Abstract
Thermal sensitivity of the cardiorespiratory oxygen supply capacity has been proposed as the cardinal link underlying the upper boundary of the temperature niche in aquatic ectotherms. Here we examined the evidence for this link in two eurythermal decapods, the Giant tiger shrimp (Penaeus monodon) and the European crayfish (Astacus astacus). We found that both species have a temperature resistant cardiorespiratory system, capable of maintaining oxygen delivery up to their upper critical temperature (Tcrit). In neither species was Tcrit reduced in hypoxia (60% air saturation) and both species showed an exponential increase in heart and gill ventilation rates up to their Tcrit. Further, failure of action potential conduction in preparations of A. astacus motor neurons coincided with Tcrit, indicating that compromised nervous function may provide the underlying determinant for Tcrit rather than oxygen delivery. At high temperatures, absolute aerobic scope was maintained in P. monodon, but reduced in A. astacus. However, A. astacus also displayed reduced exercise intensity indicating that impaired muscle performance with resulting reduced tissue oxygen demand may explain the reduced scope rather than insufficient oxygen supply capacity. This interpretation agrees with early literature on aquatic ectotherms, correlating loss of nervous function with impaired locomotion as temperatures approach Tcrit.
Due to the high thermal conductivity of water and the fundamental effects of temperature on the rates of metabolic processes1, water temperature influences physiological rates in all aquatic ectotherms including crustaceans2. In general, the effects of temperature on ectotherms are thought to be hierarchical, such that the temperature window for survival is considerably wider than for activity, while the thermal windows for growth and reproduction are narrower3,4,5,6. Given the current concerns over the ecological consequences of global warming7, considerable efforts are being devoted to identifying and understanding the physiological functions underlying thermal tolerance in aquatic animals.
One model in particular, the “Oxygen and capacity limited thermal tolerance (OCLTT) model”, has gained wide acceptance7,8 and is currently used in forecasting the effects of climate change on species distributions and future performance7,9,10. This model, based on the work of Fry and Hart11,12, proposes that cardiorespiratory system failure is the principal determinant of both upper critical temperature where animals lose equilibrium (Tcrit), and the realized environmental thermal niche8. Under the OCLTT framework, this failure at Tcrit occurs because the oxygen requirement of the mitochondria at high temperatures, necessary to meet the increasing ATP demands of the tissue, surpasses the oxygen supply capacity of the cardiovascular system. This renders the organism dependent on the much less efficient fermentation pathways for ATP production, and the animal experiences a dramatic loss of performance as energy balance becomes unsustainable.
The majority of studies supporting the OCLTT hypothesis have been performed on polar and temperate stenothermal invertebrates, including crustaceans and fish. When approaching Tcrit, these species are reported to reach a ceiling in heart and gill ventilation performance that limits their ability to increase maximum oxygen uptake rate ( O2max) above standard metabolic rate (SMR), resulting in falling blood oxygen levels and a transition to anaerobic metabolism13,14,15,16,17,18,19. However, recent studies in a number of eurythermal temperate and tropical species have demonstrated that the cardiorespiratory system (e.g., heart and gill ventilation rates) is able to increase oxygen supply proportionally with tissue oxygen demand as temperature increases to Tcrit. Hence, in these species the excess capacity for oxygen uptake beyond the SMR requirement (absolute aerobic scope), is not significantly reduced approaching the Tcrit. These animals thus avoid the transition to anaerobic metabolism20,21,22,23,24,25,26, indicating that factors other than inadequate oxygen delivery must be involved in any loss of ecological performance at high temperature. For example, growth rate in the tropical Giant freshwater shrimp (Macrobrachium rosenbergii) is reduced at a temperature below that impacting absolute aerobic scope24. It has been proposed, therefore, that eurythermal species have been evolutionarily selected for a thermally resistant cardiorespiratory system20,24. Thus, it is argued, the OCLTT model fails to explain thermal effects on species living in environments where they frequently encounter unpredictably high temperatures, such as the tropical M. rosenbergii24 and the intertidal Green crab (Carcinus maenas)20. In such high temperature or unpredictable environments, there may have been a strong evolutionary pressure to scale the cardiorespiratory system for oxygen delivery right up to Tcrit and thus other physiological parameters must account for species thermal tolerance.
O2max) above standard metabolic rate (SMR), resulting in falling blood oxygen levels and a transition to anaerobic metabolism13,14,15,16,17,18,19. However, recent studies in a number of eurythermal temperate and tropical species have demonstrated that the cardiorespiratory system (e.g., heart and gill ventilation rates) is able to increase oxygen supply proportionally with tissue oxygen demand as temperature increases to Tcrit. Hence, in these species the excess capacity for oxygen uptake beyond the SMR requirement (absolute aerobic scope), is not significantly reduced approaching the Tcrit. These animals thus avoid the transition to anaerobic metabolism20,21,22,23,24,25,26, indicating that factors other than inadequate oxygen delivery must be involved in any loss of ecological performance at high temperature. For example, growth rate in the tropical Giant freshwater shrimp (Macrobrachium rosenbergii) is reduced at a temperature below that impacting absolute aerobic scope24. It has been proposed, therefore, that eurythermal species have been evolutionarily selected for a thermally resistant cardiorespiratory system20,24. Thus, it is argued, the OCLTT model fails to explain thermal effects on species living in environments where they frequently encounter unpredictably high temperatures, such as the tropical M. rosenbergii24 and the intertidal Green crab (Carcinus maenas)20. In such high temperature or unpredictable environments, there may have been a strong evolutionary pressure to scale the cardiorespiratory system for oxygen delivery right up to Tcrit and thus other physiological parameters must account for species thermal tolerance.
Since it follows from the OCLTT model that the upper thermal limit should be reduced during hypoxia, we tested the model’s universality by measuring Tcrit in normoxia and hypoxia in two eurythermal crustacean species; the tropical Giant tiger shrimp (Penaeus monodon) and temperate European crayfish (Astacus astacus). Furthermore, we investigated the association between Tcrit and loss of oxygen delivery by quantifying absolute aerobic scope, heart rate and ventilation rate during temperature elevation. Finally, since early literature on the physiological mechanisms underlying Tcrit in aquatic ectotherms suggested that compromised neural activity may be a critical factor for thermal tolerance 27,28,29,30, we measured the impact of elevating temperature on the ability of nerve preparations from A. astacus to conduct action potentials.
Results
Oxygen uptake
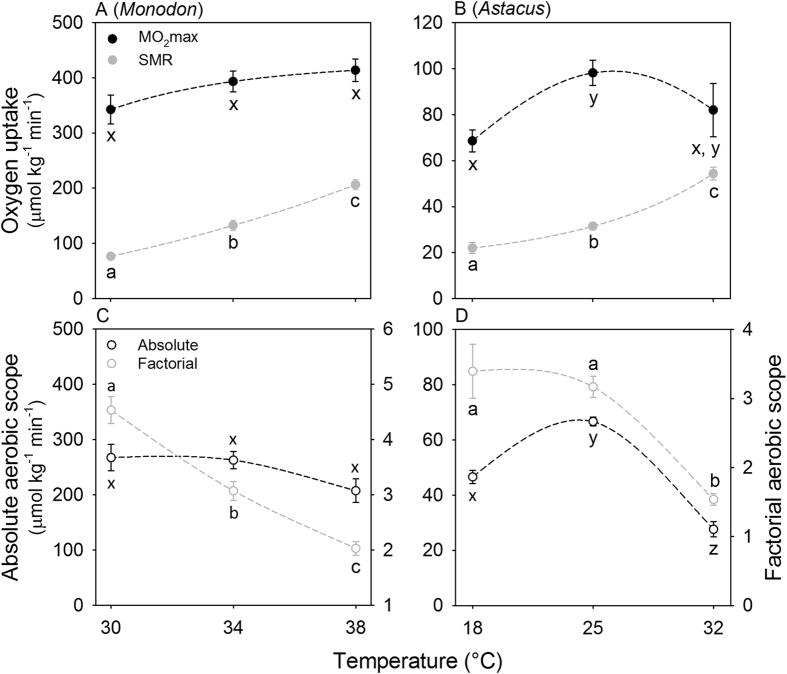
In P. monodon (n = 8), SMR increased exponentially from 30 to 38 °C (P = <0.001), while  O2max was maintained (P = 0.086) within the same temperature interval (Fig. 1A), resulting in a higher Q10 for SMR than for
O2max was maintained (P = 0.086) within the same temperature interval (Fig. 1A), resulting in a higher Q10 for SMR than for  O2max (3.5 ± 0.2 and 1.3 ± 0.2, respectively). As a consequence, absolute aerobic scope (i.e.
O2max (3.5 ± 0.2 and 1.3 ± 0.2, respectively). As a consequence, absolute aerobic scope (i.e.  O2max – SMR) was maintained (P = 0.063) as temperature rose from 30 to 38 °C and at 38 °C, 2–3 °C below Tcrit, P. monodon still retained 72% of the absolute aerobic scope that was available at 30 °C. Factorial aerobic scope (i.e.
O2max – SMR) was maintained (P = 0.063) as temperature rose from 30 to 38 °C and at 38 °C, 2–3 °C below Tcrit, P. monodon still retained 72% of the absolute aerobic scope that was available at 30 °C. Factorial aerobic scope (i.e.
 O2max / SMR), on the other hand, decreased significantly (P = <0.001) from 30 to 38 °C, with only 45% of the factorial aerobic scope available at 30 °C retained at 38 °C (Fig. 1C). In A. astacus (n = 8), SMR increased exponentially (Q10 = 1.9 ± 0.1) from 18 to 32 °C (P <0.001), while
O2max / SMR), on the other hand, decreased significantly (P = <0.001) from 30 to 38 °C, with only 45% of the factorial aerobic scope available at 30 °C retained at 38 °C (Fig. 1C). In A. astacus (n = 8), SMR increased exponentially (Q10 = 1.9 ± 0.1) from 18 to 32 °C (P <0.001), while  O2max increased significantly from 18 to 25 °C (P = 0.044) and plateaued from 25 to 32 °C (P = 0.298) (Fig. 1B). At each temperature, absolute aerobic scope of animals used in the SMR measurements, was calculated by subtracting SMR of individual animals from the mean of
O2max increased significantly from 18 to 25 °C (P = 0.044) and plateaued from 25 to 32 °C (P = 0.298) (Fig. 1B). At each temperature, absolute aerobic scope of animals used in the SMR measurements, was calculated by subtracting SMR of individual animals from the mean of  O2max, and factorial aerobic scope was calculated by dividing the mean of
O2max, and factorial aerobic scope was calculated by dividing the mean of  O2max with SMR of individual animals. Absolute aerobic scope increased significantly from 18 to 25 °C (P < 0.001) followed by a significant decrease from 25 to 32 °C (P < 0.001). At this temperature, 2–3 °C below Tcrit, the animals retained 43% of their absolute aerobic scope measured at 25 °C. Factorial aerobic scope was maintained from 18 to 25 °C (P = 0.461) followed by a significant decrease from 25 to 32 °C (P < 0.001), with 49% of the factorial aerobic scope at 18 °C retained at 32 °C (Fig. 1D).
O2max with SMR of individual animals. Absolute aerobic scope increased significantly from 18 to 25 °C (P < 0.001) followed by a significant decrease from 25 to 32 °C (P < 0.001). At this temperature, 2–3 °C below Tcrit, the animals retained 43% of their absolute aerobic scope measured at 25 °C. Factorial aerobic scope was maintained from 18 to 25 °C (P = 0.461) followed by a significant decrease from 25 to 32 °C (P < 0.001), with 49% of the factorial aerobic scope at 18 °C retained at 32 °C (Fig. 1D).
Figure 1.
Standard metabolic rate (SMR, closed grey circles) and maximum oxygen uptake rate ( O2max, closed black circles) (A, B), and absolute aerobic scope (open black circles) and factorial aerobic scope (open grey circles) (C, D) in the Giant tiger shrimp (Penaeus monodon) (n = 8) (A, C) and the European crayfish (Astacus astacus) (n = 8) (B, D). SMR in both species were fitted with an exponential growth function and
O2max, closed black circles) (A, B), and absolute aerobic scope (open black circles) and factorial aerobic scope (open grey circles) (C, D) in the Giant tiger shrimp (Penaeus monodon) (n = 8) (A, C) and the European crayfish (Astacus astacus) (n = 8) (B, D). SMR in both species were fitted with an exponential growth function and  O2max with an exponential rise and a Gaussian peak function in P. Monodon and A. Astacus, respectively. Different letters indicate a significant difference (P < 0.05). Values are means ± SEM.
O2max with an exponential rise and a Gaussian peak function in P. Monodon and A. Astacus, respectively. Different letters indicate a significant difference (P < 0.05). Values are means ± SEM.
In both species,  O2max usually occurred within 3–5 min after the exercise protocol and declined gradually to ~50% of
O2max usually occurred within 3–5 min after the exercise protocol and declined gradually to ~50% of  O2max towards the end of the measuring period (10 and 20 min for A. astacus and P. monodon, respectively). Preliminary measurements over 1 h showed a continued decline in
O2max towards the end of the measuring period (10 and 20 min for A. astacus and P. monodon, respectively). Preliminary measurements over 1 h showed a continued decline in  O2 without any delayed peaks in
O2 without any delayed peaks in  O2max. During exercise, the time until exhaustion ranged from 3–5 min at lower temperatures to 2–3 min at higher temperatures. Furthermore, both species became sluggish at higher temperatures and exhibited a reduced number of escape responses prior to exhaustion. This was especially pronounced in A. astacus where most individuals were completely unresponsive when nudged at 32 °C.
O2max. During exercise, the time until exhaustion ranged from 3–5 min at lower temperatures to 2–3 min at higher temperatures. Furthermore, both species became sluggish at higher temperatures and exhibited a reduced number of escape responses prior to exhaustion. This was especially pronounced in A. astacus where most individuals were completely unresponsive when nudged at 32 °C.
Heart rate and gill ventilation rate in normoxia
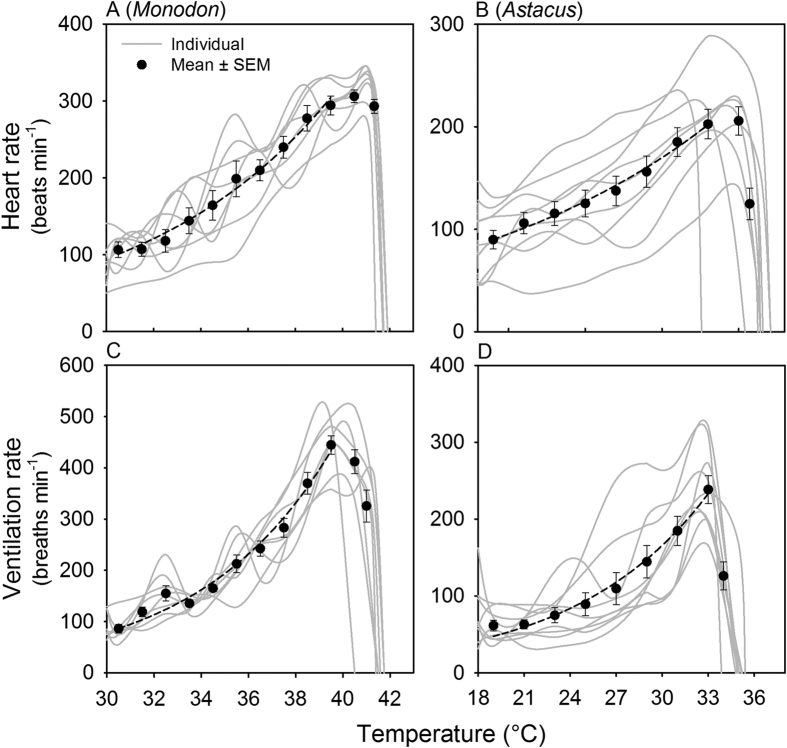
Each heartbeat in P. monodon (n = 8) and A. Astacus (n = 9) provided a single oscillation in the recorded signal. Each gill ventilation cycle consisted of a short oscillation followed by a longer oscillation, corresponding to the elevation and depression of the scaphognathite24. The heart and gill ventilation rates of both species increased exponentially with rising temperature until 2–3 °C below Tcrit, where both rates declines declined abruptly, accompanied by loss of buoyancy as the animals became clearly moribund (Fig. 2).
Figure 2.
Effects of acute temperature increase (2 °C h–1) on heart rate (A, B) and gill ventilation rate (C, D) in resting giant tiger shrimp (Penaeus monodon) (n = 8) (A, C) and European crayfish (Astacus astacus) (n = 9) (B, D). Data for each species were fitted with an exponential function. In both species, mean heart and gill ventilation rates increased exponentially with temperature up to Tcrit. P. monodon: heart: r2 = 0.98, Q10 = 3.1; gill: r2 = 0.93, Q10 = 5.0, from 30 to 39.5 °C; A. astacus: heart: r2 = 0.98, Q10 = 1.8; gill: r2 = 0.97, Q10 = 2.6, from 18 to 33 °C. Grey lines show traces for individual animals, mean ± SEM are shown in black.
Critical temperatures in normoxia and hypoxi
Tcrit in normoxia was calculated based on the heart rate measurements in normoxia described above. Heart rate measurements in hypoxia were used solely to calculate Tcrit in hypoxia. Hypoxia (60% air saturation) had no significant impact on Tcrit compared to normoxia (100% air saturation) in either species. Thus, in P. monodon Tcrit was 41.3 ± 0.1 and 41.1 ± 0.2 °C (P = 0.798, n = 8) and in A. astacus 35.7 ± 0.5 °C and 34.9 ± 0.3 °C (P = 0.052; n = 9) in normoxia and hypoxia, respectively (Fig. 3).
Figure 3.
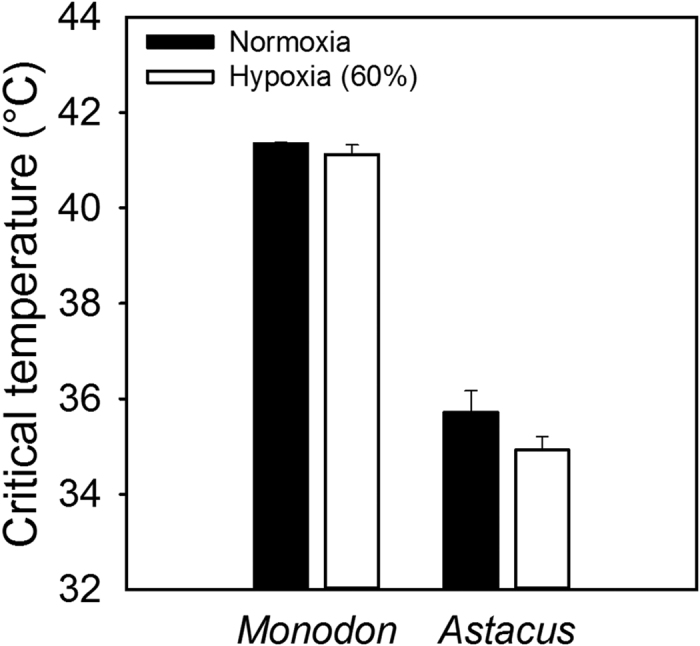
Tcrit (°C) was unaffected by reduced oxygen tension (Normoxia and 60% air saturation) in the Giant tiger shrimp (Penaeus Monodon) (n = 8) (P = 0.798) and the European crayfish (Astacus astacus) (n = 9) (P = 0.052). Columns show means ± SEM.
Action potentials in motor neurons
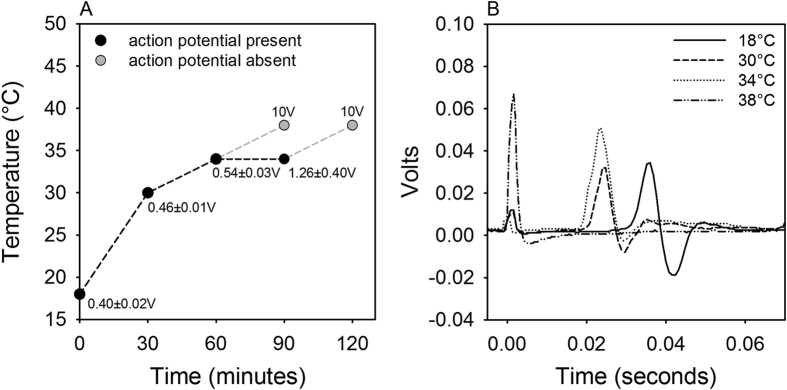
The conpduction speed of the action potentials (APs) in motor neurons from A. astacus (n = 6) exhibited a Q10 of approximately 1.5 in the temperature interval between 18–34 °C. The stimulation voltage required to evoke an AP increased slightly from 0.40 ± 0.02 V at 18 °C to 0.54 ± 0.03 V at 34 °C and more than doubled to 1.26 ± 0.35 V after 60–90 min at 34 °C, indicating a faster deterioration of this in vitro preparation at increased temperatures. The nerve bundles maintained at 34 oC for between 60 and 90 min continued to conduct APs, while an increase to 38 °C, at either 60 or 90 min, resulted in an irreversible loss of nervous function, even when the stimulation voltage was subsequently increased by more than an order of magnitude from 0.5 to 10 V. Subsequent temperature reduction from 38 to 30 °C did not restore nervous function (Fig. 4).
Figure 4.
A) Effect of time (min) and temperature (°C) on the ability of motor neurons from the legs of European crayfish (Astacus astacus) to conduct action potentials (n = 6). The stimulation voltage (± SEM) required to evoke an action potential is indicated at each step. B) Derived compound action potentials at 18, 30 and 34 °C in nerve preparations from legs of A. astacus. At 38 °C no action potentials were recorded. At time 0 stimulus artifacts from each recording are seen spikes of different amplitude depending on the stimulation voltage at each step.
Discussion
In both the tropical P. monodon and the temperate A. astacus, heart and ventilation rates increased exponentially until temperatures rose to immediately below their respective Tcrit’s (Fig. 2). That their ability to maintain oxygen supply at high temperatures is intact, was further supported by the limited loss of aerobic scope (Fig. 1) and the lack of an effect of hypoxia on Tcrit (Fig. 3). In P. monodon, the  O2max was still increasing at the highest measurement temperature, 2–3 °C below the Tcrit. Hence, this species did not show the temperature range of diminishing performance close to Tcrit predicted by the OCLTT model. A. astacus, on the other hand, appeared to provide some support for the OCLTT model with a significant reduction in
O2max was still increasing at the highest measurement temperature, 2–3 °C below the Tcrit. Hence, this species did not show the temperature range of diminishing performance close to Tcrit predicted by the OCLTT model. A. astacus, on the other hand, appeared to provide some support for the OCLTT model with a significant reduction in  O2max between 25 and 32 °C (Fig. 1). However, reduced exercise intensity during
O2max between 25 and 32 °C (Fig. 1). However, reduced exercise intensity during  O2max measurements coincided with loss of nervous function in A. astacus at high temperatures. This impaired muscle performance with associated reduced tissue oxygen demand thus appears to provide a more parsimonious explanation for the reduced
O2max measurements coincided with loss of nervous function in A. astacus at high temperatures. This impaired muscle performance with associated reduced tissue oxygen demand thus appears to provide a more parsimonious explanation for the reduced  O2max than the loss of oxygen supply capacity suggested by the OCLTT model. Both of the species tested here, therefore challenge the universality of the OCLTT model.
O2max than the loss of oxygen supply capacity suggested by the OCLTT model. Both of the species tested here, therefore challenge the universality of the OCLTT model.
The determination of Tcrit during hypoxia represents a direct test of the OCLTT model. According to the model, hypoxia causes a reduced water to blood branchial PO2 gradient and hence lower oxygen concentrations in the hemolymph returning to the heart and general circulation31. Thus, although crustaceans elevate ventilation and gill perfusion to maintain SMR in hypoxia31,32, the OCLTT model implies that these cardiorespiratory responses would already be maximized at Tcrit in normoxia, leaving no extra capacity for increasing  O2 from the water. If the model is correct, hypoxia must therefore give rise to a reduced Tcrit. This prediction of the OCLTT model was not born out in the present study, with neither species showing a significant reduction in Tcrit in 60% hypoxia compared to normoxia (Fig. 3). At constant temperatures, crustaceans have been shown to maintain arterial hemolymph oxygen saturation below 60% air saturation33. Assuming animals can also maintain oxygen saturation with rising temperatures, this might be interpreted to mean that our hypoxia level was insufficient to challenge oxygen supply. There are no data on the effects of hypoxia on arterial hemolymph oxygen tension in crustaceans approaching Tcrit. However, studies performed during normoxia show decreasing values of both arterial and venous hemolymph oxygen tensions19,34,35,36,37, indicating that as temperature rises the increasing tissue oxygen demand reduces the oxygen tension of hemolymph returning to the gills, preventing complete oxygen saturation of hemolymph leaving the gills. A 60% air saturation level should therefore present a valid challenge to the oxygen supply capacity in P. monodon and A. astacus.
O2 from the water. If the model is correct, hypoxia must therefore give rise to a reduced Tcrit. This prediction of the OCLTT model was not born out in the present study, with neither species showing a significant reduction in Tcrit in 60% hypoxia compared to normoxia (Fig. 3). At constant temperatures, crustaceans have been shown to maintain arterial hemolymph oxygen saturation below 60% air saturation33. Assuming animals can also maintain oxygen saturation with rising temperatures, this might be interpreted to mean that our hypoxia level was insufficient to challenge oxygen supply. There are no data on the effects of hypoxia on arterial hemolymph oxygen tension in crustaceans approaching Tcrit. However, studies performed during normoxia show decreasing values of both arterial and venous hemolymph oxygen tensions19,34,35,36,37, indicating that as temperature rises the increasing tissue oxygen demand reduces the oxygen tension of hemolymph returning to the gills, preventing complete oxygen saturation of hemolymph leaving the gills. A 60% air saturation level should therefore present a valid challenge to the oxygen supply capacity in P. monodon and A. astacus.
Our measurements of the cardiorespiratory responses to acute temperature elevations also indicate an adequate oxygen delivery at temperatures immediately below Tcrit. This is evident in the exponentially increasing heart rate and gill ventilation rates, measured up to temperatures immediately below Tcrit (Fig. 2). Again, this finding is not consistent with the explicit predictions of the OCLTT model19. Reduced cardiac stroke volume or tidal volume might potentially have reduced the overall oxygen supply capacity of the cardiorespiratory system. However, the SMR in both species rose exponentially (Fig. 1) with Q10-values within the range reported for other crustaceans38,39,40, indicating that oxygen delivery was sufficient to meet the rise in SMR.
Motor neuron bundles excised from A. astacus lost the ability to conduct APs at temperatures between 34 and 38 °C (Fig. 4), showing that compromised nervous function correlates with upper thermal limits. Similar conclusions were drawn in earlier studies on the physiological mechanisms underlying critical temperatures in aquatic ectotherms. In goldfish, localized heating of the brain induced the same sequence of behavioral malfunctions as those observed in whole animals during warming, and the decline in spontaneous activity of interneurons within the cerebellum occurred at similar temperatures to those disturbing behaviour27. Further, reduced spontaneous activity and behavioral disturbances were linked to thermally induced changes in the viscosity of synaptic membranes via changes in membrane phospholipid saturation30,41. In the crayfish (Astacus fluviatilis), the spontaneous spike activity of isolated neuron bundles increased from 10 to 28 °C, but decreased rapidly above 30 °C42,43. In crayfish exhibiting an impaired righting reflex between 26.7 and 30°C, the temperatures for total collapse of spontaneous activity in isolated nerve cords was between 36.4 and 38.7 °C28. Our data are consistent with these studies, as A. astacus became sluggish and unresponsive at 32 °C and lost the ability to conduct action potentials above 34 °C, a few degrees below Tcrit (36.2 ± 0.4 °C). As an alternative explanation to the OCLTT hypothesis, high temperatures may disrupt passive membrane ion permeabilities for Na+ and K+ altering cell excitability, as was the proposed explanation for early heat death in the crayfish (Astacus pallipes) at 35 °C44. Further, in muscle tissue, the breakdown of passive membrane permeability has been linked to temperature-induced inactivation of Mg2+-ATPase in the sarcolemma29. These membrane permeability disruptions can result in collapse of normal trans-membrane ion gradients, thus disrupting the resting potential and normal bioelectrical properties of excitable cells, leading to a progressive loss of function. Further, loss of nervous function can also result from enzyme denaturation with associated elevations in heat shock protein (HSP) expression. This was reported in the American lobster (Homarus americanus) with increased HSP70 levels and impaired locomotion (righting response) at 28 °C and an Arrhenius break temperature in heart rate at 30 °C, indicative of compromised cardiac function20 and hence oxygen supply. The neurogenic heart of crustaceans requires central nervous system input for contraction and maintenance of cardiac rhythm, which exerts both inhibitory and excitatory stimulation through the cardiac ganglion45,46. Heat induced deterioration of nervous functions at Tcrit therefore clearly have the potential to cause heart malfunction and thus loss of oxygen conductance.
Aerobic scope, the capacity to increase  O2max above SMR, is widely used to assess the oxygen supply capacity of the cardiorespiratory system2,47,48,49. Aerobic scope can be expressed as the absolute increase of
O2max above SMR, is widely used to assess the oxygen supply capacity of the cardiorespiratory system2,47,48,49. Aerobic scope can be expressed as the absolute increase of  O2 above SMR (i.e.
O2 above SMR (i.e.
 O2max - SMR) or as the factorial rise (i.e.
O2max - SMR) or as the factorial rise (i.e.
 O2max / SMR). Calculated with the same data, these two measures can run counter to each other and conclusions based on one value can potentially oppose those based on the other50. There is no consensus as to which of these values is most correct, but since there is a direct relationship between sustained work performed and the oxygen required, absolute aerobic scope is arguably a better measure than the proportional rise above baseline metabolism50. We therefore base our conclusions on this metric.
O2max / SMR). Calculated with the same data, these two measures can run counter to each other and conclusions based on one value can potentially oppose those based on the other50. There is no consensus as to which of these values is most correct, but since there is a direct relationship between sustained work performed and the oxygen required, absolute aerobic scope is arguably a better measure than the proportional rise above baseline metabolism50. We therefore base our conclusions on this metric.
 O2max is normally measured during, or immediately after intense exercise50 but the experimental protocol should reflect the lifestyle of the animal studied. In athletic species capable of prolonged exercise, such as tuna or migrating salmon, a
O2max is normally measured during, or immediately after intense exercise50 but the experimental protocol should reflect the lifestyle of the animal studied. In athletic species capable of prolonged exercise, such as tuna or migrating salmon, a  O2max measured during continuous swimming is normally used (e.g.47). P. monodon and A. astacus, however, are sluggish, benthic species that primarily reach peak performance during their short, intense and characteristic escape response. The post-exercise
O2max measured during continuous swimming is normally used (e.g.47). P. monodon and A. astacus, however, are sluggish, benthic species that primarily reach peak performance during their short, intense and characteristic escape response. The post-exercise  O2max protocol was therefore appropriate for these species. It has been hypothesized that in animals where Tcrit is limited by oxygen delivery,
O2max protocol was therefore appropriate for these species. It has been hypothesized that in animals where Tcrit is limited by oxygen delivery,  O2max will plateau or even decrease at high temperatures as the structural and physiological ceilings for oxygen delivery are reached by their cardiorespiratory systems8,51. Since SMR increases exponentially with temperature, absolute aerobic scope in these species decreases with rising temperatures and should, according to the OCLTT model8, be eliminated at Tcrit. Absolute aerobic scope in P. monodon was maintained immediately below Tcrit (Fig. 1C) in support of our hypothesis that eurythermal animals have evolved cardiorespiratory systems capable of delivering oxygen at high temperatures20,22,23,24. The interpretation of these data in A. astacus is slightly more involved. While its absolute aerobic scope was maintained at lower temperature, this parameter was significantly reduced at high temperatures (Fig. 1D), in accordance with the early findings of Fry and Heart11,12. At first glance this species seems to provide support to the OCLTT model8. However, because the Tcrit was not impacted by hypoxia, but was associated with loss of nerve function, we argue that the slight loss of oxygen supply capacity measured here cannot be the direct cause of the Tcrit, but is rather a secondary correlate. Hence neither of these two eurythermal species provide support for the OCLTT hypothesis.
O2max will plateau or even decrease at high temperatures as the structural and physiological ceilings for oxygen delivery are reached by their cardiorespiratory systems8,51. Since SMR increases exponentially with temperature, absolute aerobic scope in these species decreases with rising temperatures and should, according to the OCLTT model8, be eliminated at Tcrit. Absolute aerobic scope in P. monodon was maintained immediately below Tcrit (Fig. 1C) in support of our hypothesis that eurythermal animals have evolved cardiorespiratory systems capable of delivering oxygen at high temperatures20,22,23,24. The interpretation of these data in A. astacus is slightly more involved. While its absolute aerobic scope was maintained at lower temperature, this parameter was significantly reduced at high temperatures (Fig. 1D), in accordance with the early findings of Fry and Heart11,12. At first glance this species seems to provide support to the OCLTT model8. However, because the Tcrit was not impacted by hypoxia, but was associated with loss of nerve function, we argue that the slight loss of oxygen supply capacity measured here cannot be the direct cause of the Tcrit, but is rather a secondary correlate. Hence neither of these two eurythermal species provide support for the OCLTT hypothesis.
Although the tail muscle is largely powered by anaerobic ATP production52 such that the first escape response is not directly dependent of oxygen availability, both species became sluggish at higher temperatures and exhibited a reduced number of escape responses prior to exhaustion. This was especially pronounced in A. astacus where most individuals were completely unresponsive when nudged at 32 °C. The observed sluggishness of both species at high temperatures may result from compromised function of both afferent nerves from sensory systems relaying information about the mechanical stimulus and of efferent motor nerves innervating the muscles. If impaired muscle performance reduces tissue oxygen demand, the lack of responsiveness observed in A. astacus at 32 °C may explain the reduced aerobic scope in this species at 32 °C, rather than thermally induced limitations in oxygen supply capacity as suggested by the OCLTT model. If this hypothesis is correct, critical temperatures should not be affected by reduced water oxygen tension, consistent with our observations on both P. monodon and A. astacus (Fig. 3). If deterioration of nervous function impedes tail muscle tissue performance at 32 °C we might also expect diminished heart and gill movement at this temperature. An explanation for the observed lack of constrained heart and gill ventilation rates at 32 °C in A. astacus may be that the nerves innervating in these vital structures are less temperature sensitive than nerves innervating tail muscles. This is of course speculative and provides an interesting topic for future studies.
The “Multiple performances - multiple optima” (MPMO) hypothesis50 is an alternative to OCLTT, arguing that physiological functions have different optimal temperatures in the functions underlying animal fitness, and that their relative contribution to fitness varies between species. Our findings with P. monodon and A. astacus support the MPMO idea, indicating that although some functions may be impacted by oxygen supply capacity, others including those responsible for lethal temperatures appear to be dictated by nervous function. Whether or not thermal tolerance in tracheated arthropods is determined by oxygen limitations has likewise been debated53,54, and within this very diverse group it is evident that some species have evolved thermally resistant gas exchange mechanisms, while others have not55. Thus, while the oxygen supply capacity of the cardiorespiratory system does seem to be coupled to thermal tolerance in some polar and temperate stenothermal species8, there is increasing evidence in eurythermal species that this is not the case. These eurythermal animals appear to possess a more thermally resistant cardiorespiratory system23,24,26,56, adapted to provide sufficient oxygen supply in unpredictable high temperature environments. Indeed, it would seem unlikely that the upper critical temperature was universally determined by a single cardinal physiological rate across all species. It seems more plausible that failure of any of a variety of physiological systems including the cardiovascular system, the nervous system, enzyme imbalances, membrane fluidity etc., are possible, and that species differences are highly likely. General predictions of the effect of climate change on aquatic animals, based on oxygen supply capacity alone, should therefore be made with great caution.
Methods
Animals and maintenance
Giant tiger shrimp (Penaeus monodon) were obtained from a hatchery outside Can Tho (Southern Vietnam) and held in 1m3 tanks at Can Tho University at 29 ± 1 °C and salinity at 28 ppt. This salinity level matched the hatchery salinity and was reached by mixing dechlorinated tap water with concentrated (100 g L–1) seawater from The South China Sea. Water was changed regularly to ensure that NH4+-N, NO2– and NO3– concentrations never exceeded 0.25, 0.3 and 20 mg L−1, respectively. European crayfish (Astacus astacus) were obtained from a hatchery in southern Jutland (Denmark) and kept at Aarhus University (Denmark) in 1 m3 freshwater tanks (18 ± 0.1 °C), constantly supplied by particle-filtered, UV sterilized and protein skimmed water. Both species were fed to satiety on dry feed and freshly thawed shrimp every second day. To prevent the metabolic effects of molting and digestion on oxygen uptake rate ( O2), all measurements of SMR were performed in the intermolt period after 4 days of fasting. Different animal groups were used for each of the 5 experiments; oxygen uptake, gill ventilation rates in normoxia, heart rates in normoxia, heart rates in hypoxia, and evoked action potentials in motor neurons.
O2), all measurements of SMR were performed in the intermolt period after 4 days of fasting. Different animal groups were used for each of the 5 experiments; oxygen uptake, gill ventilation rates in normoxia, heart rates in normoxia, heart rates in hypoxia, and evoked action potentials in motor neurons.
Oxygen uptake
 O2 in both species was measured using computerized intermittent-flow respirometry57,58. Perforated Plexiglas plates mounted in each end of the respiration chamber ensured mixing of the circulating water during closed periods. Using equation (1),
O2 in both species was measured using computerized intermittent-flow respirometry57,58. Perforated Plexiglas plates mounted in each end of the respiration chamber ensured mixing of the circulating water during closed periods. Using equation (1),  O2 was calculated from the decline in PO2 during the closed period, with a 2 min delay from the start of the closed period:
O2 was calculated from the decline in PO2 during the closed period, with a 2 min delay from the start of the closed period:
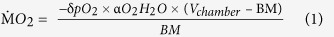 |
where  O2 is oxygen uptake rate (μmol kg–1 min–1), δpPO2 is slope of the decline in oxygen tension of the water (mmHg min-1) when the respirometer is closed, αO2H2O is the solubility of oxygen in water at the relevant temperature (μmol L−1 mmHg-1)59, Vchamber is the volume of the respirometer (L), and BM is body mass (kg). It was assumed that the animal displaced a water volume similar to its body mass (i.e., a density of 1 g ml−1).
O2 is oxygen uptake rate (μmol kg–1 min–1), δpPO2 is slope of the decline in oxygen tension of the water (mmHg min-1) when the respirometer is closed, αO2H2O is the solubility of oxygen in water at the relevant temperature (μmol L−1 mmHg-1)59, Vchamber is the volume of the respirometer (L), and BM is body mass (kg). It was assumed that the animal displaced a water volume similar to its body mass (i.e., a density of 1 g ml−1).
In P. monodon,
 O2max and SMR were measured at 30, 34 and 38 °C. At each temperature SMR and
O2max and SMR were measured at 30, 34 and 38 °C. At each temperature SMR and  O2max were measured in 8 animals, using a total of 24 animals (body mass = 33.9 ± 0.8 g). The animals were placed in the respirometer (1.4 L) at 30 ± 0.1 °C and temperature was either maintained at 30 °C or elevated to the required temperature at 4 °C h−1. Upon reaching the target temperature, animals were left undisturbed for 1h before measurement of
O2max were measured in 8 animals, using a total of 24 animals (body mass = 33.9 ± 0.8 g). The animals were placed in the respirometer (1.4 L) at 30 ± 0.1 °C and temperature was either maintained at 30 °C or elevated to the required temperature at 4 °C h−1. Upon reaching the target temperature, animals were left undisturbed for 1h before measurement of  O2 for the subsequent 4 h.
O2 for the subsequent 4 h.  O2 measurements consisted of successive 15 min time-loops of 10 min of oxygen consumption measurement and 5 min of respirometer flushing to return the respirometer PO2 to normoxia. Having completed SMR measurements,
O2 measurements consisted of successive 15 min time-loops of 10 min of oxygen consumption measurement and 5 min of respirometer flushing to return the respirometer PO2 to normoxia. Having completed SMR measurements,  O2max was measured by moving the animal to a water tank at the same temperature as the respirometer and inducing vigorous escape responses by nudging the carapace with a soft brush. When the animal became unable to perform an escape response and seemed exhausted, it was returned to the respirometer and
O2max was measured by moving the animal to a water tank at the same temperature as the respirometer and inducing vigorous escape responses by nudging the carapace with a soft brush. When the animal became unable to perform an escape response and seemed exhausted, it was returned to the respirometer and  O2 measured over the subsequent 10 min.
O2 measured over the subsequent 10 min.
In A. astacus,  O2max and SMR were measured at 18, 25 and 32 °C. SMR was measured in a total of 8 animals; individual animals being measured at all 3 temperatures (body mass = 72.8 ± 3.4 g). The animals were placed in the respirometer (1.4 L) at 18 ± 0.1 °C and left undisturbed for 1 h, after which
O2max and SMR were measured at 18, 25 and 32 °C. SMR was measured in a total of 8 animals; individual animals being measured at all 3 temperatures (body mass = 72.8 ± 3.4 g). The animals were placed in the respirometer (1.4 L) at 18 ± 0.1 °C and left undisturbed for 1 h, after which  O2 was measured over 3 h. Temperature was then elevated at 4 °C h−1 to 25 ± 0.1 °C and the animal left for 1 h before
O2 was measured over 3 h. Temperature was then elevated at 4 °C h−1 to 25 ± 0.1 °C and the animal left for 1 h before  O2 was measured again for 3 h. This 1 h delay before measurement was necessary to allow the Hamilton oxygen probes to stabilize at the new temperature for reliable PO2 measurements. The same procedure was repeated at each temperature. Each
O2 was measured again for 3 h. This 1 h delay before measurement was necessary to allow the Hamilton oxygen probes to stabilize at the new temperature for reliable PO2 measurements. The same procedure was repeated at each temperature. Each  O2 measurement comprised 30 min time loops (longer measurement period necessary with A. astacus than P. monodon because of lower metabolic rate), with a closed period of 20 min followed by 10 min of flushing.
O2 measurement comprised 30 min time loops (longer measurement period necessary with A. astacus than P. monodon because of lower metabolic rate), with a closed period of 20 min followed by 10 min of flushing.  O2max was measured separately in 8 animals at each temperature, using a total of 24 animals. Here, the animal was placed in a water tank at 18 ± 0.1 °C and the measurement initiated or the temperature elevated to the target temperature at 2 °C h−1. At the target temperature, vigorous escape responses were induced as above until the animal became sluggish, after which it was returned to the respirometer and
O2max was measured separately in 8 animals at each temperature, using a total of 24 animals. Here, the animal was placed in a water tank at 18 ± 0.1 °C and the measurement initiated or the temperature elevated to the target temperature at 2 °C h−1. At the target temperature, vigorous escape responses were induced as above until the animal became sluggish, after which it was returned to the respirometer and  O2 measured over the subsequent 20 min.
O2 measured over the subsequent 20 min.
Both species settled rapidly in the respirometers to produce  O2 traces of great consistency during SMR measurements at each temperature plateau, and the lowest of these consecutive measurements was chosen as the SMR estimate. The decline in PO2 during the
O2 traces of great consistency during SMR measurements at each temperature plateau, and the lowest of these consecutive measurements was chosen as the SMR estimate. The decline in PO2 during the  O2max measurement was divided into bins of 3 min and the bin (usually the 2nd or 3rd) with the highest δPO2 used as the
O2max measurement was divided into bins of 3 min and the bin (usually the 2nd or 3rd) with the highest δPO2 used as the  O2max estimate. During all measurements, the average decline in water PO2 in the respiration chamber was 107 ± 7 mmHg. Randomly distributed blind tests to measure bacterial respiration after the animals had been removed from the respirometers showed that background
O2max estimate. During all measurements, the average decline in water PO2 in the respiration chamber was 107 ± 7 mmHg. Randomly distributed blind tests to measure bacterial respiration after the animals had been removed from the respirometers showed that background  O2 due to bacterial respiration never exceeded 5% of SMR.
O2 due to bacterial respiration never exceeded 5% of SMR.
Heart and gill ventilation rates in normoxia
Heart and gill ventilation rates in P. monodon (n = 8) and A. astacus (n = 9) were measured in individual animals using a total of 16 (body mass = 87.9 ± 5.2 g) and 18 (body mass = 85.3 ± 4.4 g) animals, respectively. Heart and gill ventilation rates were measured using reflective infrared sensors (AMP03, Newshift Ltd, Leiria, Portugal) fitted into plastic tubes and glued onto the carapace above either the heart or the scaphognathites. The animal thus instrumented was submerged at 30 ± 0.1 °C (P. monodon) or 18 ± 0.1 °C (A. astacus) in fully oxygenated water and left overnight to obtain resting values. The following morning, water temperature was elevated linearly at 2 °C h−1, while heart or ventilation rates were recorded using Biopac MP100 data acquisition system at 200 Hz (12 bit).
Critical temperatures in normoxia and hypoxia
Tcrit during both normoxia and hypoxia was determined based on the heart rate measurements described above. Cardiac arrest was seen as a decrease in both heart rate and signal amplitude; resulting in the heart trace gradually leveling out and disappearing as the heart slowed and stopped. To ensure consistency in the critical temperature estimation, the Tcrit was defined as the first 1/6 °C temperature interval where heart rate was in clear decline. While the exact time of arrest was difficult to define, the arrest process was in all cases rapid and occurred within approximately 0.5 °C. Animals were not recoverable and therefore terminated by separating head and body at the end of each measurement. Hypoxia exposure was achieved by submerging the instrumented animal at 30 ± 0.1 °C (P. monodon) (n = 8) (body mass = 88.9 ± 4.1 g) or 18 ± 0.1 °C (A. astacus) (n = 9) (body mass = 82.1 ± 5.1 g) fully oxygenated water and left overnight. The following morning, water PO2 was reduced to 60% air saturation over 1h by bubbling with air and nitrogen mixed in a Wösthoff pump. At 60% air saturation the temperature was elevated linearly at 2 °C h−1, while heart rate was recorded. The water PO2 was maintained at 60% air saturation during the measuring periods. The duration of the measuring periods was ~6 h for P. monodon and ~9 h for A. astacus. Heart rate measurements in hypoxia was used solely to calculate Tcrit in hypoxia.
Measurements of evoked action potentials in motor neurons as a function of temperature
To investigate how temperature influenced nerve function, we measured the ability of nerve preparations to conduct action potentials (APs). Compound, extracellular APs were measured from motor neurons dissected from the legs of the A. astacus (n = 6) (body mass = 62.9 ± 8.8g). For each individual animal, two randomly chosen legs were removed and placed in a modified Krebs-Ringer’s solution consisting of 205 mM NaCl, 5.4 mM KCl, 13.5 mM CaCl2(H2O)2, 2.6 mM MgCl2(H2O)6, and 10 mM Hepes, titrated to pH 7.6 with NaOH60 at 18 °C. A small incision was made at each side of the 2nd leg joint and the two halves were separated to expose the central nerve bundle. The exposed nerve bundles were moved to a small bath of temperature controlled Krebs-Ringer solution (18 °C) and suspended between two silver coated electrodes61. One end was stimulated extracellularly with a Grass SD9 square pulse stimulator (frequency: 20s−1, duration: 0.2 ms, volts: 0.3 V–10 V), while the compound signal was recorded and amplified in the other end with a Grass P55 General Purpose AC Preamplifier (30–3000 Hz band-pass, 100 time amplification) and digitized with a HP 5506 oscilloscope triggered by the stimulator (Grass SD9). The oscilloscope averaged over 8 subsequent stimulations and 2000 data points from each averaged trace were transferred to a PC for further analysis. Temperature was regulated by circulating temperature-controlled water through copper-tubes embedded within the bottom of the bath. The embedded copper–tubes were not in direct contact with the water. The presence or absence of a derived AP was recorded at 18, 30, 34 and 38 °C. Each temperature rise was made over a 10 min period and the nerve bundle was left for 20 min at the new temperature before measurements were repeated. In one of the two nerve bundles from each crab, the presence or absence of a derived AP was measured at four temperatures. To establish whether the nervous function deteriorated at 34 °C, the second nerve bundle was studied at 18 and 30 °C, followed by two measurements at 34 °C performed 30 min apart before the temperature was elevated to 38 °C. A few drops of Krebs-Ringer solution from the bath were applied regularly to the air-exposed ends of the nerve bundles during the entire experiment to avoid desiccation.
Statistical analysis
Student’s t-test (P < 0.05) was used to test the effect of water PO2 on critical temperatures in P. monodon and A. astacus, respectively. In P. Monodon a One-Way Analysis of Variance (P < 0.05) was used to test the effect of temperature on SMR,  O2max, absolute and factorial aerobic scope, respectively. In A. astacus, a One-Way Analysis of Variance (P < 0.05) was used to test the effect of temperature on
O2max, absolute and factorial aerobic scope, respectively. In A. astacus, a One-Way Analysis of Variance (P < 0.05) was used to test the effect of temperature on  O2max and a One-Way Repeated Measures Analysis of Variance (P < 0.05) used to test the effect of temperature on SMR, absolute and factorial aerobic scopes, respectively. Statistical analyses were conducted using SigmaPlot (Systat Software, Inc., Chicago, IL, USA).
O2max and a One-Way Repeated Measures Analysis of Variance (P < 0.05) used to test the effect of temperature on SMR, absolute and factorial aerobic scopes, respectively. Statistical analyses were conducted using SigmaPlot (Systat Software, Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Ern, R. et al. Some like it hot: Thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Sci. Rep. 5, 10743; doi: 10.1038/srep10743 (2015).
Acknowledgments
The authors would like to thank Heidi M. Jensen, Per G. Henriksen and Rasmus Buchanan for animal husbandry, as well as John S. Jensen, Niels S. Bøgh, Niels U. Kristensen and Morten L. Hedegaard for technical assistance. The project is funded by the Danish International Development Agency (DANIDA), Ministry of Foreign Affairs of Denmark, as well as The Danish Research Council.
Footnotes
The authors declare no competing financial interests.
Author Contributions This study was conceived by R.E., D.T.T.H., N.T.P., P.T.M., T.W. and M.B. The experiments were designed by R.E., D.T.T.H., N.T.P., P.T.M., T.W. and M.B., and executed by R.E. and D.T.T.H. The results were interpreted by R.E., P.T.M., T.W. and M.B. The manuscript was drafted and revised by R.E., T.W. and M.B.
References
- Hochachka P. W. & Somero G. N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution. (Oxford University Press, New York, 2002). [Google Scholar]
- Brett J. R. Some principles in the thermal requirements of fishes. Q. Rev. Biol. 31, 75–87 (1956). [Google Scholar]
- Fry F. E. J. The effect of environmental factors on the physiology of fish. Fish Physiology. Vol. 6 (Academic Press, London, 1971). [Google Scholar]
- Cossins A. R. & Bowler K. Temperature Biology of Animals. (Chapman and Hall, London, 1987). [Google Scholar]
- Kingsolver J. G., Izem R. & Ragland G. J. Plasticity of size and growth in fluctuating thermal environments: comparing reaction norms and performance curves. Integr. Comp. Biol. 44, 450–460, 10.1093/Icb/44.6.450 (2004). [DOI] [PubMed] [Google Scholar]
- Wang T. & Overgaard J. The heartbreak of adapting to global warming. Science 315, 49–50, 10.1126/science.1137359 (2007). [DOI] [PubMed] [Google Scholar]
- IPCC. Pörtner H. O. et al. Ocean systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Field C. B. et al. (eds.) (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2014).
- Pörtner H. O. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893, 10.1242/Jeb.037523 (2010). [DOI] [PubMed] [Google Scholar]
- Philippart C. J. M. et al. Impacts of climate change on European marine ecosystems: Observations, expectations and indicators. J. Exp. Mar. Biol. Ecol. 400, 52–69, 10.1016/j.jembe.2011.02.023 (2011). [DOI] [Google Scholar]
- Woodin S. A., Hilbish T. J., Helmuth B., Jones S. J. & Wethey D. S. Climate change, species distribution models, and physiological performance metrics: predicting when biogeographic models are likely to fail. Ecol. Evol. 3, 3334–3346, 10.1002/Ece3.680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry F. E. J. Effects of the Environment on Animal Activity. (University of Toronto Press, Toronto, 1947). [Google Scholar]
- Fry F. E. J. & Hart J. S. Cruising speed of goldfish in relation to water temperature. J. Fish. Res. Board Can. 7b, 169–175, 10.1139/f47-018 (1948). [DOI] [Google Scholar]
- Van-Dijk P. L. M., Tesch C., Hardewig I. I. & Pörtner H. O. Physiological disturbances at critically high temperatures: a comparison between stenothermal antarctic and eurythermal temperate eelpouts (Zoarcidae). J. Exp. Biol. 202, 3611–3621 (1999). [DOI] [PubMed] [Google Scholar]
- Hardewig I., van Dijk P. L., Moyes C. D. & Pörtner H. O. Temperature-dependent expression of cytochrome-c oxidase in Antarctic and temperate fish. Am. J. Physiol. 277, R508–516 (1999). [DOI] [PubMed] [Google Scholar]
- Peck L. S., Pörtner H. O. & Hardewig I. Metabolic demand, oxygen supply, and critical temperatures in the antarctic bivalve Laternula elliptica. Physiol. Biochem. Zool. 75, 123–133 (2002). [DOI] [PubMed] [Google Scholar]
- Pörtner H. O. & Zielinski S. Environmental constraints and the physiology of performance in squids. S. Afr. J. Mar. Sci. 20, 207–221 (1998). [Google Scholar]
- Zielinski S. & Pörtner H. O. Energy metabolism and ATP free-energy change of the intertidal worm Sipunculus nudus below a critical temperature. J. Comp. Physiol. 166, 492–500 (1996). [Google Scholar]
- Pörtner H. O., Peck L., Zielinski S. & Conway L. Z. Intracellular pH and energy metabolism in the highly stenothermal Antarctic bivalve Limopsis marionensis as a function of ambient temperature. Polar Biol. 22, 17–30, 10.1007/s003000050386 (1999). [DOI] [Google Scholar]
- Frederich M. & Pörtner H. O. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, 1531–1538 (2000). [DOI] [PubMed] [Google Scholar]
- Jost J. A., Podolski S. M. & Frederich M. Enhancing thermal tolerance by eliminating the pejus range: a comparative study with three decapod crustaceans. Mar. Ecol-Prog. Ser. 444, 263–274, 10.3354/Meps09379 (2012). [DOI] [Google Scholar]
- Frederich M., O’Rourke M. R., Furey N. B. & Jost J. A. AMP-activated protein kinase (AMPK) in the rock crab, Cancer irroratus: an early indicator of temperature stress. J. Exp. Biol. 212, 722–730, 10.1242/Jeb.021998 (2009). [DOI] [PubMed] [Google Scholar]
- Clark T. D., Jeffries K. M., Hinch S. G. & Farrell A. P. Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J. Exp. Biol. 214, 3074–3081, 10.1242/Jeb.060517 (2011). [DOI] [PubMed] [Google Scholar]
- Norin T., Malte H. & Clark T. D. Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J. Exp. Biol. 217, 244-251, 10.1242/jeb.089755 (2014). [DOI] [PubMed] [Google Scholar]
- Ern R., Huong D. T. T., Phuong N. T., Wang T. & Bayley M. Oxygen delivery does not limit thermal tolerance in a tropical eurythermal crustacean. J. Exp. Biol. 217, 809–814, 10.1242/jeb.094169 (2014). [DOI] [PubMed] [Google Scholar]
- Franklin C. E., Farrell A. P., Altimiras J. & Axelsson M. Thermal dependence of cardiac function in arctic fish: implications of a warming world. J. Exp. Biol. 216, 4251–4255, 10.1242/jeb.087130 (2013). [DOI] [PubMed] [Google Scholar]
- Gräns A. et al. Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J. Exp. Biol. 217, 711–717, 10.1242/jeb.096743 (2014). [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Kotchabhakdi N. & Prosser C. L. Effects of cold and heat on behavior and cerebellar function in Goldfish. J. Comp. Physiol. 112, 19–45 (1976). [Google Scholar]
- Kivivuori L. Effects of temperature and temperature acclimation on the motor and neural functions in the crayfish Astacus astacus L. Comp. Biochem. Physiol. 65, 297–304, 10.1016/0300-9629(80)90032-8 (1980). [DOI] [Google Scholar]
- Bowler K., Duncan C. J., Gladwell R. T. & Davison T. F. Cellular heat injury. Comp. Biochem. Physiol. 45, 441–450, 10.1016/0300-9629(73)90451-9 (1973). [DOI] [Google Scholar]
- Cossins A. R., Friedlander M. J. & Prosser C. L. Correlations between behavioral temperature adaptations of goldfish and the viscosity and fatty acid composition of their synaptic membranes. J. Comp. Physiol. 120, 109–121 (1977). [Google Scholar]
- McMahon B. R. Respiratory and circulatory compensation to hypoxia in crustaceans. Resp. Physiol. 128, 349–364 (2001). [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Control and co-ordination of ventilation and circulation in crustaceans: responses to hypoxia and exercise. J. Exp. Biol. 100, 289–319 (1982). [Google Scholar]
- Hagerman L. & Weber R. E. Respiratory rate, hemolymph oxygen-tension and hemocyanin level in the shrimp Palaemon-Adspersus Rathke . J. Exp. Mar. Biol. Ecol. 54, 13–20, 10.1016/0022-0981(81)90099-X (1981). [DOI] [Google Scholar]
- Metzger R., Sartoris F. J., Langenbuch M. & Pörtner H. O. Influence of elevated CO2 concentrations on thermal tolerance of the edible crab Cancer pagurus. J. Therm. Biol. 32, 144–151, 10.1016/j.jtherbio.2007.01.010 (2007). [DOI] [Google Scholar]
- Walther K., Sartoris F. J., Bock C. & Pörtner H. O. Impact of anthropogenic ocean acidification on thermal tolerance of the spider crab Hyas araneus. Biogeosciences 6, 2207–2215 (2009). [Google Scholar]
- Giomi F. & Pörtner H. O. A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front. Physiol. 4, 1–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann A. C., Pörtner H. O. & Sartoris F. J. A role for oxygen delivery and extracellular magnesium in limiting cold tolerance of the sub-antarctic stone crab Paralomis granulosa? Physiol. Biochem. Zool. 85, 285–298, 10.1086/665328 (2012). [DOI] [PubMed] [Google Scholar]
- Manush S. M., Pal A. K., Chatterjee N., Das T. & Mukherjee S. C. Thermal tolerance and oxygen consumption of Macrobrachium rosenbergii acclimated to three temperatures. J. Therm. Biol. 29, 15–19, 10.1016/j.jtherbio.2003.11.005 (2004). [DOI] [Google Scholar]
- Spanopoulos-Hernandez M., Martinez-Palacios C. A., Vanegas-Perez R. C., Rosas C. & Ross L. G. The combined effects of salinity and temperature on the oxygen consumption of juvenile shrimps Litopenaeus stylirostris (Stimpson, 1874). Aquaculture 244, 341–348, 10.1016/j.aquaculture.2004.11.023 (2005). [DOI] [Google Scholar]
- Engel D. W. & Angelovic J. W. The influence of salinity and temperature upon the respiration of brine shrimp nauplii. Comp. Biochem. Physiol. 26, 749–752 (1968). [Google Scholar]
- Cossins A. R. Adaptation of biological membranes to temperature. The effect of temperature acclimation of goldfish upon the viscosity of synaptosomal membranes. Biochim. Biophys. Acta. 470, 395–411, 10.1016/0005-2736(77)90131-6(1977). [DOI] [PubMed] [Google Scholar]
- Kerkut G. A. & Taylor B. J. R. The effect of temperature changes on the activity of poikilotherms. Behaviour 13, 259–279 (1958). [Google Scholar]
- Prosser C. L. Action potentials in the nervous system of the crayfish IV. Influence of temperature on nerve impulses arising “spontaneously” in abdominal ganglia. J. Gen. Physiol. 19, 65–73, 10.1085/Jgp.19.1.65 (1935). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler K. A study of factors involved in acclimatization to temperature and death at high temperatures in Astacus Pallipes. 2. Experiments at tissue level. J. Cell. Comp. Physiol. 62, 133–146, 10.1002/jcp.1030620204 (1963). [DOI] [PubMed] [Google Scholar]
- Alexandrowicz J. S. The innervation of the heart of the Crustacea. I. Decapoda. Q. J. Microsc. Sci. 75, 181–249 (1932). [Google Scholar]
- Wilkens J. L. The control of cardiac rhythmicity and of blood distribution in crustaceans. Comp. Biochem. Physiol. 124, 531–538, 10.1016/S1095-6433(99)00146-4 (1999). [DOI] [Google Scholar]
- Eliason E. J. et al. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112, 10.1126/science.1199158 (2011). [DOI] [PubMed] [Google Scholar]
- Nilsson G. E., Crawley N., Lunde I. G. & Munday P. L. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob. Change Biol. 15, 1405–1412, 10.1111/j.1365-2486.2008.01767.x (2009). [DOI] [Google Scholar]
- Healy T. M. & Schulte P. M. Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic ccope in the common killifish (Fundulus heteroclitus). Physiol. Biochem. Zool. 85, 107–119, 10.1086/664584 (2012). [DOI] [PubMed] [Google Scholar]
- Clark T. D., Sandblom E. & Jutfelt F. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782, 10.1242/jeb.084251 (2013). [DOI] [PubMed] [Google Scholar]
- Farrell A. P., Eliason E. J., Sandblom E. & Clark T. D. Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 87, 835–851, 10.1139/Z09-092 (2009). [DOI] [Google Scholar]
- Onnen T. & Zebe E. Energy-metabolism in the tail muscles of the shrimp crangon-crangon during work and subsequent recovery. Comp. Biochem. Physiol. 74, 833–838, Doi 10.1016/0300-9629(83)90355-9 (1983). [DOI] [Google Scholar]
- Mölich A. B., Förster T. D. & Lighton J. R. Hyperthermic overdrive: oxygen delivery does not limit thermal tolerance in Drosophila melanogaster. J. Insect Sci. 12, 1–7, 10.1673/031.012.10901 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok C. J., Sinclair B. J. & Chown S. L. Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J. Exp. Biol. 207, 2361–2370, 10.1242/Jeb.01023 (2004). [DOI] [PubMed] [Google Scholar]
- Verberk W. C. E. P. & Bilton D. T. Respiratory control in aquatic insects dictates their vulnerability to global warming. Biology lett. 9, 1–4, 10.1098/rsbl.2013.0473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. et al. Anaemia only causes a small reduction in the upper critical temperature of sea bass: is oxygen delivery the limiting factor for tolerance of acute warming in fishes? J. Exp. Biol. 217, 4275–4278, 10.1242/Jeb.104166 (2014). [DOI] [PubMed] [Google Scholar]
- Lefevre S., Do T. T. H., Wang T., Nguyen T. P. & Bayley M. Hypoxia tolerance and partitioning of bimodal respiration in the striped catfish (Pangasianodon hypophthalmus). Comp. Biochem. Physiol. 158, 207–214, 10.1016/j.cbpa.2010.10.029 (2011). [DOI] [PubMed] [Google Scholar]
- Steffensen J. F., Johansen K. & Bushnell P. G. An automated swimming respirometer. Comp. Biochem. Physiol. 79, 437–440 (1984). [Google Scholar]
- Colt J. Computation of dissolved gas concentrations in water as functions of temperature, salinity and pressure. Am. Fish. S. (special publication) 14, 1–154 (1984). [Google Scholar]
- Van Harreveld A. A physiological solution for freshwater crustaceans. P. Soc. Exp. Biol. Med. 34, 428–432 (1936). [Google Scholar]
- Silver W. L. Recording action potentials extracellularly from earthworm giant axons. Laboratory Manual for Physiology. (Benjamin Cummings, San Francisco, 2005). [Google Scholar]