on the same mRNA. Application of a TOR inhibitor resulted in TOR inactivation and dissociation from polyribosomal profiles (Fig EV5D). Therefore, uORF‐mRNA abundance in polysomes is regulated by GTP‐bound ROP2 in a TOR‐responsive manner for several ARF‐encoded genes, and also for auxin‐unrelated bZIP11, suggesting that GTP‐bound ROP2 up‐regulates the translation capacity of reinitiation‐dependent mRNAs via TOR.
CA‐ROP2 promotes reinitiation after uORF translation and delays root response to AZD‐8055
Our results suggest that active ROP2 promotes uORF‐mRNA accumulation in polysomes in a TOR‐responsive manner. We tested whether CA‐ROP2 seedlings can drive efficient reinitiation of translation. As expected, TOR phosphorylation status was elevated in CA‐ROP2‐derived mesophyll protoplasts as compared with WT protoplasts, and nearly abolished by AZD‐8055 after overnight incubation of protoplasts (Fig 8A).
Figure 8. CA‐ROP2 promotes reinitiation after uORF translation and decreases root hair elongation inhibition by AZD‐8055.

- Phosphorylation of TOR at S2424 is augmented in CA‐ROP2‐overexpressing versus WT mesophyll protoplasts and diminished in the presence of 0.5 μM AZD‐8055. TOR and its phosphorylation levels were assayed by immunoblotting. The Western blot density bands were quantified and CA‐ROP2 values were set as 100%.
- GUS‐containing reporters with either short or uORF‐containing (ARF3 and ARF5) 5′‐UTRs were used for mesophyll protoplasts transformation.
- WT and CA‐ROP2 seedlings growing without (CA‐ROP2) or with 1 μM AZD‐8055 (CA‐ROP2 + AZD) were used to prepare mesophyll protoplasts. Both GFP fluorescence and β‐glucuronidase functional activity were analyzed in the same 96‐well microtiter plate. Functional levels of GUS expressed from pshort‐GUS normalized to corresponding GFP levels were set at 100%. GUS‐containing mRNA levels and integrity were analyzed by semiquantitative RT–PCR (sqRT–PCR); GFP levels were also analyzed by immunoblotting. LC, loading control.
- pmonoGFP and either pARF3‐GUS or pARF3Δ(AUG1+2)‐GUS were used to transform WT or CA‐ROP2‐derived protoplasts. The GUS/GFP ratio found in WT protoplasts with a uORF‐less ARF3 leader was set as 100%.
- WT mesophyll protoplasts were transfected in addition to pmonoGFP/pshort‐GUS or pARF5‐GUS with the vector expressing either myc‐tagged ROP2, CA‐ROP2, or DN‐ROP2 under the 35S promoter. Both GFP fluorescence and β‐glucuronidase functional activity were analyzed in the same 96‐well microtiter plate. GUS/GFP ratio related to the short or ARF5 5′‐UTRs was taken as 100%. GUS mRNA levels and integrity were analyzed by sqRT–PCR. ROP2 variants analyzed by immunoblotting using anti‐myc Abs (samples were run on the same gel and the solid line indicates removal of extraneous lanes).
- Dose‐dependent effect of AZD‐8055 on root growth of WT and CA‐ROP2 seedlings at 24, 48, and 72 h.
- Dose‐dependent effect of AZD‐8055 on root hair elongation of WT and CA‐ROP2 seedlings.
Next, we compared the transient expression of several reporter genes that harbor a β‐glucuronidase (GUS) ORF downstream of short 60‐nt‐, ARF3‐ or ARF5‐containing leaders (Fig 8B) in mesophyll protoplasts prepared from WT or CA‐ROP2 seedlings. Mesophyll protoplasts were transformed with one of the above plasmids, and a plasmid containing a single GFP ORF downstream of the TEV IRES (Zeenko & Gallie, 2005) as a control for transformation efficiency.
Under the conditions used, the ARF5 and ARF3 leaders fused to the GUS ORF in their authentic initiation context reduced GUS ORF translation by about 80% and 85%, respectively, compared with that of the short‐GUS mRNA (Fig 8C) in WT protoplasts. ARF3‐ and ARF5‐dependent GUS/GFP levels were dramatically increased by three‐ to fourfold in protoplasts prepared from CA‐ROP2 as compared with WT protoplasts, while GUS/GFP levels did not change significantly upon short leader‐dependent expression (Fig 8C). GUS‐containing mRNA levels as well as GFP levels in either WT or CA‐ROP2 protoplasts were similar during protoplast incubation. CA‐ROP2‐sensitive induction of ARF3‐ and ARF5‐dependent GUS ORF expression was blocked by treatment with AZD‐8055 (Fig 8C). Next, we compared the translation reinitiation efficiency of the ARF3 leader with its uORF‐removed variant in WT and CA‐ROP2‐derived protoplasts (Fig 8D). As expected, uORFs 1 and 2 inhibited translation reinitiation efficiency of ARF3‐GUS mRNA by threefold in WT protoplasts, while ARF3‐GUS mRNA translation was only 20% less efficient as compared with ARF3Δ(AUG1 + 2) translation in CA‐ROP2‐derived protoplasts. Therefore, since the initiation context of ARF3 and ARF3Δ(AUG1 + 2) 5′‐UTRs is identical, we concluded that reinitiation of translation is improved in response to active ROP2 levels.
We next monitored translation reinitiation efficiencies of short‐GUS‐ and ARF5‐GUS‐containing reporters in mesophyll protoplasts transfected with either ROP2 (WT‐ROP2), or CA‐ROP2, or DN‐ROP2‐expression vectors. As can be seen in Fig 8E, ROP2 and especially CA‐ROP2 proteins, but not DN‐ROP2, were found active in promoting reinitiation at uORFs of ARF5 mRNA. Indeed, the translation efficiency of the ARF5 leader‐containing mRNA was increased up to threefold in CA‐ROP2‐expressing protoplasts, while DN‐ROP2 GTPase failed to increase translation of either short‐GUS or ARF5‐GUS mRNAs.
It was reported that AZD‐8055 inhibits the elongation of epidermal cells and root hairs without changing the position of root hair emergence (Montane & Menand, 2013). Likewise, constitutively active GTP‐bound ROP2 (CA‐ROP2) triggered root hair elongation, but also emergence of additional misplaced hairs (Jones et al, 2002). Strikingly, treatment of WT and CA‐ROP2 seedlings with three different AZD‐8055 concentrations revealed that both primary root growth (Fig 8E) and root hair elongation inhibition (Fig 8F) were delayed in CA‐ROP2 as compared with WT seedlings, indicating that seedlings expressing higher levels of active TOR are less sensitive to AZD‐8055 inhibition. Note that AZD‐8055 specifically reduces primary root length (Fig 8F) and root hair length, while not affecting root hair position (Fig 8G).
Discussion
Our results have revealed a role for auxin in TOR signaling activation in plants (Schepetilnikov et al, 2013). Moreover, a recent publication confirmed that TOR plays an important role in auxin signaling transduction in Arabidopsis (Deng et al, 2016). These results prompted us to address the question of which TOR pathway intermediate compounds can transmit signals from auxin or other TOR upstream effectors (environmental changes, glucose, and amino acids) to promote protein synthesis via TOR in plants. Our investigations in vitro and in planta have demonstrated that the small GTPase ROP2 promotes TOR activation in response to auxin; active TOR can up‐regulate translation reinitiation at uORFs. Several lines of evidence support this conclusion: First, ROP2 interacts directly with TOR in vitro, and both proteins co‐immunoprecipitate in plant extracts (Fig 1). Second, plants characterized by high active ROP or CA‐ROP2 contain increased levels of active TOR and S6K1 (Fig 2D). Third, inactivation of TOR by AZD‐8055 abolishes ROP2 effects on TOR and its downstream signaling, but TOR is dispensable for the active status of ROP2 and other ROPs, strongly suggesting that ROP2 is upstream of TOR (Fig 2D). Importantly, TOR activation in response to auxin was abolished in rop2 rop6 ROP4 RNAi extracts (Fig 3). Fourth, GTP‐bound ROP2 dramatically stimulates translation reinitiation of uORF‐mRNAs in a manner sensitive to the TOR inhibitor AZD‐8055 (Figs 7, and 8C and D). Strikingly, CA‐ROP2 GTPase, but not the dominant‐negative DN‐ROP2, is active in reinitiation at uORFs (Fig 8E). Another interesting phenomenon revealed by our data is the connection between ROP2‐containing TOR complexes and endosome‐like structures in the cytoplasm. In addition, we found that CA‐ROP2 can somewhat suppress primary root growth (Fig 8F) and root hair inhibition by AZD‐8055 (Fig 8G).
ROPs—orthologs of mammalian Rho and Rac (Berken & Wittinghofer, 2008)—are promising candidates for regulating the TOR signaling pathway—the main growth‐related pathway in eukaryotes. In addition, a connection between auxin and ROP activation has been established (Miyawaki & Yang, 2014), placing ROPs downstream of auxin. Our data suggest that, in addition to ROP2, TOR is able to interact specifically with ROP4 and ROP6, but additional studies are needed to determine the role of these latter ROPs in TOR regulation. ROP2 is expressed in all vegetative tissues, belongs to the largest ROP subgroup (Li et al, 1998, 2001) and, according to our data, when active, contributes to TOR signaling activation in an AZD‐8055‐sensitive manner. We cannot exclude the possibility that ROP6 and ROP2, which functions in cell expansion on different sides of the membrane (Xu et al, 2010), exert differential effects on TOR signaling.
ROP2 associates with TOR or TOR‐containing complexes in vitro and in planta (Figs 1 and EV1C). According to our results, only some TOR‐binding characteristics of ROP2 resemble those of human Rho‐related GTPase Rheb, although both bind physically and activate TOR in the GTP‐bound state. However, the ROP2–TOR interaction is plant specific; ROP2 binds to the heat repeat domain of Arabidopsis TOR, while Rheb binds within the kinase domain of mTOR (Long et al, 2005). This may explain the weak interaction of Rheb with Arabidopsis TOR under our conditions. ROP2 binding to TOR can occur via the C‐terminal basic stretch of amino acids (motif I), and in vitro does not require ROP2 charging, but GTP somewhat negatively modulates this interaction (this study and Long et al, 2005). Consistently, only WT ROP2 or CA‐ROP2 are active in promoting reinitiation at uORFs in protoplasts. These results suggest that TOR/GTP‐ROP2 complex formation in Arabidopsis, albeit possibly transient, is a necessary prerequisite for the physiological activation of TOR kinase. Strikingly, Arabidopsis GFP‐TOR, when activated by ROP2, can relocate to endosome‐like structures labeled by RabC1. The closest RabC1 GTPase mammalian homologue, Rab18, is also associated with endosomes, especially in epithelial cells, and can function in recycling to the plasma membrane (Rutherford & Moore, 2002). Since the TOR cofactor LST8 was also localized on RabC1‐labeled endosomes or endosome‐like structures (Moreau et al, 2012), we suggest that endosomes are sites of TOR complex localization. Although nucleotide‐free ROP2 (DN‐ROP2) promotes targeting of TOR to endosomes (Figs 5 and 6), it remains endosome associated, indicating formation of inactive complexes, from which DN‐ROP2 is unable to dissociate. Thus, we conclude that ROP2 targeting to endosomes is an intermediate step in ROP2 recycling. In plants, the auxin‐related ROP2 pathway plays a role in the promotion of endosomal trafficking from early endosomes during PIN1 internalization (Dhonukshe et al, 2007, 2008; Nagawa et al, 2012). In turn, disruption of membrane trafficking can influence auxin signaling at the level of translation (Rosado et al, 2012). In mammals, Rag GTPases are responsible for lysosomal recruitment of mTOR by targeting its cofactor RAPTOR (Bar‐Peled & Sabatini, 2014), while our experiments did not reveal interactions between ROP2 and Arabidopsis RAPTOR (data not shown). Interestingly, although both ROP2 and human Rac1 bind TOR via their polybasic domain, ROP2 activates TOR in a way similar to human Rheb. This can reflect the situation in plants, which contain only a single family of Rho‐like GTPases.
Consistent with our findings, a subset of ROP GTPases function in auxin signaling to downstream responsive genes (Tao, 2002), indicating that the active ROP2 status can be translated into specific auxin‐dependent responses. Auxin is under the control of various environmental and developmental signals that trigger local auxin biosynthesis or its intercellular polar distribution. High auxin maxima trigger the cell transcription machinery toward expression of auxin‐responsive genes via release of repression of the ARF family (Vanneste & Friml, 2009). Recent data (Zhang et al, 2016) suggest that a subset of cell wall‐related genes was mis‐regulated in CA‐ROP2 via CTD phosphatase degradation. In the cytosol, ROP2 can trigger TOR activation in response to auxin, or other as yet uncharacterized signals, to induce translation of a highly regulated class of mRNAs containing regulatory uORFs.
Our results provide a new insight into translation regulation of a specific class of messages loaded with short uORFs within their leader regions that are responsive to small GTPase ROP2. When overexpressed in protoplasts, active ROP2 renders protoplasts high‐reinitiation‐permissive. Our results explain this phenomenon via the model presented in Fig 9. Auxin mediates recycling of ROP2‐GDP to ROP2‐GTP. ROP2 can bind TOR directly via its polybasic domain. GTP‐bound ROP2 forms a transient, but potentially active, complex with TOR, which triggers phosphorylation events and conformational changes that result in TOR activation. Although GTP‐bound ROP2 interacts somewhat weakly with TOR, the configuration of GTP‐charged ROP2 enables TOR to adopt a form that is both catalytically active and capable of producing signaling in planta. TOR activation could occur upon complex formation with ROP2 on endosomes. ROP2 then dissociates from TOR and requires recycling. Several GEFs can recycle ROPs (Oda & Fukuda, 2014). Active TOR is loaded on eIF3‐containing preinitiation complexes (Holz et al, 2005) and polysomes, where it activates S6K1, and both promote translation reinitiation of uORF‐mRNAs (Schepetilnikov et al, 2013). In our model, ROP2 is activated in response to auxin signals that are transported via an as yet uncharacterized receptor.
Figure 9. Putative model of ROP2 function in TOR activation that signals translation reinitiation.
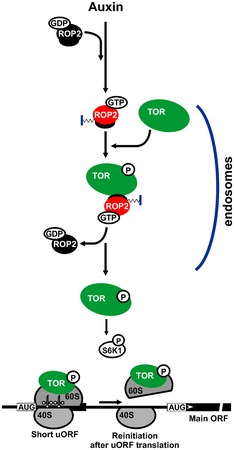
TOR was shown to be required for auxin responses, and these can converge through a small GTPase ROP2. Active ROP2 mediates TOR activation and thus controls the abundance of potent proteins in a posttranscriptional manner via selective translation mechanism—reinitiation. ROP2 recycling maintains TOR association with endosome‐like structures (see Discussion for details).
Our findings, together with the observation that TOR is required for virus‐controlled polycistronic translation—a process normally strictly prohibited in eukaryotes—in CaMV (Schepetilnikov et al, 2011) suggests that TOR up‐regulation of reinitiation at uORFs could be as harmful in plants as in mammals, where up‐regulation of the protein synthesizing machinery contributes to the development of cancer (Ruggero & Pandolfi, 2003). Notwithstanding that auxin regulates a range of distinct effectors, these findings further corroborate the idea that translation reinitiation is achieved via crosstalk between the TOR kinase and ROP2 signaling pathways. The developmental abnormalities identified in rpl24b and eif3h‐1 mutants due to defects in reinitiation at uORFs are largely similar to auxin‐related developmental defects (Zhou et al, 2010). Thus, TOR can play an important role in modulating auxin responses during plant development. Further studies are needed to understand the roles of ROP2 in TOR activation as well as to identify other TOR effectors in plants.
Materials and Methods
Cell shape analysis and chemical treatment
Interdigitation analysis of Arabidopsis pavement cells was performed as described in Appendix Supplementary Methods. For microscopic observation of GFP‐TOR in endosomes, root cells of GFP‐TOR transgenic plants were treated with 90 μM brefeldin A (BFA; Sigma) for 30 min and stained with 10 μM FM4‐64 dye (Sigma).
Time‐course experiments
To study the dynamics of TOR‐P accumulation upon auxin treatment in planta (Fig 3), 7‐dag Col0 WT and rop2 rop6 ROP4 RNAi transgenic seedlings cultured on MS agar plates were transferred into fresh liquid MS medium and incubated for 2–3 h at 24°C under constant light conditions to avoid additional stress. Seedlings were then transferred to fresh liquid MS medium with or without 100 nM NAA and samples were harvested at 0, 30, 60, 90, and 120 min after induction. The samples for TOR‐P analysis were taken 8 h after incubation with or without 100 nM NAA, or 1 μM AZD. TOR levels and phosphorylation status were determined by Western blot with specific antibodies.
In vitro kinase assay
Immunoprecipitated GFP‐TOR complexes from GFP‐TOR and GFP‐TOR/CA‐ROP2 7‐dag transgenic seedlings were compared for their phosphotransferase activity toward rec S6K1 as a substrate. Kinase reactions were stopped after 0, 5, 10, 15, and 20 min of incubation at 30°C, and incorporation of phosphate was analyzed by immunoblotting with anti‐mS6K1‐P‐T389 antibodies (Cell Signaling). For TOR immunoprecipitation details, see Appendix Supplementary Methods.
Polyribosome analysis
Polysomes were isolated from 7‐dag Arabidopsis wild‐type Col‐0 WT and CA‐ROP2 seedlings grown on MS agar plates supplemented (or not) with 0.5 μM AZD‐8055. To monitor ARFs, bZIP11, GAPC2, and ACTIN mRNA loading into polysomes, total RNA isolated from polysomal fractions as indicated was analyzed by qRT–PCR, and transcript levels for each mRNA were normalized on rRNA. The highest value of each mRNA in polysomal profiles from WT, CA‐ROP2, CA‐ROP2 + AZD‐8055 (Fig 7) and WT, WT + AZD‐8055 (Fig EV5) was set as 100%. For gene‐specific primer sequences, see Appendix Supplementary Methods.
Protoplast assays
Transient expression was analyzed in Arabidopsis mesophyll protoplasts from WT and CA‐R0P2 2‐week transgenic seedlings. For plasmid construction, transfection, and qRT–PCR protocol details, see Appendix Supplementary Methods.
Yeast two‐hybrid assay
Yeast two‐hybrid protein interaction assays were performed according to Park et al (2001). Constructs containing NTOR, CTOR, and TOR fused to the GAL4 AD‐domain and ROP1‐6, CA‐ROP2 and DN‐ROP2 fused to the BD‐domain were co‐transformed into AH109 cells. Transformants were selected onto SD‐Leu‐Trp plates. Surviving yeast colonies were picked as primary positives and transferred on SD‐Leu‐Trp‐His selection plates to score protein interaction. The assays and dilutions were performed in triplicate. For plasmid construction details, see Appendix Supplementary Methods.
GST pull‐down assay
To analyze activation of ROP2 in vivo in total plant extracts treated or not with 1 μM AZD‐8055. We utilized a biochemical assay, in which GTP‐bound active ROP2 was pulled down by use of GST‐Ric1 attached to glutathione‐agarose beads.
Binding of TOR to GST‐fused Sar1b, or Rheb, or ROP2, or ROP2∆II, or ROP2∆(I+II) or GST alone, respectively (Figs 1D and H, and EV1C) was carried out as described in Appendix Supplementary Methods.
Author contributions
MS and LAR conceived the project. MS performed the experiments and data analysis. LAR and MS wrote the manuscript. JM, OS, and AG participated in experiments. ZY provided rop mutants, and JC and PH performed MS‐MS and data analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 7
Source Data for Figure 8
Acknowledgements
We are grateful to Y. Hu for cuf1D and Y. Zhao for yuc1D lines, A. Komar (DAPCEL Inc) for recombinant TOR design, and N. Baumberger for helpful assistance in protein analysis. We thank A. von Arnim for comments on the manuscript and C. Meyer for helpful discussions. This work was supported by French Agence Nationale de la Recherché (ANR; 11‐BSV6 010 03 and ANR‐14‐CE19‐0007) funding to L.R.
The EMBO Journal (2017) 36: 886–903
References
- Andreeva AV, Zheng H, Saint‐Jore CM, Kutuzov MA, Evans DE, Hawes CR (2000) Organization of transport from endoplasmic reticulum to Golgi in higher plants. Biochem Soc Trans 28: 505–512 [PubMed] [Google Scholar]
- Bar‐Peled M, Raikhel NV (1997) Characterization of AtSEC12 and AtSAR1. Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol 114: 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Peled L, Sabatini DM (2014) Regulation of mTORC1 by amino acids. Trends Cell Biol 24: 400–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán‐Peña E, Aguilar R, Ortíz‐López A, Dinkova TD, De Jiménez ES (2002) Auxin stimulates S6 ribosomal protein phosphorylation in maize thereby affecting protein synthesis regulation. Physiol Plant 115: 291–297 [DOI] [PubMed] [Google Scholar]
- Berken A, Wittinghofer A (2008) Structure and function of Rho‐type molecular switches in plants. Plant Physiol Biochem 46: 380–393 [DOI] [PubMed] [Google Scholar]
- Betz C, Hall MN (2013) Where is mTOR and what is it doing there? J Cell Biol 203: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Hughes G, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R et al (2010) AZD8055 is a potent, selective, and orally bioavailable ATP‐competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 70: 288–298 [DOI] [PubMed] [Google Scholar]
- Cui D, Zhao J, Jing Y, Fan M, Liu J, Wang Z, Xin W, Hu Y (2013) The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet 9: e1003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski N, Hall MN (2009) TOR complex 2: a signaling pathway of its own. Trends Biochem Sci 34: 620–627 [DOI] [PubMed] [Google Scholar]
- Deng K, Yu L, Zheng X, Zhang K, Wang W, Dong P, Zhang J, Ren M (2016) Target of rapamycin is a key player for auxin signaling transduction in Arabidopsis . Front Plant Sci 7: 291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolai M, Bedu M, Robaglia C, Meyer C (2007) The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep 8: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof Y‐D, Friml J (2007) Clathrin‐mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis . Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Tanaka H, Goh T, Ebine K, Mähönen AP, Prasad K, Blilou I, Geldner N, Xu J, Uemura T, Chory J, Ueda T, Nakano A, Scheres B, Friml J (2008) Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dobrenel T, Marchive C, Sormani R, Moreau M, Mozzo M, Montané MH, Menand B, Robaglia C, Meyer C (2011) Regulation of plant growth and metabolism by the TOR kinase. Biochem Soc Trans 39: 477–481 [DOI] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F‐actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Fu Y, Xu T, Zhu L, Wen M, Yang Z (2009) A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis . Curr Biol 19: 1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K‐I, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189 [DOI] [PubMed] [Google Scholar]
- Hinnebusch A (2006) eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci 31: 553–562 [DOI] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580 [DOI] [PubMed] [Google Scholar]
- Jones MA, Shen J‐J, Fu Y, Li H, Yang Z, Grierson CS (2002) The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum‐Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J et al (2003) Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet 34: 29–31 [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim BH, Yahalom A, Chamovitz DA, von Arnim AG (2004) Translational regulation via 5′ mRNA leader sequences revealed by mutational analysis of the Arabidopsis translation initiation factor subunit eIF3h. Plant Cell 16: 3341–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M (2001) Constraints on reinitiation of translation in mammals. Nucleic Acids Res 29: 5226–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu G, Ware D, Davis KR, Yang Z (1998) Arabidopsis Rho‐related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol 118: 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z (2001) The Rop GTPase switch controls multiple developmental processes in Arabidopsis . Plant Physiol 126: 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468 [DOI] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz‐Vega S, Yonezawa K, Avruch J (2005) Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713 [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J (2009) Molecular mechanisms of mTOR‐mediated translational control. Nat Rev Mol Cell Biol 10: 307–318 [DOI] [PubMed] [Google Scholar]
- Mahfouz MM, Kim S, Delauney AJ, Verma DP (2006) Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 18: 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menand B, Desnos T, Nussaume L, Berger F, Bouchez D, Meyer C, Robaglia C (2002) Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proc Natl Acad Sci USA 99: 6422–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki KN, Yang Z (2014) Extracellular signals and receptor‐like kinases regulating ROP GTPases in plants. Front Plant Sci 5: 449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montane MH, Menand B (2013) ATP‐competitive mTOR kinase inhibitors delay plant growth by triggering early differentiation of meristematic cells but no developmental patterning change. J Exp Bot 64: 4361–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Azzopardi M, Clément G, Dobrenel T, Marchive C, Renne C, Martin‐Magniette M‐L, Taconnat L, Renou J‐P, Robaglia C, Meyer C (2012) Mutations in the Arabidopsis homolog of LST8/GβL, a partner of the target of Rapamycin kinase, impair plant growth, flowering, and metabolic adaptation to long days. Plant Cell 24: 463–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S, Xu T, Lin D, Dhonukshe P, Zhang X, Friml J, Scheres B, Fu Y, Yang Z (2012) ROP GTPase‐dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin‐dependent endocytosis. PLoS Biol 10: e1001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2014) Emerging roles of small GTPases in secondary cell wall development. Front Plant Sci 5: 428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Himmelbach A, Browning KS, Hohn T, Ryabova LA (2001) A plant viral ‘reinitiation’ factor interacts with the host translational machinery. Cell 106: 723–733 [DOI] [PubMed] [Google Scholar]
- Ren H, Dang X, Yang Y, Huang D, Liu M, Gao X, Lin D (2016) SPIKE1 activates ROP GTPase to modulate petal growth and shape. Plant Physiol 172: 358–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Geldner N, Schrader J, Wolters H, Stierhof Y‐D, Rios G, Koncz C, Robinson DG, Jürgens G (2007) Functional diversification of closely related ARF‐GEFs in protein secretion and recycling. Nature 448: 488–492 [DOI] [PubMed] [Google Scholar]
- Ren M, Venglat P, Qiu S, Feng L, Cao Y, Wang E, Xiang D, Wang J, Alexander D, Chalivendra S, Logan D, Mattoo A, Selvaraj G, Datla R (2012) Target of rapamycin signaling regulates metabolism, growth, and life span in Arabidopsis . Plant Cell 24: 4850–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A, Li R, van de Ven W, Hsu E, Raikhel NV (2012) Arabidopsis ribosomal proteins control developmental programs through translational regulation of auxin response factors. Proc Natl Acad Sci USA 109: 19537–19544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D, Pandolfi PP (2003) Does the ribosome translate cancer? Nat Rev Cancer 3: 179–192 [DOI] [PubMed] [Google Scholar]
- Rutherford S, Moore I (2002) The Arabidopsis Rab GTPase family: another enigma variation. Curr Opin Plant Biol 5: 518–528 [DOI] [PubMed] [Google Scholar]
- Saci A, Cantley LC, Carpenter CL (2011) Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell 42: 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar‐Peled L, Sabatini DM (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetilnikov M, Kobayashi K, Geldreich A, Caranta C, Robaglia C, Keller M, Ryabova LA (2011) Viral factor TAV recruits TOR/S6K1 signalling to activate reinitiation after long ORF translation. EMBO J 30: 1343–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetilnikov M, Dimitrova M, Mancera‐Martínez E, Geldreich A, Keller M, Ryabova LA (2013) TOR and S6K1 promote translation reinitiation of uORF‐containing mRNAs via phosphorylation of eIF3h. EMBO J 32: 1087–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimobayashi M, Hall MN (2014) Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15: 155–162 [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136: 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N, Gutman O, Bar E, Abu‐Abied M, Feng X, Running MP, Lewinsohn E, Ori N, Sadot E, Henis YI, Yalovsky S (2011) Differential effects of prenylation and s‐acylation on type I and II ROPS membrane interaction and function. Plant Physiol 155: 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao LZ (2002) Plant Rac‐like GTPases are activated by auxin and mediate auxin‐responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Blenis J (2005) mTOR, translational control and human disease. Semin Cell Dev Biol 16: 29–37 [DOI] [PubMed] [Google Scholar]
- Turck F, Zilbermann F, Kozma SC, Thomas G, Nagy F (2004) Phytohormones participate in an S6 kinase signal transduction pathway in Arabidopsis . Plant Physiol 134: 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Wiese A, Elzinga N, Wobbes B, Smeekens S (2004) A conserved upstream open reading frame mediates sucrose‐induced repression of translation. Plant Cell 16: 1717–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge P, Brembu T, Bones AM (1997) Cloning and characterization of Rac‐like cDNAs from Arabidopsis thaliana. Plant Mol Biol 35: 483–495 [DOI] [PubMed] [Google Scholar]
- Wu G, Li H, Yang Z (2000) Arabidopsis RopGAPs are a novel family of rho GTPase‐activating proteins that require the Cdc42/Rac‐interactive binding motif for rop‐specific GTPase stimulation. Plant Physiol 124: 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z (2001) A genome‐wide analysis of Arabidopsis Rop‐interactive CRIB motif‐containing proteins that act as Rop GTPase targets. Plant Cell 13: 2841–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Sheen J (2012) Rapamycin and glucose‐target of rapamycin (TOR) protein signaling in plants. J Biol Chem 287: 2836–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J (2013) Glucose‐TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen J‐G, Wu M‐J, Perrot‐Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface‐ and Rho GTPase‐based auxin signaling controls cellular interdigitation in Arabidopsis . Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, Wang W, Jones AM, Friml J, Patterson SE, Bleecker AB, Yang Z (2014) Cell surface ABP1‐TMK auxin‐sensing complex activates ROP GTPase signaling. Science 343: 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeenko V, Gallie DR (2005) Cap‐independent translation of tobacco etch virus is conferred by an RNA pseudoknot in the 5′‐leader. J Biol Chem 280: 26813–26824 [DOI] [PubMed] [Google Scholar]
- Zhang B, Yang G, Chen Y, Zhao Y, Gao P, Liu B, Wang H, Zheng Z‐L (2016) C‐terminal domain (CTD) phosphatase links Rho GTPase signaling to Pol II CTD phosphorylation in Arabidopsis and yeast. Proc Natl Acad Sci USA 113: E8197–E8206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase‐like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]
- Zhou F, Roy B, von Arnim AG (2010) Translation reinitiation and development are compromised in similar ways by mutations in translation initiation factor eIF3h and the ribosomal protein RPL24. BMC Plant Biol 10: 193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Roy B, Dunlap JR, Enganti R, von Arnim AG (2014) Translational control of Arabidopsis meristem stability and organogenesis by the eukaryotic translation factor eIF3h. PLoS One 9: e95396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzalla V, Stracka D, Oppliger W, Hall MN (2011) Activation of mTORC2 by association with the ribosome. Cell 144: 757–768 [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 7
Source Data for Figure 8


