Abstract
Background
Cancer cachexia and skeletal muscle wasting are related to poor survival. In this study, quantitative body composition measurements using computed tomography (CT) were investigated in relation to survival, post‐operative complications, and surgical site infections in surgical patients with cancer of the head of the pancreas.
Methods
A prospective cohort of 199 patients with cancer of the head of the pancreas was analysed by CT imaging at the L3 level to determine (i) muscle radiation attenuation (average Hounsfield units of total L3 skeletal muscle); (ii) visceral adipose tissue area; (iii) subcutaneous adipose tissue area; (iv) intermuscular adipose tissue area; and (v) skeletal muscle area. Sex‐specific cut‐offs were determined at the lower tertile for muscle radiation attenuation and skeletal muscle area and the higher tertile for adipose tissues. These variables of body composition were related to overall survival, severe post‐operative complications (Dindo–Clavien ≥ 3), and surgical site infections (wounds inspected daily by an independent trial nurse) using Cox‐regression analysis and multivariable logistic regression analysis, respectively.
Results
Low muscle radiation attenuation was associated with shorter survival in comparison with moderate and high muscle radiation attenuation [median survival 10.8 (95% CI: 8.8–12.8) vs. 17.4 (95% CI: 14.7–20.1), and 18.5 (95% CI: 9.2–27.8) months, respectively; P < 0.008]. Patient subgroups with high muscle radiation attenuation combined with either low visceral adipose tissue or age <70 years had longer survival than other subgroups (P = 0.011 and P = 0.001, respectively). Muscle radiation attenuation was inversely correlated with intermuscular adipose tissue (r p = −0.697, P < 0.001). High visceral adipose tissue was associated with an increased surgical site infection rate, OR: 2.4 (95% CI: 1.1–5.3; P = 0.027).
Conclusions
Low muscle radiation attenuation was associated with reduced survival, and high visceral adiposity was associated with an increase in surgical site infections. The strong correlation between muscle radiation attenuation and intermuscular adipose tissue suggests the presence of ectopic fat in muscle, warranting further investigation. CT image analysis could be implemented in pre‐operative risk assessment to assist in treatment decision‐making.
Keywords: Radiation attenuation, Visceral adipose tissue, Body composition, Computed tomography, Pancreatic cancer, Surgical site infection
Background
Cancer of the head of the pancreas (HOP) has a poor prognosis with 1 year and 5 years survival rates of 24% and 7%, respectively.1 The poor prognosis is partly attributable to cancer cachexia, a syndrome of severe weight loss and muscle wasting, which occurs in the vast majority of patients with pancreatic cancer and other cancers in the head region of the pancreas (e.g. ampullary carcinoma and distal cholangiocarcinoma).2, 3 Currently, surgery is the only available treatment for HOP cancer to achieve curation, usually by performing a classical pancreaticoduodenectomy (Whipple) or pylorus‐preserving pancreaticoduodenectomy. This type of surgery is very invasive and has a high risk of serious complications that considerably reduce survival, such as pancreatic fistula, gastrojejunostomy leakage, and surgical site infection (SSI).4, 5 Therefore, pre‐operative selection of patients who are less vulnerable to complications is important.
For pre‐operative risk assessment in HOP cancer, determining the degree of cachexia is particularly relevant. While weight loss usually is patient reported and therefore partly subjective, muscle and adipose tissue area can be objectively measured using the standard abdominal computer tomography (CT) scan that is routinely performed prior to surgery. Indeed, the total skeletal muscle area on a single CT slice at the third lumbar vertebra (L3) is strongly correlated with total body skeletal muscle area.6 The area of visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and intermuscular adipose tissue (IMAT) can also be accurately estimated using this approach. Although a low skeletal muscle area has been associated with poor outcome in various cancer types,7 including pancreatic cancer,8, 9 the amount of (visceral) adipose tissue seems to have more impact on outcome in patients with pancreatic cancer. Several studies found a strong association between increased VAT and post‐operative pancreatic fistula in patients with pancreatic cancer,8, 10, 11 while other studies found associations with increased major post‐operative complications12 and pulmonary complications.13 Increased fat content in muscle tissue (myosteatosis) also seems to impact clinical outcome in pancreatic cancer. In fact, there are indications that increased muscle fat content rather than low muscle mass is associated with shorter survival in patients with unresectable pancreatic cancer.14 CT scans contain information about the radio density of a specific tissue type in Hounsfield units (HUs), which is referred to as radiation attenuation. A recent review showed that muscle radiation attenuation is highly variable among patients with cancer.15 Low muscle radiation attenuation could be a reflection of increased intramyocellular triglycerides (i.e. myosteatosis) or increased water content (i.e. muscle oedema). Goodplaster et al. 16 showed an inverse association between muscle radiation attenuation and triglyceride content in muscle phantoms and muscle biopsies from healthy adults. Myosteatosis has also been associated with insulin resistance and decreased muscle activity.17, 18, 19 These changes in the composition of muscle tissue may result in diminished muscle function and strength, which, in turn, is associated with poor surgical outcome.20 We therefore aimed to assess the association of radiation attenuation, adipose tissue, and other characteristics of body composition with post‐operative survival, post‐operative complications, and SSIs in patients with HOP cancer.
Methods
Subjects
This study was a chart review of a prospective cohort of 199 patients undergoing pancreatic surgery between 2008–2013 at the Maastricht University Medical Centre (MUMC), the Netherlands. Patients were included for analysis if they had pathology‐proven or radiology‐proven cancer of the pancreatic head, ampulla, distal common bile duct, or duodenum. Exclusion criteria were the presence of a neuro‐endocrine tumour, benign or pre‐malignant disease, metastatic disease on radiology examination (e.g. liver or lung metastases), intraductal papillary mucinous neoplasm, and missing abdominal CT scan or abdominal CT scan of poor quality.
Data collection
The primary outcome parameter of this study was overall survival, measured in months from the day of surgery. Secondary outcomes included major post‐operative complications and SSIs. The pancreaticoduodenectomy composite endpoint (CEP)21 was used as outcome parameter for major post‐operative complications (Dindo–Clavien grade ≥ 3).22 The eight most important complications after a pancreaticoduodenectomy are compiled into a single variable, the CEP. The CEP is a binary variable that is given a score of 1 if a patient has experienced one or more of the eight complications; a score of 0 is given if a patient has none of the eight complications. The eight endpoints included in the CEP were intra‐abdominal abscess, sepsis, gastrojejunostomy leakage, post‐pancreaticoduodenectomy haemorrhage, bile leakage/hepaticojejunostomy leakage, pancreatic fistula/pancreatic anastomosis leakage, delayed gastric emptying, and operative mortality. Intra‐abdominal abscess was defined as any quantity of purulent fluid leaking via the abdominal drain that is culture positive or a walled‐off collection of pus in the abdominal cavity at the time of radiological imaging, reoperation, or percutaneous drainage; gastrojejunostomy leakage was defined as anastomotic incompetence documented either by confirmatory upper gastrointestinal contrast X‐rays, CT scans, or reoperation; operative mortality was defined as death within 90 days from surgery.21 Sepsis was defined as the presence of both infection and a systemic inflammatory response.23 Post‐pancreaticoduodenectomy haemorrhage, bile leakage, pancreatic fistula, and delayed gastric emptying were scored according to the International Study Group of Liver Surgery24 or International Study Group of Pancreatic Surgery criteria as applicable.25, 26, 27 Post‐operative SSIs were scored by an independent trial nurse both in‐hospital and at the outpatient department during a 90 days period after surgery. Wounds were inspected daily during hospital stay and at every visit to the outpatient department. Infections were scored as incisional or organ/space SSIs according to the Centers for Disease Control and Prevention definitions.28 Patient‐reported weight, height, and weight loss in the 6 months prior to surgery as well as age and sex were recorded at the outpatient department. Comorbidities (diabetes mellitus, cardiac, pulmonal, and renal) were retrieved from the patient's medical file. C‐reactive protein (CRP) and albumin levels at diagnosis (maximum of 1 month before surgery) were recorded from the patient's medical file (if available) as markers of inflammation, but these were not routinely measured in all patients. Measurements were performed as part of standard care by the Department of Clinical Chemistry of the MUMC. Definitive diagnosis and tumour staging were performed post‐operatively by the pathologist. In case of signs of incurable disease during surgery (e.g. peritoneal metastasis), no resection was performed, resulting in missing pathology data. These patients were included in the analysis because they are a substantial part of the surgical patient population and therefore potentially could benefit from additional pre‐operative work‐up including CT‐based body composition analysis. The study protocol was approved by the medical ethical committee of the MUMC that waived the requirement to obtain informed consent.
Computed tomography scan analysis
Pre‐operative CT scans were performed maximally 6 weeks before surgery. Abdominal CT scans were analysed in anonymized format by one blinded independent researcher trained in radiologic anatomy and body composition analysis. Firstly, a single slice of each patient's CT scan was selected at the level of the L3. CT scans with large radiation artefacts or with missing parts of muscle tissue on the ventral, dorsal, or both lateral edges of the scan were excluded. Muscles included into the analysis were the internal and external obliques, transversus abdominus, rectus abdominus, psoas, quadratus lumborum, and erector spinae muscles. CT scans were analysed using sliceOmatic 5.0 (TomoVision, Magog, Canada) software for Microsoft Windows®. Using predefined HU ranges, the total cross‐sectional area (cm2) of skeletal muscle tissue (−29 to 150 HU), VAT (−150 to −50 HU), SAT (−190 to −30 HU), and IMAT (−190 to −30 HU) was determined. In addition, the radiation attenuation for skeletal muscle was assessed by calculating the average HU value of the total muscle area within the specified range of −29 to 150 HU (i.e. this is calculated from the muscle tissue only excluding the IMAT). The total areas of skeletal muscle, VAT, and SAT were corrected for stature to calculate the L3‐muscle index, L3‐VAT index, and L3‐SAT index in cm2/m2, providing good estimates of total body skeletal muscle, VAT, and SAT mass.6
Statistical analysis
Prado et al. 29 and Martin et al. 7 previously published cut‐off values for L3‐muscle index and radiation attenuation, found by using optimal stratification. However, these cut‐offs were found in a Canadian cohort of patients with respiratory tract and gastro‐intestinal cancer and might not be comparable with the present Dutch cohort of patients with HOP cancer. Body composition varies greatly among regions and ethnicities as illustrated by the large Japanese cohort study of Fujiwara et al., which found highly different cut‐offs compared with the study of Martin (e.g. female cut‐off for L3‐muscle index at 29.6 in the Japanese cohort versus 41 in the Canadian cohort).7, 30 Therefore, we decided to set our own cut‐offs as performed by other studies with similar population sizes.31, 32, 33, 34 Optimum stratification works well for large cohorts in which the lowest P‐value will be used to set the cut‐off.35 However, in smaller cohorts, the P‐value is too unstable to use optimum stratification to find a reliable cut‐off. Because we considered our cohort was too small for cut‐point analysis by optimal stratification, we determined cut‐off values for our cohort based on tertiles. Determining the cut‐off at a tertile enables comparison between groups with a relative low/high value to be compared with the rest of the group while not forcing subjects with a value around the cut‐off value in a low or high category. Cut‐off values were set at the lowest tertile for radiation attenuation and L3‐muscle index, and at the highest tertile for L3‐VAT index, L3‐SAT index, and IMAT. Because IMAT is not uniformly distributed throughout the muscle, it was not added to the regression analysis. Body composition is highly influenced by sex; therefore, separate cut‐off values were determined for men and women. Data were analysed using IBM SPSS 23 for Microsoft Windows®. Differences in patient characteristics were analysed using the independent T‐test or Pearson χ 2 test. The Kaplan–Meier estimate and Cox regression analysis were used to assess the association of each individual body composition measurement with survival. Multiple logistic regression was used to assess the association of body composition with major complications and SSIs. For both Cox regression and multiple logistic regression analyses, two models were used. Model 1 was the unadjusted univariable analysis. Model 2 was a multivariable analysis adjusted for sex, age, and body mass index (BMI) because these are known important confounders of body composition and survival.7 In addition, any variable that generated a P‐value of <0.1 in univariable analysis was added to the multivariable analysis. For correlations, Pearson's correlation coefficient (r p) was used. Only correlations with a value of ≥0.5 or ≤−0.5 are reported. A P‐value of <0.05 was considered significant.
Results
Patient cohort
From all 199 patients of the prospective cohort, 186 were included in the present analysis. Eight patients were excluded because of benign disease, three because of intraductal papillary mucinous neoplasm, and two because of poor quality CT scans. Two patients had incomplete data on post‐operative complications and infections but were included in the survival analysis. The median follow‐up was 57.7 months. Patient characteristics are shown in Table 1. CT scan analysis was performed on all 186 patients for muscle radiation attenuation, L3‐muscle‐index, and L3‐VAT‐index. Because the tissues of some patients were outside of the CT scan range, IMAT and L3‐SAT index could be measured in 183 and 144 patients, respectively. Mean and sex‐specific cut‐off values for all CT‐derived body composition parameters are shown in Table 2.
Table 1.
General characteristics of patients with cancer of the head of the pancreas according to low and high muscle radiation attenuation
| Total (n = 186) | Low muscle radiation attenuation (n = 62) | Moderate–high muscle radiation attenuation (n = 124) | P‐value | |
|---|---|---|---|---|
| Male (n, %) | 102 (54.8%) | 34 (54.8%) | 68 (54.8%) | 1.000 |
| Age (years) | 66.5 | 69.8 ± 8.7 | 64.8 ± 9.8 | 0.001 |
| Body mass index (kg/m2) | 25.2 | 26.8 ± 5.2 | 24.4 ± 3.9 | <0.001 |
| Weight loss (%)a | 9.4 | 11.0 ± 8.4 | 8.7 ± 7.0 | 0.124 |
| Comorbidity (n, %)a | ||||
| Diabetes mellitus | 42 (22.6%) | 16 (25.8%) | 26 (21.0%) | 0.492 |
| Cardiac | 78 (41.9%) | 32 (51.6%) | 46 (37.1%) | 0.071 |
| Pulmonary | 19 (10.2%) | 7 (11.3%) | 12 (9.7%) | 0.759 |
| Renal | 9 (4.8%) | 4 (6.5%) | 5 (4.0%) | 0.488 |
| Pathology (n, %) | ||||
| Pancreatic | 73 (39.2%) | 21 (33.9%) | 52 (41.9%) | 0.288 |
| Ampullary | 28 (15.1%) | 9 (14.5%) | 19 (15.3%) | 0.885 |
| Cholangiocarcinoma | 10 (5.4%) | 1 (1.6%) | 9 (7.3%) | 0.169 |
| Duodenal carcinoma | 8 (4.3%) | 3 (4.8%) | 5 (4.0%) | 1.000 |
| Otherb | 5 (2.7%) | 1 (1.6%) | 4 (3.2%) | 0.666 |
| None available/palliative surgeryc | 62 (33.3%) | 27 (42.5%) | 35 (28.2%) | 0.037 |
| Composite endpoint (n, %)a | 88 (47.3%) | 34 (55.8%) | 54 (43.5%) | 0.130 |
| Intra‐abdominal abscess | 35 (18.8%) | 14 (22.6%) | 21 (16.9%) | 0.339 |
| Sepsis | 23 (12.4%) | 6 (9.7%) | 17 (13.7%) | 0.453 |
| Gastrojejunostomy leakage | 7 (3.8%) | 2 (3.2%) | 5 (4.0%) | 1.000 |
| Post‐pancreaticoduodenectomy haemorrhage | 22 (11.8%) | 7 (11.3%) | 15 (12.1%) | 0.872 |
| Bile leakage | 16 (8.6%) | 6 (9.7%) | 10 (8.1%) | 0.724 |
| Pancreatic fistula | 26 (14.0%) | 10 (16.1%) | 16 (12.9%) | 0.550 |
| Delayed gastric emptying | 44 (23.7%) | 17 (27.4%) | 27 (21.8%) | 0.356 |
| Operative mortality | 26 (14.0%) | 9 (14.5%) | 15 (12.1%) | 0.643 |
| Surgical site infections (n, %) | 101 (54.3%) | 34 (54.8%) | 67 (54.0%) | 0.917 |
| Incisional | 73 (39.2%) | 26 (41.9%) | 47 (37.9%) | 0.595 |
| Organ/space | 49 (26.3%) | 18 (29.0%) | 31 (25.0%) | 0.556 |
| Laboratory results (pre‐operative) | ||||
| C‐reactive protein (mg/L)a | 37.0 | 37.9 ± 50.6 | 36.5 ± 60.2 | 0.913 |
| Albumin (g/L)a | 34.4 | 31.0 ± 6.2 | 35.8 ± 6.3 | 0.006 |
Muscle radiation attenuation was measured as the average Hounsfield units of the total skeletal muscle area on a single cross‐sectional computer tomography image at the level of the third lumbar vertebra. Sex‐specific cut‐offs for muscle radiation attenuation were determent at the lower tertile (male: 33.9 HU, female: 30.9 HU).
HU, Hounsfield units.
Missing data were excluded: weight loss n = 67, comorbidity n = 2, composite endpoint n = 2, C‐reactive protein n = 97, and albumin n = 122.
Renal cell carcinoma (n = 1), malignant gastrointestinal stromal tumour (n = 1), gallbladder carcinoma (n = 1), colon carcinoma (n = 1), and leiomyosarcoma (n = 1).
Pathology was not available in cases where the surgeon decided to convert to palliative surgery because of an incurable disease (e.g. peritoneal metastases).
Table 2.
Means and sex‐specific cut‐off values for all CT‐scan measurements at the third lumbar vertebra of patients with cancer of the head of the pancreas at diagnosis
| Male (n = 102) | Female (n = 84) | Total (n = 186) | |||
|---|---|---|---|---|---|
| Mean (SD) | Cut‐off | Mean (SD) | Cut‐off | Mean (SD) | |
| Radiation attenuation (HU) | 36.38 (7.73) | 33.9 | 33.84 (9.85) | 30.9 | 35.24 (8.82) |
| L3‐muscle index (cm2/m2) | 49.13 (7.27) | 45.1 | 39.98 (6.38) | 36.9 | 45.00 (8.25) |
| L3‐visceral adipose tissue index (cm2/m2) | 55.42 (31.28) | 68.2 | 33.76 (23.51) | 39.2 | 45.64 (29.98) |
| L3‐subcutaneous adipose tissue index (cm2/m2) | 44.86 (22.76) | 49.8 | 63.41 (30.29) | 72.6 | 52.85 (27.74) |
| L3‐intermuscular adipose tissue (cm2) | 15.54 (13.38) | 16.6 | 14.81 (11.07) | 15.4 | 15.21 (12.37) |
Muscle radiation attenuation was measured as the average Hounsfield units (HU) of the total skeletal muscle area on a single cross‐sectional computer tomography (CT) image at the level of the third lumbar vertebra (L3). The L3‐muscle index, L3‐visceral adipose tissue index, and L3‐subcutaneous adipose tissue index were measured as total area at L3 level, corrected for stature. L3‐intermuscular adipose tissue was measured as total area at L3 level. Sex‐specific cut‐offs were determent at the lower tertile for muscle radiation attenuation and L3‐muscle index, and at the higher tertile for L3‐visceral adipose tissue index, L3‐subcutaneous adipose tissue index, and L3‐intermuscular adipose tissue.
SD, standard deviation.
Survival analysis
Initial survival analysis was performed using the unadjusted Kaplan–Meier estimate for all CT scan measurements. Patients with a low muscle radiation attenuation had a significantly lower survival than patients with a moderate or high radiation attenuation, median survival of 10.8 (95% CI: 8.8–12.8) vs. 17.4 (95% CI: 14.7–20.1), and 18.5 months (95% CI: 9.2–27.8), respectively (P = 0.008, Figure 1A). There were no significant differences in survival between patients within the different categories of L3‐muscle index, L3‐VAT index, and L3‐SAT index. Patients with both high muscle radiation attenuation and a low L3‐VAT index (n = 93, 50.0%) had significantly higher survival than patients with other combinations (Figure 1B, P = 0.011). Patients with both a high muscle radiation attenuation and age <70 (n = 81, 43.5%) had a higher survival compared with other combinations, while both a low muscle radiation attenuation and age ≥70 (n = 33, 17.7%) were associated with significantly lower survival (Figure 1C, P = 0.001). Results of the Cox‐regression analysis are shown in Figure 2. There was a significantly shorter survival in patients with a low muscle radiation attenuation (HR 1.57, 95% CI 1.08–2.29, P = 0.020) compared with patients with a moderate or high muscle radiation attenuation. L3‐muscle index, L3‐VAT index, and L3‐SAT index were not significantly associated with survival.
Figure 1.
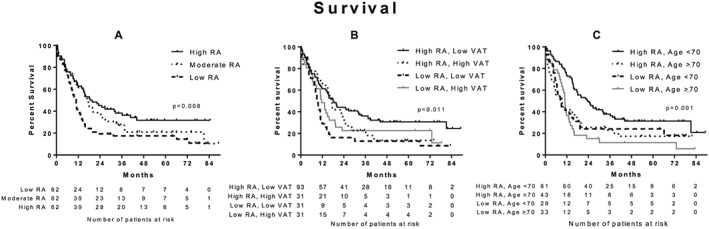
Survival is related to different risk categories in patients with cancer of the head of the pancreas. (A) Kaplan–Meier estimate (univariable analysis): patients with low muscle radiation attenuation had a significantly lower survival than patients with moderate or high radiation attenuation (log‐rank test, P = 0.002). (B) Kaplan–Meier estimate (univariable analysis): patients with both high muscle radiation attenuation and low visceral adipose tissue index had significantly higher survival than other categories (log‐rank test, P = 0.011). (C) Kaplan–Meier estimate (univariable analysis): patients with both high muscle radiation attenuation and low age had a significantly higher survival than other categories, while patients with low muscle radiation attenuation and high age had significantly lower survival (log‐rank test, P = 0.001). RA, radiation attenuation; VAT, visceral adipose tissue index.
Figure 2.
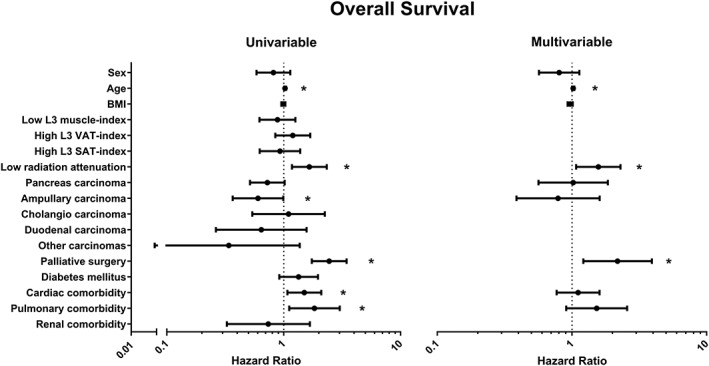
Association between computed tomography scan measurements at the third lumbar vertebra and potential confounders of patients with cancer of the head of the pancreas at diagnosis with survival using Cox‐regression analysis. Values are displayed as hazard ratio and 95% confidence interval. Sex, age, body mass index (BMI), and variables that generated a P‐value of <0.1 in univariable analysis were entered in the multivariable analysis. C‐statistic = 0.66 for multivariable analysis. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. *P < 0.05.
Post‐operative complications and surgical site infections
In total, 88 patients had one or more pancreatic surgery‐specific post‐operative complications, leading to a positive CEP: sepsis (n = 23), gastrojejunostomy leakage (n = 7), post‐pancreatectomy haemorrhage (n = 22), bile leakage (n = 16), delayed gastric emptying (n = 44), operative mortality (n = 24), intra‐abdominal abscess (n = 35), and pancreatic fistula (n = 26). Low L3‐muscle index was significantly associated with less post‐operative complications [adjusted OR: 0.48, (95% CI 0.24–0.96), P = 0.038; Figure 3]. One hundred and one patients developed an SSI (52 incisional, 28 organ/space SSIs, and 21 had both types). High L3‐VAT index was significantly associated with an increased incidence of SSIs [adjusted OR: 2.42, (95% CI 1.10–5.28), P = 0.027; Figure 4].
Figure 3.
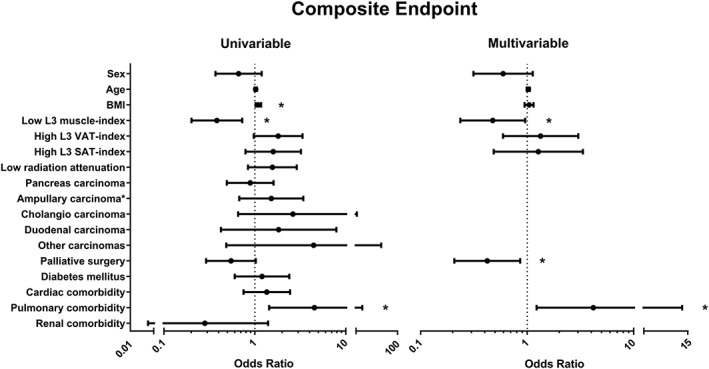
Association between computed tomography scan measurements and potential confounders with post‐operative complications using logistic regression analysis. Values are displayed as odds ratio and 95% confidence interval. Sex, age, body mass index (BMI), and variables that generated a P‐value of <0.1 in a univariable analysis were entered in the multivariable analysis. C‐statistic = 0.72 for multivariable analysis. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. *P < 0.05.
Figure 4.
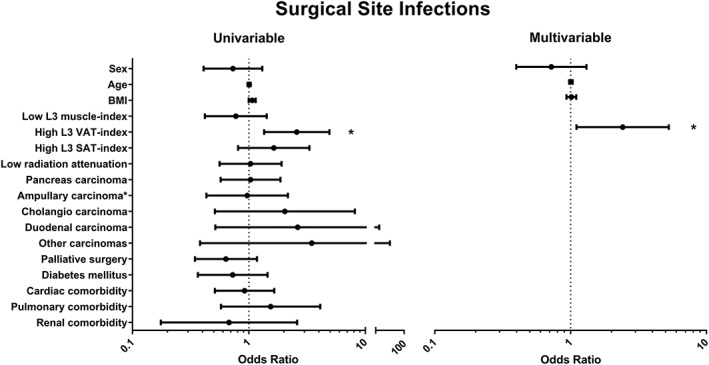
Association between computed tomography scan measurements and potential confounders with surgical site infections using logistic regression analysis. Values are displayed as odds ratio and 95% confidence interval. Sex, age, body mass index (BMI), and variables that generated a P‐value of <0.1 in univariable analysis were entered in the multivariable analysis. C‐statistic = 0.62 for multivariable analysis. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. *P < 0.05.
Comorbidities and correlations
Comorbidities for all patients are shown in Table 1. Patients with low muscle radiation attenuation did not have a higher prevalence of diabetes or cardiac, pulmonary, and renal comorbidities. Patients with low muscle radiation attenuation had significantly lower pre‐operative albumin levels compared with patients with moderate–high muscle radiation attenuation. There was no difference in pre‐operative CRP levels. Patients with an age >70 years had a significantly lower muscle radiation attenuation and L3‐muscle index than patients with an age <70 years (radiation attenuation: 32.4 ± 8.5 vs. 37.2 ± 8.5; L3‐muscle index: 43.3 ± 7.9 vs. 46.2 ± 8.3, respectively). Muscle radiation attenuation correlated inversely with IMAT area (r p = −0.697, P < 0.001). As expected, BMI correlated positively with both L3‐VAT index (r p = 0.583, P < 0.001) and SAT (r p = 0.681, P < 0.001).
Discussion
In this study, low skeletal muscle radiation attenuation was observed to be associated with worse overall survival in patients with HOP cancer. Additionally, high L3‐VAT index was related to increased incidence of SSIs.
Okumura et al. previously observed decreased overall survival and shorter recurrence‐free survival in patients with pancreatic cancer and high intramuscular adipose tissue content (in this study, this was measured as muscle radiation attenuation divided by SAT radiation attenuation) but did not investigate post‐operative complications.36 Although their results are in line with the present study, they used a different approach to define muscle quality. Firstly, we analysed all muscles at the L3‐level instead of only the psoas muscle, as was performed by Okumura et al. We evaluated all muscles at L3 because this better represents the total body skeletal muscle mass6 and has the benefit of being less susceptible to measurement errors (which will be averaged out). Secondly, we separated IMAT from skeletal muscle tissue, while Okumura et al. included IMAT in the calculation of the average psoas muscle HUs. Including the IMAT (which has a much lower HU value than muscle tissue) greatly influences the measured radiation attenuation. Because the distribution of intermuscular tissue is not uniform within muscles, the measured radiation attenuation might differ depending on which muscle or CT slice is chosen for analysis.
Joglekar et al. observed more post‐operative complications in patients with pancreatic cancer and a low radiation attenuation or low psoas muscle area but did not observe a significant association of these factors with overall survival.37 Unfortunately, patients with low radiation attenuation and low psoas muscle area were pooled for analysis in that study, making it difficult to compare the results with the present study and potentially explaining the absence of an association with survival.
To our knowledge, no studies have been reported that use similar methodology to investigate muscle radiation attenuation in patients with pancreatic cancer. When looking at studies in other malignancies with similar methodology as the present study, low muscle radiation attenuation has consistently been associated with poor survival in hepatocellular carcinoma,30, 38, 39 respiratory and colorectal cancer,7 and follicular lymphoma.40 Skeletal muscle area was not associated with outcomes in the present study, which agrees with previous findings in pancreatic cancer patients.14, 37, 41 It should be noted that the frequently reported L3‐muscle index or any other single time‐point skeletal muscle area measurement might not accurately indicate muscle wasting. Normal muscle area can be highly variable among individuals and is dependent on sex, race, age, physical fitness, and build. Moreover, a large muscle area does not necessarily mean better muscle function.42 Furthermore, given that weight loss is a much better predictor of survival than baseline weight in cachectic patients,43 information on muscle loss is likely to be important to assess the relation between sarcopenia and survival. A study by Stene et al. using CT scans before and after chemotherapy showed that muscle loss but not low muscularity at baseline was predictive for time to death in patients undergoing palliative treatment for non‐small cell lung cancer.44 Because it is impossible to assess muscle loss from the single CT scan usually available for a patient, other muscle‐related parameters should be explored as proxies for muscle loss and muscle function. In the present study, we found that muscle radiation attenuation was predictive of poor outcome. The main issue to be resolved now is to identify what (patho)physiological processes are reflected by a low radiation attenuation, and if these are related to on muscle function. Many studies have suggested that low radiation attenuation is a sign of triglyceride accumulation in muscle cells.15, 38, 39, 45 However, so far, only one study actually demonstrated a weak negative correlation between low muscle radiation attenuation and increased muscle fibre lipid content in patients with type 2 diabetes mellitus.16 The correlation between muscle radiation attenuation and IMAT that we observed supports an association between low radiation attenuation and ectopic fat in the muscle tissue (radiation attenuation is calculated from the total skeletal muscle tissue area only and does not include the IMAT area). Importantly, IMAT is independently associated with insulin resistance in healthy elderly men without diabetes.46 Hence, decreased radiation attenuation in cachectic patients might reflect insulin resistance, a frequently observed condition in this patient population.47 Low radiation attenuation has also been shown to be associated with systemic inflammation, a hallmark of cachexia.45 We also found that patients with low muscle radiation attenuation had lower albumin levels, which is associated with systemic inflammation. CRP levels were not significantly higher in patients with low radiation attenuation. However, these finding should be interpreted with caution because we had many missing data on systemic inflammation in our cohort. All in all, to identify the determinants of the reduced radiation attenuation, studies combining detailed CT image analysis with histological evaluation of the imaged muscle are required.
The rate of SSIs strongly associated with high L3‐VAT index but was not related to muscle radiation attenuation or other CT measurements in our cohort. This is in contrast to the study of Lieffers et al., which found an increase in post‐operative infections in sarcopenic patients after colorectal surgery.48 In addition, the observed relation between SSIs and high L3‐VAT index area was not observed by Lieffers et al. These discrepancies may be attributable to the fact that the type of surgery differs (pancreas vs. colorectal), and we included infections up to 90 days after surgery (which is common in pancreatic surgery because of the late occurrence of complications) instead of 30 days as performed by Lieffers et al. Furthermore, we only recorded SSIs instead of all infections. We focussed on SSIs because these are among the most relevant and most frequent infections in (pancreatic) surgery. They are usually poorly recorded making retrospective and even prospective studies a difficult task. In the present study, a trial nurse inspected the wound and medical file daily for signs of SSI, which in our opinion is the optimal way to prevent underdetection of SSIs. The increased risk of SSIs in patients with high L3‐VAT index could be related to impaired wound healing secondary to oxygenation problems, which is a common finding in large depots of adipose tissue.49 This is even more pronounced in obese individuals in whom VAT arteriolar function and angiogenesis have been shown to be impaired.50, 51 In addition, high leptin levels (as a reflection of increased adipose tissue) have been associated with an increased risk of SSIs in colorectal cancer.52 These mechanisms remain speculative and should be explored in future studies.
Surprisingly, we found that patients with a low L3‐muscle index had a reduced risk of post‐operative complications. Although many studies did not show a benefit for high muscularity with respect to complication risk,37, 41, 53, 54 the apparent benefit of low muscularity is unexpected and contrary to what was found in the vast majority of studies published in the field. The low risk of post‐operative complications was not reflected by a better survival of these patients. Therefore, this finding should be interpreted with caution because it may have been the result of uncontrolled bias or statistical error.
None of the assessed body composition measurements stand on their own, and combinations of unfavourable phenotypes can increase the impact on patient outcome. This has been indicated previously for combinations of myosteatosis with BMI,7 obesity,55 and sarcopenia.14 Although our cohort is rather small for such subgroup analyses, our survival analyses indicate that combinations of muscle radiation attenuation with visceral adiposity or age can have additional effects on survival. Exploration of patient phenotypes defined by clusters of different characteristics would provide very useful data for risk assessment in the clinical setting.
In conclusion, we showed that muscle radiation attenuation is a predictor of survival after pancreatic surgery, whereas a high L3‐VAT index is associated with the risk of SSIs. Our data therefore indicate that simple and fast analysis of a single CT image can be valuable for pre‐operative risk assessment in patients with HOP cancer. Future intervention studies are needed to investigate whether increasing muscle radiation attenuation through diet and/or exercise before surgery can improve prognosis of patients with HOP cancer.
Conflict of interest
All authors declare that they have no conflict of interest.
Acknowledgements
David van Dijk is supported as a PhD candidate by the Netherlands Organization for Scientific Research (NWO Grant 022.003.011). Martijn Bours is partly supported by grants from the Alpe d'HuZes Foundation of the Dutch Cancer Society (grant no. UM‐2012‐5633), and from the ‘Kankeronderzoekfonds Limburg’ as part of Health Foundation Limburg (grant no. 00005739).
We would like to thank Magriet Rouflart for her commitment and hard work in scoring and recording the surgical site infections and Professor Vicky Baracos for her friendly help in analysing and interpreting the results of this study. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.56
van Dijk, D. P. J. , Bakens, M. J. A. M. , Coolsen, M. M. E. , Rensen, S. S. , van Dam, R. M. , Bours, M. J. L. , Weijenberg, M. P. , Dejong, C. H. C. , and Olde Damink, S. W. M. (2017) Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. Journal of Cachexia, Sarcopenia and Muscle, 8: 317–326. doi: 10.1002/jcsm.12155.
References
- 1. Nederlandse Kankerregistratie [database on the Internet] 1989. Available from: http://cijfersoverkanker.nl/. Accessed: February 1, 2012
- 2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- 3. Dewys W, Begg C, Lavin P, Band P, Bennett J, Bertino J, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7. [DOI] [PubMed] [Google Scholar]
- 4. van der Gaag NA, Harmsen K, Eshuis WJ, Busch OR, van Gulik TM, Gouma DJ. Pancreatoduodenectomy associated complications influence cancer recurrence and time interval to death. Eur J Surg Oncol 2014;40:551–8. [DOI] [PubMed] [Google Scholar]
- 5. Sugiura T, Uesaka K, Ohmagari N, Kanemoto H, Mizuno T. Risk factor of surgical site infection after pancreaticoduodenectomy. World J Surg 2012;36:2888–94. [DOI] [PubMed] [Google Scholar]
- 6. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 7. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- 8. Kirihara Y, Takahashi N, Hashimoto Y, Sclabas GM, Khan S, Moriya T, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg 2013;257:512–9. [DOI] [PubMed] [Google Scholar]
- 9. Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo S, Takahashi D, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg 2016;20:1586–94. [DOI] [PubMed] [Google Scholar]
- 10. Tranchart H, Gaujoux S, Rebours V, Vullierme M‐PP, Dokmak S, Levy P, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg 2012;256:139–45. [DOI] [PubMed] [Google Scholar]
- 11. Park CM, Park JS, Cho ES, Kim JK, Yu JS, Yoon DS. The effect of visceral fat mass on pancreatic fistula after pancreaticoduodenectomy. J Invest Surg 2012;25:169–73. [DOI] [PubMed] [Google Scholar]
- 12. Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, et al. A high visceral adipose tissue‐to‐skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition (Burbank, Los Angeles County, Calif). 2016. [DOI] [PubMed] [Google Scholar]
- 13. Shimizu A, Tani M, Kawai M, Hirono S, Miyazawa M, Uchiyama K, et al. Influence of visceral obesity for postoperative pulmonary complications after pancreaticoduodenectomy. J Gastrointest Surg 2011;15:1401–10. [DOI] [PubMed] [Google Scholar]
- 14. Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr (Edinburgh, Scotland). 2015. [DOI] [PubMed] [Google Scholar]
- 15. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–10. [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Kuk JL, Davidson LE, Hudson R, Kilpatrick K, Graham TE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol (1985). 2005;99:1220–5. [DOI] [PubMed] [Google Scholar]
- 18. Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance‐trained older adults. Gerontology 2009;55:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross‐sectional body composition analysis. Curr Opin Support Palliat Care 2011;5:342–9. [DOI] [PubMed] [Google Scholar]
- 20. Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of sarcopenia in major surgery. Nutr Clin Pract 2015;30:175–9. [DOI] [PubMed] [Google Scholar]
- 21. Coolsen MM, Clermonts SH, van Dam RM, Winkens B, Malagó M, Fusai GK, et al. Development of a composite endpoint for randomized controlled trials in pancreaticoduodenectomy. World J Surg 2013;38:1468–75. [DOI] [PubMed] [Google Scholar]
- 22. Dindo D, Demartines N, Clavien P‐AA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530–8. [DOI] [PubMed] [Google Scholar]
- 24. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–8. [DOI] [PubMed] [Google Scholar]
- 25. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–5. [DOI] [PubMed] [Google Scholar]
- 26. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 27. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–8. [DOI] [PubMed] [Google Scholar]
- 28. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132. [PubMed] [Google Scholar]
- 29. Prado CM, Lieffers J, McCargar L, Reiman T, Sawyer M, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 30. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–40. [DOI] [PubMed] [Google Scholar]
- 31. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579–85. [DOI] [PubMed] [Google Scholar]
- 32. Antoun S, Lanoy E, Iacovelli R, Albiges‐Sauvin L, Loriot Y, Merad‐Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;119:3377–84. [DOI] [PubMed] [Google Scholar]
- 33. Kazemi‐Bajestani S, Mazurak VC, Baracos V. Computed tomography‐defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2–10. [DOI] [PubMed] [Google Scholar]
- 34. Martin L. Diagnostic criteria for cancer cachexia: data versus dogma. Curr Opin Clin Nutr Metab Care 2016;19:188–98. [DOI] [PubMed] [Google Scholar]
- 35. Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time‐to‐event outcomes. Rochester, MN, Mayo Foundation, Technical Report Series #79, 2006.
- 36. Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015;157:1088–98. [DOI] [PubMed] [Google Scholar]
- 37. Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 2014;111:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaibori M, Ishizaki M, Iida H, Matsui K, Sakaguchi T, Inoue K, et al. Effect of intramuscular adipose tissue content on prognosis in patients undergoing hepatocellular carcinoma resection. J Gastrointest Surg 2015;19:1315–23. [DOI] [PubMed] [Google Scholar]
- 39. Hamaguchi Y, Kaido T, Okumura S, Ito T, Fujimoto Y, Ogawa K, et al. Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2015;22:475–85. [DOI] [PubMed] [Google Scholar]
- 40. Chu MP, Lieffers J, Ghosh S, Belch AR, Chua NS, Fontaine A, et al. Skeletal muscle radio‐density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. PLoS One 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short‐term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 2011;13:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez‐Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–9. [DOI] [PubMed] [Google Scholar]
- 44. Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 2014;1–9. [DOI] [PubMed] [Google Scholar]
- 45. Malietzis G, Johns N, Al‐Hassi HO, Knight SC, Kennedy RH, Fearon KC, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg 2015;263:320–5. [DOI] [PubMed] [Google Scholar]
- 46. Miljkovic I, Cauley JA, Wang PY, Holton KF, Lee CG, Sheu Y, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring). 2013;21:2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tisdale M. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 48. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goossens GH, Blaak EE. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol (Lausanne) 2015;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Farb MG, Ganley‐Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, et al. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol 2012;32:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, et al. Depot‐specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 2011;123:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ortega‐Deballon P, Ménégaut L, Fournel I, Orry D, Masson D, Binquet C, et al. Are adiponectin and leptin good predictors of surgical infection after colorectal surgery? A prospective study. Surg Infect (Larchmt) 2015. [DOI] [PubMed] [Google Scholar]
- 53. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross‐sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015;17:6. [DOI] [PubMed] [Google Scholar]
- 54. Kuroki LM, Mangano M, Allsworth JE, Menias CO, Massad LS, Powell MA, et al. Pre‐operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol 2015;22:972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tan B, Birdsell L, Martin L, Baracos V, Fearon K. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–9. [DOI] [PubMed] [Google Scholar]
- 56. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


