Abstract
One major, yet poorly studied, change in the environment is nocturnal light pollution, which strongly alters habitats of nocturnally active species. Artificial night lighting is often considered as driving force behind rapid moth population declines in severely illuminated countries. To understand these declines, the question remains whether artificial light causes only increased mortality or also sublethal effects. We show that moths subjected to artificial night lighting spend less time feeding than moths in darkness, with the shortest time under light conditions rich in short wavelength radiation. These findings provide evidence for sublethal effects contributing to moth population declines. Because effects are strong under various types of light compared with dark conditions, the potential of spectral alterations as a conservation tool may be overestimated. Therefore, restoration and maintenance of darkness in illuminated areas is essential for reversing declines of moth populations.
Keywords: nocturnal light pollution, feeding behaviour, Lepidoptera, moth population declines, sublethal effect
1. Introduction
The majority of global terrestrial biodiversity is nocturnally active [1,2]. In recent decades, however, nocturnal animals are confronted with increasing illumination of nightscapes by artificial lighting [3]. Artificial lighting can have a strong impact on survival and, consequently, population sizes and biodiversity [2,4]. However, despite the continuous rise in global levels of artificial night lighting (6% average annual increase [2]), effects on behaviour of nocturnal animals remain poorly studied to date.
Moths (Lepidoptera) represent a large, diverse, geographically widespread and largely nocturnal species group and are well-known to be strongly attracted to artificial light (phototaxis) [5,6]. Owing to phototaxis, artificial light is considered as one of the driving forces behind observed moth population declines in strongly illuminated countries [4,7]. Moths have important functional roles in food webs, as bulk-food for many birds and bats, as herbivores and as pollinators [1]. Therefore, moth population declines can have severe consequences for ecosystem functioning. Although phototaxis can have a direct lethal effect, it is unlikely that this effect alone can explain moth population declines. Sublethal effects, such as changes in behaviour [8] and physiology [9] that may underlie artificial light-induced moth declines, are hardly known to date.
Feeding behaviour of adult moths may be disturbed by artificial light with potential major consequences for population dynamics. In this study we focus on feeding behaviour of moths that are experimentally subjected to artificial light. Because moths are particularly strongly attracted to light that is relatively rich in short wavelength radiation (i.e. ultraviolet, blue and green light), especially larger species [6], application of longer wavelength light (i.e. amber and reddish light) is often proposed as tool for moth conservation in illuminated areas [6,10]. To test the effects of this conservation tool, we conducted an experiment testing effects of white (broad spectrum radiation), green (rich in short wavelength radiation), red (rich in long wavelength radiation) and no (dark control) artificial night lighting on feeding behaviour of four moth species. We hypothesized that moths reduce their feeding frequency because of artificial night lighting, especially larger species.
2. Methods
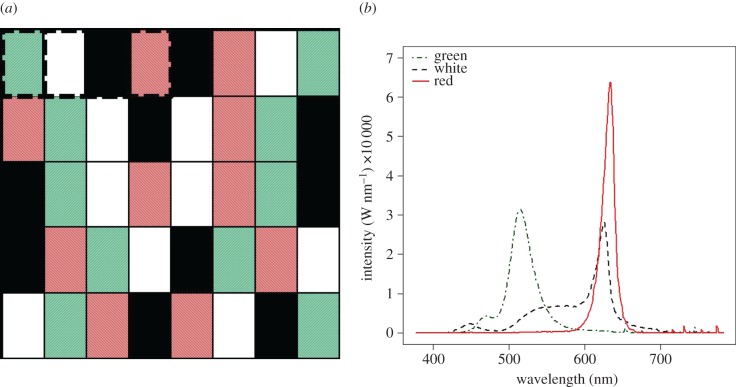
The effect of artificial light on feeding behaviour was tested during night time. We used a shelf of 60 cm depth with 40 compartments (30 × 25 cm). The compartments were divided over 10 blocks (figure 1a), in which the four light treatments were replicated once (figure 1b). Lamps for this experiment were custom-made (1 W Deco-LED lamps, Philips Lighting, Eindhoven, The Netherlands) and were mechanically filtered to achieve the different spectra (green: 78% green, 16% cool white and 6% blue light; red: 69% red and 31% warm white light; white: 100% warm white). The radiant power was respectively 36.5, 42.3 and 76.3 mWatt m−2 for the green, white and red light. The lamps were installed in 19.4 cm diameter, 16 cm high white containers and covered with several layers of cotton as diffuser. Cotton has very low absorbance for the spectral composition of our lamps (400–770 nm [11]). Light pollution of surrounding compartments was prevented by curtains. Light was applied at intensities of 15 ± 1 lux (mean ± s.d., measured with the diffuser; typical streetlight intensities range from 10 to 60 lux at street level [10]). Light levels in the dark control were 5.6 × 10−3 ± 2 × 10−3 lux.
Figure 1.
(a) Experimental design: the four light treatments (red, green, and white light, and dark) were divided over 10 blocks (indicated by the dashed square). Each compartment was 60 cm deep, 30 cm long and 25 cm wide. (b) Spectral compositions (intensities per wavelength) of the used light sources.
The moth species were: Mamestra brassicae (Linnaeus 1758) (Noctuidae, average forewing length (FWL), from www.vlindernet.nl: 18 mm), Rivula sericealis (Scopoli 1763) (Eribidae, FWL: 14 mm), Idaea biselata (Hufnagel 1767) (Geometridae, FWL: 10.5 mm) and Dysstroma truncata (Hufnagel 1767) (Geometridae, FWL: 16.5 mm). Mamestra brassicae moths were obtained from mass rearing, which was maintained at room temperature (20 ± 2°C) under natural day/night rhythm with natural daylight. For M. brassicae, we used each night a batch of raised moths of the same age (n = 60). The other species were wild-caught with light traps (100 and 50 Watt HPL Mercury with Magnesium Arsenate Phosphor, Philips Lighting, Eindhoven, The Netherlands), kept under natural light and tested the subsequent night. Moths were caught in mixed forests in Wageningen, The Netherlands (51°58.950′ N, 5°39.413′ E) and Epen, The Netherlands (50°46.159′ N, 5°56.342′ E): I. biselata on 1 August 2012 in Epen (n = 20), R. sericealis on 13 August 2012 in Wageningen (n = 20), and D. truncata on 9 September 2012 in Wageningen (n = 15). By collecting the moths the night before the experiment (both the wild-caught moths and the ones from the laboratory) and starving them until they were randomly assigned to a given light treatment, we controlled for the potential effect of age and physiological state.
Moths were placed individually in 12 cm high, 10 cm diameter transparent plastic cups, capped with white insect mesh. Per night, we tested 20 individuals of M. brassicae together with 20 individuals of one of the three other species, i.e. n = 5 per species per treatment, except for D. truncata with n = 4 for green, red and dark control and n = 3 for white owing to mortality. Male : female ratio was 46 : 14 in M. brassicae, 12 : 8 in I. biselata, 8 : 12 in R. sericealis and 8 : 7 in D. truncata. Species and sex were allocated randomly over the compartments at 22.30 h. Moths were provided with a (1 : 10) sugar-water soaked piece of cotton wool for feeding at 22.50 h. Lamps were turned on, and observations started at 23.00 h, i.e. approximately 2 h into scotophase. All moths were observed 10 times per hour until 5.00 h, i.e. approximately 8 h into scotophase. Each observation per moth took a few seconds to record feeding behaviour (yes or no). The compartments were checked in a fixed order. A Sony DCR-SR85 infrared-sensitive camera was used for observations of moths in darkness (data can be found in [12]).
We used a generalized linear mixed model (GLMM, the function glmer of the package ‘lme4’ in R [13]) with a binomial distribution and logit link function to test the differences in feeding events (yes/no) between the treatments. As we followed each individual 60 times per night, we treated individual as subject variable and subsequent measurements as the repeated variable. We tested the differences between the two small species and the two large species (small: FWL < 15 mm, and large: FWL ≥ 15 mm) as large moth species were found to more be attracted to light than small species [6]. Hence, treatment, size, sex and the interactions treatment × sex and treatment × size were used as fixed factors in the GLMM, and the ID of each individual, species, night and block as random variables. The GLMM was followed by the Tukey–Kramer post hoc test (using the ‘multcomp’ package in R [14]).
3. Results
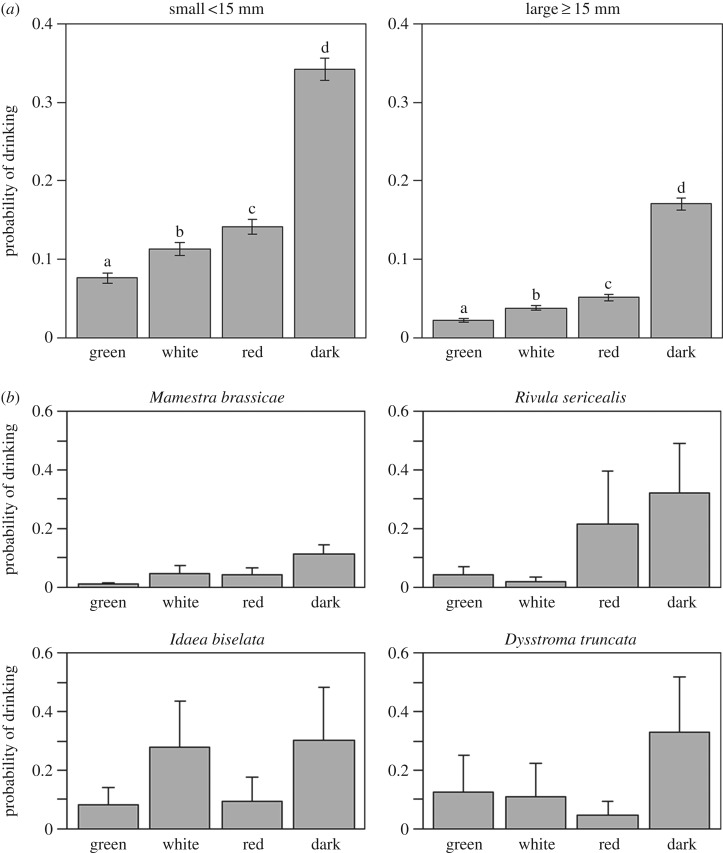
We found that the probability of feeding was significantly different among the treatments (F3 = 4.131, p = 0.006, n = 6900). Feeding probability was higher in darkness compared with red, green and white light, and lowest under the green light (figure 2a). The small species had higher feeding probability than the large species (F1 = 6.969, p = 0.008). We could not significantly add treatment × size, sex and treatment × sex to the model. The observed mean feeding probability per lamp type per species (random factor in the model) is given in figure 2b. When assuming each count to be representative for 6 min (10 observations per hour), small and large moths were feeding respectively on average 123 ± 10 and 61 ± 6 min in darkness, 27 ± 5 and 8 ± 1 min under green, 41 ± 6 and 14 ± 2 min under white and 51 ± 7 and 18 ± 3 min under red light (means ± 95% confidence interval). Therefore, green light reduces feeding activity on average with 82% (range: 78–85%), white light with 72% (range: 67–77%) and red light with 63% (range: 58–69%) compared with the dark control.
Figure 2.
(a) Differences in feeding behaviour of the small and large moth species between lamps that differ in spectral composition (estimated marginal means ± 95% CI). The size classes are defined based on the average length of the forewing. Letters indicate significant differences between the treatments per panel. (b) Differences in feeding behaviour of the four species between lamps that differ in spectral composition (mean ± standard error). Means are calculated based on the observations (hence variation owing to the random factors in the generalized linear mixed model is still included).
4. Discussion
The results from our experiment clearly demonstrate for the first time to our knowledge that artificial night lighting not only attracts moths, but also reduces their feeding frequency. We used species that are known to be attracted by light, and it has been shown that especially this group of moths declines in The Netherlands [15]. The sublethal effect of artificial light on feeding contributes to a mechanistic explanation for these moth population declines. We found that smaller species had higher feeding probability than large species, but there was no interaction between treatment and size class, suggesting that smaller species did not react differently to the lamps than the larger species (in contrast to [6]). Moreover, our results show that both sexes are equally strongly negatively affected by artificial light (in contrast to [5]). Reduced feeding of both females and males owing to artificial light, especially rich in short wavelength radiation (green treatment), results in shorter longevity [16,17] and subsequently a shorter effective reproduction period. Moreover, it results in reduced fertility, e.g. starved females of Cydia pomonella (Tortricidae) laid fewer eggs than fed females [18]. Also sex pheromone production in Heliothis virescens (Noctuidae) [19] and duration of pheromone excretion in Spodoptera littoralis (Noctuidae) [17] reduces when females feed less. Therefore, fewer males will be attracted for mating. As males frequently have greater flight activity than females and males are primarily responsible for dispersion and thus gene flow between populations [20], reduced feeding decreases flight distance [16], artificial night lighting probably leads to reduced exchange rates of genes.
To prevent and reverse population declines in moths, lamps that attract fewer insects have been applied. Several studies found that light rich in shorter wavelengths attract, and thus negatively affect, moths more than light rich in longer wavelengths [6,21]. Indeed, we found that effects on feeding behaviour are strongest under green light, although feeding frequency is also severely reduced under white and red light. Apparently, application of longer-wavelength radiation light (i.e. red) is less effective as conservation tool than previously thought [1,6,10]. Therefore, we conclude that restoration and maintenance of darkness is an essential requirement to stop and reverse the rapid declines in moth populations.
Acknowledgements
We thank Koert van Geffen for his contribution and the WUR Laboratory of Entomology for providing M. brassicae pupae.
Data accessibility
Data supporting this article can be found at the Dryad repository (http://dx.doi.org/10.5061/dryad.8v212) [12].
Authors' contributions
F.v.L. conceived the idea; F.v.L. and T.P.M.F. designed the experiment; T.P.M.F. did the measurements; F.v.L. and T.P.M.F. analysed the data; F.v.L. wrote the initial manuscript. All authors contributed to improve the paper, approved the final version of this manuscript and are in agreement to be accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
Funded by NWO-STW grant 11110.
References
- 1.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 2.Hölker F, Wolter C, Perkin EK, Tockner K. 2010. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25, 681–682. ( 10.1016/j.tree.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 3.Cinzano P, Falchi F, Elvidge CD. 2001. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 128, 689–707. ( 10.1046/j.1365-8711.2001.04882.x) [DOI] [Google Scholar]
- 4.Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP. 2006. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol. Conserv. 132, 279–291. ( 10.1016/j.biocon.2006.04.020) [DOI] [Google Scholar]
- 5.Altermatt F, Baumeyer A, Ebert D. 2009. Experimental evidence for male biased flight-to-light behavior in two moth species. Entomol. Exp. Appl. 130, 259–265. ( 10.1111/j.1570-7458.2008.00817.x) [DOI] [Google Scholar]
- 6.Van Langevelde F, Ettema J, Donners M, Wallis de Vries MF, Groenendijk D. 2011. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 144, 2274–2281. ( 10.1016/j.biocon.2011.06.004) [DOI] [Google Scholar]
- 7.Groenendijk D, Ellis WN. 2011. The state of the Dutch larger moth fauna. J. Insect Conserv. 15, 95–101. ( 10.1007/s10841-010-9326-y) [DOI] [Google Scholar]
- 8.Van Geffen KG, Van Eck E, De Boer RA, Van Grunsven RHA, Salis L, Berendse F, Veenendaal EM. 2015. Artificial light at night inhibits mating in a Geometrid moth. Insect Conserv. Divers. 8, 282–287. ( 10.1111/icad.12116) [DOI] [Google Scholar]
- 9.Van Geffen KG, Groot AT, Van Grunsven RHA, Donners M, Berendse F, Veenendaal EM. 2015. Artificial night lighting disrupts sex pheromone production in a Noctuid moth. Ecol. Entomol. 40, 401–408. ( 10.1111/een.12202) [DOI] [Google Scholar]
- 10.Gaston KJ, Davies TW, Bennie J, Hopkins J. 2012. Reducing the ecological consequences of night-time light pollution: options and developments. J. Appl. Ecol. 49, 1256–1266. ( 10.1111/j.1365-2664.2012.02212.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almer J, Mcansh E, Doupe B. 2010. Forensic fibre analysis by UV-visible microspectrophotometry. Can. Soc. Forensic Sci. J. 43, 16–30. ( 10.1080/00085030.2010.10757617) [DOI] [Google Scholar]
- 12.Van Langevelde F, van Grunsven R, Veenendaal E, Fijen T. 2017. Data from: Artificial night lighting inhibits feeding in moths. Dryad Digital Repository. ( 10.5061/dryad.8v212) [DOI] [PMC free article] [PubMed]
- 13.Bates D, Maechler M, Bolker B. 2011. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-39. See http://CRAN.R-project.org/package=lme4.
- 14.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 15.Van Langevelde F, Annegarn M, Huigens ME, Ellis WN, Van Grunsven RHA, Vos RA, Wallis de Vries MF. In preparation. Population declines in moths due to light pollution.
- 16.Shirai Y. 2006. Flight activity, reproduction, and adult nutrition of the beet webworm, Spoladea recurvalis (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 41, 405–414. ( 10.1303/aez.2006.405) [DOI] [Google Scholar]
- 17.Sadek MM. 2012. Changes in the calling behaviour of female Spodoptera littoralis (Lepidoptera: Noctuidae) as a function of body weight and adult feeding. Eur. J. Entomol. 109, 103–109. ( 10.14411/eje.2012.013) [DOI] [Google Scholar]
- 18.Wenninger EJ, Landolt PJ. 2011. Apple and sugar feeding in adult codling moths, Cydia pomonella: effects on longevity, fecundity and egg fertility. J. Insect Sci. 11, 161 ( 10.1673/031.011.16101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster SP, Johnson CP. 2010. Feeding and hemolymph trehalose concentration influence sex pheromone production in virgin Heliothis virescens moths. J. Insect Physiol. 56, 1617–1623. ( 10.1016/j.jinsphys.2010.06.002) [DOI] [PubMed] [Google Scholar]
- 20.Nieminen M. 1996. Migration of moth species in a network of small islands. Oecologia 108, 643–651. ( 10.1007/BF00329038) [DOI] [PubMed] [Google Scholar]
- 21.Cowan T, Gries G. 2009. Ultraviolet and violet light: attractive orientation cues for the Indian meal moth, Plodia interpunctella. Entomol. Exp. Appl. 131, 148–158. ( 10.1111/j.1570-7458.2009.00838.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Van Langevelde F, van Grunsven R, Veenendaal E, Fijen T. 2017. Data from: Artificial night lighting inhibits feeding in moths. Dryad Digital Repository. ( 10.5061/dryad.8v212) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data supporting this article can be found at the Dryad repository (http://dx.doi.org/10.5061/dryad.8v212) [12].