Abstract
Cigarette smoke has been considered a major contributor to the pathogenesis of chronic obstructive pulmonary disease (COPD). In COPD patients, the airway smooth muscle layer has been observed to be markedly thickened and the proliferation of airway smooth muscle cells (ASMCs) was therefore used by the present study as a model to assess the impact of cigarette smoke extract (CSE). ASMCs were exposed to various concentrations of CSE and the proliferation of the cells was analyzed by an MTT assay. Furthermore, the expression levels of calreticulin and CCAAT/enhancer-binding protein alpha (C/EBP-α) in CSE-stimulated ASMCs were determined by polymerase chain reaction and western blot analyses. In addition, the effects of RNA interference (RNAi) to knockdown calreticulin and/or C/EBP-α on ASMC proliferation were studied. CSE was found to promote the proliferation of ASMCs, which was associated with increased expression of calreticulin and decreased expression of C/EBP-α. Knockdown of calreticulin resulted in the upregulation of C/EBP-α and inhibition of cell proliferation, while simultaneous knockdown of C/EBP-α promoted cell proliferation. The present study revealed that CSE promoted the proliferation of ASMCs, which was mediated by inhibition of C/EBP-α. These findings shed new light on airway remodeling in COPD and may provide novel approaches for therapies.
Keywords: cigarette smoke extract, airway smooth muscle cell, cell proliferation, calreticulin, CCAAT/enhancer-binding protein α
Introduction
Chronic obstructive pulmonary disease (COPD) is a major chronic disease with increasing morbidity and mortality worldwide and is characterized by reversible airflow limitation (1). Pathologically, persistent airway inflammation and airway remodeling are two key factors of airway obstruction in COPD (1,2). In airway remodeling, airway smooth muscle cells (ASMCs) act as the main effector cells and their proliferation represents a major characteristic of airway remodeling in COPD (3,4). Studies in animal models and on human patients have shown that cigarette smoke is one of the most important risk factors for the development of COPD. In addition, studies have demonstrated that cigarette smoke extract (CSE) can stimulate the proliferation of ASMCs (5,6). The ASM layer was markedly thickened in COPD patients and in a rat model of cigarette smoking (7,8).
CCAAT/enhancer-binding protein alpha (C/EBP-α), a member of the C/EBP family, was recently implicated in the pathogenesis of COPD. It belongs to the basic leucine zipper class of transcription factors and has essential roles in the regulation of cell cycle progression, differentiation and pro-inflammatory gene expression. C/EBP-α-deficient mice displayed histopathological and inflammatory characteristics of COPD (9). In asthma patients, the expression levels of C/EBP-α are markedly decreased in lung ASMCs (10). In addition, a previous study has revealed that C/EBP-α inhibited cell proliferation by directly suppressing cyclin-dependent kinase (Cdk) 2 and Cdk4 (11).
A previous study reported that calreticulin, a Ca2+-binding chaperone in the endoplasmic reticulum (ER) of eukaryotic cells, transcriptionally regulated C/EBP-α through a cis-regulatory CNG-rich loop in the mRNA of C/EBP-α (12). In the ER lumen, calreticulin acts as a chaperone, which controls newly synthesized proteins and glycoproteins and regulates intracellular Ca2+ homeostasis to affect a variety of cell processes such as adipocyte differentiation, cardiogenesis and cell stress responses (13). Besides, calreticulin is also involved in the regulation of wound healing, tumorigenesis and immunity. Studies have also assessed the function of calreticulin in cell proliferation, revealing that it is highly expressed in several cancer types such as hepatoma, colon cancer and oral squamous cell carcinoma with increased cell proliferation (14,15). Furthermore, manipulation of calreticulin levels affected cancer cell proliferation, angiogenesis and differentiation (15). However, another study performed by Miglino et al (12) demonstrated that calreticulin negatively regulates the proliferation of bronchial (B) SMCs. These controversial findings inspired our group to investigate the regulatory role of calreticulin in cell proliferation.
It was demonstrated that the interaction of calreticulin with stem-loop structures of C/EBP-β and C/EBP-α mRNAs leads to inhibition of translation of C/EBP proteins, indicating the potential involvement of calreticulin in the post-transcriptional processing of certain GC-rich mRNAs via regulation of C/EBP protein expression (16). In the present study, the expression of calreticulin and C/EBP-α in a CSE-treated cell model was examined and the effect of CSE on the proliferation of human ASMCs was assessed. In addition, the molecular mechanism by which CSE controls cell proliferation through inhibiting C/EBP-α was investigated.
Materials and methods
Cell culture and treatments
Normal human ASMCs were purchased from the American Type Tissue Collection (Manassas, VA, USA). ASMCs were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) and maintained in a humidified atmosphere with 5% CO2 at 37°C. The subcultures of ASMCs between passage 4 and 6 were used in the experiments. For cell treatment, ASMCs were seeded in 6-well plates and then stimulated with various concentrations of CSE (0, 2.5, 5, 10, 15 and 20%) for 24, 48 or 72 h. For all experiments, cells were made quiescent by incubation in serum-free medium overnight prior to exposure to CSE.
Preparation of CSE
CSE was freshly generated under standardized conditions as previously described (17). In brief, aqueous CSE was obtained by combustion of two University of Kentucky 3R4F research cigarettes (filters removed) purchased from the University of Kentucky (Lexington, KT, USA) and passing the resulting smoke through 25 ml DMEM using a peristaltic pump, followed by filtering through a 0.22-µm pore filter. The obtained solution was referred to as having 100% strength.
Cell proliferation assay
The proliferation of ASMCs was determined by a colorimetric assay using MTT. Following CSE treatment, the supernatant was removed and 150 µl MTT (AMRESCO, LLC., Cleveland, OH, USA) was added to each well. Following incubation at 37°C for 4 h, the reaction product of MTT was extracted with dimethyl sulfoxide (DMSO). The absorbance was measured at 570 nm using the multiskan MK3 (Thermo Fisher Scientific, Inc.) with DMSO as a blank.
Cell apoptosis assay
For detection of apoptosis, human ASMCs from each group were stained with Annexin V conjugated to fluorescein isothiocyanate (FITC) as well as propidium iodide (PI) using the FITC Annexin V/Dead Cell Apoptosis kit (Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. In brief, subsequent to treatment, human ASMCs were suspended and incubated in buffer containing Annexin V and PI for 5 min at room temperature in the dark. The cells were then assessed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and the data were analyzed using FlowJo software 7.6 (Tree Star, Inc., Ashland, OR, USA).
Immunofluorescence
ASMCs were prepared on chambered slides (BD Falcon; BD Biosciences) and exposed to 10% CSE for 24 h. Cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min. Subsequently, cells were permeabilized with methanol and then blocked with 3% bovine serum albumin (BSA, Gibco; Thermo Fisher Scientific, Inc.) in PBS at room temperature for 1 h. The cells were incubated with anti-C/EBP-α primary antibody (dilution, 1:300; no. ab40761; Abcam, Cambridge, UK) at 4°C overnight. Detection was performed with goat anti-rabbit immunoglobulin (Ig) G secondary antibody (dilution, 1:500; no. ab175471; Abcam) labeled with Alexa Fluor 568 (red) at 37°C for 1 h, using ProLong Gold anti-fade with DAPI (blue). Images were obtained using a fluorescence microscope (Axiovert 200; Carl Zeiss AG, Oberkochen, Germany).
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells of each group using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). Complementary (c)DNA was synthesized using the PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan), according to the manufacturer's instructions. The cDNA obtained served as a template for PCR using PCR Master Mix (Promega Corp., Madison, WI, USA) and the following gene-specific primers: C/EBP-α forward, 5′-GGCGGCGACTTTGACTACC-3′ and reverse, 5′-CTGCTTGGCTTCATCCTCCTC-3′; β-actin forward, 5′-ACACTGTGCCCATCTACGACG-3′ and reverse, 5′-AGGGGCCGGACTCCTCATACT-3′ (Shanghai GenePharma Co., Ltd., Shanghai, China). PCR conditions were set as follows: 5 min at 95°C, followed by 35 cycles of 30 sec at 94°C, 40 sec at 54°C and 40 sec at 72°C, and a final extension at 72°C for 6 min (on an ABI9700. PCR products were separated on a 1.5% agarose gel, mRNA levels were captured and then calculated using the comparative cycle threshold method and normalized to the expression of β-actin mRNA as a control (18).
Western blot analysis
Following treatment with CSE, the medium was removed and cell protein was extracted using the Total Protein Extraction kit (cat. no. AR0103; Boster Biological Technology, Wuhan, China) and then quantified using the BSA method (17). Whole-cell lysate (20 µg) was loaded onto an 8% Tris-glycine gel (Invitrogen; Thermo Fisher Scientific, Inc.). Following electrophoresis, proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% BSA in Tris-buffered saline containing Tween-20 for 1 h at room temperature. Blots were then incubated at 4°C overnight with primary antibodies against calreticulin (dilution, 1:1,000; no. ab2908), C/EBP-α (dilution, 1:1,000; no. ab40761) and β-actin (dilution, 1:5,000; no. ab8227), followed by the secondary antibody anti-mouse IgG (dilution, 1:1,000; no. ab131368; Abcam) at 37°C for 1 h. All antibodies were purchased from Abcam. An enhanced chemiluminescence detection system (ECL) western blot reagent (RPN210; GE Healthcare, Chalfont, UK) was used to detect the signals on the membranes with the aid of a Benchtop Ultraviolet Transilluminator (VWR, Radnor, PA, USA).
RNA interference
ASMCs were seeded into 6-well plates and incubated for 24 h prior to transfection with 50 nM control small interfering siRNA vector, siRNA calreticulin (5′-GGAGGAUGAUGAGGACAAATT-3′) or siRNA C/EBP-α (5′-GACAAGAACAGCAACGAGUTT-3′; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The sequence of siRNA used for negative control was 5′- UUC UCC GAA CGU GUC ACG UTT-3. Transfected cells were cultured in DMEM medium and incubated at 37°C for 24 h. Following treatment with 10% CSE for 24 h, ASMCs were collected for analysis using the aforementioned assays.
Statistical analysis
Values are expressed as the mean ± standard deviation. Statistical analyses were performed using one-way analysis of variance (for multiple-group comparisons) or Student's t-test (for comparison between two groups). SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of CSE on ASMC proliferation
To investigate the effect of CSE on cell proliferation, ASMCs were treated with 0, 2.5, 5, 10, 15 or 20% CSE for 24, 48 or 72 h and subjected to the MTT colorimetric assay. As shown in Fig. 1, treatment with 2.5–10% CSE increased ASMC proliferation compared with the control in a dose-dependent manner, with the increase being significant with 10% CSE (P<0.05) compared with 0–5% CSE. By contrast, CSE at 15 and 20% significantly decreased ASMC proliferation compared with the control (P<0.05), which may have been due to the cytotoxic effect of high concentrations of CSE (Fig. 1).
Figure 1.
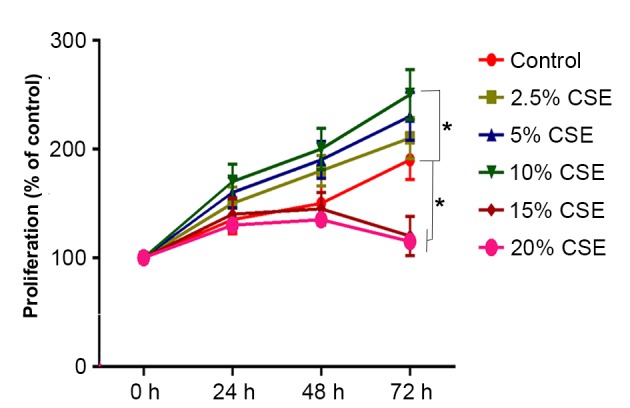
Effect of various concentrations of CSE on cell proliferation of human ASMCs. ASMCs were treated with various concentration of CSE (0, 2.5, 5, 10, 15 or 20%) for 24, 48 or 72 h. Cell proliferation was assessed using an MTT assay. Values are expressed as the mean ± standard deviation. *P<0.05 vs. Control. AMSC, airway smooth muscle cell; CSE, cigarette smoke extract.
Effects of CSE on expression of calreticulin and C/EBP-α
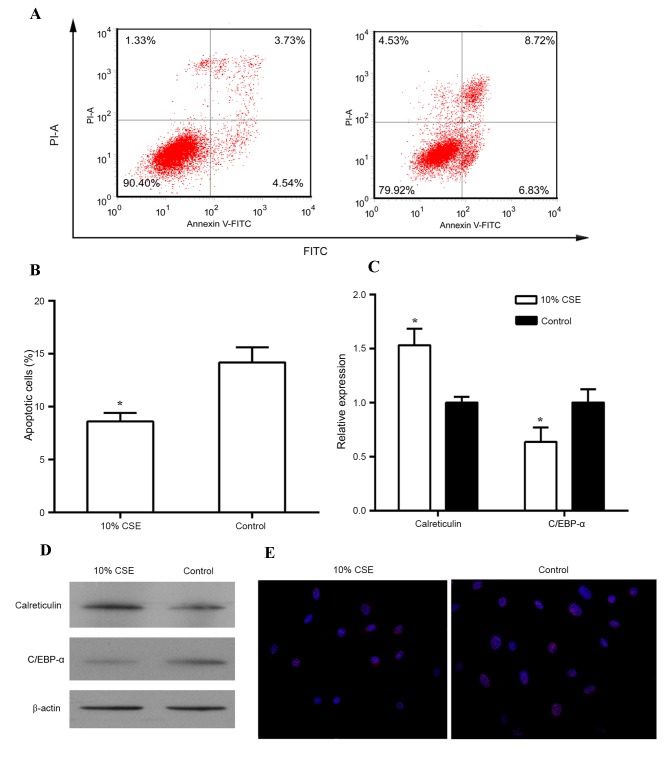
Since the stimulating effect of CSE on cell proliferation was greatest at the concentration of 10% of CSE, this concentration was used for the subsequent experiments. When stimulated with 10% CSE, the proliferation of human ASMCs was significantly increased from 24 h onwards as compared with that in the control group (P<0.05; Fig. 1B). In addition, treatment with 10% CSE reduced the apoptotic rate of human ASMCs in comparison to that of untreated cells (Fig. 2A and B). A previous study demonstrated that C/EBP-α and its regulator calreticulin are implicated in the control of cell proliferation (16). Thus, the expression of calreticulin and C/EBP-α was then examined in these cells treated with CSE. As shown in Fig. 2C and D, treatment of human ASMCs with 10% CSE resulted in an increase in mRNA and protein levels of calreticulin but caused a decrease in mRNA and protein levels of C/EBP-α as compared to those in the control cells (all P<0.05). In addition, immunostaining with antibodies against C/EBP-α revealed that 10% CSE suppressed the expression of C/EBP-α in the nuclei of human ASMCs (Fig. 2E).
Figure 2.
Effect of CSE on the expression of calreticulin and C/EBPα. (A) Proliferation of human ASMCs treated with 10% CSE for 24, 48 and 72 h was analyzed by an MTT assay. (A-E) Human ASMCs were treated with 10% CSE for 24 h. and cell apoptosis was analyzed using the FITC Annexin V/Dead Cell Apoptosis kit; (B) Bar chart showing the percentage of apoptotic cells with or without 10% CSE treatment for 24 h; (C) Reverse transcription-quantitative polymerase chain reaction and (D) western blots analyses were performed to detect mRNA and protein levels, respectively, of calreticulin and C/EBP-α in human ASMCs; (E) Immunostaining of ASMCs was performed using fluorescent-labeled antibodies against C/EBP-α (red); nuclei were counter-stained with DAPI (blue). Magnification, ×200). Values are expressed as the mean ± standard deviation. *P<0.05 vs. control. AMSC, airway smooth muscle cell; C/EBP-α, CCAAT/enhancer-binding protein alpha; CSE, cigarette smoke extract; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Knockdown of calreticulin suppresses the proliferation of human ASMCs
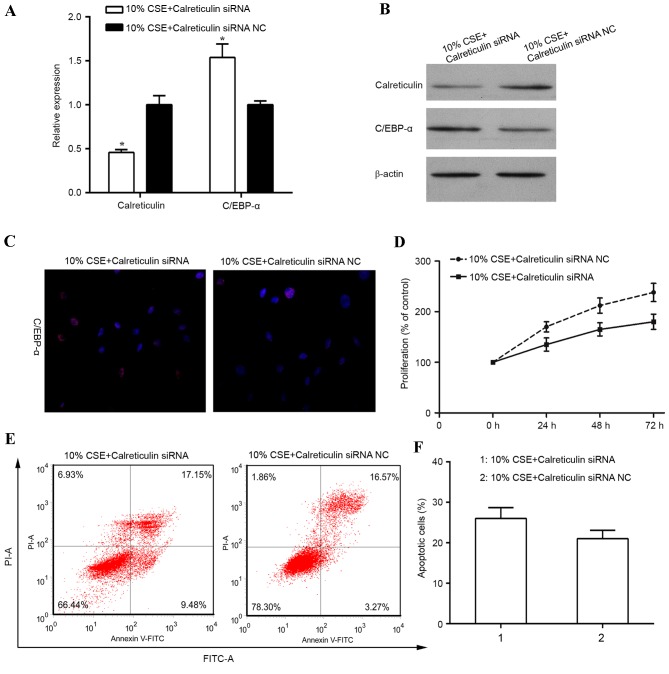
To investigate the role of calreticulin in the regulation of cell proliferation in human ASMCs treated with CSE, knockdown of calreticulin was performed and the effect on cell proliferation and apoptosis was examined. RT-qPCR and western blot analysis revealed the calreticulin knockdown efficiency (Fig. 3A and B). When ASMCs were transfected with calreticulin siRNA, the expression of calreticulin was significantly diminished at the mRNA level (P<0.05) and also suppressed at the protein level, compared with that in control siRNA-transfected ASMCs stimulated with 10% CSE. A previous study reported that calreticulin transcriptionally regulates C/EBP-α through a cis-regulatory CNG-rich loop in the mRNA of C/EBP-α (19). In the present study, knockdown of calreticulin resulted in an upregulation of the mRNA (P<0.05) and protein levels of C/EBP-α, and an increase in C/EBP-α expression in the nucleus in human ASMCs stimulated with 10% CSE (Fig. 3A-C). In addition, knockdown of calreticulin was found to suppress ASMC proliferation induced by 10% CSE (Fig. 3D). Furthermore, the percentage of apoptotic cells was increased in CSE-stimulated human ASMCs with calreticulin knockdown (Fig. 3E and F).
Figure 3.
Knockdown of calreticulin suppresses proliferation of human ASMCs. Human ASMCs were transfected with calreticulin siRNA or siRNA NC for 24 h and then stimulated with 10% of CSE for 24 h prior to analysis. (A) Reverse transcription -quantitative polymerase chain reaction and (B) western blot analyses were performed to determine the mRNA and protein levels of calreticulin and C/EBP-α in the ASMCs. (C) Immunostaining of human ASMCs was performed using antibody against C/EBP-α (red); nuclei were counter-stained with DAPI (blue). Magnification, ×200. (D) Cell proliferation was assessed by MTT assay. (E) Cell apoptosis was analyzed using the FITC Annexin V/Dead Cell Apoptosis kit. (F) Bar chart showing the percentage of apoptotic cells after 10% CSE treatment for 24 h. Values are expressed as the mean ± standard deviation. *P<0.05 vs. control. AMSC, airway smooth muscle cell; C/EBP-α, CCAAT/enhancer-binding protein alpha; CSE, cigarette smoke extract; siRNA, small interfering RNA; siRNA NC, scrambled control siRNA; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Simultaneous knockdown of calreticulin and C/EBP-α increases the proliferation of human ASMCs
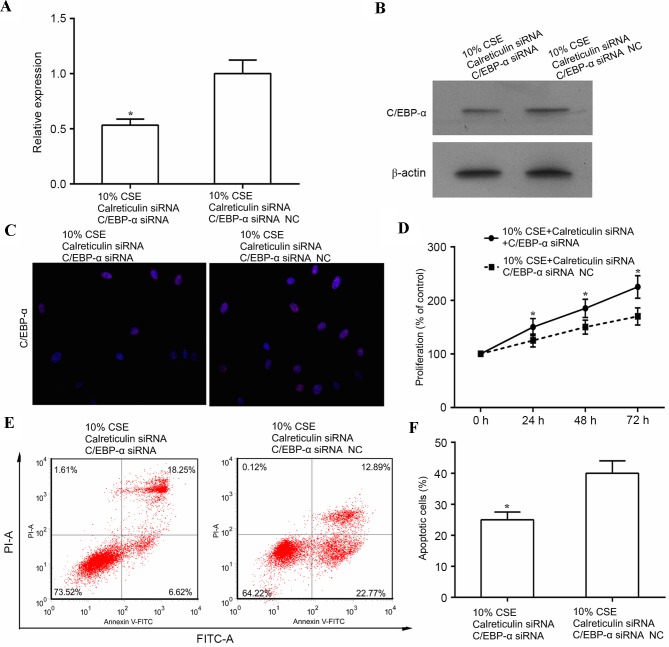
The aforementioned results indicated that CSE (10%) promoted cell proliferation, which was associated with increased expression of calreticulin and decreased expression of C/EBP-α. In addition, knockdown of calreticulin upregulated C/EBP-α and suppressed cell proliferation. This evidence led to the hypothesis that CSE promotes ASMC proliferation through suppressing of C/EBP-α via upregulation of calreticulin. To further test this, human ASMCs were co-transfected with calreticulin siRNA, and either C/EBP-α siRNA or control siRNA, and cell proliferation and apoptosis was examined in these cells following treatment with 10% CSE. As shown in Fig. 4A and B, the expression of C/EBP-α was significantly decreased at the mRNA level (P<0.05) and markedly suppressed at the protein level by simultaneous knockdown of calreticulin and C/EBP-α as compared with that in the control siRNA (knockdown of calreticulin only) in CSE-treated human ASMCs. In addition, double knockdown of calreticulin and C/EBP-α reduced the expression of C/EBP-α in the nuclei of ASMCs compared with that in ASMCs subjected to knockdown of calreticulin alone (Fig. 4C). Of note, double knockdown of calreticulin and C/EBP-α led to increased cell proliferation (P<0.05) and reduced apoptosis (P<0.05) in the calreticulin knockdown only group (Fig. 4D-F).
Figure 4.
Knockdown of C/EBP-α and calmodulin enhances ASMC proliferation. Human ASMCs were transfected with calreticulin siRNA together with either C/EBP-α siRNA or control siRNA for 24 h and then stimulated with 10% CSE for 24 h prior to the following analyses. (A) Reverse transcription-quantitative polymerase chain reaction and (B) western blot analyses were performed to determine mRNA and protein levels of C/EBP-α in the ASMCs. (C) Immunostaining of human ASMCs was performed using antibody against C/EBP-α (red); nuclei were counter-stained with DAPI (blue). Magnification, ×200. (D) Cell proliferation was analyzed by MTT assay. (E) Cell apoptosis was analyzed using the FITC Annexin V/Dead Cell Apoptosis kit. (F) Bar chart showing the percentage of apoptotic cells after 10% CSE treatment for 24 h. *P<0.05 vs. control. AMSC, airway smooth muscle cell; C/EBPα, CCAAT/enhancer-binding protein α; CSE, cigarette smoke extract; siRNA, small interfering RNA; siRNA NC, scrambled control siRNA; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Discussion
Cigarette smoke has been considered a major factor in the pathogenesis of COPD, which causes inflammatory injury (20,21). The present study showed that CSE promoted ASMC proliferation, which is a key factor of airway remodeling in COPD (3,4,22). The effect of CSE at 0–10% on ASMC proliferation was concentration-dependent; however, CSE at >10% had a cytotoxic effect. This observation is in agreement with that of previous studies reporting that CSE caused necrosis of neonatal vascular SMCs and this toxicity was mainly mediated by volatile components such as acrolein and acetaldehyde, possibly in association with nitric oxide and carbon monoxide (23). In the present study, C/EBP-α was identified as a molecular target of CSE. Treatment with CSE significantly decreased C/EBP-α protein levels, particularly the biologically reactive form, which resides in the nucleus. These findings were consistent with those of other studies suggesting that CSE promoted ASMC proliferation (5,6,24) and inhibited C/EBP-α (9,10). Additionally, a previous study indicated that CSE can significantly induce proliferation, with C/EBP-α and C/EBP-β proteins upregulated simultaneously (25). However, to the best of our knowledge, the present study was the first to propose that CSE stimulated ASMC proliferation through C/EBP-α.
The present study further investigated the upstream signaling of C/EBP-α. C/EBP-α can be detected in the liver, adipose tissue, intestine, lung, adrenal gland as well as myeloid and placental cells. Studies on BSMCs and abiogenesis revealed that C/EBP-α is regulated by calreticulin (12,26). Thus, in the present system, calreticulin levels were assessed after CSE treatment its association with C/EBP-α was assessed. Following treatment with 10% CSE calreticulin was significantly increased at the RNA and protein level. Induction of calreticulin was inversely correlated with C/EBP-α. Thus, the present study hypothesized that calreticulin, which was upregulated by CSE, functioned as a negative regulator of C/EBP-α. To verify this hypothesis, siRNA-mediated knockdown of calreticulin and C/EBP-α was performed. Cells' proliferation capacity impaired by calreticulin knockdown was restored by simultaneous knockdown of C/EBP-α. These results further supported the finding that CSE promotes ASMC proliferation through inhibition of C/EBP-α. By contrast, knockdown of calreticulin only suppressed ASMC proliferation. Furthermore, cells with calreticulin knockdown had significant higher C/EBP-α mRNA and protein levels. This result provided evidence that calreticulin functions as negative regulator of C/EBP-α.
The results of the present study revealed that the induction of C/EBP-α is more profound at the protein level than at the RNA level. This may be explained by the mechanism of regulation, as C/EBP-α is predominantly regulated at the translational level. A previous study reported that calreticulin transcriptionally regulated C/EBP-α through a cis-regulatory CNG-rich loop in the mRNA of C/EBP-α (16). Calreticulin binds to a stem loop within the C/EBP-α mRNA, which is formed by internal base-pairing of the (GC)n repeat motif. When calreticulin is bound to this loop, translation of the full-length C/EBP-α (p42) is inhibited.
It has been demonstrated that intervention with anisodamine, an antagonist of muscarinic acetylcholine receptors, via increasing the expression of cyclin D1, prevents smoking-induced ASMC proliferation, exerting a protective and reversing effect regarding CSE-induced changes (27,28). It has been suggested that inhibition of four and a half LIM domains protein 1 limited the CSE-induced proliferation of pulmonary arterial SMCs and may represent a potential therapeutic target for pulmonary hypertension (19). Furthermore, the results of the present study revealed that CSE promoted the proliferation of ASMCs via induction of calreticulin, which inhibits the expression of C/EBP-α. This result provided the molecular link for airway remodeling in COPD and may provide insight into the molecular pathogenetic mechanism as well as possible therapeutic approaches with calreticulin or C/EBP-α as key targets.
Acknowledgements
The present study was supported by the Natural Science Foundation of Hainan Province (grant no. 807081).
References
- 1.Gorska K, Krenke R, Kosciuch J, Korczynski P, Zukowska M, Domagala-Kulawik J, Maskey-Warzechowska M, Chazan R. Relationship between airway inflammation and remodeling in patients with asthma and chronic obstructive pulmonary disease. Eur J Med Res. 2009;14(Suppl 4):S90–S96. doi: 10.1186/2047-783X-14-S4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yawn BP. Differential assessment and management of asthma vs chronic obstructive pulmonary disease. Medscape J Med. 2009;11:20. [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 4.Pini L, Pinelli V, Modina D, Bezzi M, Tiberio L, Tantucci C. Central airways remodeling in COPD patients. Int J Chron Obstruct Pulmon Dis. 2014;9:927–932. doi: 10.2147/COPD.S52478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pera T, Gosens R, Lesterhuis AH, Sami R, van der Toorn M, Zaagsma J, Meurs H. Cigarette smoke and lipopolysaccharide induce a proliferative airway smooth muscle phenotype. Respir Res. 2010;11:48. doi: 10.1186/1465-9921-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He F, Li B, Zhao Z, Zhou Y, Hu G, Zou W, Hong W, Zou Y, Jiang C, Zhao D, Ran P. The pro-proliferative effects of nicotine and its underlying mechanism on rat airway smooth muscle cells. PLoS One. 2014;9:e93508. doi: 10.1371/journal.pone.0093508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Wu YN, Zhang W, Zhang XM, Ding X, Li HQ, Geng M, Xie ZQ, Wu HM. Monocarboxylate transporter 4 facilitates cell proliferation and migration and is associated with poor prognosis in oral squamous cell carcinoma patients. PLoS One. 2014;9:e87904. doi: 10.1371/journal.pone.0087904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seow CY, Schellenberg RR, Paré PD. Structural and functional changes in the airway smooth muscle of asthmatic subjects. Am J Respir Crit Care Med. 1998;158:S179–S186. doi: 10.1164/ajrccm.158.supplement_2.13tac160. [DOI] [PubMed] [Google Scholar]
- 9.Didon L, Roos AB, Elmberger GP, Gonzalez FJ, Nord M. Lung-specific inactivation of CCAAT/enhancer binding protein alpha causes a pathological pattern characteristic of COPD. Eur Respir J. 2010;35:186–197. doi: 10.1183/09031936.00185008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth M, Johnson PR, Borger P, Bihl MP, Rüdiger JJ, King GG, Ge Q, Hostettler K, Burgess JK, Black JL, Tamm M. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med. 2004;351:560–574. doi: 10.1056/NEJMoa021660. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ, Timchenko NA. C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell. 2001;8:817–828. doi: 10.1016/S1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- 12.Miglino N, Roth M, Lardinois D, Tamm M, Borger P. Calreticulin is a negative regulator of bronchial smooth muscle cell proliferation. J Allergy (Cairo) 2012;2012:783290. doi: 10.1155/2012/783290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol. 2012;44:842–846. doi: 10.1016/j.biocel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Chiang WF, Hwang TZ, Hour TC, Wang LH, Chiu CC, Chen HR, Wu YJ, Wang CC, Wang LF, Chien CY, et al. Calreticulin, an endoplasmic reticulum-resident protein, is highly expressed and essential for cell proliferation and migration in oral squamous cell carcinoma. Oral Oncol. 2013;49:534–541. doi: 10.1016/j.oraloncology.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Lu YC, Weng WC, Lee H. Functional roles of calreticulin in cancer biology. Biomed Res Int. 2015;2015:526524. doi: 10.1155/2015/526524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timchenko LT, Iakova P, Welm AL, Cai ZJ, Timchenko NA. Calreticulin interacts with C/EBPalpha and C/EBPbeta mRNAs and represses translation of C/EBP proteins. Mol Cell Biol. 2002;22:7242–7257. doi: 10.1128/MCB.22.20.7242-7257.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pera T, Atmaj C, van der Vegt M, Halayko AJ, Zaagsma J, Meurs H. Role for TAK1 in cigarette smoke-induced proinflammatory signaling and IL-8 release by human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L272–L278. doi: 10.1152/ajplung.00291.2011. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Pu G, Chen C, Yang L. Inhibition of FHL1 inhibits cigarette smoke extract-induced proliferation in pulmonary arterial smooth muscle cells. Mol Med Rep. 2015;12:3801–3808. doi: 10.3892/mmr.2015.3787. [DOI] [PubMed] [Google Scholar]
- 20.Mortaz E, Kraneveld AD, Smit JJ, Kool M, Lambrecht BN, Kunkel SL, Lukacs NW, Nijkamp FP, Folkerts G. Effect of cigarette smoke extract on dendritic cells and their impact on T-cell proliferation. PLoS One. 2009;4:e4946. doi: 10.1371/journal.pone.0004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu XJ, Luo GX, Zeng X, Lan LL, Ning Q, Xu YJ, Zhao JP, Xie JG. Genotoxicity and reduced heat shock protein 70 in human airway smooth muscle cells exposed to cigarette smoke extract. J Huazhong Univ Sci Technolog Med Sci. 2013;33:827–833. doi: 10.1007/s11596-013-1206-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen QW, Edvinsson L, Xu CB. Cigarette smoke extract promotes human vascular smooth muscle cell proliferation and survival through ERK1/2- and NF-κB-dependent pathways. ScientificWorldJournal. 2010;10:2139–2156. doi: 10.1100/tsw.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambalavanan N, Carlo WF, Bulger A, Shi J, Philips JB., III Effect of cigarette smoke extract on neonatal porcine vascular smooth muscle cells. Toxicol Appl Pharmacol. 2001;170:130–136. doi: 10.1006/taap.2000.9094. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XY, Xu YJ, Liu XS, Zhang ZX. Cigarette smoke extract promotes proliferation of airway smooth muscle cells in asthmatic rats via regulating cyclin D1 expression. Chin Med J (Engl) 2010;123:1709–1714. [PubMed] [Google Scholar]
- 25.Helbling D, Mueller BU, Timchenko NA, Schardt J, Eyer M, Betts DR, Jotterand M, Meyer-Monard S, Fey MF, Pabst T. CBFB-SMMHC is correlated with increased calreticulin expression and suppresses the granulocytic differentiation factor CEBPA in AML with inv (16) Blood. 2005;106:1369–1375. doi: 10.1182/blood-2004-11-4392. [DOI] [PubMed] [Google Scholar]
- 26.Miglino N, Roth M, Lardinois D, Sadowski C, Tamm P, Borger P. Cigarette smoke inhibits lung fibroblast proliferation by translational mechanisms. Eur Respir J. 2012;39:705–711. doi: 10.1183/09031936.00174310. [DOI] [PubMed] [Google Scholar]
- 27.Xu GN, Yang K, Xu ZP, Zhu L, Hou LN, Qi H, Chen HZ, Cui YY. Protective effects of anisodamine on cigarette smoke extract-induced airway smooth muscle cell proliferation and tracheal contractility. Toxicol Appl Pharmacol. 2012;262:70–79. doi: 10.1016/j.taap.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Zeng DX, Xu YJ, Liu XS, Wang R, Xiang M. Cigarette smoke extract induced rat pulmonary artery smooth muscle cells proliferation via PKCα-mediated cyclin D1 expression. J Cell Biochem. 2011;112:2082–2088. doi: 10.1002/jcb.23131. [DOI] [PubMed] [Google Scholar]