Abstract
Social interactions are often characterized by cooperation within groups and conflict or competition between groups. In certain circumstances, however, cooperation can arise between social groups. Here, we examine the circumstances under which inter-group cooperation is expected to emerge and present examples with particular focus on groups in two well-studied but dissimilar taxa: humans and ants. Drivers for the evolution of inter-group cooperation include overarching threats from predators, competitors or adverse conditions, and group-level resource asymmetries. Resources can differ between groups in both quantity and type. Where the difference is in type, inequalities can lead to specialization and division of labour between groups, a phenomenon characteristic of human societies, but rarely seen in other animals. The ability to identify members of one's own group is essential for social coherence; we consider the proximate roles of identity effects in shaping inter-group cooperation and allowing membership of multiple groups. Finally, we identify numerous valuable avenues for future research that will improve our understanding of the processes shaping inter-group cooperation.
Keywords: cooperation, conflict, social organization, tolerance, humans, social insects
1. Introduction
Across taxa, group-living organisms tend to behave differently towards members of their own group (in-group) than towards members of other groups (out-groups) (table 1). This characteristically involves two behaviours that are distinct but often co-occur: (i) cooperation with in-group members and (ii) conflict with out-groups [7]. While inter-group conflict is undoubtedly common, it is often assumed to be the default scenario, meaning that other types of inter-group interactions (tolerance and cooperation) may be overlooked [8,9]. In this review, we integrate inter-group cooperation into the broader picture of both within-group cooperation and inter-group conflict, using two taxonomically distinct organisms as case studies (humans and an ant species: table 2). We illustrate key properties of inter-group cooperation and highlight areas for future research.
Table 1.
Definitions of key terms used in the text.
| term | definition |
|---|---|
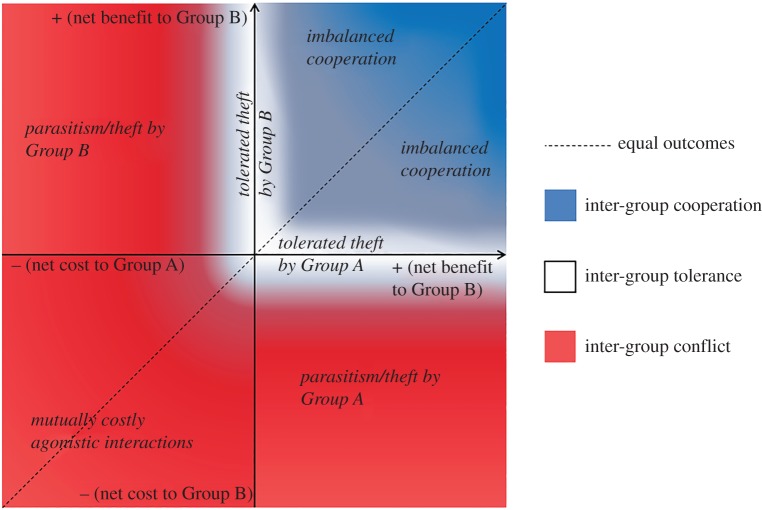
| inter-group cooperation | the transfer of benefits from one group to one or more other groups of conspecifics, resulting in net benefits shared by multiple members of the groups involved (although benefits may not be equal in size: figure 1). This includes benefits that evolved as a by-product of non-cooperative activities and excludes cooperation that occurs (i) between groups of different species, and (ii) on an individual basis between members of different groups (e.g. [1]) |
| inter-group conflict | the imposition of costs from one group on one or more other groups of conspecifics, resulting in a net cost to members of at least one group. This includes both actively inflicted costs from direct combat and passive or indirect conflict (scramble competition) |
| inter-group tolerance | a state in which groups neither incur a net cost nor receive a net benefit as a result of interaction with other groups |
| inter-group interaction | reciprocal action or influence of multiple groups on each other |
| group-level identity cues | features of group members that carry information about their group membership [2] |
| in-group and out-group members | members of one's own group and of other groups, respectively [3,4] |
| group | aggregation of cooperating individuals that is stable with respect to the timescale of cooperation. We use this definition for the purpose of this review, noting that ‘group’ is defined in several different ways in the literature (e.g. [5]) and that in some cases the term is used as a heuristic without definition |
| cooperation | the transfer of benefits from one party to another, ultimately resulting in direct or indirect fitness benefits to both parties (modified from [6] to include behaviours with a non-cooperative evolutionary origin) |
Table 2.
A summary of the properties of cooperative groups and the interactions between them in two case-study examples of resource-based inter-group cooperation: human trading groups and a well-studied polydomous ant species.
| human trading groups | polydomous ant (Formica lugubris) colonies | |
|---|---|---|
| what is the nature of a group? | ‘group’ is used heuristically to include the people who interact with each other to cooperatively produce or acquire a resource, but is often based on location | a group is all the ants usually resident in a certain nest; several socially connected but spatially separated nests make up a polydomous colony (box 1) |
| how stable is group membership? | people can move between groups, but this generally happens less frequently than the time taken to cooperatively produce the resource and exchange it for one from another group | worker ants can move between nests, but most show high fidelity to one ‘home nest’ (e.g. [10]) making groups stable with respect to the timescale of cooperation |
| what resources are shared between groups? | many, e.g. food and natural resources such as metals, stone and shells [11–13] | primarily carbohydrate food; also protein and nest material [14] |
| how do resources differ between groups? | both amount and kind (resources tend to be abundant locally but patchy over a larger scale [15]) | groups differ in the amount of foraging they perform; therefore, the amount of carbohydrate resource available within each nest differs [14] |
| how are cooperation partners chosen? | people cooperate with other groups that have the required resources (e.g. trade between people on the coast and inland [15]) repeated interactions occur between the same groups, reducing the likelihood of exploitation [12,13,16] |
ants cooperate only with other groups from the same wider colony and cooperate most strongly when resource asymmetry between groups is high [17] |
| what is the outcome of resource exchange? | benefits of the exchange are shared within each group. Note that: (i) trade can occur between individuals as well as between groups, but it is only inter-group cooperation when the benefits are shared among members of a given group (table 1); (ii) the benefits from such inter-group interactions are not necessarily shared equally among group members [18] | recipient ants share carbohydrate with other members of their close in-group (nest-mates) [19]; the group benefits from the resource acquired |
| is there division of labour between groups? | yes: groups specialize on locally abundant resources and those which they can produce or acquire most efficiently [20] | at the group level, nests may specialize in producing just one reproductive sex [21], but there is no evidence of resource type (e.g. protein/carbohydrate) specialization at the group (nest) level [14] instead, some groups specialize on resource collection while others appear to focus on exploring the resource environment [14], suggesting an ‘exploration/exploitation’ division of labour analogous to individual-level ‘scout versus recruit’ specializations seen widely in social insects (e.g. [22]) |
| how balanced is the exchange of benefits? | generally balanced. If not, expect repeated interactions and/or cultural institutions such as rituals to maintain reciprocity [16,23,24], or expect inter-group cooperation to break down into tolerance or conflict, e.g. if one group surrenders its resources [25], sometimes under duress [12] | resource transfer can be strongly directional where there is variation in need [17] but donors may benefit indirectly because the resources are going to their kin [26] |
Inter-group conflict (table 1) is thought to have been a key feature in human evolutionary history (e.g. [27]) and has been extensively studied in other animals, particularly primates, e.g. [28–31]. Inter-group conflict is expected to occur when individuals have shared interests with in-group members (interdependence, e.g. via kinship [26,32]) but not out-group members, and resources are distributed such that groups can attempt to both defend resources and take resources from others. For example, inter-group conflict can occur over access to food and male access to females, as in primates [33,34].
In other situations, groups may coexist without conflict, for example maintaining adjacent territories without aggression or feeding from a common food source [35]. We expect inter-group tolerance (table 1) to occur when costs of aggression are high, and when resources are abundant and/or not in defensible patches (so there will not be local competition, e.g. [36,37]). Moving beyond tolerance, in some cases, inter-group interactions may involve the transfer of fitness benefits (inter-group cooperation: table 1). For social animals, cooperation is generally at an individual level, generating benefits that may be shared within a group. In addition, cooperation can occur at a group level, where benefits are shared across group boundaries; such inter-group cooperation (table 1) is our focus here.
Inter-group interactions can be classified on the basis of the resulting costs and benefits (figure 1). In this review, we consider the factors that affect where interactions fall within this classification, focusing on inter-group cooperation. Most of the current literature on inter-group interactions focuses on conflict (e.g. [7,33]), so we know relatively little about when inter-group cooperation may occur. As inter-group cooperation is not simply the absence of conflict [23], the mechanisms that shift interactions from conflict to tolerance may be different from those that shift interactions from tolerance to cooperation. For example, within-group collective action problems reduce the likelihood of inter-group conflict [38], but we do not predict them necessarily to increase the likelihood of inter-group cooperation. Here, we examine two ultimate drivers of inter-group cooperation (threats and resource asymmetries) and discuss the proximate effects of identity on the form that inter-group cooperation takes.
Figure 1.
Summary of inter-group interactions assuming only two groups are involved, Group A and Group B. Outcomes (net cost/net benefit) at the group level are taken to include both direct and indirect fitness benefits across all group members. Above the dotted line, Group B gains a higher benefit or pays a lower cost than does Group A.
2. Why cooperate with other groups?
The two main benefits that groups gain by cooperating with other groups appear to be protection and resource-sharing. Protection from the threats of predation, competition and harsh environmental conditions is a major driver of the initial formation of social groups [5]. When multiple groups face an increased and large-scale threat, they may respond by fusing, forming a larger group that is better able to withstand attack or harsh conditions (e.g. in wolves [39], spotted hyenas [35], elephants [40], ants [41] and humans [42]). Fusion is not inter-group cooperation under our definition (table 1), but rather can be seen as inter-group tolerance or a temporary conceptual expansion of the ‘in-group’ to encompass a wider group of individuals. The original in-group may still be treated differently, although in humans group fusion is often accompanied by mechanisms to promote cohesion across the new, larger group [16].
Alternatively, rather than responding to threats by fusing, groups could maintain their own in-group identities, but actively cooperate with other groups to diminish a threat. All groups may play the same role in protection against threats: here, the benefits of cooperation arise simply from having more individuals contribute. This can occur when the threat is from predators or enemies that affect all groups equally. For example, in the ant Iridomyrmex purpureus, multiple nests of a polydomous colony (box 1) appear to engage in combined defensive activities when any one of the nests faces the threat of echidna predation [45].
Box 1. Polydomous ants.
In many ant species, each colony occupies a single nest (monodomy). Other species of ants spread their colonies across several spatially separate nests, each containing workers and brood, which remain socially connected (polydomy) [41]. Workers usually show fidelity to a particular ‘home’ nest within a polydomous colony [41,43]. Polydomous species can have a single queen (monogynous) or multiple queens (polygynous), and the polydomous nesting strategy has evolved many times in the ants [41]. Polydomy may confer colony-level advantages in resource exploitation, risk-spreading and colony ergonomics [44]. In its most extreme case, polydomous species may form ‘unicolonial’ populations, where all ants in a population behave as part of one huge polydomous colony. Unicoloniality usually occurs in invasive species; most polydomous species are ‘multicolonial’, that is, each colony is formed of a group of socially connected nests that functions independently from other neighbouring multi-nest colonies and usually is hostile towards them [44].
In other cases, different groups may play different roles in protection from threats, for example ‘risk-pooling’ by exchange of resources in times of shortfall for each group [11,16]. Groups may also differ in the magnitude of risk experienced or vulnerability to the threat, for example from climate change [46]. Differences in vulnerability may arise through differences in group size, with smaller groups more at risk. In general the groups that face the higher threat should invest more in inter-group cooperation, although this effect interacts with the amount of resources groups have [47].
In summary, although external threats can sometimes promote inter-group conflict [16,48], they can also promote inter-group cooperation when groups have some degree of shared interests or interdependence. When groups fuse, the interaction between groups is qualitatively similar to within-group cooperation; however, when groups remain distinct, even if all groups play the same role in defence against threats, identity effects (see below) may mean that there are qualitative differences from within-group cooperation. As groups do not necessarily behave as additive aggregations of their individual members [49], modelling groups as individual players responding to threats may be misleading.
In addition to benefits of protection, a second potential driver of inter-group cooperation is the acquisition of resource-related benefits through cooperation with other groups. Many asymmetries or inequalities among groups are related to the resources that a group acquires or uses. For example, asymmetries in group size may result in differences in the amount of resources that different groups hold: larger groups may benefit from economies of scale, but may also suffer from greater free-riding [50]. In turn, resource asymmetry may be a driver of further inter-group asymmetries, for example in fighting ability.
Asymmetry in resources can take two forms: groups may differ in the amount of a given resource, or in the type of resource they hold. Inequalities in resource quantity can arise via differing abilities to produce a certain resource, or via differing needs for that resource. The literature on the effects of this form of resource inequality on cooperation in humans is equivocal (e.g. [51]). For example, theory predicts that wealth inequality between groups can in some cases make inter-group cooperation more likely, with greater cooperation among groups with unequally versus equally distributed resources [52]. However, other models predict that resource inequality between groups can be a driver of inter-group conflict [53].
Whether cooperation or conflict occurs between unequal groups may depend on the cost for a resource-rich group to ‘subsidize’ a resource-poor group, and whether there is some overarching process that provides a global benefit to redistributing the resources: possibly a large-scale threat [23]. Resource inequality can interact with inequality in risks from a threat, where rich groups contribute more than poor groups when the rich groups are more at risk but not when poor groups are more vulnerable [47]. More likely in non-human animals is the linkage of (inclusive) fitness across groups caused by high relatedness [26]. One situation in which unequally resourced highly related groups can occur is polydomous ant colonies (box 1), where food resources are redistributed from successfully foraging nests to poorly provisioned ones [17,19] (table 2). This process of resource redistribution shapes the large-scale colony structure and dynamics [54].
The second form of resource asymmetry is in the type, rather than abundance, of resources. Exchange of different types of resources between groups has been important throughout human evolution, with widespread archaeological and current evidence of inter-group trade [11–13,16] (table 2).
Inter-group exchanges can be beneficial when access to necessary resources varies spatially, meaning that groups are interdependent with regard to those resources and thus have interests in common [32]. Groups that are more efficient at acquiring one type of resource may specialize on that resource, leading to group-level division of labour: this ‘comparative advantage’ principle explains both the economics of international trade and resource exchange between species [20]. When the scale at which resources vary and specialization occurs is large, an entire nation could be considered to act as a ‘group’. In other cases, specialization occurs at a smaller scale, for example among different human ethnic groups: this may arise due to conflict avoidance, analogous to niche differentiation [42,55], leading to further opportunities for exchange. The potential for exploitation in inter-group resource exchange is generally high [56], but can be reduced by two mechanisms. First, there are often repeated interactions between the same groups, leading to long-term inter-group relationships [11,16]. Second, behaviour can be regulated by strong cultural norms (institutions) [23]. For example, inter-group exchange is generally associated with ‘balanced reciprocity’, i.e. with the expectation of immediate return [55], and with rituals establishing inter-group partnerships [16].
While human societies engage in inter-group resource exchange to a remarkable extent (probably facilitated by the ability to establish cultural institutions: [24]), in non-human animals there is little evidence of group-level resource exchange or division of labour. Resource-related division of labour is common at the individual-level among social insects, for example between foragers specializing in protein and carbohydrate (e.g. [57]); the different resources are then shared at the nest. At the group-level, however, nests of polydomous ant colonies do not appear to show resource-type specialization [14] (table 2).
Resource asymmetries are known to play a role in within-group cooperation and inter-group conflict (e.g. [18]), and we suggest that they also affect inter-group cooperation. Whether the effects of resource asymmetries on inter-group cooperation are qualitatively or quantitatively different from their effects on within-group cooperation depends in part on the spatial scale of the relevant resource distribution. For example, within-group asymmetries can affect inter-group interactions [18], meaning that inter-group cooperation may be affected by two levels of asymmetry, potentially leading to qualitative differences from within-group cooperation. Qualitative differences may also arise owing to differences in type of resources at the group versus the individual level, for example if a group's resources are only available when its members contribute.
3. How do identity effects modulate inter-group cooperation?
Once inter-group cooperation arises from threats and resource asymmetries, its form is mediated by group identity effects, which may make inter-group cooperation qualitatively different from within-group cooperation. In this section, we first discuss how the capacity to recognize group membership opens the door to differential treatment of in-group versus out-group members, generally manifested as in-group favouritism [58]. Secondly, we ask how identity effects operate when individuals can be members of more than one group.
Humans can recognize multiple categories of group membership, facilitated by cues and signals that function to display group commitment (e.g. [59]). However, many other animals can also discriminate in-group from out-groups (e.g. by self-referent phenotype matching [2]). In social insects, colony-mates are recognized via matching to odour cues derived primarily from the shared nest environment. For polydomous species (box 1), strong nest fidelity and limited local dispersal can mean individuals experience differing local environments within a colony. Thus, in addition to colony/non-colony discrimination, some polydomous ants can discriminate their own nest-mates from other colony members [60], or distinguish more local and more distant colony members [61]. Certain social insects are thus able to achieve up to three levels of discrimination (nest-mate, colony-mate, stranger); we expect multilevel discrimination to be a pre-requisite for inter-group cooperation (table 2).
Identity effects facilitated by group membership recognition are taxonomically widespread, and generally involve some form of in-group favouritism. For example, social insects exhibit greater aggression towards members of other colonies [2], while humans are more cooperative with in-group members than out-group members. This is the case not only for ‘real’ groups such as religious communities [62] but also for ‘minimal’ groups experimentally created on the basis of arbitrary characteristics [3]. Most explanations for in-group favouritism take a proximate approach (social psychology, economics, e.g. [58,63]). The most likely ultimate explanation is that people expect reciprocity to occur within groups only (‘bounded generalized reciprocity’), and thus benefit from cooperating with in-group, but not out-group, members [64,65]. We thus predict that stronger group identity effects would reduce the likelihood of inter-group cooperation, and that the strength of these effects would be mediated by opportunities for repeated beneficial interactions between groups.
The strength and nature of identity effects interact with the potential to change group membership. Changing group membership can be costly: for example, many signals of group identity function by removing opportunities for beneficial interactions with out-group members, thus honestly advertising commitment to within-group cooperation [66] and decreasing the potential for inter-group cooperation. In social insects, transfer to another colony is usually rare, because colony-membership is associated with high relatedness—although in some contexts individuals may move to neighbouring colonies (e.g. [67]). However, in many cases, animals, including social insects, do change their group identity. In polydomous ant colonies, where between-group relatedness is high, changes to group membership are much more common: nest fidelity, while often high, is rarely complete (e.g. [43]). Fission-fusion societies are characterized by highly flexible group membership; decisions to change group are influenced by many factors, including sex, dominance rank, reproductive status and local environment [35,68]. Many mammals disperse to new groups throughout adulthood [69], and in humans, group membership is frequently fluid, even for groups based on ethnicity [70]. The potential to change group membership could increase interdependence between groups, making inter-group cooperation more likely. In some circumstances, exchange of group members could even be a form of inter-group cooperation, although in most cases change of group membership will be driven by benefits at the individual level.
Identity effects are also influenced by the degree to which it is possible to be a member of multiple groups simultaneously. In non-human animals, where membership of multiple groups occurs, it is generally hierarchical, e.g. polydomous ant colonies or family subgroups within a larger society [40,44], and group identity is based primarily on kinship and/or locality. Humans also belong to nested hierarchical groups determined by relatedness and place (e.g. family, regional identity, nationality), but in addition humans form non-nested groups defined along many orthogonal axes, e.g. religion and language [71] and professional and recreational affiliations. People who consider themselves to be members of a greater number of non-overlapping groups are more tolerant towards members of other groups [4]. Strength of identification with any particular group varies depending on circumstances and context, e.g. when faced by overarching threats common to multiple groups, people shift to a broader ‘superordinate’ group identity [72]. In the latter example, the interdependence between groups probably becomes more salient, explaining why people with a more ‘globalized’ group identity are more willing to cooperate with individuals from other groups [73].
4. Discussion and open questions for further study
Here, we show that cooperation between groups, although rare relative to inter-group conflict, can arise in a wide range of animal taxa and ecological contexts. Although in this paper we focus on groups of organisms, it is important to note that organisms themselves are groups of cells, and that inter-group cooperation is also relevant to the issue of organismality [74]. Cooperation between groups is most likely when multiple groups face an overarching threat, or when groups can benefit from mutual resource exchange. The likelihood of cooperation between two groups is also higher when individuals in those two groups are not competing with each other but are competing with individuals in other groups in the population; however, unlike for individual-level cooperation, this has received little theoretical attention. Many models demonstrate that when both competition and cooperation are at a local scale, individual-level cooperation is generally hindered [36,37,75]. However, this theory has not considered the opportunity for group-level cooperation at a global scale, an area ripe for future study.
Our review reveals many additional areas where further study would be valuable. These include fundamental issues of group identity and the nature of interactions between groups. For example, where animals belong to nested groups (a close in-group and a wider in-group), how do cooperative interactions differ between these two levels of ‘in-group’? How widespread (and reliable) is the ability to distinguish between members of these groups? Is inter-group cooperation more likely to evolve when groups are linked by membership of a wider in-group, or can the formation of a wider in-group be a consequence of inter-group cooperation? To what extent does discrimination depend on individual recognition and memory, as opposed to group-level identity cues? This has implications for an animal's capacity to change group membership, and for the cognitive characteristics we might predict would be associated with inter-group cooperation.
There are potential costs to being identified as a member of particular group, e.g. the risk of attracting aggression, but some form of recognition of group identity is necessary for within-group cooperation. Group-level identity cues that do not rely on individual recognition are particularly open to exploitation by cheats, so we would predict that these will be used only where group identity is strongly associated with high relatedness or where identity cues are high cost or difficult to fake. Sub-group identity cues may also be lost if cooperation—or even tolerance—between groups leads to group fusion; this is expected to happen when competition, or other threats, occur at a large scale [36]. More research is needed on the circumstances under which loss of sub-group identity occurs, and whether fusion of cooperative groups is more likely than of groups which are simply mutually tolerant.
The distinction between cooperation and other inter-group interactions is not clear cut. For example, if one group has a large competitive advantage, a competitively inferior group may concede resources to avoid conflict, i.e. ‘tolerated inter-group theft’ [25] (figure 1). Alternatively, tolerance of the inferior group could be viewed as extending a benefit, in the sense that the weaker group is being given access to a space (or resource) from which they could easily be excluded. Further development of cooperation theory is needed to explore the relative roles of selection for direct and indirect fitness benefits in the evolution of inter-group interactions. Where tolerated theft occurs, there is no clear direct beneficial return to the donor group (or individual), so the relationship could be viewed as parasitic, rather than cooperative. Conversely, if the interaction were controlled by the donor group, this could even be viewed as group-level altruism, although we would expect this to be very rare. Group-level altruism/parasitism are additional under-studied areas of research. In interspecies mutualisms, it is well established that the same interaction can be cooperative in one context but parasitic in another [76,77], and this probably applies also to group-level interactions. Here, we discuss cooperation between conspecific groups, but interspecific cooperation could be viewed as a special case of inter-group cooperation with low relatedness and typically involving resource exchange or shared protection against large-scale threats [78]. Many of the open questions we highlight here also apply to interspecific cooperation.
One major benefit of within-group cooperation is that individuals can obtain resources from others in times of shortfall [16]. In human inter-group cooperation this buffering effect can occur also at the group level [12,16], but it is unclear what role buffering plays in other animal group interactions. Buffering often requires delayed reciprocity, which is vulnerable to cheating; thus, we might expect to see this only where relatedness is high or where reputation effects are strong. Resource distribution may modulate the advantages of inter-group cooperation. Inter-group division of labour should be more likely where resources are spatially segregated, and when resource transfer is common, as in humans and the social insects. In other animals, some forms of resource transfer, e.g. food sharing, are actually rare (except between mates or with dependent offspring), whereas information sharing is more common. How inter-group cooperation may function with currencies other than physical resource exchange is an interesting area for future study.
Both the ecological context (e.g. resource distribution, harshness of environmental conditions, level of competition) and group characteristics (e.g. size, resource holding potential, need for a particular resource) will affect the dynamics of inter-group cooperation. Within-group heterogeneity may mean the consequences of inter-group cooperation differ greatly among members of the same group. For example, if group members differ in the extent to which they value inter-group cooperation (e.g. owing to kinship with out-group members), within-group conflict can arise [79]. In addition, sex-differences in within-group cooperation and inter-group conflict occur widely [33,34], so group sex composition would be predicted to affect inter-group cooperation dynamics too—another fruitful area for future study.
Our review implicitly focuses on pairwise interactions. While many non-human group-level interactions are likely to be pairwise, human inter-group interactions can require agreement among many groups, and this can constrain inter-group cooperation [80]. Cooperative interactions between multiple contributing groups would be predicted to be subject to the same risks of defection and clique-formation as seen in within-group cooperation; this is especially true if producing a public good critically requires participation by all groups, e.g. protection of clean water. Many major issues suffer when a breakdown in human cooperation occurs—including climate change initiatives, conservation and immigration management—and such issues span regional or national boundaries [81]. Further research on inter-group cooperation will thus not only increase our understanding of the evolution of inter-group interactions but can also shed light on developing strategies to promote cooperation among human groups in the face of these global challenges.
Acknowledgements
We thank Trine Bilde's group, Lauren Brent, Sam Ellis, Elly Power and Caitlin Stern, as well as Andy Gardner, Alberto Micheletti and two anonymous reviewers, for valuable comments on the manuscript.
Competing interests
We declare that we have no competing interests.
Funding
J.L.B. was supported by the European Commission Horizon 2020 and Aarhus Institute of Advanced Studies Marie Curie COFUND Fellowship.
References
- 1.Pisor AC, Gurven M. 2016. Risk buffering and resource access shape valuation of out-group strangers. Sci. Rep. 6, 30435 ( 10.1038/srep30435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeve HK. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. ( 10.1086/284926) [DOI] [Google Scholar]
- 3.Tajfel H, Billig MG, Bundy RP, Flament C. 1971. Social categorization and intergroup behaviour. Eur. J. Soc. Psychol. 1, 149–178. ( 10.1002/ejsp.2420010202) [DOI] [Google Scholar]
- 4.Brewer MB, Pierce KP. 2005. Social identity complexity and outgroup tolerance. Personal Soc. Psychol. Bull. 31, 428–437. ( 10.1177/0146167204271710) [DOI] [PubMed] [Google Scholar]
- 5.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.West SA, Griffin AS, Gardner A. 2007. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432. ( 10.1111/j.1420-9101.2006.01258.x) [DOI] [PubMed] [Google Scholar]
- 7.Rusch H. 2014. The evolutionary interplay of intergroup conflict and altruism in humans: a review of parochial altruism theory and prospects for its extension. Proc. R. Soc. B 281, 20141539 ( 10.1098/rspb.2014.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry DP, Söderberg P. 2014. Myths about hunter-gatherers redux: nomadic forager war and peace. J. Aggress. Confl. Peace Res. 6, 255–266. ( 10.1108/JACPR-06-2014-0127) [DOI] [Google Scholar]
- 9.Boehm C. 2012. Ancestral hierarchy and conflict. Science 336, 844–847. ( 10.1126/science.1219961) [DOI] [PubMed] [Google Scholar]
- 10.Procter DS, Cottrell J, Watts K, A'Hara S, Hofreiter M, Robinson EJH. 2016. Does cooperation mean kinship between spatially discrete ant nests? Ecol. Evol. 6, 8846–8856. ( 10.1002/ece3.2590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiessner P. 2002. Hunting, healing, and hxaro exchange: a long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evol. Hum. Behav. 23, 407–436. ( 10.1016/S1090-5138(02)00096-X) [DOI] [Google Scholar]
- 12.Oka R, Kusimba CM. 2008. The archaeology of trading systems, part 1: towards a new trade synthesis. J. Archaeol. Res. 16, 339–395. ( 10.1007/s10814-008-9023-5) [DOI] [Google Scholar]
- 13.Wiessner P. 2002. The vines of complexity: egalitarian structures and the institutionalization of inequality among the Enga. Curr. Anthropol. 43, 233–269. ( 10.1086/338301) [DOI] [Google Scholar]
- 14.Ellis S, Robinson EJH. 2015. The role of non-foraging nests in polydomous wood ant colonies. PLoS ONE 10, e0138321 ( 10.1371/journal.pone.0138321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop CA. 1987. Coast-interior exchange: the origins of stratification in Northwestern North America. Arctic Anthropol. 24, 72–83. [Google Scholar]
- 16.Zori C, Brant E. 2012. Managing the risk of climatic variability in late prehistoric northern Chile. J. Anthropol. Archaeol. 31, 403–421. ( 10.1016/j.jaa.2012.03.005) [DOI] [Google Scholar]
- 17.Ellis S, Franks DW, Robinson EJH. 2014. Resource redistribution in polydomous ant nest networks: local or global? Behav. Ecol. 25, 1183–1191. ( 10.1093/beheco/aru108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker JL, Loope KJ, Reeve HK. 2016. Asymmetry within social groups: division of labour and intergroup competition. J. Evol. Biol. 29, 560–571. ( 10.1111/jeb.12805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis S, Robinson EJH. 2016. Inter-nest food sharing in wood ant colonies: resource redistribution behavior in a complex system. Behav. Ecol. 27, 660–668. ( 10.1093/beheco/arv205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz MW, Hoeksema JD. 1998. Specialization and resource trade: biological markets as a model of mutualisms. Ecology 79, 1029–1038. ( 10.1890/0012-9658(1998)079[1029:SARTBM]2.0.CO;2) [DOI] [Google Scholar]
- 21.Pamilo P, Seppä P, Helanterä H. 2016. Population genetics of wood ants. In Wood ant ecology and conservation (eds Stockan JA, Robinson EJH), pp. 51–77. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.de Biseau JC, Pasteels JM. 2000. Response thresholds to recruitment signals and the regulation of foraging intensity in the ant Myrmica sabuleti (Hymenoptera, Formicidae). Behav. Process. 48, 137–148. ( 10.1016/S0376-6357(99)00077-7) [DOI] [PubMed] [Google Scholar]
- 23.Fry DP. 2012. Life without war. Science 336, 879–884. ( 10.1126/science.1217987) [DOI] [PubMed] [Google Scholar]
- 24.Powers ST, van Schaik CP, Lehmann L. 2016. How institutions shaped the last major evolutionary transition to large-scale human societies. Phil. Trans. R. Soc. B 371, 20150098 ( 10.1098/rstb.2015.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rusch H. 2014. The two sides of warfare. Hum. Nat. 25, 359–377. ( 10.1007/s12110-014-9199-y) [DOI] [PubMed] [Google Scholar]
- 26.Hamilton WD. 1964. The genetical evolution of social behaviour. I & II. J. Theor. Biol. 7, 1–52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 27.Bowles S. 2009. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293–1298. ( 10.1126/science.1168112) [DOI] [PubMed] [Google Scholar]
- 28.Manson JH, Wrangham RW. 1991. Intergroup aggression in chimpanzees and humans. Curr. Anthropol. 32, 369–390. ( 10.1086/203974) [DOI] [Google Scholar]
- 29.Harcourt AH, de Waal FBM (eds). 1992. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 30.Radford AN, Fawcett TW. 2014. Conflict between groups promotes later defense of a critical resource in a cooperatively breeding bird. Curr. Biol. 24, 2935–2939. ( 10.1016/j.cub.2014.10.036) [DOI] [PubMed] [Google Scholar]
- 31.Reeve HK, Hölldobler B. 2007. The emergence of a superorganism through intergroup competition. Proc. Natl Acad. Sci. USA 104, 9736–9740. ( 10.1073/pnas.0703466104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts G. 2005. Cooperation through interdependence. Anim. Behav. 70, 901–908. ( 10.1016/j.anbehav.2005.02.006) [DOI] [Google Scholar]
- 33.McDonald MM, et al. 2012. Evolution and the psychology of intergroup conflict: the male warrior hypothesis. Phil. Trans. R. Soc. B 367, 670–679. ( 10.1098/rstb.2011.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fashing PJ. 2001. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behav. Ecol. Sociobiol. 50, 219–230. ( 10.1007/s002650100358) [DOI] [Google Scholar]
- 35.Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE. 2008. Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Anim. Behav. 76, 619–636. ( 10.1016/j.anbehav.2008.05.001) [DOI] [Google Scholar]
- 36.West SA, Gardner A, Shuker DM, Reynolds T, Burton-Chellow M, Sykes EM, Guinnee MA, Griffin AS. 2006. Cooperation and the scale of competition in humans. Curr. Biol. 16, 1103–1106. ( 10.1016/j.cub.2006.03.069) [DOI] [PubMed] [Google Scholar]
- 37.Taylor PD. 1992. Altruism in viscous populations: an inclusive fitness model. Evol. Ecol. 6, 352–356. ( 10.1007/BF02270971) [DOI] [Google Scholar]
- 38.Willems EP, van Schaik CP. 2015. Collective action and the intensity of between-group competition in nonhuman primates. Behav. Ecol. 26, 625–631. ( 10.1093/BEHECO/ARV001) [DOI] [Google Scholar]
- 39.Metz MC, Vucetich JA, Smith DW, Stahler DR, Peterson RO. 2011. Effect of sociality and season on gray wolf (Canis lupus) foraging behavior: implications for estimating summer kill rate. PLoS ONE 6, e17332 ( 10.1371/journal.pone.0017332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wittemyer G, Douglas-Hamilton I, Getz WM. 2005. The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 69, 1357–1371. ( 10.1016/j.anbehav.2004.08.018) [DOI] [Google Scholar]
- 41.Debout G, Schatz B, Elias M, McKey D. 2007. Polydomy in ants: what we know, what we think we know, and what remains to be done. Biol. J. Linn. Soc. 90, 319–348. ( 10.1111/j.1095-8312.2007.00728.x) [DOI] [Google Scholar]
- 42.Holly DHJ. 2005. The place of ‘Others’ in hunter-gatherer intensification. Am. Anthropol. 107, 207–220. ( 10.1525/aa.2005.107.2.207) [DOI] [Google Scholar]
- 43.Denis D, Chameron S, Costille L, Pocheville A, Châline N, Fresneau D. 2008. Workers agonistic interactions in queenright and queenless nests of a polydomous society. Anim. Behav. 75, 791–800. ( 10.1016/j.anbehav.2007.06.016) [DOI] [Google Scholar]
- 44.Robinson EJH. 2014. Polydomy: the organisation and adaptive function of complex nest systems in ants. Curr. Opin. Insect Sci. 5, 37–43. ( 10.1016/j.cois.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 45.Van Wilgenburg E, Elgar MA. 2007. Colony characteristics influence the risk of nest predation of a polydomous ant by a monotreme. Biol. J. Linn. Soc. 92, 1–8. ( 10.1111/j.1095-8312.2007.00868.x) [DOI] [Google Scholar]
- 46.Srinivasan UT, et al. 2008. The debt of nations and the distribution of ecological impacts from human activities. Proc. Natl Acad. Sci. USA 105, 1768–1773. ( 10.1073/pnas.0709562104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton-Chellew MN, May RM, West SA. 2013. Combined inequality in wealth and risk leads to disaster in the climate change game. Clim. Change 120, 815–830. ( 10.1007/s10584-013-0856-7) [DOI] [Google Scholar]
- 48.Schleussner C-F, Donges JF, Donner RV, Schellnhuber HJ. 2016. Armed-conflict risks enhanced by climate-related disasters in ethnically fractionalized countries. Proc. Natl Acad. Sci. USA 113, 9216–9221. ( 10.1073/pnas.1601611113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kugler T, Kausel EE, Kocher MG. 2012. Are groups more rational than individuals? A review of interactive decision making in groups. Wiley Interdiscip. Rev. Cogn. Sci. 3, 471–482. ( 10.1002/wcs.1184) [DOI] [PubMed] [Google Scholar]
- 50.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081 ( 10.1098/rspb.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fung JMY, Au W. 2014. Effect of inequality on cooperation: heterogeneity and hegemony in public goods dilemma. Organ. Behav. Hum. Decis. Process. 123, 9–22. ( 10.1016/j.obhdp.2013.10.010) [DOI] [Google Scholar]
- 52.Vasconcelos VV, Santos FC, Pacheco JM, Levin SA. 2014. Climate policies under wealth inequality. Proc. Natl Acad. Sci. USA 111, 2212–2216. ( 10.1073/pnas.1323479111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Besançon ML. 2005. Relative resources: inequality in ethnic wars, revolutions, and genocides. J. Peace Res. 42, 393–415. ( 10.1177/0022343305054086) [DOI] [Google Scholar]
- 54.Cook Z, Franks DW, Robinson EJH. 2014. Efficiency and robustness of ant colony transportation networks. Behav. Ecol. Sociobiol. 68, 509–517. ( 10.1007/s00265-013-1665-8) [DOI] [Google Scholar]
- 55.Cashdan E. 2001. Ethnic diversity and its environmental determinants: effects of climate, pathogens, and habitat diversity. Am. Anthropol. 103, 968–991. ( 10.1525/aa.2001.103.4.968) [DOI] [Google Scholar]
- 56.Cashdan E. 2001. Ethnocentrism and xenophobia: a cross-cultural study. Curr. Anthropol. 42, 760–765. ( 10.1086/323821) [DOI] [Google Scholar]
- 57.Fewell JH, Bertram SM. 1999. Division of labor in a dynamic environment: response by honeybees (Apis mellifera) to graded changes in colony pollen stores. Behav. Ecol. Sociobiol. 46, 171–179. ( 10.1007/s002650050607) [DOI] [Google Scholar]
- 58.Hewstone M, Rubin M, Willis H. 2002. Intergroup bias. Annu. Rev. Psychol. 53, 575–604. ( 10.1146/annurev.psych.53.100901.135109) [DOI] [PubMed] [Google Scholar]
- 59.Sosis R, Kress HC, Boster JS. 2007. Scars for war: evaluating alternative signaling explanations for cross-cultural variance in ritual costs. Evol. Hum. Behav. 28, 234–247. ( 10.1016/j.evolhumbehav.2007.02.007) [DOI] [Google Scholar]
- 60.Pirk CWW, Neumann P, Moritz RFA, Pamilo P. 2001. Intranest relatedness and nestmate recognition in the meadow ant Formica pratensis (R.). Behav. Ecol. Sociobiol. 49, 366–374. ( 10.1007/s002650000315) [DOI] [Google Scholar]
- 61.Holzer B, Chapuisat M, Kremer N, Finet C, Keller L. 2006. Unicoloniality, recognition and genetic differentiation in a native Formica ant. J. Evol. Biol. 19, 2031–2039. ( 10.1111/j.1420-9101.2006.01133.x) [DOI] [PubMed] [Google Scholar]
- 62.Silva AS, Mace R. 2014. Cooperation and conflict: field experiments in Northern Ireland. Proc. R. Soc. B 281, 20141435 ( 10.1098/rspb.2014.1435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Habyarimana J, Humphreys M, Posner DN, Weinstein JM. 2009. Coethnicity: diversity and the dilemmas of collective action. New York, NY: Russell Sage Foundation. [Google Scholar]
- 64.Yamagishi T, Jin N, Kiyonari T. 1999. Bounded generalized reciprocity: ingroup boasting and ingroup favoritism. Adv. Gr. Process. 16, 161–197. [Google Scholar]
- 65.Balliet D, Wu J, De Dreu CKW. 2014. Ingroup favoritism in cooperation: a meta-analysis. Psychol. Bull. 140, 1556–1581. ( 10.1037/a0037737) [DOI] [PubMed] [Google Scholar]
- 66.Fessler DMT, Quintelier K. 2013. Suicide bombings, weddings, and prison tattoos: an evolutionary perspective on subjective commitment and objective commitment. In Cooperation and its evolution (eds Sterelny K, Joyce R, Calcott B, Fraser B), pp. 459–483. Cambridge, MA: MIT Press. [Google Scholar]
- 67.Sumner S, Lucas E, Barker J, Isaac N. 2007. Radio-tagging technology reveals extreme nest-drifting behavior in a eusocial insect. Curr. Biol. 17, 140–145. ( 10.1016/j.cub.2006.11.064) [DOI] [PubMed] [Google Scholar]
- 68.Aureli F, et al. 2008. Fission-fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. ( 10.1086/586708) [DOI] [Google Scholar]
- 69.Mabry KE, et al. 2013. Social mating system and sex-biased dispersal in mammals and birds: a phylogenetic analysis. PLoS ONE 8, e57980 ( 10.1371/journal.pone.0057980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moya C, Scelza B. 2015. The effect of recent ethnogenesis and migration histories on perceptions of ethnic group stability. J. Cogn. Cult. 15, 131–173. ( 10.1163/15685373-12342144) [DOI] [Google Scholar]
- 71.Moya C, Boyd R. 2015. Different selection pressures give rise to distinct ethnic phenomena. Hum. Nat. 26, 1–27. ( 10.1007/s12110-015-9224-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kramer RM, Brewer MB. 1984. Effects of group identity on resource use in a simulated commons dilemma. J. Pers. Soc. Psychol. 46, 1044–1057. ( 10.1037/0022-3514.46.5.1044) [DOI] [PubMed] [Google Scholar]
- 73.Buchan NR, Grimalda G, Wilson R, Brewer M, Fatas E, Foddy M. 2009. Globalization and human cooperation. Proc. Natl Acad. Sci. USA 106, 4138–4142. ( 10.1073/pnas.0809522106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strassmann JE, Queller DC. 2010. The social organism: congresses, parties, and committees. Evolution 64, 605–616. ( 10.1111/j.1558-5646.2009.00929.x) [DOI] [PubMed] [Google Scholar]
- 75.Lehmann L, Rousset F. 2010. How life history and demography promote or inhibit the evolution of helping behaviours. Phil. Trans. R. Soc. B 365, 2599–2617. ( 10.1098/rstb.2010.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bronstein JL. 1994. Conditional outcomes in mutualistic interactions. Trends Ecol. Evol. 9, 214–217. ( 10.1016/0169-5347(94)90246-1) [DOI] [PubMed] [Google Scholar]
- 77.Barker JL, Bronstein JL. 2016. Temporal structure in cooperative interactions: what does the timing of exploitation tell us about its cost? PLoS Biol. 14, e1002371 ( 10.1371/journal.pbio.1002371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bronstein JL. (ed). 2015. Mutualism. Oxford, UK: Oxford University Press. [Google Scholar]
- 79.Gardner A. 2014. Genomic imprinting and the units of adaptation. Heredity (Edin). 113, 104–111. ( 10.1038/hdy.2013.128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santos FC, Pacheco JM. 2011. Risk of collective failure provides an escape from the tragedy of the commons. Proc. Natl Acad. Sci. USA 108, 10 421–10 425. ( 10.1073/pnas.1015648108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dietz T, Ostrom E, Stern PC. 2003. The struggle to govern the commons. Science 302, 1907–1912. ( 10.1126/science.1091015) [DOI] [PubMed] [Google Scholar]