Abstract
The nucleus accumbens core (AcbC) is a key brain region known to regulate the discriminative stimulus/interoceptive effects of alcohol. As such, the goal of the present work was to identify AcbC projection regions that may also modulate sensitivity to alcohol. Accordingly, AcbC afferent projections were identified in behaviorally naïve rats using a retrograde tracer which led to the focus on the medial prefrontal cortex (mPFC), insular cortex (IC) and rhomboid thalamic nucleus (Rh). Next, to examine the possible role of these brain regions in modulating sensitivity to alcohol, neuronal response to alcohol in rats trained to discriminate alcohol (1 g/kg, intragastric [IG]) vs. water was examined using a two-lever drug discrimination task. As such, rats were administered water or alcohol (1g/kg, IG) and brain tissue was processed for c-Fos immunoreactivity (IR), a marker of neuronal activity. Alcohol decreased c-Fos IR in the mPFC, IC, Rh, and AcbC. Lastly, site-specific pharmacological inactivation with muscimol+baclofen (GABAA agonist+GABAB agonist) was used to determine the functional role of the mPFC, IC and Rh in modulating the interoceptive effects of alcohol in rats trained to discriminate alcohol (1 g/kg, IG) vs. water. mPFC inactivation resulted in full substitution for the alcohol training dose, and IC and Rh inactivation produced partial alcohol-like effects, demonstrating the importance of these regions, with known projections to the AcbC, in modulating sensitivity to alcohol. Together, these data demonstrate a site of action of alcohol and the recruitment of cortical/thalamic regions in modulating sensitivity to the interoceptive effects of alcohol.
Introduction
Despite the well-known deleterious effects of alcohol, its consumption among the general population remains high, with approximately 2 billion people worldwide consuming alcohol (WHO, 2004) and 57% of Americans consuming at least one alcoholic beverage within the past month (SAMHSA, 2014). Thus, understanding the neurobiological mechanisms that modulate sensitivity to alcohol, especially the subjective/interoceptive (discriminative stimulus) effects of alcohol, is important given that interoceptive drug cues can impact drug-related behaviors from onset of drug use and throughout dependence (Koob & Volkow, 2010; Verdejo-Garcia et al., 2012; Bevins & Besheer, 2014; Paulus & Stewart, 2014).
Drug discrimination procedures are commonly used to assess the interoceptive/discriminative stimulus effects of drugs of abuse in animal models (Solinas et al., 2006) and these procedures have identified several receptor systems that modulate the interoceptive effects of alcohol ([gamma]-aminobutyric acid type A [GABAA], N-methyl-D-aspartate [NMDA], serotonin, metabotropic glutamate, opioid; Grant & Barrett, 1991; Grant & Colombo, 1993; Grant et al., 1997; Hodge & Cox, 1998; Maurel et al., 1998; Kostowski & Bienkowski, 1999; Shelton & Grant, 2002; Vivian et al., 2002; Besheer & Hodge, 2005; Helms et al., 2009; Besheer et al., 2010; Platt & Bano, 2011; Jaramillo et al., 2015). Additionally, the existing literature heavily implicates the nucleus accumbens core (AcbC; and possible projections to the AcbC) as a central region in modulating sensitivity to the interoceptive effects of alcohol (Hodge & Alken, 1996; Hodge & Cox, 1998; Besheer et al., 2003; Besheer et al., 2010).
The goal of the present work was to broaden understanding of potential AcbC-related neural circuitry modulating the interoceptive effects of alcohol by identifying brain regions with projections to the AcbC and whether these regions may regulate sensitivity to alcohol. Thus, in behaviorally naïve male Long-Evans rats, projections to the AcbC were identified using a neuronal retrograde tracer. Second, neuronal response to alcohol was examined in alcohol discrimination-trained rats based on the selected brain regions that were identified to have projections to the AcbC. Lastly, to determine the functional role of these brain regions in modulating sensitivity to alcohol pharmacological inactivation was used (intra-brain regional administration of GABAA+GABAB agonists - muscimol+baclofen; Lasseter et al., 2011; Chaudhri et al., 2013; Willcocks & McNally, 2013). The present retrograde tracing study identified and led to the focus of three regions of interest with projections to the AcbC: the prelimbic subdivision of the prefrontal cortex (mPFC); the anterior insular cortex (IC), and the rhomboid thalamic nucleus (Rh). These regions were selected for the following reasons. 1) Previous work has determined that activation of GABAA receptors within the mPFC elicits partial substitution for the discriminative stimulus effects of alcohol (Hodge & Cox, 1998), suggesting that neural inhibition in this region produces some effects that are similar to alcohol. Therefore, we hypothesized that pharmacological inactivation of the mPFC would result in full substitution for alcohol. 2) The IC is proposed to integrate internal and external stimuli into interoceptive states to drive motivated behavior, which has extensive implications for drug addiction (Craig, 2009; Paulus & Stewart, 2014) and various preclinical studies have determined a functional role for the IC in modulating self-administration of several drugs of abuse (Di Pietro et al., 2008; Hollander et al., 2008; Pushparaj & Le Foll, 2015). Thus, we hypothesized that the IC is involved in modulating sensitivity to alcohol and that pharmacological inactivation would disrupt expression of the discriminative stimulus effect of alcohol. 3) The Rh is implicated in modulating behavioral inhibition and motivation (Cassel et al., 2013; Cholvin et al., 2013; Prasad et al., 2013; Prasad et al., 2016), and has been proposed to integrate and modulate arousal and attention (Cassel et al., 2013), all of which are key behavioral components in drug use and may have implications for modulating sensitivity to the interoceptive effects of alcohol. Accordingly, we hypothesized, that similar to the IC, pharmacological inactivation of the Rh would disrupt expression of the discriminative stimulus effects of alcohol.
Materials and methods
Animals
This study used single-housed male Long-Evans rats (Harlan Sprague–Dawley, Indianapolis, IN). All rats were weighed and handled daily for at least 1 week before the start of training. Food intake was restricted to maintain body weight (325–340 g) for all experiments. Water was available ad libitum in the home cage unless noted. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
Apparatus
All behavioral experiments occurred in chambers (Med Associates, Georgia, VT) measuring 31 × 32 × 24 cm. The right wall of the chamber contained a liquid dipper receptacle, two retractable response levers, and stimulus lights (mounted above each lever). Lever press responses activated a dipper mechanism that presented 0.1 mL of a 10% (w/v) sucrose solution for 4 seconds. All chambers were equipped with infrared beams that divided the chamber into 4 parallel zones to measure general locomotor data during the sessions. Each chamber was located in a sound-attenuating cubicle equipped with an exhaust fan that provided both ventilation and masking of external sounds. Additionally, chambers were interfaced (Med Associates) to a computer programmed to control sessions and record lever responses and locomotor data.
Discrimination training
Daily training sessions (Monday–Friday) were identical to those previously described (Besheer et al., 2015; Jaramillo et al., 2015; Randall et al., 2015). Briefly, following administration of water or alcohol (1 g/kg) by intragastric gavage (IG), rats were placed in the chambers for a 10-min timeout period. Next, both levers were introduced into the chamber and the house light was illuminated signaling commencement of the 15-min session. During an alcohol session, completion of a fixed ratio 10 (FR10) on the alcohol-appropriate lever (e.g., left lever) resulted in sucrose delivery. Alternatively, during a water session, completion of an FR10 on the water-appropriate lever (e.g., right lever) resulted in the delivery of sucrose reinforcer. During both alcohol and water sessions, responding on the inappropriate lever was recorded but had no programmed consequence. Alcohol- and water-associated levers were counterbalanced across animals and training days varied on a double alternation schedule (alcohol, alcohol, water, water,..). Testing began once the following criteria were met: the percentage of appropriate lever responses before the first reinforcer, and during the entire session was >80% for at least 8 out of the 10 consecutive days.
Discrimination Testing
Test sessions began following a 10-min delay and were similar to training sessions except they were 2-min in duration. Additionally, an FR10 on either lever resulted in sucrose delivery, thus sucrose reinforcement was delivered independent of lever-appropriate responding so as not to bias lever selection and to allow for the analysis of the effects of treatments on overall response rates (internal measure of nonspecific motor effects). Prior to the start of testing in all rats, a cumulative alcohol curve (0.1, 0.3, 1.0, and 1.7 g/kg) was generated to confirm discriminative stimulus control by alcohol (Schechter, 1997) as described in detail (Besheer et al., 2012b; Besheer et al., 2014). Briefly, rats initially received 0.1 g/kg alcohol and were placed in the chamber for the test session (i.e., 10-min pre-session delay and 2 min test session). At the conclusion of the session, rats received a subsequent alcohol administration of 0.2 g/kg and immediately began another test session. This procedure was repeated with two subsequent administrations of 0.7 g/kg alcohol, thus administration of alcohol was additive to produce the stated dose range (0.1, 0.3, 1.0, and 1.7 g/kg). Once discriminative stimulus control by alcohol was confirmed experimental testing began. In Experiment 3, testing was interspersed with training sessions and only occurred when accuracy criteria was met during 3 of 4 previous training sessions. No more than two test sessions were conducted per week.
Cannulae Implantation Surgery and Microinjection Procedures, and Verification
Site-specific microinjections were delivered by a microinfusion pump (Harvard Apparatus, MA) through 1.0 μl Hamilton syringes connected to 33-gauge injectors (Plastics One, VA). For Experiment 1, anesthetized rats received a unilateral microinjection of FG into the AcbC (AP +1.7, ML +1.5, DV −6.8 from skull) at a volume of 0.5 μl across 8-min. The injector remained in place for an additional 4-min to allow for diffusion. For Experiment 3, anesthetized rats received implantation of 26-gauge guide cannulae (Plastics One, Roanoke, VA) aimed to terminate 2 mm above the prelimbic region of the PFC (mPFC; bilateral coordinates: AP +3.2, ML ±0.6 mm, DV −2.0 mm), the anterior IC (bilateral coordinates: AP +3.2, ML ±4.0 mm, DV −4.0 mm) or Rh (unilateral coordinates: AP −2.3, ML −1.7 mm (15° angle), DV −5.2 mm). Coordinates were based on (Paxinos & Watson, 2007). Muscimol+baclofen microinjections were delivered through injectors extending 2 mm below the guide cannulae at a volume of 0.5 μl/side across 1 min. The injector(s) remained in place for an additional 2 -min after the infusion to allow for diffusion. Additional microinjection procedures are described in detail in (Cannady et al., 2011; Besheer et al., 2014). At the end of Experiment 3, brain tissue was stained with cresyl violet to verify cannulae placement. Only data from rats with cannulae/injector tracts determined to be in the target brain regions were used in the analyses. For bilateral cannulae (mPFC and IC), both cannulae had to be in the target region. As such, for the IC, three rats had a confirmed cannula on one side (depicted as solid circles on Figure 4A), but the cannula for the opposite side was outside of the target region or we were unable to visibly confirm the injector tract and thus, were considered misses (depicted as solid triangles on Figure 4A). Data from these rats and others with cannulae determined to be out of the other target brain regions were combined and analyzed to serve as anatomical controls.
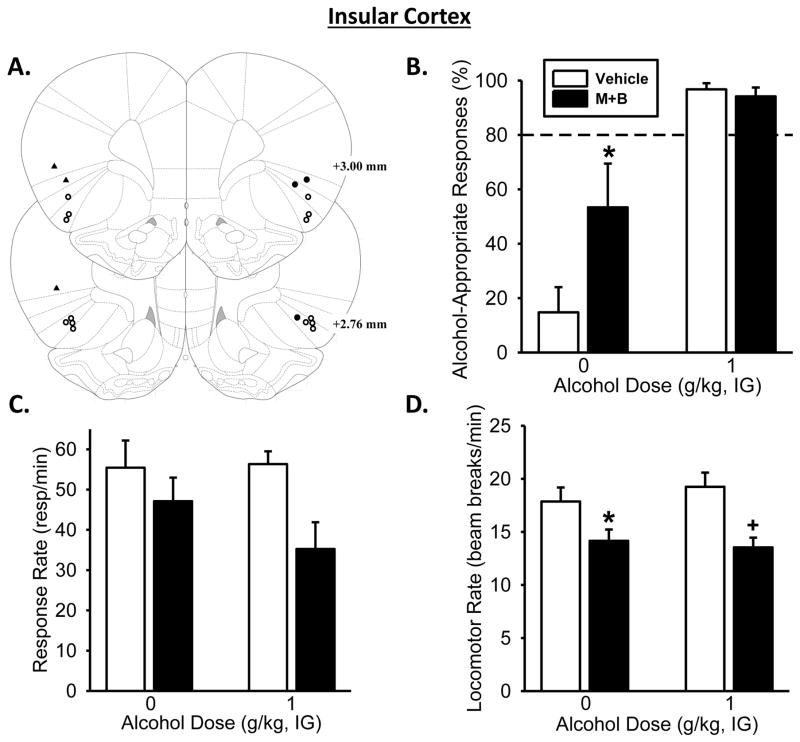
Figure 4. Pharmacological inactivation of the insular cortex partially substitutes for the discriminative stimulus effects of the alcohol training dose.
(A) Insular cortex bilateral injector tip placements from individual discrimination-trained rats with accurate placements (depicted as open circles) and inaccurate placements (depicted as solid triangles/circles). (B) Pharmacological inactivation of the insular cortex, through bilateral infusion of muscimol+baclofen (M+B), significantly increased mean (±SEM) percentage of alcohol-appropriate responses following Water (IG). However, IC inactivation had no effect on alcohol-appropriate responses following the training dose of alcohol (1 g/kg, IG). (C) M+B infusion did significantly decrease response rate relative to vehicle. (D) Locomotor rate was significantly decreased with M+B infusion following Water and 1 g/kg (IG). Dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. * significant difference from vehicle in the Water condition (i.e., 0 g/kg; Tukey, p<0.05;n= 7). Values on graphs represent mean ± SEM.
Immunohistochemistry Procedure and Quantification
To obtain brain tissue for Experiment 2, rats were deeply anesthetized with pentobarbital and perfused with 0.1 M PBS, followed by 4% paraformaldehyde, 4°C; pH=7.4. The brains were removed from the skull and placed in the same fixative solution for approximately 24 h. Next, they were transferred to 30% (w/v) sucrose in a 0.1 M PBS solution, and subsequently sliced on a freezing microtome into 40 μm coronal sections. Tissue was then stored in cryoprotectant (−20°C) until immunohistochemistry (IHC) processing. IHC staining and quantification procedures were similar to those we have previously described (Cannady et al., 2011; Besheer et al., 2012a; Besheer et al., 2014). Free-floating coronal sections were incubated in rabbit anti-Fluorogold antibody (1:8,000; Millipore) for 24 h or rabbit anti-c-Fos antibody (1:20,000; Millipore) for 48 h at 4 °C with agitation. The brain regions examined were the prelimbic region of the medial prefrontal cortex (mPFC; AP +4.2 to +3.2 mm), anterior insular cortex (IC; +2.8 to +1.9 mm), and nucleus accumbens core (AcbC; AP −2.3 to −1.3) and rhomboid thalamic nucleus (Rh; AP −1.8 to −3.2 mm), according to (Paxinos & Watson, 2007). Images were acquired utilizing Olympus CX41 light microscope (Olympus America) and analyzed utilizing Image-Pro Premier image analysis software (Media Cybernetics, MD). IR data (c-Fos positive pixels/mm2) were acquired from a minimum of three sections/brain region/animal, and the data were averaged to obtain a single value per subject.
Experimental Procedures
Experiment 1: Confirmation of incoming AcbC projections utilizing a neuronal retrograde tracer
To confirm afferent neuronal projections to the AcbC, a region known to modulate the discriminative stimulus effects of alcohol, and to determine anatomical coordinates for those brain sites of interest for the discrimination studies (i.e., the c-Fos analyses and the inactivation studies, Experiments 2 and 3, respectively), behaviorally naïve rats (n=6) received a unilateral microinjection of the neuronal retrograde tracer Fluoro-Gold (2%; FG) aimed at the AcbC. One week following injection, allowing time for recovery and diffusion of the tracer, brain tissue was collected and analyzed for FG expression using IHC.
Experiment 2: Alcohol-induced neuronal activation in mPFC, IC, and Rh in discrimination-trained rats
After identifying the regions of interest with projections to the AcbC (i.e., mPFC, IC, and Rh), we sought to investigate whether those regions and the nucleus accumbens would show changes in neuronal activity following alcohol in rats whose behavior was under the discriminative control of alcohol. As such, discrimination-trained rats were administered water or alcohol (1 g/kg, IG; n=4–5/group) and underwent a standard 2-min discrimination test session. 90-min after the end of the test, rats were sacrificed and brain tissue was collected and processed for c-Fos IR. c-Fos IR in the nucleus accumbens (core and shell), mPFC, IC, and Rh was then analyzed.
Experiment 3: Examination of the functional role of mPFC, IC, and Rh on the discriminative stimulus effects of alcohol, through pharmacological inactivation
Discrimination-trained rats were implanted with bilateral cannulae aimed at the mPFC (n=8). A second group was implanted with bilateral cannulae aimed at the IC and a unilateral cannula aimed at the Rh (n=11). Dual cannulae implantation in this latter group was conducted to minimize the number of animals required for this study. Cannulae implantation coordinates were based on FG expression from Experiment 1 and previous work (Kesner & Gilbert, 2007; Besheer et al., 2010; Cholvin et al., 2013; Cosme et al., 2015). To determine the functional role of each brain region in modulating the discriminative stimulus effects of alcohol, each region was independently inactivated with a muscimol+baclofen cocktail infusion prior to a discrimination test session. For the IC and Rh group, testing was interspersed between both regions. On test days, rats received vehicle or microinjection of muscimol+baclofen, 15-min prior to receiving water or the alcohol training dose (1 g/kg, IG). Rats were then placed in the chamber for a 2-min test session (following the 10 min time out period).
Drugs
Alcohol (95% w/v) was diluted in distilled water to a concentration of 20% (v/v) and administered IG, with volumes varied by weight to obtain the desired dose. Fluoro-Gold (FG; Fluorochrome, LLC, Denver, Colorado) was dissolved in 0.9% saline (w/v)/2% (v/v) FG per manufacturer instructions (Schmued & Fallon, 1986). Muscimol and baclofen (R&D systems, Minneapolis, Minnesota) were dissolved in sterile 0.9% saline to produce a cocktail of 0.1mM muscimol + 1mM baclofen, and the doses were chosen based on previous work and our own pilot studies (Lasseter et al., 2011; Chaudhri et al., 2013).
Data Analysis
For the discrimination experiments, response accuracy was expressed as the percentage of alcohol-appropriate lever responses upon delivery of the first reinforcer. Complete expression of the discriminative stimulus effects of alcohol (i.e., full substitution) was defined as ≥80% alcohol-appropriate responding and partial substitution was defined as >40% and <80% alcohol-appropriate responses (Solinas et al., 2006; Besheer et al., 2015). If an animal did not complete an FR10 during these test sessions, data from that animal were not included in the response accuracy analysis, but were included in the response rate analysis. Response rate (responses/min) and general locomotor rate (beam breaks/min) were analyzed for the entire session and served as an index of motor activity. Group differences in discrimination behavior and c-Fos IR for Experiment 2 were determined by t-test. In Experiments 2 and 3, one or two-way repeated measures analysis of variance (RM ANOVA) were used to analyze response accuracy, response rate, and locomotor rate data. Tukey post hoc analyses were used to explore significant interactions. Significance was declared at p ≤ 0.05. Injector tip placements are shown in Figures 4A, 5A, 6A and only animals with accurate bilateral cannulae placements (mPFC and IC groups) or unilateral placement (Rh) were included in the analyses. Data from the rats with inaccurate cannulae placements were analyzed sparately and served as anatomical controls.
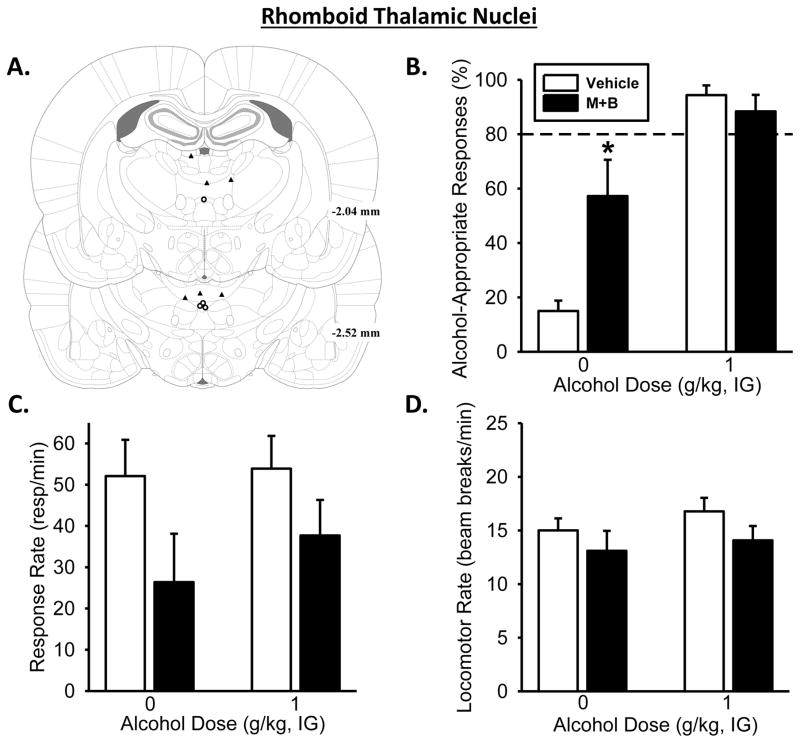
Figure 5. Pharmacological inactivation of the rhomboid thalamic nucleus partially substitutes for the discriminative stimulus effects of the alcohol training dose.
(A) Rhomboid thalamic nucleus unilateral injector tip placements from individual discrimination-trained rats with accurate placements. (B) Temporary inactivation of the rhomboid thalamic nucleus, through unilateral infusion of muscimol+baclofen (M+B), increased mean (±SEM) percentage of alcohol-appropriate responses following Water (IG) but had no effect following the training dose of alcohol (1 g/kg, IG). (C) Response rate was significantly decreased with M+B infusion relative to vehicle. (D) However there was no effect on locomotor rate. Dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. *significant difference from vehicle in the Water condition (i.e., 0 g/kg; Tukey, p ≤ 0.05;n= 4). Values on graphs represent mean ± SEM.
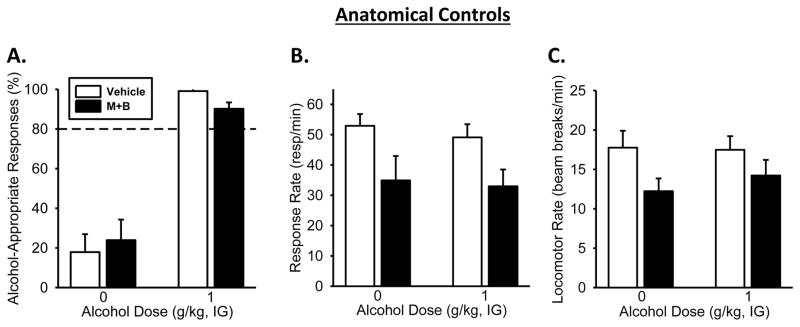
Figure 6. Pharmacological inactivation of anatomical controls/misses produced no effects on the discriminative stimulus effects of the alcohol training dose.
(A) Alcohol significantly increased the mean (±SEM) percentage of responding on the alcohol-appropriate lever relative to Water. However, infusion of muscimol+baclofen (M+B) had no effect alcohol-appropriate responses following Water or alcohol (1g/kg, IG). (B) Response rate and (C) locomotor rate were significantly lowered with M+B infusion, relative to vehicle. Dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. (Tukey, p<0.05; n=10) Values on graphs represent mean ± SEM.
Results
Experiment 1: Confirmation of incoming AcbC projections utilizing a neuronal retrograde tracer
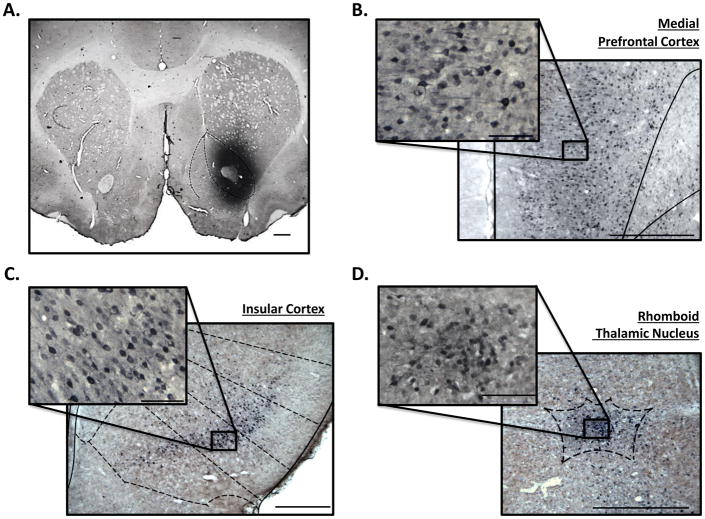
Injection of FG, a neuronal retrograde tracer, in the AcbC (Figure 1A) resulted in dense FG IR in the mPFC (Figure 1B), IC (Figure 1C), and Rh (Figure 1D). FG IR was also found in other regions (e.g., amygdala, hippocampus, etc.); however, the focus of the present study was on the mPFC, IC, and Rh.
Figure 1. FG immunoreactivity identifies incoming neuronal projections to the nucleus accumbens core.
Representative photomicrograph to show (A) unilateral FG infusion into the nucleus accumbens core (1.25X) and FG expression in the (B) medial prefrontal cortex (8X), (C) insular cortex (5X), and (D) rhomboid thalamic nucleus (10X). Photomicrograph insets in panels B, C, D represent FG-positive cells within the regions (B-C=32X, D=40X). Scale bars represent 250 μm in pictographs, insets represent 50 μm.
Experiment 2: Alcohol-induced neuronal activation in mPFC, IC, and Rh in discrimination-trained rats
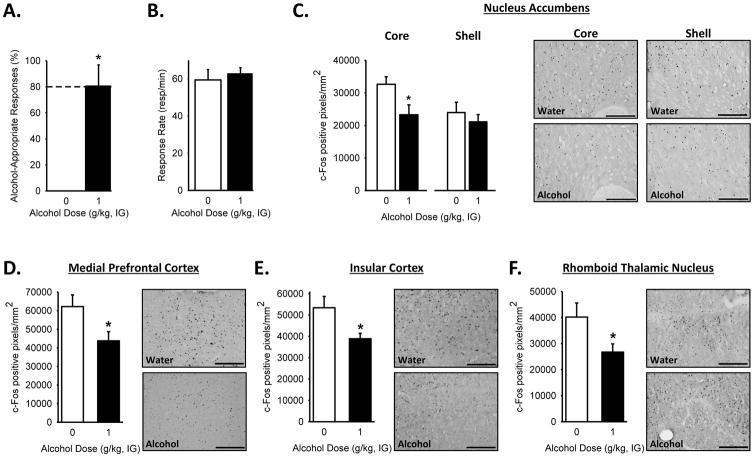
Alcohol stimulus control was confirmed by testing a cumulative alcohol dose response curve. Alcohol- appropriate responding increased with the alcohol dose as confirmed by the one-way RM ANOVA [F(3,30)=54.639, p<0.001], with higher alcohol-appropriate responding at the training dose (1 g/kg) and the highest dose (1.7 g/kg) relative to the lowest dose (0.1 g/kg; p<0.001; Table 1). No effects on response rate were observed (Table 1). However, a significant decrease in locomotor rate[F(3,10)=9.70, p<0.001] was observed for all the alcohol doses relative to the lowest dose (0.1 g/kg; p<0.002; Table 1). Discrimination accuracy performance on the final test showed a significant increase in responding on the alcohol-appropriate lever following the alcohol training dose (1 g/kg; t=4.46, p=0.002; Figure 2A). There were no significant differences in response rate (Figure 2B) or locomotor rate (beam breaks– Water: 272.10±21.84; Alcohol 271.92±31.52), suggesting that any group differences in c-Fos expression is likely not related to a change in response output or general motor behavior. IHC analysis of the brain tissue demonstrated a decrease in c-Fos IR following alcohol (1 g/kg) in the AcbC (t=2.36, p=0.04; but not shell, Figure 2C), the mPFC (Figure 2D; t=2.35, p=0.04), the IC (Figure 2E; t=2.61, p<0.03), and the Rh (Figure 2F; t=2.25, p=0.05).
Table 1.
Performance during the initial cumulative alcohol discrimination test to confirm discriminative control (mean ± S.E.M.)
| Alcohol-appropriate Responses (%) | Response Rate (resp/min) | Locomotor Rate (beam breaks/min) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Alcohol Dose (g/kg, IG) | Cumulative Alcohol Dose (g/kg, IG) | Cumulative Alcohol Dose (g/kg, IG) | ||||||||||
|
|
||||||||||||
| 0.1 | 0.3 | 1.0 | 1.7 | 0.1 | 0.3 | 1.0 | 1.7 | 0.1 | 0.3 | 1.0 | 1.7 | |
| Exp 2 | 9.1±3.9 | 17.6±8.9 | 86.7±8.9* | 98.4±1.1* | 54.7±3.7 | 48.0±3.4 | 59.0±3.5 | 48.1±3.9 | 17.7±1.4 | 13.2±0.7* | 12.5±0.8* | 12.7±1.2* |
| Exp 3 | ||||||||||||
| mPFC | 7.4±3.3 | 19.4±12.1 | 94.5±3.3* | 85.3 ±12.3* | 43.9±4.7 | 51.0±5.1 | 51.1±5.2 | 37.8±6.0 | 21.8±3.4 | 16.8±3.6 | 13.9±161.1* | 12.3±1.4* |
| IC & Rh | 11.4±2.4 | 34.9±10.6 | 77.5±8.8* | 92.5±4.5* | 55.4±4.2 | 53.6±6.2 | 46.5±4.3 | 40.6±5.9* | 21.5±2.2 | 15.4±1.5* | 10.2±1.2* | 9.1±0.8* |
p<0.05 vs. lowest alcohol dose (0.1 g/kg)
Figure 2. Decreased brain regional neuronal activity in response to the training dose of alcohol.
(A) Increased alcohol-appropriate responses following the training dose of alcohol (1 g/kg) with no effect on (B) response rate on the terminal test prior to sacrifice. c-Fos IR, following the discrimination test, shows a significant decrease in c-Fos-positive cells in response to the training dose of alcohol (1 g/kg) in the (C) nucleus accumbens core, but not shell, (D) medial prefrontal cortex, (E) insular cortex and (F) and rhomboid thalamic nucleus. Representative photomicrographs (20X) to show c-Fos positive cells for each brain region. Scale bars represent 250 μm. Dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol.* p<0.05, significant difference from water (i.e., 0 g/kg; t-test; n=4–5/group).Values on graphs represent mean ± SEM.
Experiment 3: Examination of the functional role of mPFC, IC, and Rh on the discriminative stimulus effects of alcohol, through pharmacological inactivation
Confirmation of stimulus control
Alcohol stimulus control was confirmed for the cannulated mPFC group and the dual cannulated IC/Rh group with a cumulative alcohol curve as shown in Table 1. One-way RM ANOVA showed an increase in alcohol-appropriate lever responding for both the mPFC [F(3,21)=31.69, p<0.001] and the IC/Rh group [F(3,30)=29.20, p<0.001], at the training dose (1 g/kg) and the highest dose (1.7 g/kg) relative to the lowest dose (0.1 g/kg; p<0.001). No change in response rate was observed for the mPFC group; however in the IC/Rh group [F(3,30)=3.81, p=0.02] a significant reduction was observed at the highest dose (1.7 g/kg) relative to the lowest dose (0.1 g/kg; p<0.03). In the mPFC and the IC/Rh groups, locomotor rate was significantly decreased [F(3,21)=5.70, p=0.005, F(3,30)=32.33, p<0.001, respectively] at the two highest doses (1.0 and 1.7 g/kg) relative to the lowest dose (0.1 g/kg; p ≤ 0.02) in the mPFC group, and at all doses (0.3, 1.0, and 1.7 g/kg) relative to the lowest dose (0.1 g/kg; p ≤ 0.001), in the IC/Rh group.
Pharmacological inactivation of the medial prefrontal cortex
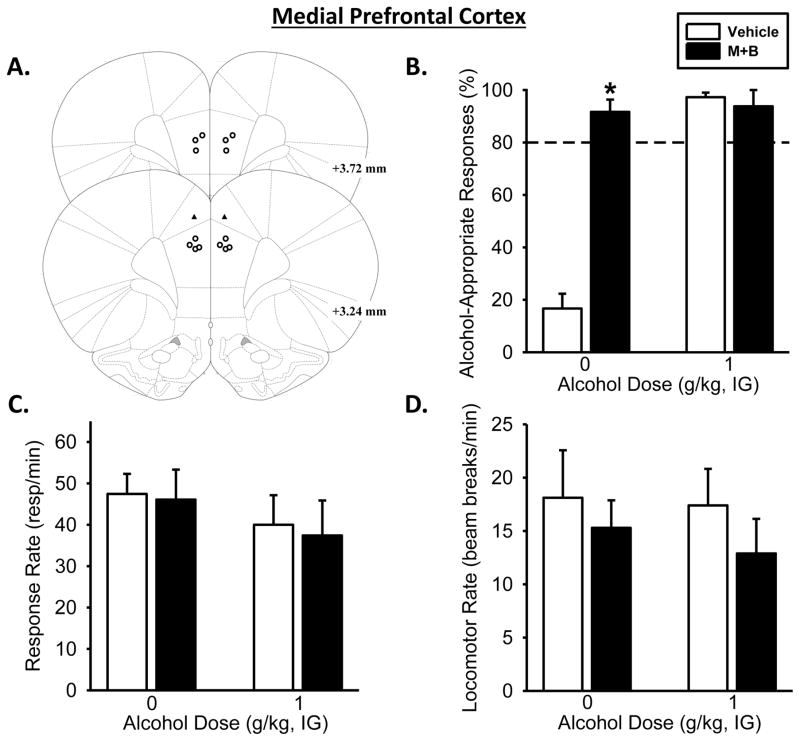
Muscimol+baclofen treatment significantly affected alcohol-appropriate responding as the two-way RM ANOVA showed a significant main effect of alcohol dose [F(1,6)=66.11, p<0.001], of muscimol+baclofen treatment [F(1,6)=42.44, p<0.001], and a significant interaction between alcohol dose and muscimol+baclofen treatment [F(1,5)=74.24, p<0.001; Figure 3B]. As would be expected, under vehicle conditions, a significant increase in alcohol-appropriate responding following the training dose (1 g/kg) was observed (p<0.001). Interestingly, mPFC inactivation followed by water administration resulted in a significant increase in alcohol-appropriate responding relative to vehicle (p<0.001), which resulted in full substitution for the alcohol training dose. mPFC inactivation prior to alcohol (1 g/kg) administration did not affect alcohol-appropriate responding, likely due to a ceiling effect (i.e., full substitution). Two-way ANOVA showed no effects of alcohol dose or treatment on response rate (Figure 3C) or locomotor rate (Figure 3D)
Figure 3. Pharmacological inactivation of the medial prefrontal cortex substitutes for the discriminative stimulus effects of the alcohol training dose.
(A) Medial prefrontal cortex bilateral injector tip placements from individual discrimination-trained rats with accurate placements (depicted as open circles) and inaccurate placements (depicted as solid triangles). (B) Temporary inactivation of the medial prefrontal cortex, through bilateral infusion of muscimol+baclofen (M+B), increased mean (±SEM) percentage of alcohol-appropriate responses following Water (IG) but had no effect following the training dose of alcohol (1 g/kg, IG). (C) Response rate and (D) locomotor activity were unaffected. Dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. * significant difference from vehicle in the Water condition (i.e., 0 g/kg; Tukey, p <0.05; n=7). Values on graphs represent mean ± SEM.
Pharmacological inactivation of the insular cortex
The two-way RM ANOVA analysis on alcohol-appropriate responding following IC inactivation (Figure 4A), showed a significant main effect of alcohol dose [F(1,6)=19.81, p=0.004] and muscimol+baclofen treatment [F(1,6)=7.38, p<0.04], and a significant interaction ([F(1,6)=5.95, p=0.05]; Figure 4B). IC inactivation prior to water administration induced increased alcohol-appropriate responding (p=0.004), resulting in partial substitution for the 1 g/kg alcohol training dose. IC inactivation prior to the alcohol-training dose (1 g/kg) did not affect discrimination performance, again as behavior was likely at a ceiling effect. One rat did not complete an FR10 following IC inactivation and thus was not included in the response accuracy measure, but was included in the response rate analysis. Two-way RM ANOVA of response rate as shown in Figure 4C showed a significant main effect of muscimol+baclofen treatment [F(1,7)=10.18, p<0.015], with lower response rates following inactivation relative to vehicle and there was a trend for an interaction (p<0.07). Muscimol+baclofen treatment significantly affected locomotor rate [F(1,7)=34.84, p<0.001; Figure 4D] and a significant interaction between alcohol dose and treatment was also observed [F(1,7)=6.62, p<0.04], with significantly decreased locomotor rate compared to vehicle following water (p=0.002) and alcohol (p<0.001).
Pharmacological inactivation of the rhomboid thalamic nucleus
The two-way RM ANOVA analysis of Rh inactivation (Figure 5A) on alcohol-appropriate responding showed a main effect of alcohol dose [F(1,3)=185.63, p<0.001] and a significant alcohol dose by muscimol+baclofen treatment interaction [F(1,3)=28.39, p=0.01]. Interestingly, Rh inactivation prior to Water resulted in a significant increase in alcohol-appropriate responding relative to Water under vehicle conditions (p<0.05), resulting in partial substitution for the training dose. However, Rh inactivation prior to administration of the alcohol-training dose (1 g/kg) did not affect discrimination performance. One rat did not complete an FR10 following Rh inactivation and thus was not included in the response accuracy measure, but was included in the response rate analysis. There was a significant main effect of muscimol+baclofen treatment on response rate [F(1,4)=23.26, p=0.009], but no significant main effect of alcohol or interaction (Figure 5B–C). Additionally, Rh inactivation produced no effect on locomotor rate(Figure 5D).
Pharmacological inactivation of anatomical control s/misses
Following verification of cannulae implantation, data from animals considered to be outside the target regions (n=10), as depicted by triangles in each of the figures (Figures 3A, 4A, 5A), were considered misses and not included in the analyses of that brain region. As such, the data from this group of animals were combined to serve as anatomical controls. Discrimination performance was analyzed with a two-way RM ANOVA which demonstrated a significant main effect of alcohol dose (Figure 6A; [F(1,9)=65.29, p<0.001]) with a significant increase in alcohol-appropriate lever responding following alcohol (1 g/kg) relative to water, as would be expected. No significant main effect of muscimol+baclofen treatment was observed. Two-way RM ANOVA analysis of response rate demonstrated a significant main effect of muscimol+baclofen treatment (Figure 6B; [F(1,9)=21.34, p<0.001]), with a decreased response rates following inactivation relative to vehicle. There was no main effect of alcohol dose or interaction. Additionally, two-way RM ANOVA analysis also showed a significant main effect of muscimol+baclofen treatment on locomotor rate (Figure 6C; [F(1,9)=5.80, p<0.04]), with significantly less locomotor activity following muscimol+baclofen relative to vehicle condition.
Discussion
The findings from the present work demonstrate that the mPFC, IC, and Rh are targets of alcohol (1 g/kg), as measured by c-Fos IR in rats trained to discriminate alcohol (1 g/kg) from water, suggesting that these brain regions may be recruited in modulating sensitivity to alcohol. Indeed, we confirm the functional involvement of these regions as temporary pharmacological inactivation of the IC or Rh partially substitutes, while mPFC inactivation fully substitutes, for the discriminative stimulus effects of a moderate alcohol dose (1 g/kg). While the data patterns in the IC and Rh are contrary to our original hypotheses, the findings from the present work identify the functional role of the mPFC, IC, and Rh in modulating sensitivity to alcohol, which is an important and novel contribution to the literature.
Neuronal response as measured by c-Fos expression has been widely used to determine the brain regional site of action of alcohol (see: Vilpoux et al., 2009). A previous study utilizing a higher alcohol dose (1.5 g/kg, IP) found an increase in c-Fos IR in the IC, both in alcohol-naïve and -experienced rats, an effect not seen with a lower alcohol dose (0.5 g/kg; Ryabinin et al., 1997). Increases in c-Fos IR have also been reported in the PFC (specifically the infralimbic cortex), following a 1.5 g/kg alcohol dose (IP) in alcohol-naïve rats (Ryabinin et al., 1997; Hansson et al., 2008) and following a 0.5 g/kg dose (IP) in alcohol-experienced rats (Ryabinin et al., 1997). Additionally, increases in c-Fos IR in the PFC (Knapp et al., 1998; Chen et al., 2009; George et al., 2012), and specifically the mPFC(Kozell et al., 2005) have been reported following alcohol withdrawal. In the present work, decreases in c-Fos IR within the AcbC, mPFC, IC, and the Rh were observed following alcohol in discrimination-trained animals, suggesting that these regions may be recruited when the animal is using the alcohol interoceptive cue to guide behavior. The animals were tested following a discrimination session as we sought to examine the brain response in conjunction with the discrimination behavior; therefore, it would be interesting to determine whether a similar pattern of c-Fos response would occur if the rats were sacrificed without undergoing the behavioral session on the final session, as it is possible that basal levels of c-Fos IR are elevated, in general, as a consequence of engaging in the behavior. Additionally, the alcohol-induced decrease in c-Fos IR was observed in the AcbC, but not the nucleus accumbens shell. This data pattern is consistent with the observed decrease in the AcbC projection regions (mPFC, IC, and Rh) as confirmed by the FG retrograde tracer study. Analysis of FG positive cells that co-express c-Fos would allow for determination of whether the alcohol-induced decreases in neuronal activity are specific to projection neurons from the mPFC, IC, or Rh to the AcbC. This strategy was not implemented in the present work as the FG retrograde tracer study (Experiment 1) was conducted in naïve rats in order to identify projection regions to the AcbC and not in the discrimination-trained rats that were used for the c-Fos analyses (Experiment 2), but will be an interesting future direction. Importantly, in the present study, the alcohol-induced decrease in c-Fos IR in these brain regions is likely not due to differences in motor output (i.e., lever responding), as response rates were similar between the groups that received water or alcohol on the test (Figure 2B). Given that only one alcohol training dose (1 g/kg) was examined it will be interesting for future work to broaden the range of alcohol training doses, as these studies may identify dose-related effects on these anatomical sites of action of alcohol.
In general, as reflected in the alcohol discrimination literature, pharmacological manipulations that result in CNS inhibition (e.g., GABAA agonists, NMDA antagonist) tend to have “alcohol-like” effects (Hiltunen & Jarbe, 1989; Grant & Colombo, 1993; Hodge & Alken, 1996; Hodge & Cox, 1998; Hodge et al., 2001). Thus, while utilization of a muscimol+baclofen cocktail is commonly used as a tool by which to “temporarily inactivate” a specific brain region, and was used for that purpose in the present work, this pharmacological strategy also allows for a mechanistic interpretation. That is, while co- activation of GABAA and GABAB receptors (i.e., muscimol+baclofen cocktail infusions) in the IC, Rh, and mPFC intrinsically “inactivate” the brain regions, we are also able to conclude that these receptors in these brain regions contribute, in part, to the discriminative stimulus effects of alcohol, as full substitution (mPFC) and partial substitution (Rh and IC) for alcohol was observed. Therefore, the present results mechanistically implicate the importance of GABAA and GABAB receptors and indicate that activating these receptors is critical for the expression of the discriminative stimulus effects of alcohol. Although, pharmacological inactivation of the Rh resulted in a decrease in response rate, responding on the alcohol-appropriate lever was not altered following the training dose of alcohol (e.g., appropriate accuracy performance). Additionally, pharmacological inactivation of the mPFC or the IC did not alter response rates, confirming that changes in discrimination performance were not due to nonspecific changes in motor output, or motivation to respond for the sucrose reinforcer. This latter point suggests that there was also no change in sucrose palatability which is important given that the IC (albeit further posterior IC than that targeted in the present work) has been implicated in food-seeking and taste processing (Carleton et al., 2010; Kusumoto-Yoshida et al., 2015).
Previous work has shown that activation of intra-mPFC GABAA receptors by muscimol, results in partial substitution for the discriminative stimulus effects of alcohol (1 g/kg; Hodge & Cox, 1998). Here, we demonstrate that intra-mPFC co-activation of GABAA and GABAB receptors results in full substitution for alcohol (1 g/kg), confirming the importance of this region in the modulating sensitivity to alcohol and also implicating a functional role for intra-mPFC GABAB receptors. Interestingly, previous work has shown that GABAB activation substitutes for the discriminative stimulus effects of gamma-hydroxybutyric acid (Lobina et al., 1999), which has been shown to generalize to alcohol (1 g/kg, IG; Colombo et al., 1995). Therefore, it will be interesting for future work to investigate the role of intra-mPFC GABAB receptors alone in modulating sensitivity to alcohol. In contrast to the full substitution observed in the mPFC following GABAA and GABAB activation, this pharmacological manipulation in the IC and Rh resulted in partial substitution for the discriminative stimulus effects of alcohol (1 g/kg). Even though full substitution was not observed, these findings implicate, in part, the functional importance of the IC and Rh and activation of GABAA and GABAB receptors within these brain regions in modulating sensitivity to alcohol. These findings are highly novel given that, to date, these brain regions have not been previously examined in terms of modulating sensitivity to the interoceptive effects of alcohol in an animal model. Further, it is possible that GABAA and GABAB activation in the IC and Rh may potentiate the effects of low alcohol doses (e.g., 0.3 or 0.5 g/kg), resulting in full substitution. Unfortunately, this was not tested in the present study, but will be important for future work to determine. Moreover, these findings also suggest that co-activation of GABAA and GABAB receptors only constitute a partial target site of action in the IC and Rh as other receptor systems are likely also recruitedin modulating interoceptive sensitivity to alcohol.
Many studies suggest a motivational network involving the IC, mPFC and the AcbC (Cardinal et al., 2002; Rangel et al., 2008; Kouneiher et al., 2009; Pessoa, 2009). Both the mPFC and IC have been implicated in regulating motivationally relevant events (Damasio, 1996; Clithero et al., 2011), which is highly relevant for drug-related stimuli. Therefore, it is not surprising that in human imaging studies both the IC and mPFC respond to alcohol-related cues in individuals with alcohol-use disorders (Filbey et al., 2008) and among at-risk individuals (Ray et al., 2010; Ihssen et al., 2011), an effect absent in social drinkers (George et al., 2001; Myrick et al., 2004; Tapert et al., 2004). Further pre-clinical data also implicates the role of the IC and the mPFC in modulating compulsive alcohol drinking, in which optogenetic inactivation of IC and mPFC projections to the AcbC decreased aversion-resistant alcohol intake (Seif et al., 2013). Taken together, the current findings lend further support for the importance of the IC and mPFC in modulating sensitivity to alcohol.
Interestingly, there is relatively little literature on the functional role of the Rh, especially in relation to drug and alcohol-related behaviors. The Rh receives dense projections from the brainstem and shares reciprocal projections with the cortices (Ohtake & Yamada, 1989; Vertes, 2002; Vertes et al., 2006); see: Cassel et al., 2013; Vertes et al., 2015). Historically, the Rh is studied with the reuniens ventral thalamic nucleus, as together they form the ventral midline nuclei (Cassel et al., 2013). Inactivation and lesions to the RhRe implicate their role in modulating behavioral flexibility (Cholvin et al., 2013; Prasad et al., 2013; Prasad et al., 2016). Additionally lesions to the RhRe increase accuracy, decrease number of omitted responses and latency to obtain reward during behavioral tasks, suggesting a role for RhRe in motivation and executive control (Prasad et al., 2013; Prasad et al., 2016). The presence of these known connections along with the current findings, suggest that RhRe integrate cognitive and arousal processes to induce behavioral flexibility in a changing environment (Cassel et al., 2013). The majority of those studies attribute the Re/Rh with the role of modulating working-memory particularly with reference to spatial context (Hembrook & Mair, 2011; Cholvin et al., 2013; Hallock et al., 2013; Layfield et al., 2015; Prasad et al., 2016). Therefore, it is possible that pharmacological inactivation of these regions may induce memory impairments. Indeed, a memory impairment in a two-lever discrimination task, would be reflected by 50% responding on either lever. While this was the behavioral pattern observed following inactivation under the water condition (i.e., ~50% alcohol-appropriate responding), alcohol-appropriate responding under the alcohol condition was unaffected by inactivation (i.e., similar to the control condition). Therefore, this accurate discrimination performance would argue against a memory impairment (Figure 5B). To date the role of the Rh in drug-related behaviors has been understudied, however there is growing interest in this midline thalamic nucleus especially given its projections to limbic structures such as the mPFC, hippocampus, nucleus accumbens and its role in cognitive function (see: Vertes et al., 2015). The present findings implicating the Rh in modulating sensitivity to alcohol suggest the importance of future work to examine the role of this brain region in modulating other alcohol- and drug-related behaviors. However, it is important to consider the small sample size in the Rh inactivation studies, which was the consequence of several inaccurate cannula placements primarily due to the location and the small target area. Therefore, it will be important for future work to replicate this finding.
One of the goals of the present work was to focus on upstream regions to the AcbC, as general inhibition in the AcbC has been shown to modulate sensitivity to alcohol (Hodge & Alken, 1996; Hodge & Cox, 1998; Hodge et al., 2001; Besheer et al., 2003). It is important to consider that infusion of muscimol+baclofen into these regions inactivates all of the regions’ outgoing projections. Thus, the partial and full substitution of alcohol obtained through pharmacological inactivation may not be specific to inactivation of the outgoing AcbC projections but rather of a widespread regional effect. In addition to projecting to the AcbC, the mPFC, IC, and Rh all share reciprocal projections (Ohtake & Yamada, 1989; Sesack et al., 1989; Vertes et al., 2006). Thus, the present findings may be an indirect result of communication within these regions and may explain the partial vs. full substitution of “alcohol-like” effects. Further, while the FG study led to the focus on the mPFC, IC, and Rh as being AcbC-projecting regions, which is consistent other findings (Wright & Groenewegen, 1996; Ding et al., 2001; Vertes et al., 2006), it is important to consider that FG diffusion into the proximal shell or caudate nucleus may have occurred. Therefore, it will be important for future studies to isolate the specific neural circuitry modulating sensitivity to alcohol, and whether projections from the mPFC, IC, Rh to the AcbC are functionally involved.
The present findings provide evidence that GABAA+GABAB receptor systems in the IC, Rh, and mPFC functionally modulate, in part, the interoceptive effects of alcohol. Studies also utilizing muscimol+baclofen infusions in the IC demonstrate decreased alcohol self-administration (Pushparaj & Le Foll, 2015) while infusions in the mPFC decrease reinstatement of alcohol (Willcocks & McNally, 2013). Thus, it is possible that the decrease in alcohol self-administration and seeking (Willcocks & McNally, 2013; Pushparaj & Le Foll, 2015) may be related to “alcohol-like” effects induced by the pharmacological inactivation. In conclusion, the current results have identified novel brain regional involvement in modulation of the discriminative stimulus effects of alcohol.
Acknowledgments
This work was supported, in part, by grant AA019682 to JB from the National Institute on Alcohol Abuse and Alcoholism and by the Bowles Center for Alcohol Studies at the University of North Carolina at Chapel Hill. AAJ was supported, in part, by an NSF Graduate Research Fellowship (DGE-1144081) and then by F31AA024973 while working on the writing of this manuscript. The authors would like to thank Dr. Thomas Kash for helpful discussions regarding the utilization of the retrograde tracer and to Dr. Reginald Cannady for help with the conduct of that study. The authors have no conflict of interest to declare.
Abbreviations
- AcbC
Nucleus accumbens core
- FG
Fluoro-Gold
- FR
Fixed Ratio
- GABAA
[gamma]-aminobutyric acid type A
- GABAB
[gamma]-aminobutyric acid type B
- IC
Insular cortex
- IG
Intragastric
- IHC
Immunohistochemistry
- IR
Immunoreactivity
- mPFC
Medial prefrontal cortex
- NMDA
n-methyl-D-aspartate
- Rh
Rhomboid thalamic nucleus
- RM ANOVA
repeated measures analysis of variance
References
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Cannady R, Grondin JJ, Hodge CW. Intra-amygdala inhibition of ERK(1/2) potentiates the discriminative stimulus effects of alcohol. Behav Brain Res. 2012a;228:398–405. doi: 10.1016/j.bbr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJ, Cannady R, Hodge CW. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology (Berl) 2012b;220:809–822. doi: 10.1007/s00213-011-2533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology. 2014;39:2376–2386. doi: 10.1038/npp.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Frisbee S, Randall PA, Jaramillo AA, Masciello M. Gabapentin potentiates sensitivity to the interoceptive effects of alcohol and increases alcohol self-administration in rats. Neuropharmacology. 2015;101:216–224. doi: 10.1016/j.neuropharm.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Interoception and learning: import to understanding and treating diseases and psychopathologies. ACS Chem Neurosci. 2014;5:624–631. doi: 10.1021/cn5001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology. 2011;36:2328–2338. doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci. 2013;38:2751–2761. doi: 10.1111/ejn.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43:411–420. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci. 2013;33:8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, Huettel SA. Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Front Hum Neurosci. 2011;5:87. doi: 10.3389/fnhum.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Fadda F, Gessa GL. Symmetrical generalization between the discriminative stimulus effects of gamma-hydroxybutyric acid and ethanol: occurrence within narrow dose ranges. Physiol Behav. 1995;57:105–111. doi: 10.1016/0031-9384(94)00215-q. [DOI] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, LaLumiere RT. The Dorsal Agranular Insular Cortex Regulates the Cued Reinstatement of Cocaine-Seeking, but not Food-Seeking, Behavior in Rats. Neuropsychopharmacology. 2015;40:2425–2433. doi: 10.1038/npp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology (Berl) 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DC, Gabbott PL, Totterdell S. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res. 2001;917:81–89. doi: 10.1016/s0006-8993(01)02912-2. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Barrett JE. Blockade of the discriminative stimulus effects of ethanol with 5-HT3 receptor antagonists. Psychopharmacology (Berl) 1991;104:451–456. doi: 10.1007/BF02245648. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993;264:1241–1247. [PubMed] [Google Scholar]
- Grant KA, Colombo G, Gatto GJ. Characterization of the ethanol-like discriminative stimulus effects of 5-HT receptor agonists as a function of ethanol training dose. Psychopharmacology (Berl) 1997;133:133–141. doi: 10.1007/s002130050383. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci. 2013;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rogers LS, Grant KA. Antagonism of the ethanol-like discriminative stimulus effects of ethanol, pentobarbital, and midazolam in cynomolgus monkeys reveals involvement of specific GABA(A) receptor subtypes. J Pharmacol Exp Ther. 2009;331:142–152. doi: 10.1124/jpet.109.156810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU. Discriminative stimulus properties of ethanol: effects of cumulative dosing and Ro 15-4513. Behav Pharmacol. 1989;1:133–140. [PubMed] [Google Scholar]
- Hodge CW, Alken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1996;20:1221–1228. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25:1441–1447. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–1415. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Frisbee S, Fisher KR, Besheer J. Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol. 2015;49:525–532. doi: 10.1016/j.alcohol.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostowski W, Bienkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17:63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kozell LB, Hitzemann R, Buck KJ. Acute alcohol withdrawal is associated with c-Fos expression in the basal ganglia and associated circuitry: C57BL/6J and DBA/2J inbred mouse strain analyses. Alcohol Clin Exp Res. 2005;29:1939–1948. doi: 10.1097/01.alc.0000187592.57853.12. [DOI] [PubMed] [Google Scholar]
- Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, Bonci A. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A. 2015;112:1190–1195. doi: 10.1073/pnas.1416573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2011;36:711–720. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, Griffin AL. Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiol Learn Mem. 2015;125:163–167. doi: 10.1016/j.nlm.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobina C, Agabio R, Reali R, Gessa GL, Colombo G. Contribution of GABA(A) and GABA(B) receptors to the discriminative stimulus produced by gamma-hydroxybutyric acid. Pharmacol Biochem Behav. 1999;64:363–365. doi: 10.1016/s0091-3057(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Maurel S, Schreiber R, De Vry J. Role of 5-HT1B, 5-HT2A and 5-HT2C receptors in the generalization of 5-HT receptor agonists to the ethanol cue in the rat. Behav Pharmacol. 1998;9:337–343. [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Yamada H. Efferent connections of the nucleus reuniens and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci Res. 1989;6:556–568. doi: 10.1016/0168-0102(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Bano KM. Opioid receptors and the discriminative stimulus effects of ethanol in squirrel monkeys: Mu and delta opioid receptor mechanisms. Eur J Pharmacol. 2011;650:233–239. doi: 10.1016/j.ejphar.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Abela AR, Chudasama Y. Midline thalamic reuniens lesions improve executive behaviors. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Pushparaj A, Le Foll B. Involvement of the caudal granular insular cortex in alcohol self-administration in rats. Behav Brain Res. 2015;293:203–207. doi: 10.1016/j.bbr.2015.07.044. [DOI] [PubMed] [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hanson C, Hanson SJ, Bates ME. fMRI BOLD response in high-risk college students (Part 1): during exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol Alcohol. 2010;45:437–443. doi: 10.1093/alcalc/agq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health (NSDUH) 2014. [Google Scholar]
- Schechter MD. Discrete versus cumulative dosing in dose-response discrimination studies. Eur J Pharmacol. 1997;326:113–118. doi: 10.1016/s0014-2999(97)85404-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Discriminative stimulus effects of ethanol in C57BL/6J and DBA/2J inbred mice. Alcohol Clin Exp Res. 2002;26:747–757. [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl- D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology (Berl) 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- WHO. Global Status Report on Alcohol 2004. 2004. [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience. 1996;73:359–373. doi: 10.1016/0306-4522(95)00592-7. [DOI] [PubMed] [Google Scholar]