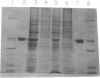
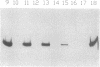
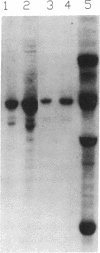
Abstract
Human adenosine deaminase (EC 3.5.4.4), a key purine salvage enzyme essential for immune competence, has been overproduced in Spodoptera frugiperda cells and in Trichoplusia ni (cabbage looper) larvae infected with recombinant baculovirus. The coding sequence of human adenosine deaminase was recombined into a baculovirus immediately downstream from the strong polyhedrin gene promoter. Approximately 60 hr after infection of insect cells with the recombinant virus, maximal levels of intracellular adenosine deaminase mRNA, protein, and enzymatic activity were detected. The recombinant human adenosine deaminase represented 10% of the total cellular protein and exhibited a specific activity of 70 units/mg of protein in crude homogenate. This specific activity is 70-350 times greater than that exhibited by the enzyme in homogenates of the two most abundant natural sources of human adenosine deaminase, thymus and leukemic cells. When the recombinant virus was injected into insect larvae, the maximum recombinant enzyme was produced 4 days postinfection and represented about 2% of the total insect protein with a specific activity of 10-25 units/mg of protein. The recombinant human adenosine deaminase was purified to homogeneity from both insect cells and larvae and demonstrated to be identical to native adenosine deaminase purified from human cells with respect to molecular weight, interaction with polyclonal anti-adenosine deaminase antibody, and enzymatic properties. A pilot purification yielded 8-9 mg of homogeneous enzyme from 22 larvae. The production of large quantities of recombinant human adenosine deaminase in insect larvae is inexpensive and rapid and eliminates the need for specialized facilities for tissue culture. This method should be applicable to large-scale production of many recombinant proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Spector T., Parks R. E., Jr Tight-binding inhibitors--IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977 Mar 1;26(5):359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J. Micromethod for quantitation of adenosine deaminase activity in cells from human peripheral blood. Biochem Med. 1975 May;13(1):46–55. doi: 10.1016/0006-2944(75)90139-8. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Kelley W. N. Human adenosine deaminase. Stoichiometry of the adenosine deaminase-binding protein complex. Biochim Biophys Acta. 1979 Oct 24;580(2):302–311. doi: 10.1016/0005-2795(79)90143-0. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Kati W. M., Wolfenden R. Contribution of a single hydroxyl group to transition-state discrimination by adenosine deaminase: evidence for an "entropy trap" mechanism. Biochemistry. 1989 Sep 19;28(19):7919–7927. doi: 10.1021/bi00445a055. [DOI] [PubMed] [Google Scholar]
- Kurz L. C., Frieden C. Adenosine deaminase converts purine riboside into an analogue of a reactive intermediate: a 13C NMR and kinetic study. Biochemistry. 1987 Dec 15;26(25):8450–8457. doi: 10.1021/bi00399a063. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Philips A. V., Coleman M. S., Maskos K., Barkley M. D. Time-resolved fluorescence spectroscopy of human adenosine deaminase: effects of enzyme inhibitors on protein conformation. Biochemistry. 1989 Mar 7;28(5):2040–2050. doi: 10.1021/bi00431a012. [DOI] [PubMed] [Google Scholar]
- Philips A. V., Robbins D. J., Coleman M. S., Barkely M. D. Immunoaffinity purification and fluorescence studies of human adenosine deaminase. Biochemistry. 1987 May 19;26(10):2893–2903. doi: 10.1021/bi00384a034. [DOI] [PubMed] [Google Scholar]
- Price P. M., Reichelderfer C. F., Johansson B. E., Kilbourne E. D., Acs G. Complementation of recombinant baculoviruses by coinfection with wild-type virus facilitates production in insect larvae of antigenic proteins of hepatitis B virus and influenza virus. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1453–1456. doi: 10.1073/pnas.86.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. P., Stacy A. R. Purification and subunit structure of adenosine deaminase from human kidney. J Biol Chem. 1977 Sep 25;252(18):6409–6415. [PubMed] [Google Scholar]
- Van der Weyden M. B., Kelley W. N. Human adenosine deaminase. Distribution and properties. J Biol Chem. 1976 Sep 25;251(18):5448–5456. [PubMed] [Google Scholar]
- Wiginton D. A., Coleman M. S., Hutton J. J. Purification, characterization and radioimmunoassay of adenosine deaminase from human leukaemic granulocytes. Biochem J. 1981 May 1;195(2):389–397. doi: 10.1042/bj1950389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. K., Rudolph F. B., Harrison M. L., Kellems R. E., Quiocho F. A. Preliminary X-ray analysis of crystals of murine adenosine deaminase. J Mol Biol. 1988 Apr 5;200(3):613–614. doi: 10.1016/0022-2836(88)90549-9. [DOI] [PubMed] [Google Scholar]