Abstract
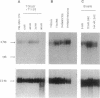
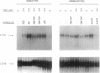
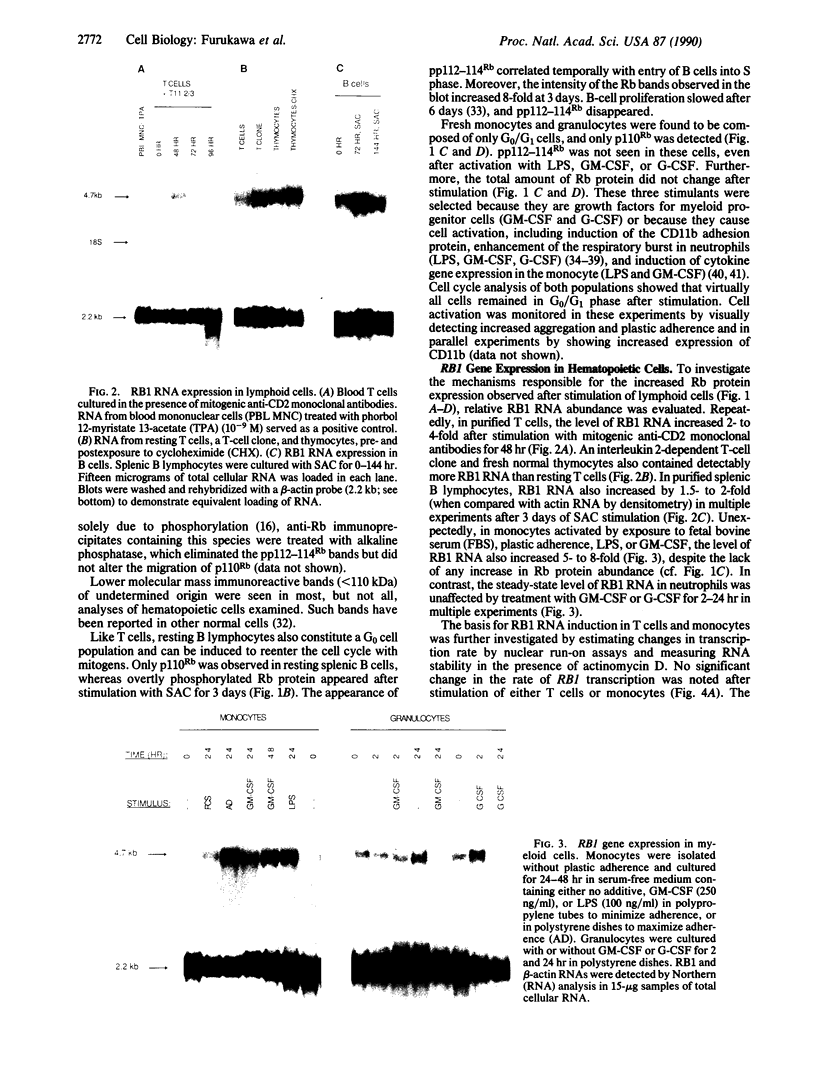
The product of the retinoblastoma susceptibility gene RB1 (Rb) is likely to function as an inhibitor of cell growth. Previous studies have suggested that certain growth-suppressing effects of Rb are exerted in G0/G1 phase and that phosphorylation can inactivate these functions. We tested this hypothesis by examining the expression and state of phosphorylation of Rb in several lineages of primary hematopoietic cells that spontaneously arrest in G0 phase. Resting lymphocytes were found to express only unphosphorylated Rb, but phosphorylation of Rb occurred as the cells entered S phase in response to mitogens. In contrast, although monocytes and granulocytes also expressed high levels of unphosphorylated Rb, these terminally differentiated cells did not phosphorylate Rb, nor could they exit from G1 phase in response to growth factors. Thus, Rb phosphorylation appears linked to the ability of a cell to synthesize DNA. In T and B lymphocytes, Rb protein increased 8-fold after stimulation, while RB1 RNA levels increased 2- to 4-fold. Nuclear run-on assays and measurement of RB1 RNA half-life in T cells suggested that the increased RNA abundance was, at least in part, due to increased RNA stability. By contrast, Rb protein levels did not increase in either monocytes or granulocytes after stimulation, although RB1 RNA levels did increase in monocytes. Thus, there are lineage-specific differences in both the regulation of Rb phosphorylation and RB1 gene expression in lymphoid and myeloid cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Wang E. A., Clark S. C., Sieff C. A. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J Clin Invest. 1986 Aug;78(2):597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Shackleford G. M., Schackleford G. M., Gerber M. R., Horowitz J. M., Friend S. H., Schartl M., Bogenmann E., Rapaport J. M., McGee T. Structure and expression of the murine retinoblastoma gene and characterization of its encoded protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6474–6478. doi: 10.1073/pnas.86.17.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. W., Anderson K. C., Freedman A. S., Fisher D. C., Slaughenhoupt B., Schlossman S. F., Nadler L. M. Studies of in vitro activation and differentiation of human B lymphocytes. I. Phenotypic and functional characterization of the B cell population responding to anti-Ig antibody. J Immunol. 1985 Mar;134(3):1516–1523. [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistra S. A., Griffin J. D. Regulation of the production and function of granulocytes and monocytes. Semin Hematol. 1988 Jul;25(3):173–188. [PubMed] [Google Scholar]
- Cannistra S. A., Vellenga E., Groshek P., Rambaldi A., Griffin J. D. Human granulocyte-monocyte colony-stimulating factor and interleukin 3 stimulate monocyte cytotoxicity through a tumor necrosis factor-dependent mechanism. Blood. 1988 Mar;71(3):672–676. [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprey S. P., Boettiger D. Developmental regulation of c-myb in normal myeloid progenitor cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6937–6941. doi: 10.1073/pnas.82.20.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Eierman D. F., Johnson C. E., Haskill J. S. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989 Mar 15;142(6):1970–1976. [PubMed] [Google Scholar]
- Ernst T. J., Ritchie A. R., Demetri G. D., Griffin J. D. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989 Apr 5;264(10):5700–5703. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M., Anfossi G., Venturelli D., Valpreda S., Sims R., Calabretta B. G1/S transition in normal human T-lymphocytes requires the nuclear protein encoded by c-myb. Science. 1989 Jul 14;245(4914):180–183. doi: 10.1126/science.2665077. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Yuo A., Souza L. M., Saito M., Miura Y., Takaku F. Recombinant human granulocyte colony-stimulating factor enhances superoxide release in human granulocytes stimulated by the chemotactic peptide. Biochem Biophys Res Commun. 1987 May 14;144(3):1143–1146. doi: 10.1016/0006-291x(87)91430-6. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- McGee T. L., Yandell D. W., Dryja T. P. Structure and partial genomic sequence of the human retinoblastoma susceptibility gene. Gene. 1989 Aug 1;80(1):119–128. doi: 10.1016/0378-1119(89)90256-4. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Vadas M. A., Lopez A. F., Williamson D. J., Wong G. G., Clark S. C., Wang E. A. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986 Jan;67(1):37–45. [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Ohta M., Furukawa Y., Ide C., Akiyama N., Utakoji T., Miura Y., Saito M. Establishment and characterization of four human monocytoid leukemia cell lines (JOSK-I, -S, -M and -K) with capabilities of monocyte-macrophage lineage differentiation and constitutive production of interleukin 1. Cancer Res. 1986 Jun;46(6):3067–3074. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sisson S. D., Dinarello C. A. Production of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988 Oct;72(4):1368–1374. [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (first of three parts). N Engl J Med. 1974 Mar 28;290(13):717–723. doi: 10.1056/NEJM197403282901306. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Melnick D. A., Malech H. L., Meshulam T., Simons E. R., Lazzari K. G., Proto P. J., Gadenne A. S., Leavitt J. L., Griffin J. D. The effects of phorbol myristate acetate and chemotactic peptide on transmembrane potentials and cytosolic free calcium in mature granulocytes evolve sequentially as the cells differentiate. J Biol Chem. 1987 Jan 25;262(3):1274–1281. [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Kwan L., Golde D. W., Gasson J. C. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987 Jan;69(1):18–21. [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Hashimoto T., Takahashi R., Benedict W. F. The retinoblastoma susceptibility gene product: a characteristic pattern in normal cells and abnormal expression in malignant cells. Oncogene. 1989 Jun;4(6):807–812. [PubMed] [Google Scholar]