Abstract
Uremia results in a relatively immunocompromised status, and patients under chronic dialysis have an elevated risk of developing herpes zoster (HZ). We sought to investigate the relationship between vitamin D status and immunity to varicella-zoster virus (VZV). A multicenter prevalent hemodialysis cohort was assembled between 2012 and 2013. We assayed the biochemical parameters, 25-hydroxy- (25-OH-D) and 1,25-dihydroxyvitamin D, vitamin D-binding protein levels in the sera. VZV immunity was quantitated using VZV-specific glycoprotein IgG and IgM titers. Eighty-eight patients were enrolled and their sera were analyzed. Chronic hemodialysis patients with 25-OH-D < 30 ng/ml (insufficiency or deficiency) had significantly lower VZV-IgG than those with sufficient 25-OH-D (p = 0.04). This discrepancy became more prominent if active vitamin D users alone were analyzed (p = 0.01). Generalized additive modeling showed that those with 25-OH-D higher than 27.8 ng/ml or bioavailable 25-OH-D higher than 3.88 ng/ml had significantly higher VZV-IgG levels than those with lower values. Linear regression suggested that both total and bioavailable 25-OH-D were significantly associated with higher VZV-IgG levels (p = 0.003 [total] and 0.01 [bioavailable]), whereas patients with cancer had lower VZV-IgG. Vitamin D may therefore be a potentially useful choice for raising VZV immunity in chronic dialysis patients.
Non-communicable diseases, especially chronic kidney disease (CKD) and end-stage renal disease (ESRD), are emerging global threats for public health and constitute significant burden to healthcare systems1. ESRD patients often have impaired immune systems and manifest susceptibility to infections2. Patients under chronic hemodialysis have a 40-50-fold increased risk of mortality from sepsis compared with the general population3,4.
ESRD impairs immunity in multiple ways. First, innate immunity is compromised, as monocytes and polymorphonuclear leukocytes display defective phagocytosis and increased apoptosis during uremia5,6. Second, adaptive immunity is dysregulated in ESRD patients: antigen-presenting cells are found to have poor antigen-presenting capabilities, and mitogen-induced T cell activation is suppressed7. Moreover, the depletion of dendritic cells, naïve and central memory CD4+/CD8+ T cells further illustrates the relative immune-deficient status of uremic patients8,9. B-cell lymphopenia and reduced antibody production during uremia indicates the concurrent impairment of humoral immunity as well10,11.
Weakened immunity brought by uremia potentially leads to higher incidences of both bacterial and viral infections in ESRD patients. Among those infections, herpes zoster (HZ) reactivation (from varicella zoster virus [VZV]) is an important category because HZ episodes are associated with the subsequent development of post-herpetic neuralgia (PHN) and long-term elevated risks for cardiovascular and stroke events12,13. Indeed, recent studies discovered that CKD as well as ESRD patients have 60% and nearly 200% higher risks of developing HZ compared with age/gender-matched population, respectively14,15. Together, these findings signifies that uremic patients have impaired immunity against VZV.
Despite this, the risk factors for HZ reactivation in chronic hemodialysis patients remain unknown. We previously identified that active vitamin D use was associated with a lower risk of HZ reactivation16. However, vitamin D statuses were not determined in such retrospective study, and whether the association with lower HZ risk was mediated by vitamin D deficiency or active vitamin D use was unclear. In the current study, we analyzed the association between VZV-specific antibody titers and circulating vitamin D forms, to test whether serum vitamin D levels play an important role in determining VZV immunity.
Methods
Study design, setting, and clinical data collection
The current study was conducted prospectively in National Taiwan University Hospital (NTUH) and its affiliated branches in Yun-Lin County, between 2012 and 2013. ESRD patients undergoing maintenance hemodialysis for more than three months were identified and recruited from these centers. Exclusion criteria included patients with a history of renal transplantation, those who were pregnant, and those who did not provide signed consent. The study was approved by the Institutional Review Board of NTUH (NO. 201208069RIC), and all participants provided written inform consent. This study was performed in compliance with the declaration of Helsinki.
Demographic information, including age, gender, and dialysis vintage was collected through chart abstraction or electronic medical record verification. We recorded patients' comorbidities and ESRD etiologies at the time of recruitment. The comorbidities were defined as follows: patients were classified as having DM and hypertension if their medical records had evidence of past/current use of hypoglycemic medications and anti-hypertensive agents, respectively. Heart failure was defined according to evidence of echocardiographic findings (left ventricular ejection fraction <50%) with compatible symptoms from medical records. Cirrhosis was identified with abdominal imaging studies. A history of malignancy and was confirmed by histopathologic findings, and rheumatologic diseases were confirmed with positive serology and clinical diagnoses by rheumatologists.
Laboratory assay
Blood samples before dialysis were collected and immediately transported for analysis of routine hemogram and biochemistry assays. In addition, blood samples were centrifuged to obtain plasma. Samples were all frozen at −80°C until assays were performed. Serum biochemistry assays included albumin, azotemic levels (urea nitrogen), creatinine, and electrolytes (sodium, potassium, calcium, phosphate). Calcium levels were corrected for serum albumin concentrations. Dialysis adequacy was calculated using the Daugirdas formula. Intact parathyroid hormone (PTH, 1-84) was measured by the LIAISON (DiaSorin, Stillwater, MN, USA) assay in the central laboratory.
Total 25-hydroxy-vitamin D (25-OH-D) and 1,25-dihydroxy-vitamin D [1,25-(OH)2-D] were measured using a commercially available radioimmunoassay (DiaSorin, Stillwater, MN, USA) within 3 months of sample collection. Inter-assay coefficients of variation for 25-OH-D and 1,25-(OH)2-D were less than 5% at values < 30 ng/ml and < 30 pg/ml, respectively. We also examined vitamin D-binding protein (DBP) by commercial ELISA (R&D Systems, Minneapolis, MN, USA), with an inter-assay coefficient of variation < 5%.
Serum antibody titers including VZV-specific IgG and IgM were utilized as surrogate markers for immunity against VZV. VZV-specific glycoprotein IgG has been demonstrated in past studies and recognized by U.S. Food and Drug Administration (FDA) as an alternative measure for vaccine efficacy and potential VZV immunity17,18, owing to the convenience and availability of this assay, compared with those for cell-mediated immunity. VZV-specific IgM, when detectable, indicates recent exposure to the virus, including primary infection, re-infection, or reactivation, but discrimination could be difficult. On the other hand, VZV-specific IgG starts to rise after IgM wanes, but persists for years even after primary infection/reactivation subsided.
VZV-specific antibodies were measured with a previously validated quantitative ELISA method, which detected antibodies against VZV glycoproteins prepared from human fibroblasts infected by VZV19. Both VZV-specific IgG and IgM were determined (VIRION/SERION ELISA Classic, Wurzburg, Germany). Rheumatoid factor-absorbent was added into dilution buffer to prevent false positivity during the VZV-gpELISA IgM assay, according to the manufacturer's suggestion.
Calculation of different circulating vitamin D forms
The formulae for the calculation of bioavailable forms of 25-OH-D and 1,25-(OH)2-D have been proposed and validated previously20,21. Briefly, both 25-OH-D and 1,25-(OH)2-D contain forms binding to albumin and DBP in the circulation, and total vitamin D concentrations (both 25 and 1,25) may be divided into albumin-bound, DBP-bound, and free (unbound) forms. The free form can be calculated using total forms, affinity constants (Kalb, between vitamin D and albumin; KDBP, between vitamin D and DBP) and albumin concentrations. The bioavailable form is calculated from the free form in conjunction with Kalb/albumin. Equations used in the current study are provided below20,21:
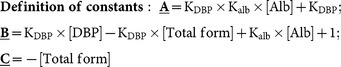 |
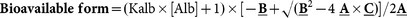 |
Statistical Analysis
Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as median value with interquartile ranges, with comparisons by Mann-Whitney U-test. Categorical variables were expressed as the number and percentage and analyzed with the chi-square test. Spearman's correlation analysis was utilized for assessing linear associations between VZV-specific antibodies with clinical and laboratory parameters. We then determined VZV antibody titers among different 25-OH-D and 1,25-(OH)2-D statuses, according to an existing guideline. Multivariate linear regression analyses and generalized additive modeling (GAM) were undertaken to assess the relationship between different vitamin D forms and VZV antibody titers.
Results
Clinical features of the participants
A total of 88 chronic hemodialysis patients were enrolled consecutively. The baseline characteristics of these patients are summarized in Table 1. The median age was over 65 years, and most patients had comorbidities such as hypertension. Chronic glomerulonephritis and DM nephropathy were the predominant causes of dialysis. Approximately half of the patients were using active vitamin D, which in this cohort consisted of 1-α-calcidol (44 [97.8%]) and calcitriol (1 [2.2%]). All active vitamin D users received at least 3 months of therapy before blood tests, and the 1-α-calcidol dosage was 3.4 ± 3.8 μg/week. All the participants were enrolled during the warm seasons (March to June).
Table 1. Characteristics of study cohort (n = 88).
| Median (IQR) or n (%) | |
|---|---|
| Demographic data | |
| Age (years) | 67.5 (58–75) |
| Gender (Male) | 54 (61) |
| Dialysis vintage (years) | 3.5 (2.3–8.4) |
| Comorbidities | |
| Hypertension | 76 (86) |
| Diabetes mellitus | 33 (38) |
| Heart failure | 3 (3) |
| Cirrhosis | 4 (5) |
| Malignancy | 12 (14) |
| Rheumatology illness | 5 (6) |
| Etiology of ESRD | |
| CGN | 30 (34) |
| DM nephropathy | 25 (28) |
| Hypertensive nephropathy | 4 (5) |
| SLE | 3 (3) |
| Others | 27 (31) |
| Laboratory profiles | |
| Albumin (g/dl) | 4.0 (3.8–4.2) |
| Hemoglobin (mg/dl) | 10.2 (9.2–10.9) |
| Corrected Calcium (mg/dl) | 9.3 (8.8–9.8) |
| Phosphorus (mg/dl) | 4.7 (3.9–5.7) |
| Parathyroid hormone (pg/ml) | 454.8 (196.5–652.9) |
| Total 25-OH-D (ng/ml) | 17.7 (11.6–23.4) |
| Total 1,25-(OH)2-D (pg/ml) | 17.6 (11.1–26.9) |
| Alkaline phosphatase (mg/dl) | 77.5 (60–98) |
| Dialysis parameters | |
| Kt/V | 1.66 (1.44–1.86) |
| Urea reduction ratio | 74.9 (70.0–78.9) |
| Medications | |
| Activated vitamin D use (any form) | 45 (51) |
Abbreviation: CGN, chronic glomerulonephritis; DM, diabetes mellitus; ESRD, end-stage renal disease; IQR, inter-quartile range; SLE, systemic lupus erythematosus.
For the past history of VZV, only 3 (3.4%) patients recalled experiences of developing zoster-like lesions before, all of whom belonged to the 25-OH-D deficiency category (total 25-OH-D < 20 ng/ml). One event occurred within one year before blood test, while the others occurred more than two years ago. None of the participants received VZV vaccines before.
Mineral metabolism and hormone status
Parameters pertaining to mineral and bone metabolism are also provided in Table 1. Since other active vitamin analogs were unavailable in the participating institutes, and both 1-α-calcidol and calcitriol were converted to 1,25-(OH)2-D, the measured 25-OH-D and 1,25-(OH)2-D levels were representative of the true vitamin D metabolite concentrations within the body. We tested the association between total 25-OH-D, 1,25-(OH)2-D and relevant mineral-bone metabolism parameters among non-vitamin D users only, since the correlation between 25-OH-D/1,25-(OH)2-D and serum calcium/phosphate could be attenuated in active vitamin D users from the disproportionate influence of active vitamin D on measured vitamin D concentrations and divalent ion levels. Serum calcium/phosphate might be influenced by other factors including hyperparathyroidism, types of renal osteodystrophy, use of calcium/non-calcium-based phosphate binders, dietary calcium/phosphate intake amount, etc
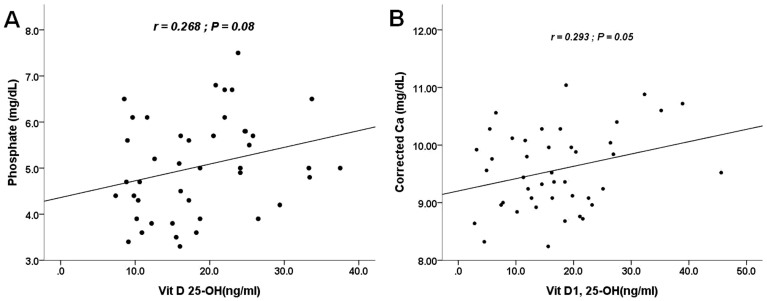
Active vitamin D users were found to have significantly higher 1,25-(OH)2-D levels (Table 2), but 25-OH-D levels did not differ between users and non-users (p = 0.87). Corrected serum calcium and phosphate levels were each associated with total 1,25-(OH)2-D (r = 0.29, p = 0.05) and borderline with 25-OH-D (r = 0.27; p = 0.08) among non-active vitamin D users, respectively (Figure 1). Alkaline phosphatase was neither associated with total 25-OH-D (r = 0.02, p = 0.83) nor 1,25-(OH)2-D (r = 0.05, p = 0.63).
Table 2. Different forms of vitamin D levels in the current cohort.
| Median | IQR | p-value* | |
|---|---|---|---|
| Vitamin D binding protein (μg/ml) | 236.0 | 174.4–280.0 | |
| 25-OH-D | |||
| Total form (ng/ml) | 17.7 | 11.6–23.4 | 0.87 |
| Users | 18.2 | 11.9–22.4 | |
| Non-users | 17.2 | 10.6–24.1 | |
| Bioavailable form (ng/ml) | 2.1 | 1.5–3.0 | 0.92 |
| Users | 2.0 | 1.5–3.0 | |
| Non-users | 2.1 | 1.4–3.2 | |
| 1,25-(OH)2-D | |||
| Total form (pg/ml) | 17.6 | 11.1–26.9 | <0.01 |
| Users | 18.9 | 11.1–41.2 | |
| Non-users | 15.7 | 9.4–21.1 | |
| Bioavailable form (pg/ml) | 3.6 | 2.1–5.5 | <0.01 |
| Users | 4.0 | 2.2–8.1 | |
| Non-users | 3.1 | 1.7–3.9 | |
Formulae for calculation were adapted from Vermeulen equations (J Clin Endocrinol Metab 1999;84:3666-72) originally and later affirmed in vitamin D forms determination with consistent results (reference 21).
*Comparison between active vitamin D users and non-users.
Abbreviations: IQR, inter-quartile range.
Figure 1. Scatter plot illustrating the correlations between 25-OH-D, 1,25-(OH)2-D and serum phosphate, corrected calcium levels.
(A) A significant association between 25-OH-D and phosphate was found; (B) A borderline significant association was noted over 1,25-(OH)2-D and serum calcium.
VZV immunoglobulin status and clinical variables
Next, we investigated the associations between serum VZV-IgG, IgM levels and enrollees' clinical variables (Table 3). Correlations between VZV immunoglobulin and mineral bone parameters were analyzed owing to anecdotal evidence regarding the impact of hyperparathyroidism on immune system, and calcium related VZV gene regulation22,23. VZV-IgG levels were negatively associated with the presence of malignancy (p = 0.02), marginally associated with male gender (p = 0.06), whereas VZV-IgM levels were positively associated with higher hemoglobin levels (p = 0.05) but negatively associated with the presence of heart failure in chronic dialysis patients (p = 0.04).
Table 3. Correlation between different VZV-specific antibodies and clinical as well as laboratory parameters in the current cohort.
| Correlation coefficient | VZV-IgG (IU/ml) | p value | VZV-IgM (IU/ml) | p value |
|---|---|---|---|---|
| Age (years) | −0.01 | 0.92 | 0.01 | 0.89 |
| Gender (male) | 0.20 | 0.06 | −0.15 | 0.18 |
| Dialysis vintage (years) | 0.05 | 0.67 | 0.18 | 0.09 |
| Comorbidities | ||||
| Hypertension | −0.07 | 0.50 | 0.18 | 0.10 |
| Diabetes mellitus | 0.08 | 0.45 | 0.02 | 0.86 |
| Heart failure | 0.18 | 0.10 | −0.22 | 0.04 |
| Cirrhosis | −0.18 | 0.09 | −0.18 | 0.10 |
| Malignancy | −0.25 | 0.02 | −0.10 | 0.36 |
| Rheumatology illness | 0.07 | 0.51 | 0.08 | 0.47 |
| Laboratory parameters | ||||
| Albumin (g/dl) | −0.15 | 0.16 | −0.01 | 0.97 |
| Hemoglobin (mg/dl) | 0.03 | 0.81 | 0.22 | 0.05 |
| Corrected calcium (mg/dl) | 0.01 | 1.00 | 0.13 | 0.24 |
| Phosphorus (mg/dl) | −0.01 | 0.94 | 0.04 | 0.74 |
| Intact parathyroid hormone (pg/ml) | 0.03 | 0.76 | −0.03 | 0.76 |
| Alkaline phosphatase (mg/dl) | −0.03 | 0.82 | 0.01 | 0.96 |
| Dialysis parameters | ||||
| Kt/V | 0.02 | 0.86 | 0.07 | 0.52 |
| Urea reduction ratio | 0.06 | 0.60 | 0.05 | 0.66 |
| Medications | ||||
| Active vitamin D use | −0.03 | 0.80 | 0.05 | 0.63 |
Relationship between VZV immunoglobulin and vitamin D statuses
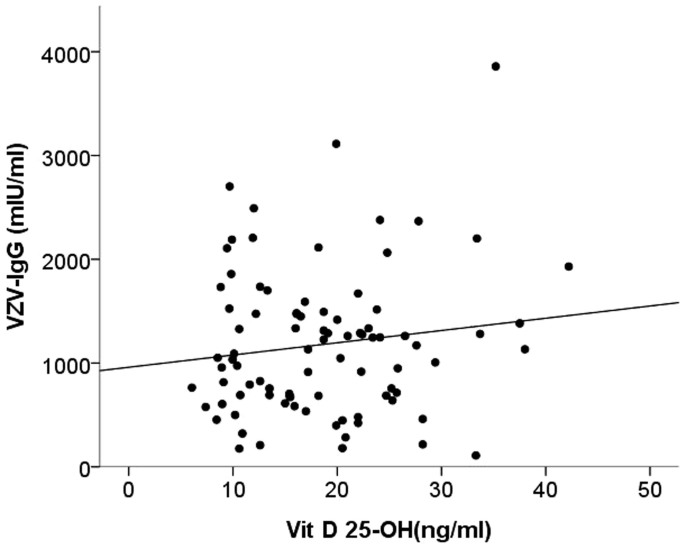
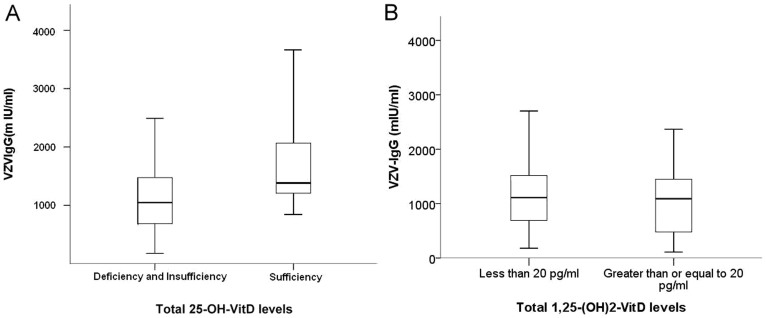
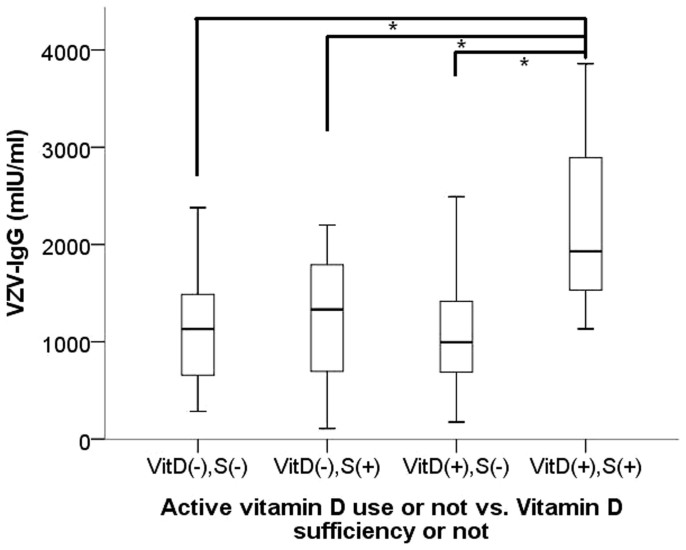
We subsequently divided patients according to their serum 25-OH-D levels, based upon the Endocrine Society's guideline (deficiency, < 20 ng/ml; insufficiency, 20 – 30 ng/ml; sufficiency, ≥ 30 ng/ml)24. A total of 52 (59.1%) patients were categorized with 25-OH-D deficiency, 29 patients (33%) with 25-OH-D insufficiency, with only 7 patients (8%) found to be 25-OH-D sufficient. The relationship between VZV-IgG levels and 25-OH-D is shown in Figure 2. We discovered that chronic dialysis patients with vitamin D deficiency or insufficiency (serum 25-OH-D <30 ng/ml) had significantly lower VZV-IgG levels than those with sufficient 25-OH-D (deficiency/insufficiency vs. sufficiency, 1133 mIU/ml vs. 1699 mIU/ml, p = 0.04) (Figure 3), while no significant difference existed between those with deficient and insufficient 25-OH-D (deficiency vs. insufficiency, 1056 mIU/ml vs. 1179 mIU/ml, p = 0.43). VZV-IgG levels did not differ between patients with higher 1,25-(OH)2-D (defined as ≥ 20 pg/ml) and those with low 1,25-(OH)2-D (p = 0.65) (Figure 3). VZV-IgM levels did not differ among different vitamin D statuses. In order to discern the relative impact of active vitamin D and vitamin D statuses on the observed VZV-IgG levels, we subdivided the entire cohort according to active vitamin D use or not and 25-OH-D status (Figure 4). We found that only the subgroup with sufficient 25-OH-D and under active vitamin D use possessed significantly higher VZV-IgG levels (p = 0.01). The subgroup with sufficient 25-OH-D but no active vitamin D use displayed an insignificantly higher VZV-IgG levels compared with those with deficient/insufficient 25-OH-D and no active vitamin D use (the former vs. the latter, 1243 mIU/ml vs. 1136 mIU/ml, p = 0.72).
Figure 2. Scatter plot showing the positive association between VZV-IgG and 25-OH-D levels.
Figure 3.
(A) Box-and-whisker plot showing significantly higher VZV-IgG levels among patients with sufficient total 25-OH-D (≥ 30 ng/ml) (B) VZV-IgG levels did not differ between patients with high or low 1,25-(OH)2-D levels. Sample size: (A) Deficiency/Insufficiency (n = 81) vs. Sufficiency (n = 7) (B) 1,25-(OH)2-D < 20 pg/ml (n = 54) vs. ≥ 20 pg/ml (n = 34). Abbreviations: IgG, immunoglobulin G, VZV, varicella-zoster virus.
Figure 4. Significantly higher VZV-IgG levels were observed only among the subgroup of patients with sufficient total 25-OH-D (≥ 30 ng/ml) and concurrent active vitamin D use.
Sample size: VitD(-),S(-) (n = 39) vs. VitD(-),S(+) (n = 4) vs. VitD(+),S(-) (n = 42) vs. VitD(+),S(+) (n = 3). Abbreviation: S, sufficiency; VitD, active vitamin D. *: p = 0.01 for all three comparisons.
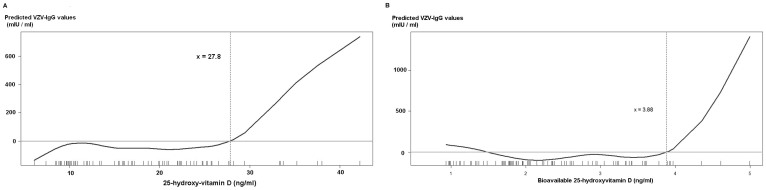
Furthermore, to clarify the relationship between different vitamin D forms and VZV immunity, we calculated the bioavailable forms of 25-OH-D and 1,25-(OH)2-D using validated formulae (Table 2)20,21. DBP levels did not differ between patients with sufficient or insufficient/deficient 25-OH-D levels (252 ± 46.1 vs. 224 ± 78.3 μg/ml, p = 0.36), and between patients receiving active vitamin D or not (227 ± 67.6 vs. 227 ± 85.5 μg/ml, p = 0.99). Bioavailable 1,25-(OH)2-D levels were significantly higher among active vitamin D users (p < 0.01). GAM models showed that serum 25-OH-D values higher than 27.8 ng/ml were significantly more likely to be associated with higher VZV-IgG levels, whereas serum bioavailable 25-OH-D values higher than 3.88 ng/ml demonstrated a similarly positive association with VZV-IgG (Figure 5). Excluding the three patients with previous experiences of zoster, GAM still revealed a cut-off value of 27 ng/ml, close to the original results.
Figure 5. The predictive values of VZV-specific IgG according to (A) total and (B) bioavailable 25-OH-D levels among the entire dialysis cohort, constructed with the generalized additive model (GAM).
Threshold values of (A) 27.8 ng/ml and (B) 3.88 ng/ml were chosen for further analysis. Abbreviations: IgG, immunoglobulin G, VZV, varicella-zoster virus.
Finally, multivariate linear regression analyses incorporating demographic data, comorbidities, laboratory profiles, dialysis parameters, use of active vitamin D or not, and different vitamin D forms (both total/bioavailable 25-OH-D and 1,25-(OH)2-D) were performed to determine the associations between different vitamin D forms and VZV antibody titers. VZV-IgM showed no significant associations with both total and bioavailable 25-OH-D levels. In contrast, regression analyses revealed that VZV-IgG levels were positively affected by the presence of total 25-OH-D levels ≥ 27.8 ng/ml (p = 0.03), whereas bioavailable 25-OH-D ≥ 3.88 ng/ml also significantly predicted higher VZV-IgG levels (p = 0.01) (Table 4). However, after adjustment, both total and bioavailable 1,25-(OH)2-D levels were not significantly associated with VZV-IgG levels (p = 0.08 and 0.06, respectively). Active vitamin D use did not significantly correlate with higher VZV-IgG levels. Sensitivity analysis excluding the three patients with VZV experiences also showed that VZV-IgG levels were positively affected by the presence of 25-OH-D higher than 27 ng/ml (p = 0.036).
Table 4. Multivariate-adjusted linear regression analysis showing relationships between different 25-OH-D forms and VZV gp-ELISA antibody levels.
| VZV-IgG | ||||
|---|---|---|---|---|
| Model * | Parameter estimates | Standard error | t-value | p value |
| Intercept | 524 | 200.9 | 2.61 | 0.01 |
| Total 25-OH-D > 27.8 | 14.1 | 6.3 | 2.24 | 0.03 |
| Cancer | −453.5 | 198.4 | −2.29 | 0.02 |
| Intercept | 2528.3 | 786.3 | 3.22 | <0.01 |
| Bioavailable 25-OH-D > 3.66 | 149 | 56.5 | 2.64 | 0.01 |
| Cancer | −639.2 | 197.8 | −3.23 | <0.01 |
*Adjusted for demographic data, comorbiditis, albumin, dialysis parameters, vintage, and use of active vitamin D.
Abbreviations: ELISA, enzyme-linked immuno-sorbent assay; VZV, varicella zoster virus.
Discussion
In the current study, we demonstrated that serum vitamin D levels were positively associated with immunity against VZV. Both total and bioavailable 25-OH-D sufficiency were associated with higher VZV IgG levels, whereas neither total nor bioavailable 1,25-(OH)2-D levels demonstrated such associations. Furthermore, the identified threshold value of total 25-OH-D, 27.8 ng/ml, for predicting higher VZV-IgG values, was close to the guideline-specified 25-OH-D sufficiency (30 ng/ml).
Vitamin D is renowned for its pleiotropic effects. Vitamin D traditionally plays an important role in divalent ion regulation and skeletal mineralization, and its deficiency is associated with a higher risk of fractures25. In addition, vitamin D has non-skeletal functions including cardiovascular, metabolic, and most importantly, immune-modulatory effects26,27. Indeed, a 25-OH-D level lower than 20 ng/ml confers increased risk of immune disorders such as type 1 DM and multiple sclerosis28,29. Because vitamin D receptors (VDRs) reside in neutrophils, T-cells and antigen-presenting cells (APCs)30, and vitamin D exerts its action through VDRs, vitamin D would be expected to participate in the regulation of immunity.
In innate immunity, vitamin D may affect APC maturation, whereas cytokines released by activated immunocytes upregulate CYP27B1 (1-α hydroxylase) expression31. Vitamin D also induces the production of human cathelicidin, a potent antimicrobial peptide against bacterial infections30. In adaptive immunity, evidence suggests that the activation of human B and T-cells resulted in VDR expression32. 1,25-(OH)2-D also modulates T-cell maturation and increases the proportion of regulatory T cells33. Moreover, 1,25-(OH)2-D fine-tunes interactions between pathogens and their targets and potentially limits the extent of inflammation triggered by microbial invasion30,34. Consequently, vitamin D may play an even more important role in calibrating immunity among the relatively immunocompromised ESRD patients. Existing studies did find that vitamin D levels correlated with circulating cathelicidin concentrations in ESRD patients, and lower cathelicidin levels were associated with higher mortality from sepsis35. Additionally, 25-OH-D deficiency was associated with impaired cellular immunity in hemodialysis patients, whereas the administration of vitamin D restored T-cell IL-2 production and NK cell function36,37. Based on these data, vitamin D potentially exerts a potentially immune-enhancing role in ESRD patients, and variations in vitamin D status may underlie the susceptibility to infection among this population.
Our finding that insufficient/deficient 25-OH-D correlated with lower VZV-IgG levels, or lower VZV immunity, is noteworthy (Table 4). The associations between vitamin D statuses and circulating antibody levels have been described only among rheumatologic disorders in the literature. For example, 25-OH-D is found to negatively correlate with autoantibody levels in patients with thyroid diseases38. For pathogen-specific antibodies, 25-OH-D levels often display positive associations. There is evidence that suggests that 25-OH-D levels are positively linked with pneumococcal antibody titers, whereas sufficient 25-OH-D (>30 ng/ml) is predictive of higher EBV, CMV and HSV antibody titers in patients with multiple sclerosis39,40. Supplementation of cholecalciferol in lupus patients significantly raises tetanus-specific IgG41. Nonetheless, such connection had not been reported before in ESRD patients. Our finding would be the first to demonstrate that the vitamin D-antibody relationship exists in ESRD patients. Furthermore, we discovered that this relationship exists for both total and bioavailable forms of 25-OH-D. This issue deserves further investigation because nutritional vitamin D use may bear theoretical potential for boosting VZV immunity. The absence of an association between 1,25-(OH)2-D levels and VZV-IgG may be partially explained by the tissue-specific nature of 1,25-(OH)2-D biologic effects, or by the relatively modest case number in this study.
Our finding that active vitamin D might simultaneously play a role in enhancing VZV-immunity (Figure 4) also merits attention. In unadjusted analysis, the subgroup with sufficient 25-OH-D and concurrent vitamin D use manifested significantly higher VZV-IgG levels than the other subgroups. However, multivariate regression analyses identified only 25-OH-D levels (total or bioavailable) as significant predictors of VZV-IgG, while active vitamin D did not (Table 4). Taking into account the lack of an association between 1,25-(OH)2-D (the physiological product of active vitamin D in this study) and VZV-IgG, we propose that active vitamin D use might play a permissive role in VZV-IgG enhancement. It is more likely that 25-OH-D sufficiency was still the major driving force, whereas active vitamin D potentially boosted the efficacy. However, a larger sample size would be needed to characterize this phenomenon. In addition, although subgroup analyses (univariate) (Figure 4) showed that only dialysis patients with active vitamin D use and sufficient 25-OH-D has significantly higher VZV-IgG levels than all other patients, in multivariate analyses, it is the dialysis patients with sufficient 25-OH-D that demonstrated significantly higher VZV-IgG levels than the others. A further sub-analysis dividing the entire cohort according to 25-OH-D quartiles (N = 22) showed that VZV-IgG levels disclosed similar trend with our GAM results (stratum 1 [lowest] vs. 2 vs. 3. vs. 4 [highest], 1102 vs. 1148 vs. 1132 vs. 1333 mIU/ml). Patients with the highest quartile of 25-OH-D possessing the highest mean VZV-IgG levels; however, the difference did not reach significance (p = 0.38). These findings are compatible with our original findings, since the significantly elevated VZV-IgG levels only occurred in those patients with high 25-OH-D (25-OH-D > 27.8 ng/ml). Expanding the subgroup to encompass more dialysis patients with 25-OH-D < 27.8 ng/ml significantly attenuated the observed difference in VZV-IgG levels.
The VZV-specific immunity could potentially be influenced by the patients' medical history of zoster. However, reports of vaccination immunologic efficacy described that VZV-specific IgG levels did not alter significantly for at least two years after vaccination, except an initial surge within 6 weeks of vaccination17,42. In line with this, remote episodes of zoster expectedly won't influence the VZV-IgG levels, since vaccination and recent infection were both stimulators of VZV-IgG productions. If a great majority of our cohort did not have a recent zoster episode, their VZV-IgG status might not be altered significantly at the time of blood testing. In addition, none of participants ever received VZV vaccines before, and vaccination-induced antibody enhancement would not affect our results. Consequently, we believed that medical history of zoster in our cohort did not influence our findings.
Another notable finding of this study lies in the concordance of the biologic effects of bioavailable and total vitamin D (Table 4). Since the discovery that genetic heterogeneity of vitamin D binding protein levels is responsible for racial differences in vitamin D levels43, subsequent studies sought to clarify the role of bioavailable vitamin D, mostly within the context of mineral-bone studies. Bhan et al. determined that bioavailable 25-OH-D displayed a greater association with divalent ions and parathyroid hormone levels in incident ESRD patients21. However, most vitamin D studies still rely on the measurement of total vitamin D alone, and conflicting results often ensue. It is then likely that some reported absence of relationship between vitamin D status and pathogen-specific immunity44 may result from a failure to consider the bioavailable forms. In this regard, utilizing bioavailable vitamin D may be both important and practical for the study of vitamin D effects.
There may be several reasons for the increase in HZ incidence among ESRD patients. The defense against VZV reactivation lies mostly within the intactness of cellular immunity, and 25-OH-D deficiency has been known to aggravate CD8 T-cell shortage45, thus predisposing patients to developing HZ. The association between 25-OH-D insufficiency and poorer humoral immunity against VZV may then be reflective of defective cellular immunity simultaneously because VZV-IgG titers reportedly correlate well with VZV cellular immunity17,18. It may be worthwhile to investigate the utility of vitamin D in ESRD patients with experiences of HZ. Moreover, nutritional vitamin D may serve as a potentially useful adjuvant in patients who wish to receive the globally marketed VZV vaccines.
Our study has several strengths. This is the first study to clarify the associations between vitamin D levels and viral immunity in ESRD patients, and this finding could be potentially therapeutic. The identified 25-OH-D threshold correlated fairly well with the existing guideline. However, there are still limitations to this study. First, the cellular immunity index was not tested in our cohort, and thus the associations between cell-mediated immunity and vitamin D status could not be confirmed. Because such an index is feasible only in highly specialized laboratories, and past studies derived similar trends of change in VZV-IgG and cell immunity index, we believed this issue was of less importance. Second, the size of our cohort is inadequate to draw definite conclusions. Further large-scale studies would be required to confirm our findings.
Conclusion
Vitamin D presumably has an immunoregulatory role and is involved for dialysis patients in the defense against infection. We found that 25-OH-D ≥ 27.8 ng/ml was positively associated with VZV-IgG levels in ESRD patients, suggesting potentially greater VZV-specific immunity. In addition, both higher total and bioavailable 25-OH-D correlated with higher VZV-IgG levels, whereas 1,25-(OH)2-D did not demonstrate such association. Judging from these findings, nutritional vitamin D may be a promising agent in our armamentarium by boosting immunity against herpes zoster in chronic dialysis patients.
Acknowledgments
We are grateful to the staff of the Second Core Lab of the department of Medical Research of NTUH for their technical support.
Funding Disclosure: This study is financially supported by NTUH (project NO.102-N2249 and project NO.103-N2525).
Footnotes
The authors declare no competing financial interests.
Author Contributions C.T.C. and J.W.H. were responsible for study design; C.T.C., C.J.Y., J.W.H., C.K.C. and K.Y.H. conducted data analysis; C.T.C., S.Y.L., W.S.Y., C.J.Y., J.W.H., C.K.C. and K.Y.H. drafted the manuscript; All authors have read and approved the submission of this manuscript.
References
- Stevens P. E. & Levin A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 158, 825–830 (2013). [DOI] [PubMed] [Google Scholar]
- Eleftheriadis T., Antoniadi G., Liakopoulos V., Kartsios C. & Stefanidis I. Basic Science and Dialysis: Disturbances of Acquired Immunity in Hemodialysis Patients. Semin. Dial. 20, 440–451 (2007). [DOI] [PubMed] [Google Scholar]
- Sarnak M. J. & Jaber B. L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney. Int. 58, 1758–1764 (2000). [DOI] [PubMed] [Google Scholar]
- Patel P. R., Kallen A. J. & Arduino M. J. Epidemiology, Surveillance, and Prevention of Bloodstream Infections in Hemodialysis Patients. Am. J. Kidney. Dis. 56, 566–577 (2010). [DOI] [PubMed] [Google Scholar]
- Alexiewicz J. M., Smogorzewski M., Fadda G. Z. & Massry S. G. Impaired phagocytosis in dialysis patients: studies on mechanisms. Am. J. Nephrol. 11, 102–111 (1991). [DOI] [PubMed] [Google Scholar]
- Massry S. G. & Smogorzewski M. Dysfunction of polymorphonuclear leukocytes in uremia: Role of parathyroid hormone. Kidney. Int. 59, S195–S196 (2001). [DOI] [PubMed] [Google Scholar]
- Sester U. et al. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol. Dial. Transplant. 15, 1217–1223 (2000). [DOI] [PubMed] [Google Scholar]
- Agrawal S., Gollapudi P., Elahimehr R., Rahl M. V. & Vaziri N. D. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol. Dial. Transplant. 25, 737–746 (2010). [DOI] [PubMed] [Google Scholar]
- Yoon J. W., Gollapudi S., Pahl M. V. & Vaziri N. D. Naive and central memory T-cell lymphopenia in end-stage renal disease. Kidney. Int. 70, 371–376 (2006). [DOI] [PubMed] [Google Scholar]
- Smogorzewski M. & Massry S. G. Defects in B-cell function and metabolism in uremia: Role of parathyroid hormone. Kidney. Int. 59, S186–S189 (2001). [DOI] [PubMed] [Google Scholar]
- Pahl M. V. et al. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transplant. 25, 205–212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. Y., Lin C. L., Sung F. C., Chou T. C. & Lee Y. T. Increased risk of cardiovascular events in patients with herpes zoster: A population-based study. J. Med. Virol. 86, 772–777 (2014). [DOI] [PubMed] [Google Scholar]
- Kim S. R., Khan F. & Tyring S. K. Varicella zoster: an update on current treatment options and future perspectives. Expert. Opin. Pharmacother. 15, 61–71 (2014). [DOI] [PubMed] [Google Scholar]
- Wu M. Y., Hsu Y. H., Su C. L., Lin Y. F. & Lin H. W. Risk of Herpes Zoster in CKD: A Matched-Cohort Study Based on Administrative Data. Am. J. Kidney. Dis. 60, 548–552 (2012). [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Lee C. T., Lee I. M., Ho S. C. & Yang C. Y. Risk of Herpes Zoster in Patients Treated With Long-term Hemodialysis: A Matched Cohort Study. Am. J. Kidney. Dis. 59, 428–433 (2012). [DOI] [PubMed] [Google Scholar]
- Chao C. T., Lai C. F. & Huang J. W. Risk factors for herpes zoster reactivation in maintenance hemodialysis patients. Eur. J. Intern. Med. 23, 711–715 (2012). [DOI] [PubMed] [Google Scholar]
- Levin M. J. et al. Varicella-zoster virsu-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 197, 825–835 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. B. et al. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J. Infect. Dis. 4, 279; 10.1093/infdis/jiu279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond O. et al. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J. Med. Virol. 78, 1679–1687 (2006). [DOI] [PubMed] [Google Scholar]
- Bikle D. D. et al. Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein. J. Clin. Endocrinol. Metab. 63, 954–959 (1986). [DOI] [PubMed] [Google Scholar]
- Bhan I. et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney. Int. 82, 84–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. et al. RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation. PLoS. Pathog. 10, e1003896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geara A. S. et al. Effects of parathyroid hormone on immune function. Clin. Dev. Immunol. 2010, 418695 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930 (2011). [DOI] [PubMed] [Google Scholar]
- van. Schoor N. M. et al. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone. 42, 260–266 (2008). [DOI] [PubMed] [Google Scholar]
- Pittas A. G., Lau J., Hu F. B. & Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 92, 2017–2029 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobnig H. et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 168, 1340–1349 (2008). [DOI] [PubMed] [Google Scholar]
- Duan S. et al. Vitamin D status and the risk of multiple sclerosis: A systematic review and meta-analysis. Neurosci. Lett. 570, 108–113 (2014). [DOI] [PubMed] [Google Scholar]
- Abd-Allah S. H., Pasha H. F., Hagrass H. A. & Alghobashy A. A. Vitamin D status and vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Egyptian children. Gene. 536, 430–434 (2014). [DOI] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and the Immune System: New Perspectives on an Old Theme. Endocrinol. Metab. Clin. N. Am. 39, 365–379 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Pillai S., Gee E. & Hincenbergs M. Tumor Necrosis Factor-α Regulation of 1,25-Dihydroxy vitamin D Production by Human Keratinocytes. Endocrinology. 129, 33–38 (1991). [DOI] [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J. & Manolagas S. C. 25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 221, 1181–1183 (1983). [DOI] [PubMed] [Google Scholar]
- Bikle D. Nonclassic Actions of Vitamin D. J. Clin. Endocrinol. Metab. 94, 26–34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Equils O. et al. 1,25-Dihydroxyvitamin D3 inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin. Exp. Immunol. 141, 58–64 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A. F. et al. Low Plasma Level of Cathelicidin Antimicrobial Peptide (hCAP18) Predicts Increased Infectious Disease Mortality in Patients Undergoing Hemodialysis. Clin. Infect. Dis. 48, 418–424 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T. et al. In vivo Effect of 1α-Hydroxyvitamin D<sub>3</sub> on Interleukin-2 Production in Hemodialysis Patients. Nephron. 50, 295–298 (1988). [DOI] [PubMed] [Google Scholar]
- Quesada J. M. et al. Calcitriol effect on natural killer cells from hemodialyzed and normal subjects. Calcif. Tissue. Int. 56, 113–117 (1995). [DOI] [PubMed] [Google Scholar]
- Bozkurt N. et al. The Association Between Severity of Vitamin D Deficiency and Hashimoto's Thyroiditis. Endocrine. Pract. 19, 479–484 (2013). [DOI] [PubMed] [Google Scholar]
- Ryoo E., Kumar R., Kita H. & Juhn Y. J. Serum 25-hydroxyvitamin D concentrations and waning pneumococcal antibody titers among individuals with atopy. Allergy. Asthma. Proc. 34, 370–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry E. M., James J. A., Krupp L. B. & Waubant E. Vitamin D status and antibody levels to common viruses in pediatric-onset multiple sclerosis. Mult. Scler. J. 17, 666–671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine G. et al. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur. J. Clin. Nutr. 65, 329–334 (2011). [DOI] [PubMed] [Google Scholar]
- Irwin M. R. et al. Recipients with major depression and the impact of antidepressant medications. Clin. Infect. Dis. 56, 1085–1093 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe C. E. et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. New. Engl. J. Med. 369, 1991–2000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M. E. et al. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 31, 2057–2061 (2013). [DOI] [PubMed] [Google Scholar]
- Pender M. P. CD8+ T-Cell Deficiency, Epstein-Barr Virus Infection, Vitamin D Deficiency, and Steps to Autoimmunity: A Unifying Hypothesis. Autoimmune. Dis. 2012, 189096 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]