Abstract
We report, for the first time, a multimodal, oxidation-responsive contrast agent for magnetic resonance imaging and photoacoustic imaging that uses the differences in the properties between Eu in the +2 and +3 oxidation states. The enhancement of contrast in T1-weighted magnetic resonance and photoacoustic imaging was observed in the +2 but not in the +3 oxidation state, and the complex is a known chemical exchange saturation transfer agent for magnetic resonance imaging in the +3 oxidation state.
Introduction
Perturbation of redox homeostasis has been correlated with pancreatic, colorectal, prostate, and lung cancers;1−9 neurodegenerative diseases;10−14 colitis and inflammatory bowel disease;15,16 and liver diseases.17,18 Because changes in redox environments are associated with many diseases, there is value in detecting these changes. Multimodal imaging agents that combine magnetic resonance imaging (MRI) and photoacoustic imaging are capable of producing images that provide molecular information,19−42 which is of potential utility in the imaging of oxidative stress. MRI and photoacoustic imaging have complementary properties: MRI enables scans over large volumes with practically unlimited depth penetration, and photoacoustic imaging can produce detailed, real-time images of areas that are accessible by sources of light.43 Orthogonal imaging techniques that use multimodal imaging agents are potentially useful with respect to validation and the avoidance of misinterpretation of data from a single imaging technique. However, reported multimodal MRI and photoacoustic contrast agents do not report changes in redox environments. EuII-containing complexes have been reported as oxidation-responsive contrast agents for MRI that shorten the T1 relaxation times of protons on exchanging water,44−49 and we hypothesized that a subset of these complexes that change absorbance in the visible region of the electromagnetic spectrum as a function of oxidation state45,49,50 could serve as oxidation-responsive, multimodal contrast agents for magnetic resonance imaging and photoacoustic imaging. Here, we report the photoacoustic properties of Eu-containing species 1 and 2 (Figure 1) that are contrast agents for T1-weighted and chemical exchange saturation transfer MRI, respectively.45,51−65
Figure 1.
(Top) EuII-containing complex 1 and EuIII-containing complex 2. Counterions are not shown for clarity. (Middle) Normalized absorbance spectra of 1 (••) and 2 (—). (Bottom) Absorbance at 410 nm vs concentrations of 1 and 2. The slopes of the lines of best fit are molar absorptivities (M–1 cm–1).
Eu-containing complexes have different magnetic and absorptive properties depending on the ligand and the oxidation state of Eu: EuII shortens T1 relaxation times, and some complexes of EuII absorb visible light; EuIII does not measurably shorten T1 relaxation times, and many complexes of EuIII do not absorb visible light. The influence of the oxidation state of Eu and ligand structure on T1-weighted MRI is well-documented,44−49,66−72 and this influence on MRI together with the ability to shift absorbance into the visible range of the electromagnetic spectrum45,49,50 suggested a potential use in responsive photoacoustic imaging. Photoacoustic imaging relies on the photoacoustic effect that is initiated by the absorption of visible light and detected via nonradiative decay in the form of acoustic waves using an ultrasound detector. As potential responsive contrast agents for MRI and photoacoustic imaging, we chose to study complexes 1 and 2 because of the visible-light-absorbing properties of 1 (Figure 1) and the contrast-enhancement properties in MRI documented with 1 and 2.45,51−65
Results and Discussion
The pair of complexes 1 and 2 has been previously reported as an oxidation-responsive contrast agent for MRI.45 Complex 1 has a relaxivity of 3.73 ± 0.04 mM–1 s–1, and complex 2 has a relaxivity of zero but acts as a contrast agent for chemical exchange saturation transfer MRI.45,51−65 This difference in relaxivity is amenable to the visualization of oxidation from EuII to EuIII. Neither complex has been characterized with respect to photoacoustic imaging.
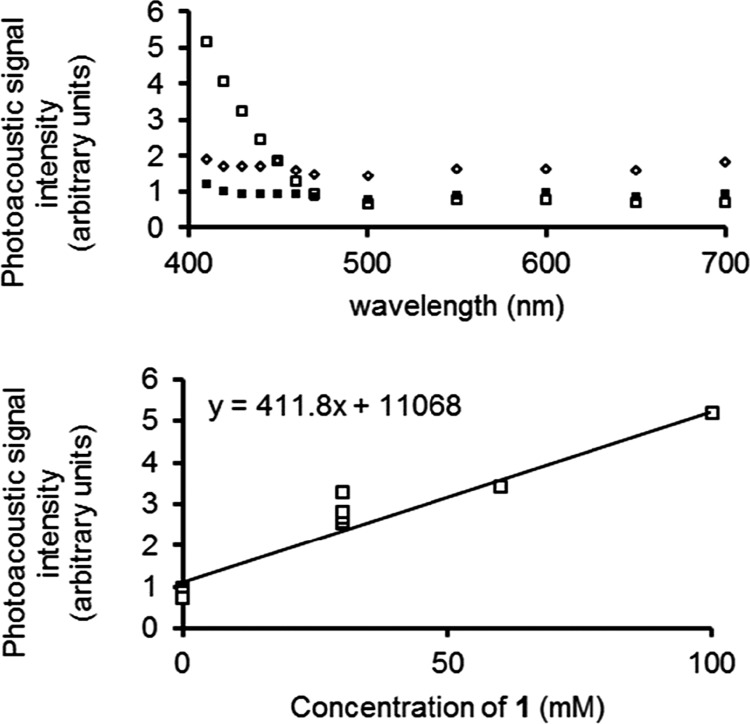
To study the optical absorption properties relevant to photoacoustic imaging, molar absorptivity values were calculated. The molar absorptivity at 410 nm was calculated because this wavelength is near the wavelength where the EuII-containing complexes in this study has the highest absorption on the photoacoustic imaging system used (Figure 1). To determine the molar absorptivity at 410 nm, UV–visible spectra were measured before and after oxidation, and molar absorptivity values were calculated from the spectra. The molar absorptivity of EuII-containing complex 1 was 3.0 × 102 mM–1 cm–1, and there were no absorptions above the noise for EuIII-containing species 2 at 410 nm (molar absorptivity of 0 mM–1 cm–1); therefore, the difference in molar absorptivity between the oxidation states of Eu in complexes 1 and 2 was stark. Because photoacoustic imaging agents rely on the absorption of visible light, we expected that complex 1 but not complex 2 would act as a contrast agent for photoacoustic imaging.
To determine whether measurable photoacoustic responses could be observed that changed with the oxidation state of Eu, we measured the photoacoustic response (25 °C, 14.5 mJ cm−2 at 410 nm) of 1 and 2 from 410 to 700 nm. Glass tubes with thin walls (0.35 mm) were filled with solutions of complex 1 under an inert atmosphere, and the same samples were exposed to air to yield 2. The samples were placed in a bath of distilled water and irradiated with light, and the photoacoustic signal amplitude was recorded (Figure 2). The data revealed that complex 1 had a photoacoustic response at 410 nm, which was 4.3× higher than oxidized complex 2. To verify that the signal observed was different from the blank (phosphate-buffered saline), we calculated the minimum detectable concentration of 1. First, the photoacoustic signal amplitude was plotted as a function of concentration for 1 (Figure 2), and then the photoacoustic intensities of the lowest point of 2 (30 mM) and the blank were each measured using seven independently prepared samples. The minimum detectable concentration was calculated to be 3σ/m,73 where σ is the standard deviation of the seven repeated measurements at 30 mM and m is the slope of the best fit line in Figure 2. The minimum detectable concentration for 1 was determined to be 15 mM. To place this value in context, another discrete molecule has been imaged with photoacoustic imaging in mice with an injection concentration of 33.3 mM.74 The photoacoustic response data demonstrate that the Eu-containing species studied show detectable photoacoustic response in the reduced form (+2 oxidation state of Eu) and do not show photoacoustic response in the oxidized form (+3 oxidation state of Eu).
Figure 2.
Photoacoustic signal amplitude as a function of wavelength for 1 (□), 2 (■), and phosphate-buffered saline (pH 7.4) (◇); (bottom) photoacoustic intensity vs concentration of 1.
To visualize the photoacoustic response before and after oxidation, samples of 1 and 2 were imaged with photoacoustic imaging (410 nm, 25 °C, Figure 3). Images of 1 and 2 were collected at 30, 60, and 100 mM because these were the concentrations used in calculating the minimum detectable concentration (Figure 2). The photoacoustic images of 1 appeared bright relative to those of 2, as expected based on the photoacoustic signal amplitudes in Figure 2. Furthermore, T1-weighted images showed positive contrast enhancement for complex 1 that contained EuII, and no visible contrast enhancement for samples of 2 that contained EuIII (Figure 3), as expected based on reported images for lower concentrations.52−55,57,61,62 EuII-containing complex 1 showed visible photoacoustic responses in addition to contrast enhancement in T1-weighted MRI. These imaging properties ceased upon oxidation to EuIII-containing complex 2, which is an agent for chemical exchange saturation transfer MRI.45,51−65
Figure 3.
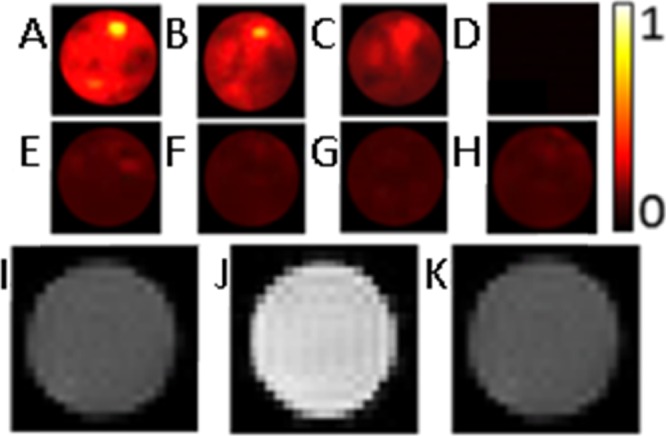
Photoacoustic images of (A) 1 (100 mM in phosphate-buffered saline, pH 7.4); (B) 1 (60 mM in phosphate-buffered saline, pH 7.4); (C) 1 (30 mM in phosphate-buffered saline, pH 7.4); (D) optical glass tube with a wall thickness of 0.35 mm in a bath of distilled water, demonstrating an absence of scattering due to the glass tube; (E) 2 (100 mM in phosphate-buffered saline, pH 7.4); (F) 2 (60 mM in phosphate-buffered saline, pH 7.4); (G) 2 (30 mM in phosphate-buffered saline, pH 7.4); and (H) phosphate-buffered saline (pH 7.4). The color map shows the normalized intensity for photoacoustic images; and T1-weighted images of (I) phosphate-buffered saline (pH 7.4); (J) 1 (30 mM in phosphate-buffered saline, pH 7.4); and (K) 2 (30 mM in phosphate-buffered saline, pH 7.4). The samples for MRI and photoacoustic imaging were in tubes with diameters of 3 and 5 mm, respectively.
In conclusion, we demonstrated, for the first time, an example of an oxidation-responsive, multimodal contrast agent for MRI and photoacoustic imaging. Limitations of the proof-of-concept relative to the reported multimodal, photoacoustic imaging agents, which are capable of picomolar limits of detection, use near-IR light, or both,19−32 include the relatively high detection limit and use of relatively high energy visible light in our system. We are currently pursuing ways to decrease the limit of detection and red-shift the wavelength of Eu-based systems by forming aggregates of the Eu-containing complexes in solution and changing the ligand field to shift absorbance of the EuII-containing species. These findings are a step toward multimodal sensing of redox environments using photoacoustic imaging.
Experimental Procedures
Commercially available chemicals were of reagent-grade purity or better and were used without further purification unless otherwise noted. Water was purified using a PURELAB Ultra Mk2 water purification system (ELGA). Phosphate-buffered saline (10×) was purchased from Fisher BioReagents. EuIII–DOTA-4AmC [DOTA-4AmC = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis-(acetamidoacetate)] (2) was purchased from Macrocyclics.
Samples of 1 were prepared in a wet glove box (water was allowed but not molecular oxygen) under an atmosphere of N2. Complex 1 was prepared following a published procedure.45 UV–visible spectra were recorded using an Ocean Optics spectrophotometer (STS-UV-L-25-400-SMA) coupled to a high-power DH-MINI deuterium tungsten halogen source (Ocean Optics) or on a Shimadzu UVmini-1240 spectrophotometer. Extinction coefficients were calculated by plotting absorbance intensity at 410 nm as a function of concentration of 1 or 2. The UV–visible spectra of samples of 1 were measured under an atmosphere of N2 in a wet glove box or in airtight cuvettes sealed with paraffin wax inside of the glove box and then removed from the glove box and measured within an hour of taking the samples out of the glove box.
Photoacoustic imaging was performed in the Department of Biomedical Engineering at Wayne State University with a photoacoustic imaging system consisting of a programmable ultrasound scanner (Verasonics Vantage) equipped with a linear array transducer (L114-v), a 30 Hz pulsed and tunable Nd:YAG pump/OPO laser (Spectra Physics PRO270/Verascan), and a field-programmable gate array (FPGA) control system. The images and amplitudes were acquired at 25 °C in a bath of distilled water. The samples of 1 (100, 60, and 30 mM) and 2 (100, 60, and 30 mM) were in 1× phosphate-buffered saline (pH 7.4). The solutions were imaged in optical glass tubes with a wall thickness of 0.35 mm. These tubes showed no observable signal in the absence of Eu-containing samples (see Figure 3). For samples of 1, the optical glass tubes were plugged with silica glue, waxed, and imaged within 1 h of preparation.
MRI was performed using a Bruker ClinScan small animal scanner (300.43 MHz, 7.06 T) equipped with a 30 cm bore magnet. The samples of 1 (30 mM) and 2 (30 mM) were in 1× phosphate-buffered saline (pH 7.4) under an atmosphere of N2, sealed with a cap and then paraffin wax, and measured within an hour of taking the samples out of the glove box. The T1-weighted images were acquired at ambient temperature (21 °C) with an echo time of 3.26 ms, a repetition time of 21 ms, flip angles of 5°, 10°, 20°, 30°, 40°, 50°, and 60°, 16 slices at 2 mm thickness, a field of view of 35 × 70 mm2, and a matrix size of 128 × 256.
Concentrations of Eu were determined using inductively coupled plasma mass spectrometry (ICP-MS) or energy-dispersive X-ray fluorescence (EDXF) spectroscopy. ICP-MS measurements were acquired on an Agilent Technologies 7700 series ICP-MS instrument in the Lumigen Instrument Center in the Department of Chemistry at Wayne State University. All dilutions were performed with 2% aqueous HNO3, which was also used for blank samples during calibration. The calibration curves were created using the 153Eu isotope ion count for a 1–200 ppb concentration range (diluted from Fluka ICP standard solution, 100 mg of Eu L–1). EDXF measurements were recorded using a Shimadzu EDX-7000 spectrometer (Shimadzu Scientific Instruments) in the Lumigen Instrument Center in the Department of Chemistry at Wayne State University. The calibration curves were created using the Eu fluorescence intensity at 5.845 keV for a 25–100 ppm concentration range (diluted from Fluka ICP standard solution, 100 mg of Eu L–1).
Acknowledgments
The authors acknowledge Maryam Basij for assistance with photoacoustic image processing. We acknowledge research support from the National Institutes of Health grant EB013663 (M.J.A.).
The authors declare no competing financial interest.
References
- Facciabene A.; Peng X.; Hagemann I. S.; Balint K.; Barchetti A.; Wang L.-P.; Gimotty P. A.; Gilks C. B.; Lal P.; Zhang L.; Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011, 475, 226–230. 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- Ishikawa K.; Takenaga K.; Akimoto M.; Koshikawa N.; Yamaguchi A.; Imanishi H.; Nakada K.; Honma Y.; Hayashi J.-I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science 2008, 320, 661–664. 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Shweiki D.; Itin A.; Soffer D.; Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Chandel N. S.; Heiden M. G. V.; Thompson C. B.; Schumacker P. T. Redox regulation of p53 during hypoxia. Oncogene 2000, 19, 3840–3848. 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M.; Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakura N.; Kobayashi M.; Horiuchi I.; Suzuki A.; Wang J.; Chen J.; Niizeki H.; Kawamura K.-i.; Hosokawa M.; Asaka M. Constitutive Expression of Hypoxia-Inducible Factor-1α Renders Pancreatic Cancer Cells Resistant to Apoptosis Induced by Hypoxia and Nutrient Deprivation. Cancer Res. 2001, 61, 6548–6554. [PubMed] [Google Scholar]
- Koukourakis M. I.; Giatromanolaki A.; Sivridis E.; Pitiakoudis M.; Gatter K. C.; Harris A. L. Beclin 1 over- and underexpression in colorectal cancer: Distinct patterns relate to prognosis and tumour hypoxia. Br. J. Cancer 2010, 103, 1209–1214. 10.1038/sj.bjc.6605904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H.; Agani F.; Baccala A. A.; Laughner E.; Rioseco-Camacho N.; Isaacs W. B.; Simons J. W.; Semenza G. L. Increased Expression of Hypoxia Inducible Factor-1α in Rat and Human Prostate Cancer. Cancer Res. 1998, 58, 5280–5284. [PubMed] [Google Scholar]
- Giatromanolaki A.; Koukourakis M. I.; Sivridis E.; Pastorek J.; Wykoff C. C.; Gatter K. C.; Harris A. L. Expression of Hypoxia-Inducible Carbonic Anhydrase-9 Relates to Angiogenic Pathways and Independently to Poor Outcome in Non-Small Cell Lung Cancer. Cancer Res. 2001, 61, 7992–7998. [PubMed] [Google Scholar]
- Park L.; Zhou P.; Pitstick R.; Capone C.; Anrather J.; Norris E. H.; Younkin L.; Younkin S.; Carlson G.; McEwen B. S.; Iadecola C. Nox2-Derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 1347–1352. 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. T.; Beal M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Nunomura A.; Tamaoki T.; Motohashi N.; Nakamura M.; McKeel D. W.; Tabaton M. Jr.; Lee H.-g.; Smith M. A.; Perry G.; Zhu X. The Earliest Stage of Cognitive Impairment in Transition from Normal Aging to Alzheimer Disease is Marked by Prominent RNA Oxidation in Vulnerable Neurons. J. Neuropathol. Exp. Neurol. 2012, 71, 233–241. 10.1097/nen.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham K. J.; Masters C. L.; Bush A. I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discovery 2004, 3, 205–214. 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Eltzschig H. K.; Carmeliet P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhausen J.; Furuta G. T.; Tomaszewski J. E.; Johnson R. S.; Colgan S. P.; Haase V. H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 2004, 114, 1098–1106. 10.1172/jci200421086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. M. Jr.; Thomes P. G. Ethanol-induced oxidant stress modulates hepatic autophagy and proteasome activity. Redox Biol. 2014, 3, 29–39. 10.1016/j.redox.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P.; Lindor K. D. Non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002, 17, S186–S190. 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- Bouchard L.-S.; Anwar M. S.; Liu G. L.; Hann B.; Xie Z. H.; Gray J. W.; Wang X.; Pines A.; Chen F. F. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 4085–4089. 10.1073/pnas.0813019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Li Y.; Li X.; Jing L.; Deng Z.; Yue X.; Li C.; Dai Z. PEGylated Polypyrrole Nanoparticles Conjugating Gadolinium Chelates for Dual-Modal MRI/Photoacoustic Imaging Guided Photothermal Therapy of Cancer. Adv. Funct. Mater. 2015, 25, 1451–1462. 10.1002/adfm.201402338. [DOI] [Google Scholar]
- Yang H.-W.; Liu H.-L.; Li M.-L.; Hsi I.-W.; Fan C.-T.; Huang C.-Y.; Lu Y.-J.; Hua M.-Y.; Chou H.-Y.; Liaw J.-W.; Ma C.-C. M.; Wei K.-C. Magnetic gold-nanorod/PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials 2013, 34, 5651–5660. 10.1016/j.biomaterials.2013.03.085. [DOI] [PubMed] [Google Scholar]
- Yu J.; Yang C.; Li J.; Ding Y.; Zhang L.; Yousaf M. Z.; Lin J.; Pang R.; Wei L.; Xu L.; Sheng F.; Li C.; Li G.; Zhao L.; Hou Y. Multifunctional Fe5C2 Nanoparticles: A Targeted Theranostic Platform for Magnetic Resonance Imaging and Photoacoustic Tomography-Guided Photothermal Therapy. Adv. Mater. 2014, 26, 4114–4120. 10.1002/adma.201305811. [DOI] [PubMed] [Google Scholar]
- Mou J.; Liu C.; Li P.; Chen Y.; Xu H.; Wei C.; Song L.; Shi J.; Chen H. A facile synthesis of versatile Cu2–xS nanoprobe for enhanced MRI and infrared thermal/photoacoustic multimodal imaging. Biomaterials 2015, 57, 12–21. 10.1016/j.biomaterials.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Gao D.; Sheng Z.; Liu Y.; Hu D.; Zhang J.; Zhang X.; Zheng H.; Yuan Z. Protein-Modified CuS Nanotriangles: A Potential Multimodal Nanoplatform for in Vivo Tumor Photoacoustic/Magnetic Resonance Dual-Modal Imaging. Adv. Healthcare Mater. 2017, 6, 1601094. 10.1002/adhm.201601094. [DOI] [PubMed] [Google Scholar]
- Guo T.; Lin Y.; Li Z.; Chen S.; Huang G.; Lin H.; Wang J.; Liu G.; Yang H.-H. Gadolinium oxysulfide-coated gold nanorods with improved stability and dual-modal magnetic resonance/photoacoustic imaging contrast enhancement for cancer theranostics. Nanoscale 2017, 9, 56–61. 10.1039/c6nr08281e. [DOI] [PubMed] [Google Scholar]
- An Q.; Liu J.; Yu M.; Wan J.; Li D.; Wang C.; Chen C.; Guo J. Multifunctional Magnetic Gd3+-Based Coordination Polymer Nanoparticles: Combination of Magnetic Resonance and Multispectral Optoacoustic Detections for Tumor-Targeted Imaging in Vivo. Small 2015, 11, 5675–5686. 10.1002/smll.201501491. [DOI] [PubMed] [Google Scholar]
- Yu J.; Yin W.; Zheng X.; Tian G.; Zhang X.; Bao T.; Dong X.; Wang Z.; Gu Z.; Ma X.; Zhao Y. Smart MoS2/Fe3O4 Nanotheranostic for Magnetically Targeted Photothermal Therapy Guided by Magnetic Resonance/Photoacoustic Imaging. Theranostics 2015, 5, 931–945. 10.7150/thno.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Jing L.; Peng D.; Li Y.; Tian J.; Dai Z. Manganese(II) Chelate Functionalized Copper Sulfide Nanoparticles for Efficient Magnetic Resonance/Photoacoustic Dual-Modal Imaging Guided Photothermal Therapy. Theranostics 2015, 5, 1144–1153. 10.7150/thno.11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L.-Y.; Yang X.-Q.; An J.; Zhang L.; Zhao K.; Qin M.-Y.; Fang B.-Y.; Li C.; Xuan Y.; Zhang X.-S.; Zhao Y.-D.; Ma Z.-Y. Multifunctional magnetic-hollow gold nanospheres for bimodal cancer cell imaging and photothermal therapy. Nanotechnology 2015, 26, 315701–315713. 10.1088/0957-4484/26/31/315701. [DOI] [PubMed] [Google Scholar]
- Song X.-R.; Wang X.; Yu S.-X.; Cao J.; Li S.-H.; Li J.; Liu G.; Yang H.-H.; Chen X. Co9Se8 nanoplates as a new theranostic platform for photoacoustic/magnetic resonance dual-modal-imaging-guided chemo-photothermal combination therapy. Adv. Mater. 2015, 27, 3285–3291. 10.1002/adma.201405634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D.; Liu C.; Song L.; Cui H.; Gao G.; Liu P.; Sheng Z.; Cai L. Indocyanine green-loaded polydopamine–iron ions coordination nanoparticles for photoacoustic/magnetic resonance dual-modal imaging-guided cancer photothermal therapy. Nanoscale 2016, 8, 17150–17158. 10.1039/c6nr05502h. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Kang N.; Lv J.; Zhou Z.; Zhao Q.; Ma L.; Chen Z.; Ren L.; Nie L. Deep Photoacoustic/Luminescence/Magnetic Resonance Multimodal Imaging in Living Subjects Using High-Efficiency Upconversion Nanocomposites. Adv. Mater. 2016, 28, 6411–6419. 10.1002/adma.201506460. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Dong Y.; Huang L.; Yang S.; Chen W. Study of cerebrovascular reserve capacity by magnetic resonance perfusion weighted imaging and photoacoustic imaging. Magn. Reson. Imaging 2009, 27, 155–162. 10.1016/j.mri.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Qin C.; Cheng K.; Chen K.; Hu X.; Liu Y.; Lan X.; Zhang Y.; Liu H.; Xu Y.; Bu L.; Su X.; Zhu X.; Meng S.; Cheng Z. Tyrosinase as a multifunctional reporter gene for photoacoustic/MRI/PET triple modality molecular imaging. Sci. Rep. 2013, 3, 1490–1497. 10.1038/srep01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M. F.; de la Zerda A.; Jokerst J. V.; Zavaleta C. L.; Kempen P. J.; Mittra E.; Pitter K.; Huang R.; Campos C.; Habte F.; Sinclair R.; Brennan C. W.; Mellinghoff I. K.; Holland E. C.; Gambhir S. S. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H.; Zhou T.; Yang S.; Chen Q.; Xing D. Gadolinium(III)–gold nanorods for MRI and photoacoustic imaging dual-modality detection of macrophages in atherosclerotic inflammation. Nanomedicine 2013, 8, 1611–1624. 10.2217/nnm.12.168. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Wu M.; Zeng Y.; Liao N.; Cai Z.; Liu G.; Liu X.; Liu J. Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. J. Mater. Chem. B. 2016, 4, 589–599. 10.1039/c5tb01827g. [DOI] [PubMed] [Google Scholar]
- Sivakumar B.; Aswathy R. G.; Romero-Aburto R.; Mitcham T.; Mitchel K. A.; Nagaoka Y.; Bouchard R. R.; Ajayan P. M.; Maekawa T.; Sakthikumar D. N. Highly versatile SPION encapsulated PLGA nanoparticles as photothermal ablators of cancer cells and as multimodal imaging agents. Biomater. Sci. 2017, 5, 432–443. 10.1039/C6BM00621C. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yang X.; Huang Z.; Huang P.; Zhang Y.; Deng L.; Wang Z.; Zhou Z.; Liu Y.; Kalish H.; Khachab N. M.; Chen X.; Nie Z. Magneto-Plasmonic Janus Vesicles for Magnetic Field-Enhanced Photoacoustic and Magnetic Resonance Imaging of Tumors. Angew. Chem., Int. Ed. 2016, 55, 15297–15300. 10.1002/anie.201608338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia A. B. E.; Ho C. J. H.; Chandrasekharan P.; Balasundaram G.; Tay H. C.; Burton N. C.; Chuang K.-H.; Ntziachristos V.; Olivo M. Multispectral optoacoustic and MRI coregistration for molecular imaging of orthotopic model of human glioblastoma. J. Biophotonics 2016, 9, 701–708. 10.1002/jbio.201500321. [DOI] [PubMed] [Google Scholar]
- Bogdanov A. A. Jr.; Dixon A. J.; Gupta S.; Zhang L.; Zheng S.; Shazeeb M. S.; Zhang S.; Klibanov A. L. Synthesis and Testing of Modular Dual-Modality Nanoparticles for Magnetic Resonance and Multispectral Photoacoustic Imaging. Bioconjugate Chem. 2016, 27, 383–390. 10.1021/acs.bioconjchem.5b00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y.; Kamisugi R.; Narazaki M.; Matsuda T.; Tabata Y.; Toshimitsu A.; Kondo T. Size-controlled and biocompatible Gd2O3 nanoparticles for dual photoacoustic and MR imaging. Adv. Healthcare Mater. 2012, 1, 657–660. 10.1002/adhm.201200103. [DOI] [PubMed] [Google Scholar]
- Yang J.-M.; Favazza C.; Chen R.; Yao J.; Cai X.; Maslov K.; Zhou Q.; Shung K. K.; Wang L. V. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 2012, 8, 1297–1302. 10.1038/nm.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanger L. A.; Ali M. M.; Allen M. J. Oxidation-responsive Eu2+/3+-liposomal contrast agent for dual-mode magnetic resonance imaging. Chem. Commun. 2014, 50, 14835–14838. 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanger L. A.; Mills D. R.; Ali M. M.; Polin L. A.; Shen Y.; Haacke E. M.; Allen M. J. Spectroscopic Characterization of the 3+ and 2+ Oxidation States of Europium in a Macrocyclic Tetraglycinate Complex. Inorg. Chem. 2016, 55, 9981–9988. 10.1021/acs.inorgchem.6b00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanger L. A.; Polin L. A.; Shen Y.; Haacke E. M.; Martin P. D.; Allen M. J. A EuII-Containing Cryptate as a Redox Sensor in Magnetic Resonance Imaging of Living Tissue. Angew. Chem., Int. Ed. 2015, 54, 14398–14401. 10.1002/anie.201507227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. J. Aqueous Lanthanide Chemistry in Asymmetric Catalysis and Magnetic Resonance Imaging. Synlett 2016, 27, 1310–1317. 10.1055/s-0035-1561363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanger L. A.; Polin L. A.; Shen Y.; Haacke E. M.; Allen M. J. Evaluation of EuII-based positive contrast enhancement after intravenous, intraperitoneal, and subcutaneous injections. Contrast Media Mol. Imaging 2016, 11, 299–303. 10.1002/cmmi.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanger L. A.; Basal L. A.; Allen M. J. The Role of Coordination Environment and pH in Tuning the Oxidation Rate of Europium(II). Chem.—Eur. J. 2017, 23, 1145–1150. 10.1002/chem.201604842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuda-Wedagedara A. N. W.; Wang C.; Martin P. D.; Allen M. J. Aqueous EuII-Containing Complex with Bright Yellow Luminescence. J. Am. Chem. Soc. 2015, 137, 4960–4963. 10.1021/jacs.5b02506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime S.; Barge A.; Castelli D. D.; Fedeli F.; Mortillaro A.; Nielsen F. U.; Terreno E. Paramagnetic lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magn. Reson. Med. 2002, 47, 639–648. 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Malloy C. R.; Sherry A. D. MRI Thermometry Based on PARACEST Agents. J. Am. Chem. Soc. 2005, 127, 17572–17573. 10.1021/ja053799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov E.; Soesbe T. C.; Balschi J. A.; Sherry A. D.; Lenkinski R. E. pCEST: Positive Contrast Using Chemical Exchange Saturation Transfer. J. Magn. Reson. 2012, 215, 64–73. 10.1016/j.jmr.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B.; Sheth V. R.; Howison C. M.; Douglas M. J. K.; Pineda C. T.; Maine E. A.; Baker A. F.; Pagel M. D. Detection of in vivo enzyme activity with CatalyCEST MRI. Magn. Reson. Med. 2014, 71, 1221–1230. 10.1002/mrm.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman D.; Kiefer G. E.; Rothman D. L.; Sherry A. D.; Hyder F. A lanthanide complex with dual biosensing properties: CEST (chemical exchange saturation transfer) and BIRDS (biosensor imaging of redundant deviation in shifts) with europium DOTA-tetraglycinate. NMR Biomed. 2011, 24, 1216–1225. 10.1002/nbm.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evbuomwan O. M.; Merritt M. E.; Kiefer G. E.; Sherry A. D. Nanoparticle-based PARACEST agents: The quenching effect of silica nanoparticles on the CEST signal from surface-conjugated chelates. Contrast Media Mol. Imaging 2012, 7, 19–25. 10.1002/cmmi.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. M.; Bhuiyan M. P.; Janic B.; Varma N. R. S.; Mikkelsen T.; Ewing J. R.; Knight R. A.; Pagel M. D.; Arbab A. S. A nano-sized PARACEST-fluorescence imaging contrast agent facilitates and validates in vivo CEST MRI detection of glioma. Nanomedicine 2012, 7, 1827–1837. 10.2217/nnm.12.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evbuomwan O. M.; Lee J.; Woods M.; Sherry A. D. The Presence of Fast-Exchanging Proton Species in Aqueous Solutions of paraCEST Agents Can Impact Rate Constants Measured for Slower Exchanging Species When Fitting CEST Spectra to the Bloch Equations. Inorg. Chem. 2014, 53, 10012–10014. 10.1021/ic501290q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumas C.; Fernando W. S.; Zhao P.; Regueiro-Figueroa M.; Kiefer G. E.; Martins A. F.; Platas-Iglesias C.; Sherry A. D. Unexpected Changes in the Population of Coordination Isomers for the Lanthanide Ion Complexes of DOTMA–Tetraglycinate. Inorg. Chem. 2016, 55, 9297–9305. 10.1021/acs.inorgchem.6b01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-Y.; Yadav N. N.; Friedman J. I.; Ratnakar J.; Sherry A. D.; van Zijl P. C. M. Using Frequency-Labeled Exchange Transfer to Separate Out Conventional Magnetization Transfer Effects From Exchange Transfer Effects When Detecting ParaCEST Agents. Magn. Reson. Med. 2012, 67, 906–911. 10.1002/mrm.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. M.; Yoo B.; Pagel M. D. Tracking the Relative in Vivo Pharmacokinetics of Nanoparticles with PARACEST MRI. Mol. Pharm. 2009, 6, 1409–1416. 10.1021/mp900040u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime S.; Carrera C.; Castelli D. D.; Crich S. G.; Terreno E. Tunable Imaging of Cells Labeled with MRI-PARACEST Agents. Angew. Chem., Int. Ed. 2005, 44, 1813–1815. 10.1002/anie.200462566. [DOI] [PubMed] [Google Scholar]
- Aime S.; Castelli D. D.; Terreno E. Novel pH-Reporter MRI Contrast Agents. Angew. Chem., Int. Ed. 2002, 41, 4334–4336. . [DOI] [PubMed] [Google Scholar]
- Soesbe T. C.; Merritt M. E.; Green K. N.; Rojas-Quijano F. A.; Sherry A. D. T2 Exchange Agents: A New Class of Paramagnetic MRI Contrast Agent that Shortens Water T2 by Chemical Exchange Rather Than Relaxation. Magn. Reson. Med. 2011, 66, 1697–1703. 10.1002/mrm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. T.; Ren J.; Lubag A. J. M.; Ratnakar J.; Vinogradov E.; Hancu I.; Lenkinski R. E.; Sherry A. D. A Concentration-Independent Method to Measure Exchange Rates in PARACEST Agents. Magn. Reson. Med. 2010, 63, 625–632. 10.1002/mrm.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burai L.; Tóth É.; Moreau G.; Sour A.; Scopelliti R.; Merbach A. E. Novel Macrocyclic EuII Complexes: Fast Water Exchange Related to an Extreme M–Owater Distance. Chem.—Eur. J. 2003, 9, 1394–1404. 10.1002/chem.200390159. [DOI] [PubMed] [Google Scholar]
- Burai L.; Scopelliti R.; Tóth É. EuII-Cryptate with optimal water exchange and electronic relaxation: A synthon for potential pO2 responsive macromolecular MRI contrast agents. Chem. Commun. 2002, 2366–2367. 10.1039/B206709A. [DOI] [PubMed] [Google Scholar]
- Burai L.; Tóth É.; Seibig S.; Scopelliti R.; Merbach A. E. Solution and Solid-State Characterization of EuII Chelates: A Possible Route Towards Redox Responsive MRI Contrast Agents. Chem.—Eur. J. 2000, 6, 3761–3770. . [DOI] [PubMed] [Google Scholar]
- Garcia J.; Allen M. J. Interaction of biphenyl-functionalized Eu2+-containing cryptate with albumin: Implications to contrast agents in magnetic resonance imaging. Inorg. Chim. Acta 2012, 393, 324–327. 10.1016/j.ica.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.; Kuda-Wedagedara A. N. W.; Allen M. J. Physical Properties of Eu2+-Containing Cryptates as Contrast Agents for Ultrahigh-Field Magnetic Resonance Imaging. Eur. J. Inorg. Chem. 2012, 2135–2140. 10.1002/ejic.201101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.; Neelavalli J.; Haacke E. M.; Allen M. J. EuII-containing cryptates as contrast agents for ultra-high field strength magnetic resonance imaging. Chem. Commun. 2011, 47, 12858–12860. 10.1039/C1CC15219J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravan P.; Tóth É.; Rockenbauer A.; Merbach A. E. Nuclear and Electronic Relaxation of Eu2+(aq): An Extremely Labile Aqua Ion. J. Am. Chem. Soc. 1999, 121, 10403–10409. 10.1021/ja992264v. [DOI] [Google Scholar]
- Harris D. C.Quantitative Chemical Analysis, 8th ed.; W. H. Freeman and Company: New York, 2010; pp 103–104. [Google Scholar]
- Luciano M.; Erfanzadeh M.; Zhou F.; Zhu H.; Bornhütter T.; Röder B.; Zhu Q.; Brückner C. In vivo photoacoustic tumor tomography using a quinoline-annulated porphyrin as NIR molecular contrast agent. Org. Biomol. Chem. 2017, 15, 972–983. 10.1039/c6ob02640k. [DOI] [PMC free article] [PubMed] [Google Scholar]