Abstract
Hot days in summer (involving a few hours at particularly high temperatures) are expected to become more common under climate change. How such events at different life stages affect survival and reproduction remains unclear in most organisms. Here, we investigated how an exposure to 40 °C at different life stages in the global insect pest, Plutella xylostella, affects immediate survival, subsequent survival and reproductive output. First-instar larvae showed the lowest survival under heat stress, whereas 3rd-instar larvae were relatively heat resistant. Heat exposure at the 1st-instar or egg stage did not influence subsequent maturation success, while exposure at the 3rd-instar larval stage did have an effect. We found that heat stress at developmental stages closer to adult stage caused greater detrimental effects on reproduction than heat stress experienced at earlier life stages. The effects of hot events on insect populations can therefore depend critically on the timing of the event relative to an organism’s life-cycle.
Global climate change will not only lead to a substantial increase in average temperature but also in the frequency of hot events1. Maximum temperatures will often be high for short periods in summer in many regions around the world2,3,4. For example, summer daily maximum temperatures in Brassica fields at Wuhan (30.62N, 114.13E) in China often climb to 40 °C or more for several hours, and the number of such hot days has been increasing in the last 10 years (Fig. S1). Insects and other ectotherms in fields are increasingly likely to experience these short hot periods under global warming5,6. Species with relatively short generation times might experience hot days only at certain developmental stages. This raises the question of whether populations of organisms are susceptible to hot periods, which in turn will depend on the sensitivity of the life stage that is exposed.
Effects of hot events on demographics are likely to be stage specific because physiological responses to temperature change during development7,8. Despite recent progress in understanding the sensitivity of different developmental stages to heat stress measured by immediate survival in insects and other small invertebrates9, there is limited information on the longer-term consequences of hot events. Some studies have considered effects of heat stress in early stages on subsequent adult reproduction10,11,12,13,14. However, heat exposure on only one stage was usually considered, such as the egg stage10,11, 1st-instar larva12, or a more extended period of development13,14,15. The impacts of heat stress at different stages have only been compared in a few insects16,17.
Effects of heat stress are likely to differ depending on the nature of the species being considered. For instance, in aphids like Metoplophium dirhodum where the effects of heat stress have been considered16, there is the possibility that adults can repair and compensate the effects of heat stress at an earlier developmental stage through adult feeding. In contrast, Lepidoptera usually do not take in nitrogen sources at the adult stage, limiting the extent to which they might be able to repair damage due to heat stress at earlier developmental stages.
Here we investigate this issue in the diamondback moth, Plutella xylostella, the most destructive pest of cruciferous crops around the world. This species is well known for its ability to persist in stressed environments, such as under extreme high temperatures18,19,20, cold conditions21,22,widely fluctuating temperatures10 and in the presence of insecticides23,24. In our study site at Wuhan (30.62N, 114.13E), peak densities of Plutella usually occur from early May to early June and consist of multiple overlapping generations. During this time when hot events occur, all life stages are therefore present in the field.
We consider how 40 °C during different development stages of Plutella affects survival and reproduction. The following questions are considered. (1) Do heat effects on survival vary between development stages? (2) What is the impact of heat exposures at different stages on adult reproduction? (3) Does heat exposure in the most thermally sensitive stage (the most vulnerable to heat stress in terms of immediate survival) generate the largest reduction in adult egg production? To answer these questions, we investigated the effects of heat stress during five stages (egg, 1st-instar larva, 3rd-instar larva, pupa and both female and male adult) at 40 °C for 4–24 hrs exposures on immediate survival, subsequent survival to adulthood and fecundity.
Materials and Methods
Insect rearing
The population of Plutella larvae and pupae were originally collected on Brassica crops in fields at the experiment station of the Hubei Academy of Agricultural Sciences, China in May 2008. Plutella larvae were reared on artificial diet at 25 ± 1 °C, 60–80% RH and 15L: 9D, as described by Zhang et al. (2013)18. A total of ~6,000 eggs (≤6 hrs old) were collected for the experiment.
Experimental protocol
We identified the effects of heat stress during five stages (egg, 1st-instar larva, 3rd-instar larva, pupa and both female and male adult) on immediate survival, subsequent survival (maturation success) and fecundity after exposure to 40 °C. Firstly, eggs were divided into six groups and each group was reared to different development stages at 25 ± 1 °C, 60–80% RH and 15L: 9D. For each stage, individuals were randomly assigned to six temperature treatments (40 °C for 4, 8, 12, 16, 20 or 24 hrs with 60–80% RH) or 25 °C in a climate chamber. Each temperature treatment involved 3 replications (eggs) or 4 replications (other stages). For each replication, we used 19–51 eggs (≤18 hrs old), 20–65 1st-instar larvae (newly hatched larvae,≤6 hrs old), 20–30 3rd-instar larvae (newly molt, ≤12 hrs old), 20–30 pupae (newly pupation,≤12 hrs old) or 10–20 females or males (newly emerged, ≤12 hrs old).
During heat stress, individuals were maintained in a Petri dish (9 cm diameter), and supplied with fresh artificial food (Southland Products Incorporated, USA) in the case of the larval treatment. Newly emerged females or males were stressed singly in a Petri dish (6 cm diameter). After heat stress, all individuals tested were removed to 25 °C for further rearing. Food was renewed every 3 days to assure that development and growth was not food limited. Once Plutella eclosed, a total of 16 surviving males and females from a given temperature treatment were paired, or all pairs were used if there were fewer survivors than 16 pairs. Adults were allowed to oviposit in a Petri dish (9 cm diameter), and held at 25 °C, 60–80% RH and 15L: 9D. Adults received a piece of fresh cabbage leaf (4 × 2 cm) for egg laying. Fresh cabbage leaves and Petri dishes were renewed every day. Temperature and humidity in the chamber were monitored (Taiwan Hengxin AZ Co., AZ-8829, Taizhong, China Taiwan); temperature variation for all treatments was within ±1 °C.
Measurements
After heat stress, hatching status of eggs was checked (with a stereo microscope) twice daily at 08:00 and 20:00. For larva treatments, 1st-instar or 3rd-instar larval survival was checked after recovery at 25 °C for 24 hrs following the stress. Larvae were considered dead if their body did not move after touching them with a brush. The survivors of 1st-instar or 3rd-instar larvae after heat treatments continued to be observed daily at 08:00 am until all adults emerged. In pupal treatments, stressed pupae were checked twice daily until all adults emerged or pupae died (based on lack of adult emergence). For adult treatments, adult survival was checked 24 hrs after they were stressed. After pairing, we counted egg numbers (laid on the leaf and inner surface of the dish) daily across the first 7 days.
Statistical analysis
All analyses were run with SAS V9.2. We compared immediate survival after heat stress between different development stages. For 1st-instar larvae, 3rd-instar larvae and adults, immediate survival was defined as the survival rate measured following a recovery at 25 °C for 24 hrs after heat stress. For eggs and pupae, immediate survival was measured as egg hatching success and pupal emergence rate when these stages were returned to 25 °C after heat stress. The relative impact of heat exposure of 40 °C at the different stages was assessed through the exposure time for 50% mortality (LT50 in hrs) to be observed. LT50 was estimated by fitting time–mortality data to logit models. LT50 values between the stage treatments were compared by examining their 95% confidence intervals. If the limits overlapped, lethal times were not considered to differ significantly25.
We explored the effect of heat exposures on subsequent survival (maturation success) after egg, 1st- or 3rd-instar larval heat stress. For egg treatments, maturation success was defined as the proportion of emerged adults from hatched eggs. For the 1st-instar or 3rd-instar larval treatment, maturation success was measured as the proportion of emerged adults from 1st- or 3rd-instars surviving after heat treatment. To compare treatments, we ran ANOVAs on survival data (with the proportion survival of each replicate group of eggs and larvae treated as data points). This was followed by post hoc Tukey B tests to determine which means differed significantly.
Adult egg production was determined as the total number of eggs laid across 7 days. Because egg production of the controls for the different developmental stages did not differ significantly, we compared heat treatments to a pooled set of controls. For each developmental stage, the relationship between exposure time and adult egg production was analyzed by fitting a linear regression model. We compared the slopes and intercepts of the regression lines by one-way analysis of covariance (ANCOVA)26. To further test the stage-specific heat effect on adult egg production, we compared the difference of adult egg production between different stage treatments in each exposure time, using one-way ANOVAs followed by Tukey B post hoc comparisons.
To compare the impacts of exposure time on the egg laying pattern, we calculated a midpoint of egg production (in days) for each individual, which is the period of time required for 50% of total egg production, For each stage, the relationships between midpoint of egg production and exposure time were analyzed through linear regression. A positive slope reflects postponed oviposition due to the heat stress, while a negative slope reflects an accelerated rate of oviposition.
Results
Immediate survival rate
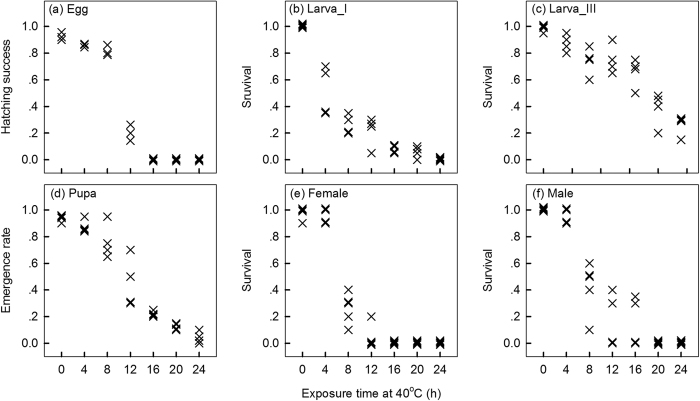
Overall, survival rate declined with an increase of exposure time at 40 °C at all stages (Fig. 1). Significant differences in LT50 were apparent between development stages of Plutella (indicated by non-overlapping 95% confidence intervals) (Fig. 2). The 3rd-instar larvae proved to be the most heat tolerant, with an LT50 of 17.5 hrs. Pupae had a relatively higher heat tolerance with an LT50 of 10.4 hrs followed by egg (8.6 hrs) and male adult (8.1 hrs) stages, and 1st-instar larvae were the least heat tolerant with an LT50 of 5.0 hrs. Female adults displayed a lower LT50 than male adults, although the 95% confidence intervals overlapped.
Figure 1.
Immediate survival of each stage of Plutella after different exposures at 40 °C. Replications are shown separately. For (b) 1st-instar larva, (c) 3rd-instar larva, (e) female and (f) male, immediate survival is defined as the survival rate measured followed by a recovery at 25 °C for 24 hrs after heat stress. For (a) egg and (d) pupa, immediate survival is measured as the egg hatching success and pupal emergence rate respectively, when reared at 25 °C after heat stress.
Figure 2.
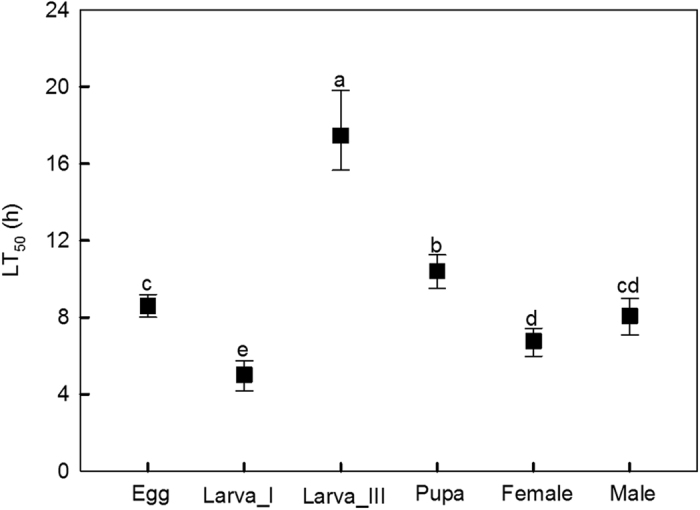
Lethal time (LT50 in hrs) and confidence interval (95% CI) of each stage of Plutella after heat exposure at 40 °C. Significant differences in LT50 were apparent between development stages of Plutella (indicated by non-overlapping 95% confidence intervals). Different letters indicate non overlapping confidence intervals.
Subsequent survival (maturation success)
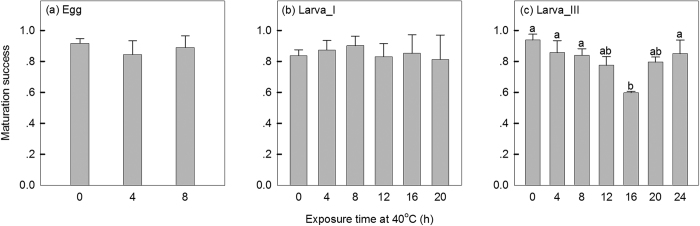
For egg treatments, heat exposure for 4 or 8 hrs did not result in a subsequent decrease in the number of adults that emerged from the hatched eggs compared to the control group, with a mean maturation success (±SE) of 0.88 ± 0.04 (Fig. 3a; F2,6 = 0.29, P = 0.756). Hatchlings from eggs exposed to 12 hrs heat stress could not survive to the adult stage. Heat exposures on 1st-instar larvae did not influence the number of adults that emerged from the surviving larvae (Fig. 3b; F5,17 = 0.13, P = 0.984). A complex pattern emerged for 3rd-instar larval treatments (Fig. 3c). As the exposure time increased from 0 to 16 hrs, maturation success decreased and was lowest (0.60) with the 16 hrs heat exposure (Fig. 3c; F6,21 = 4.38, P = 0.011). However, maturation success increased as exposure time increased from 16 to 24 hrs, with a high survival (0.85, N = 24) by those few that survived the 24 hrs treatment (Fig. 3c).
Figure 3.
Maturation success (mean ± SE) after egg and larval exposures at 40 °C. Different letters above each bar indicate significant differences between exposure times at 40 °C (P < 0.05) based on post hoc tests. No eggs hatched when egg heat exposure exceeded 12 hrs. All 1st-instar larvae died after heat exposure for 24 hrs. Therefore maturation success could not be assessed for these treatments.
Female egg production
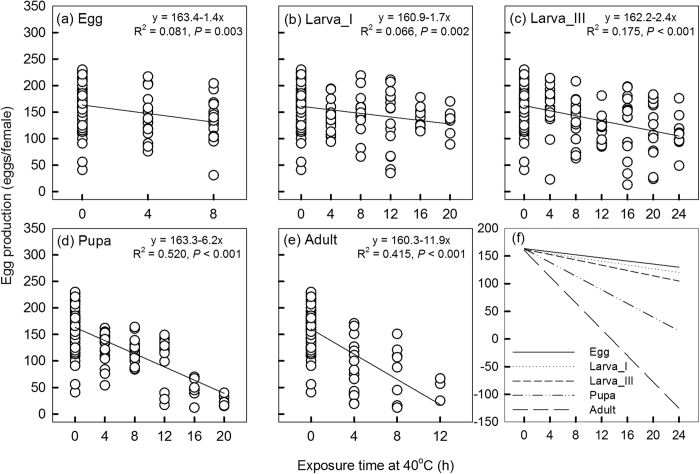
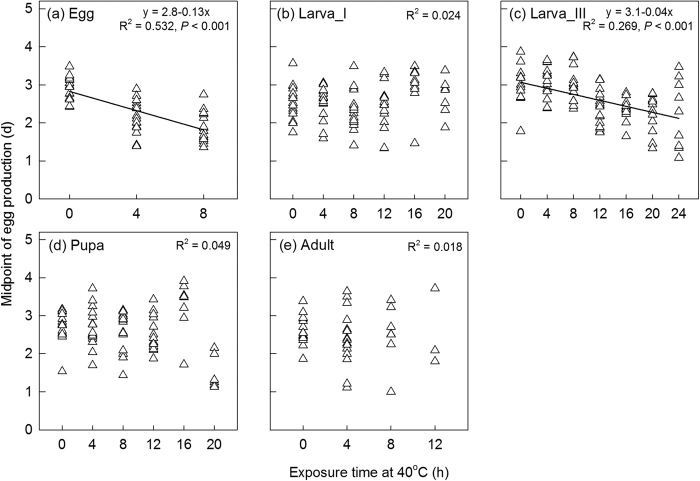
Overall, egg production in the first 7 days declined with an increase in exposure time at 40 °C across all stages (Fig. 4). Although only conditions where sufficient numbers of individuals survived could be considered, there is a consistent pattern in that regression slopes become steeper as later developmental stages are heat stressed, resulting in significant differences between slopes (Fig. 4f; regression slopes: F4,661 = 21.41, P < 0.001; intercepts: F4,661 = 0.10, P = 0.981). The adult treatment showed the fastest decline in egg production when the duration of thermal stress increased, followed by the pupal treatment. The decline in egg and 1st-instar larva treatments appeared to be slower than for the 3rd-instar larva treatment, but we found no significant difference in slopes between these treatments (Fig. 4f, F2,401 = 1.61, P = 0.201). We examined different stage effects on adult egg production for each exposure time (Fig. S2). Stressed adults always produced the lowest egg numbers compared to pupal and larval treatments regardless of exposure time (Fig. S2, a-c, P < 0.019). Adults from the pupal treatment always laid lower numbers of eggs than those from the 1st- or the 3rd-instar larval treatment (Fig. S2, a, d, e, P < 0.002), although differences were not significant with exposures of 8 hrs (Fig. S2, b) or 12 hrs (Fig. S2, c). Heat treatment of 3rd-instar larvae tended to decrease egg production further than heat treatment of 1st-instar larvae, although these differences were small (Fig. S2, a-e). Regression analyses indicated no effect of exposure time on the midpoint of egg production of 1st-instar larval (Fig. 5b), pupal (Fig. 5d) or adult (Fig. 5e) treatments. However heat exposure time at the egg (Fig. 5a) and 3rd-instar larval treatments (Fig. 5c) did decrease the midpoint of egg production, reflecting faster egg laying by females from treatments with longer exposure times.
Figure 4.
Relationship between egg production and exposure time at 40 °C of each development stage treatment. Linear regression lines and R2 values are included in (a)-(e). (f) Comparison of the slopes of regression lines from the different stage treatments.
Figure 5.
Relationship between midpoint of egg production and exposure time at 40 °C of each development stage treatment. The midpoint of egg production (in days) was determined as the period of time required for each individual to complete 50% of its oviposition. R2 values are included in (a)-(e) and linear regression lines are provided where patterns are significant.
Discussion
This study considered the impacts of hot events experienced at different development stages in Plutella moths; a period at 40 °C was used because this stress commonly occurs in agricultural production areas. To summarize the main findings, first instar larvae showed the lowest survival following exposure, whereas 3rd-instar larvae were relatively heat resistant. Heat exposure at early stages did not influence subsequent survival to adulthood (maturation success), while maturation success was affected by the 3rd-larval stage. Fecundity was affected most strongly by heat exposure at life stages closest to the adult stage rather than exposure at the earlier stage.
Stage-specific survival under heat stress
Older instar larvae (or nymphs) often survive better than adults in many insects27,28,29,30. Older larvae may rapidly balance water loss through mass feeding29,31, while adult survival might be depressed if there is a trade-off between survival under stress and reproductive output32,33. Our finding that older larvae of P. xylostella showed higher heat tolerance than pupae or adults is also consistent with other Plutella studies which have shown this life stage to have low resistance under different protocols, including constant warm temperatures34 and heat shock in a ramping regime19, suggesting that the relative sensitivity of different ontogenetic stages may not depend on the method used to test for heat responses in Plutella.
Eggs, young larvae or pupae often have a relatively high level of heat tolerance. The pupae and eggs of Drosophila buzzatii35, Otiorhynchus sulcatus36 and Wyeomyia smithii17, as well as the early instar larvae of Bombyx mori37 and M. dirhodum28 are relatively tolerant of heat stress, perhaps because these stages have low mobility and are unable to evade a heat stress. In contrast, we found that first-instar larvae, eggs, and pupae of Plutella had relatively low heat resistance compared to other larval stages. This pattern has also been found for other Plutella studies19,34. Stress resistance may be affected by past selection pressures depending on the environment where different developmental stages are found7,38. Plutella eggs, first instar larvae and pupae usually occur on the underside of leaves39, where temperatures are cooler in hot days40,41, and this may help explain the relative sensitivity of these stages in contrast to the pattern in other insects.
Heat stress effects on reproduction
The finding that heat stress on a life history stage closer to the adult stage is more detrimental for reproduction rather than heat applied at earlier stages may also apply to other taxa. Reproduction of the Homoptera insect, M. dirhodum, is reduced further by heat stress during late stages (4th-instar and adult stage) than early stages (2nd- and 3rd-instar)16. Egg production of the Diptera species, W. smithii appears to be depressed further by heat stress during the pupal stage than the egg or larval stages17. Lepidopteran studies involving Plutella10 and Manduca sexta11 also point to heat stress in the egg stage failing to influence adult reproduction. In addition to reproduction, morphological traits were impacted more by heat stress closer to adult stage than at earlier stages. In the Coleoptera species, Harmonia axyridis, adult body size and coloration were affected by heat stress at the 4th-instar larval or pupal stages but not at early development stages42. Moreover, in the butterfly, Bicyclus anynana, wing pattern was sensitive only to late larval stage temperatures43.
Stage-specific heat effects on reproduction might depend on whether enough time has elapsed for recovery to occur. Adult stresses often influence egg maturation and oviposition due to direct damage44,45. In addition, adult Lepidoptera may allocate less nitrogen and other resources to maturing eggs when they are stressed; stresses at the adult or late larval stage may reduce availability of these resources46,47, particularly as nutrition and water intake is required by insects to compensate for adverse conditions. Stresses on larval stages well before the adult stage may not have much impact on reproduction because holometabolous insects can repair and restructure morphology and physiological metabolism through metamorphosis48,49 to reduce the long-term effects on subsequent life-stages10,11.
Stressful conditions often induce adults to lay eggs as early as possible to avoid possible detrimental impacts later in life50. We found that heat stress applied to eggs or 3rd-instar larvae led to a faster rate of oviposition in Plutella. Body injuries have previously been reported to accelerate egg-laying50. Potential heat injury in eggs and 3rd-instar larvae might be carried over to the adult stage and lead to a more rapid rate of egg laying.
Potential applications
Understanding how hot days affect population dynamics is likely to be important for pest management. To date, most studies on climate change have investigated effects of changes in mean temperature51 and fluctuating temperatures52 during an entire life cycle on organism performance. Ambient temperatures are rarely constant or rarely fluctuate in a fixed cycle. Instead in species with relatively short generation times, it is possible that any developmental stage might experience an extremely hot day.
Clearly the timing of hot events is likely to have important consequences for population size and local pest pressures involving Plutella due to effects on survival (immediate and through influencing maturation success) and reproduction. The number of surviving adults was reduced much more by a single hot event (e.g. 40 °C for 8 hrs) at the adult stage (>74%) than at other stages (<32%). In addition, reproduction was depressed furthest by a single hot event at the adult stage (55%), followed by pupal (28%), 3rd-instar (20%) and 1st-instar larval (12%) stages. If pests in a crop are mostly at late larval stage, heat stress may have less impact on population size because survival and reproduction are not affected much by such a stress. On the other hand, exposures at a later stage might depress population size and reduce pest pressures. Our results therefore highlight the importance of understanding how hot days affect survival and reproduction performance of the population with complex age/stage structure when predicting population dynamics under extreme events increasing in frequency under climate change.
Additional Information
How to cite this article: Zhang, W. et al. Impact of hot events at different developmental stages of a moth: the closer to adult stage, the less reproductive output. Sci. Rep. 5, 10436; doi: 10.1038/srep10436 (2015).
Supplementary Material
Acknowledgments
This work was supported by the Public Welfare Project from Ministry of Agriculture (201103021), 948 Project of the Ministry of Agriculture of the People’s Republic of China (2011-G9) and National Natural Science Foundation of China (31471764). We thank reviewers Bob Krebs and Toomas Tammaru for constructive criticisms that helped improve this paper.
Footnotes
The authors declare no competing financial interests.
Author Contributions C.S.M., X.Q.C. and S.Z. designed the study, C.S.M. and S.Z. contributed reagents, materials and analysis tools. X.Q.C. performed the experiments. W.Z., A.A.H., C.S.M. and X.Q.C performed the statistical analysis. W.Z., C.S.M., A.A.H. and X.Q.C. wrote the first draft of the manuscript and all authors contributed substantially to revisions.
References
- Hartmann D. L. et al. Chapter 2: Observations: atmosphere and surface. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change ( Hurrell J., Marengo J., Tangang F. & Viterbo P. ed. ), pp. 1–163 (Cambridge University Press 2013). [Google Scholar]
- Huang D. Q. Qian Y. F. & Zhu J. Trends of temperature extremes in China and their relationship with global temperature anomalies. Adv. Atmos. Sci. 27, 937–946 (2010). [Google Scholar]
- Barriopedro D., Fischer E. M., Luterbache J., Trigo R. M. & García-Herrera R. The hot summer of 2010: redrawing the temperature record map of Europe. Science 332, 220–224 (2011). [DOI] [PubMed] [Google Scholar]
- Pezza A. B., Van Rensch P. & Cai W. J. Severe heat waves in Southern Australia: synoptic climatology and large scale connections. Clim. Dyn. 38, 209–224 (2012). [Google Scholar]
- Deutsch C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. 105, 6668–6672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey R. B. et al. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver J. G. et al. Complex life cycles and the responses of insects to climate change. Integr. Comp. Biol. 51, 719–732 (2011). [DOI] [PubMed] [Google Scholar]
- Moran N. A. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 25, 573–600 (1994). [Google Scholar]
- Bowler K. & Terblanche J. S. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol. Rev. 83, 339–355 (2008). [DOI] [PubMed] [Google Scholar]
- Xing K., Hoffmann A. A. & Ma C. S. Does thermal variability experienced at the egg stage influence life history traits across life cycle stages in a small invertebrate? PLoS ONE 9, e99500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter K. A., Davidowitz G. & Woods H. A. Cross-stage consequences of egg temperature in the insect Manduca sexta. Funct. Ecol. 25, 548–556 (2011). [Google Scholar]
- Piyaphongkul J., Pritchard J. & Bale J. Heat stress impedes development and lowers fecundity of the brown planthopper Nilaparvata lugens (Stål). PLoS ONE 7, e47413 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey R. B., Wakefield T., Crill W. D. & Gilchrist G. W. Within- and between-generation effects of temperature on early fecundity of Drosophila melanogaster. Heredity 74, 216–223 (1995). [DOI] [PubMed] [Google Scholar]
- Stillwell R. C. & Fox C. W. Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology 86, 924–934 (2005). [Google Scholar]
- Vasudeva R., Deeming D. C. & Eady P. E. Developmental temperature affects the expression of ejaculatory traits and the outcome of sperm competition in Callosobruchus maculatus. J. Evol. Biol. 27, 1811–1818 (2014). [DOI] [PubMed] [Google Scholar]
- Ma C. S., Hau B. & Poehling H. M. Effects of pattern and timing of high temperature exposure on reproduction of the rose grain aphid, Metopolophium dirhodum. Entomol. Exp. Appl. 110, 65–71 (2004). [Google Scholar]
- Zani P. A., Cohnstaedt L. W., Corbin D., Bradshaw W. E. & Holzapfel C. M. Reproductive value in a complex life cycle: heat tolerance of the pitcher-plant mosquito, Wyeomyia smithii. J. Evol. Biol. 18, 101–105 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhao F., Hoffmann A. A. & Ma C. S. A single hot event that does not affect survival but decreases reproduction in the diamondback moth, Plutella xylostella. PLoS ONE 8, e75923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X. Q., Ma C. S., Zhang S. & Lv L. Thermal tolerance of diamondback moth Plutella xylostella. Chin. J. Appl. Ecol. 23, 772–778 (2012). [PubMed] [Google Scholar]
- Nguyen C., Bahar M. H., Baker G. & Andrew N. R. Thermal tolerance limits of diamondback moth in ramping and plunging assays. PLoS ONE 9, e87535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C. S., Ma G. & Yang H. P. Overwintering of the diamondback moth, Plutella xylostella in temperate countries. Acta. Ecologica. Sinica. 30, 3628–3636 (2010). [Google Scholar]
- Gu H. N. Cold tolerance and overwintering of the diamondback moth (Lepidoptera: Plutellidae) in Southeastern Australia. Environ. Entomol. 38, 524–529 (2009). [DOI] [PubMed] [Google Scholar]
- Ferré J. & Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47, 501–533 (2002). [DOI] [PubMed] [Google Scholar]
- Groeters F. R., Tabashnik B. E., Finson N. & Johnson M. W. Fitness costs of resistance to Bacillus thuringiensis in the diamondback moth (Plutella xylostella). Evolution 48, 197–201 (1994). [DOI] [PubMed] [Google Scholar]
- Sokal R. R. & Rohlf F. J. Biometry: the principle and practice of statistics in biological research 3rd edn, (WH Freeman and Company 1995). [Google Scholar]
- McDonald J. H. Tests for multiple measurement variables: Analysis of covariance. In Handbook of Biological Statistics (3rd edn), pp. 220–228 (Sparky House Publishing 2014). [Google Scholar]
- Lyons C. L., Coetzee M., Terblanche J. S. & Chown S. L. Thermal limits of wild and laboratory strains of two African malaria vector species, Anopheles arabiensis and Anopheles funestus. Malar. J. 11, 1–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C. S, Hau B & Poehling H. M. The effect of heat stress on the survival of the rose grain aphid, Metopolophium dirhodum (Hemiptera: Aphididae). Eur. J. Entomol. 101, 327–331 (2004). [Google Scholar]
- Klok C. J. & Chown S. L. Critical thermal limits, temperature tolerance and water balance of a sub-Antarctic kelp fly, Paractora dreuxi (Diptera: Helcomyzidae). J. Insect Physiol. 47, 95–109 (2001). [DOI] [PubMed] [Google Scholar]
- Focks D. A., Haile D. G., Daniels E. & Mount G. A. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): analysis of the literature and model development. J. Med. Entomol. 30, 1003–1017 (1993). [DOI] [PubMed] [Google Scholar]
- Benoit J. B. et al. Mechanisms to reduce dehydration stress in larvae of the Antarctic midge, Belgica antarctica. J. Insect Physiol. 53, 656–667 (2007). [DOI] [PubMed] [Google Scholar]
- Marshall K. E. & Sinclair B. J. Repeated stress exposure results in a survival–reproduction trade-off in Drosophila melanogaster. Proc. Biol. Sci. 277, 963–969 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M. et al. Experimental evidence for physiological costs underlying the trade-off between reproduction and survival. Funct. Ecol. 24, 1262–1269 (2010). [Google Scholar]
- Liu S. S., Chen F. Z. & Zalucki M. P. Development and survival of the diamondback moth (Lepidoptera: Plutellidae) at constant and alternating temperatures. Environ. Entomol. 31, 221–231 (2002). [Google Scholar]
- Krebs R. A & Loeschcke V. Resistance to thermal stress in preadult Drosophila buzzatii: variation among populations and changes in relative resistance across life stages. Biol. J. Linn. Soc. 56, 517–531 (1995). [Google Scholar]
- Son Y. & Lewis E. E. Modelling temperature-dependent development and survival of Otiorhynchus sulcatus (Coleoptera: Curculionidae). Agric. For. Entomol. 7, 201–209 (2005). [Google Scholar]
- Chavadi V. B., Sosalegowda A. H. & Boregowda M. H. Impact of heat shock on heat shock proteins expression, biological and commercial traits of Bombyx mori. Insect Sci. 13, 243–250 (2006). [Google Scholar]
- Chown S. L. & Nicolson S. W. Insect Physiological Ecology: Mechanisms and Patterns. (Oxford 2004). [Google Scholar]
- Talekar N. & Shelton A. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 38, 275–301 (1993). [Google Scholar]
- Potter K., Davidowitz G. & Woods H. A. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 212, 3448–3454 (2009). [DOI] [PubMed] [Google Scholar]
- Pincebourde S., Sinoquet H., Combes D. & Casas J. Regional climate modulates the canopy mosaic of favourable and risky microclimates for insects. J. Anim. Ecol. 76, 424–438 (2007). [DOI] [PubMed] [Google Scholar]
- Knapp M. & Nedvěd O. Gender and timing during ontogeny matter: effects of a temporary high temperature on survival, body size and colouration in Harmonia axyridis. PLoS ONE 8, e74984 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooi R. E. & Brakefield P. M. The critical period for wing pattern induction in the polyphenic tropical butterfly Bicyclus anynana (Satyrinae). J. Insect Physiol. 45, 201–212 (1999). [DOI] [PubMed] [Google Scholar]
- Berger D., Walters R. & Gotthard K. What limits insect fecundity? Body size- and temperature-dependent egg maturation and oviposition in a butterfly. Funct. Ecol. 22, 523–529 (2008). [Google Scholar]
- Mironidis G. K. & Savopoulou-Soultani M. Effects of heat shock on survival and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) adults. J. Therm. Biol. 35, 59–69 (2010). [DOI] [PubMed] [Google Scholar]
- Cerdá X., Retana J. & Cros S. Critical thermal limits in Mediterranean ant species: trade-off between mortality risk and foraging performance. Funct. Ecol. 12, 45–55 (1998). [Google Scholar]
- Kingsolver J. G. & Woods H. A. Thermal sensitivity of growth and feeding in Manduca sexta caterpillars. Physiol. Biochem. Zool. 70, 631–638 (1997). [DOI] [PubMed] [Google Scholar]
- Consoulas C., Restifo L. L. & Levine R. B. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J. Neurosci. 22, 4906–4917 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert A. W., et al. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biol. Rev. 87, 330–345 (2012). [DOI] [PubMed] [Google Scholar]
- Javoiš J, Tammaru T. Reproductive decisions are sensitive to cues of life expectancy: the case of a moth. Anim. Behav. 68, 249–255 (2004). [Google Scholar]
- Cannon R. J. C. The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Glob. Chang. Biol. 4, 785–796 (1998). [Google Scholar]
- Zhao F., Zhang W., Hoffmann A. A. & Ma C. S. Night warming on hot days produces novel impacts on development, survival and reproduction in a small arthropod. J. Anim. Ecol. 83, 769–778 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.