Abstract
Background
While much cancer research focuses on tumours and their microenvironment, malignancies cause widespread physiologic changes. Cancer and treatment‐related sarcopenia, measured with quantitative imaging or as a decrease in overall body mass, are indicative of poor prognosis in elderly diffuse large B‐cell lymphoma (DLBCL) patients, skeletal muscle radiodensity (SMD) may be a better prognostic marker. SMD, a measure of muscle radiation attenuation on CT imaging, is more prognostic than sarcopenia or International Prognostic Index (IPI) scores in follicular lymphoma and multiple solid organ malignancies. Low SMD appears to correlate with fat accumulation in muscle and is associated with inflammation. This study set out to examine SMD's prognostic ability in DLBCL.
Methods
All DLBCL patients treated with rituximab‐containing therapy between 2004 and 2009 were compared to determine SMD's prognostic ability in this single centre, retrospective study. Pre‐treatment CT scans were used to measure SMD and muscle cross‐sectional area. Primary endpoints included progression free (PFS) and overall survival (OS) while objective response rates (ORR) were secondary.
Results
Of 224 evaluable patients, 116 were identified as having low SMD. Low SMD predicted poorer 5 year PFS, 60 vs. 81% (p = 0.001) and OS, 58 vs. 86% (p < 0.0001). SMD's prognostic ability retained significance in multivariate analysis taking into consideration the Revised International Prognostic Index (R‐IPI) and sex. Although high SMD was not predictive of ORR (95.4 vs. 91.4%, p = 0.17), it was strongly associated with radiographic complete response (85 vs. 66%, p = 0.0007). Contrary to previous findings, sarcopenia did not predict for poorer OS but suggested improved OS in elderly DLBCL patients (HR 0.38, p = 0.01).
Conclusions
SMD is a novel prognostic (and potentially treatment predictive) marker independent of R‐IPI in DLBCL. It presents an inexpensive yet complementary assessment to R‐IPI for prognosticating DLBCL outcomes.
Keywords: Skeletal muscle radiodensity, Sarcopenia, Diffuse large B‐cell lymphoma, Cachexia
Introduction
Skeletal muscle (radio)density (SMD) is reported as the mean radiation attenuation in Hounsfield Units (HU) on computed tomography (CT) imaging. Although SMD is measured within the range of −29 to 150 HU, most of the tissue area typically falls between 10 and 40 HU.1 SMD can vary greatly, and muscle with lower SMD has increased fat production or infiltration.2 In diabetes, inflammation is a proposed mechanism behind accumulation of excess intermuscular adipose tissue.3 Ectopic fatty infiltration of muscle may also be a phenomenon that contributes to muscle mass loss (sarcopenia).4 Clinically, sarcopenia and cachexia are related in cancer patients, both of which have been shown to be poor prognostic indicators.5 Low SMD, though, appears to be more prognostic than sarcopenia in both solid and haematologic cancers.6, 7, 8 It was previously observed that SMD was prognostic of survival in follicular lymphoma, an indolent form of non‐Hodgkin lymphoma. SMD has not yet been evaluated in the commonest form of aggressive non‐Hodgkin lymphoma, diffuse large B‐cell lymphoma (DLBCL). Because weight loss is described to be prognostic of survival in DLBCL,9 we hypothesized that this disease may be characterized by reduced SMD with likely prognostic significance.
After the advent of rituximab, a monoclonal antibody targeting CD20,10, 11 weight loss is less viewed as an important prognostic factor in DLBCL. Rather, older age is associated with poor prognosis in DLBCL based on the Revised International Prognostic Index (R‐IPI).12 Lanic et al..13 have recently reviewed elderly patients with DLBCL to examine what aspects of that population may relate to poorer survival. Sarcopenia appeared to be a defining feature predictive of not only higher R‐IPI scores but also poorer overall survival (OS). However, Lanic et al. used a cut‐off of ≥70 years of age to define their elderly population whereas the R‐IPI uses an age of 60 years—highlighting that age cut‐offs can be difficult to define. Conceptually, the poor prognosis associated with sarcopenia should not be limited by age.1 Like SMD, sarcopenia in a wider DLBCL population has not been investigated.
Differences in muscle mass may also play a factor in DLBCL, sex‐specific outcomes. Males were identified as a DLBCL subgroup in the RICOVER60 study that did not have as large of an improvement in both progression free survival (PFS) and OS compared with females.14 In evaluating rituximab's pharmacokinetics, it was thought that males' higher weight (and by extension, muscle mass) increased rituximab's clearance thereby correlating with its decreased efficacy. Subsequent retrospective data confirmed a survival advantage favouring females.15 It appears contradictory that lower muscle mass in females compared with males would improve rituximab's ability to linger in circulation and therefore lead to a better prognosis while at the same time lower muscle mass is an indicator of poor prognosis in elderly DLBCL patients. A more unifying explanation would be that prognosis is more related to SMD than to age and sex. SMD does not differ between sexes as does skeletal muscle mass.1 This study explored whether underlying sex and age outcome differences in DLBCL were related to SMD and sarcopenia.
Materials and methods
Patients
Following institutional ethics review approval; DLBCL patients treated at a centralized, single institution of Northern Alberta (catchment population of >1.8 million) from 2004 to 2009 were reviewed. Patient demographics including age, stage, R‐IPI score at diagnosis, sex, height, weight, and performance status were collected. The chemotherapy regimen they received, number of cycles received, and best response to first‐line therapy were then documented. PFS and OS were collected as primary endpoints. Objective response rates (ORR) to chemoimmunotherapy was the secondary endpoint.
Body composition analysis
Height and weight at the first cycle of treatment were documented. Body composition was assessed using each patient's pre‐treatment, staging CT scans. To be included in review, CT scans must have been within 30 days prior to beginning therapy. To ensure image quality, CT scanner calibration was performed daily at start up with using air in the CT scanner gantry, then dynamically during scanning for individual patients using air as a negative control. Therefore, although CT tube current may fluctuate between patients, variability is minimal yielding the following CT parameters for each patient: contrast enhanced or unenhanced, 5 mm slice thickness, 120 kVp, and 290 mA. Two adjacent images at the L3 vertebral body level were used to measure total muscle surface area (cm2) and averaged. This vertebral landmark is chosen based on its linear correlation to total body lean body mass.16, 17 Muscles were quantified within a range of −29 to 150 HU using Slice‐O‐Matic software (version 5.0, TomoVision, Magog, Quebec, Canada). Measurements were normalized to patient's height in metres squared and expressed as skeletal muscle index (reported as cm2/m2).
SMD was quantified as mean muscle radiation attenuation (HU) of the muscle cross‐sectional area across the L3 vertebral body level was assessed between −29 and +150 HU.18
Statistical analysis
The general principle of SMD and skeletal muscle index analysis is that there is a threshold value (cut point) within these continuous variables, below which there is a significant increase in risk of mortality. Such statistically defined cut points have been described in large populations of patients with solid tumours, but mortality‐related cut point values for haematological malignancies are lacking.1 For that reason, this exploratory analysis in DLBCL adopted two approaches: (i) testing SMD and skeletal muscle index cut points defined by various sources in the literature including Lanic et al.,13 Prado et al.,19 and Martin et al.;1 and (ii) evaluation of cut point values within our population by a cut‐point analysis using minimal p‐value approach.20 The continuous variable was divided into different cut‐points and the cut‐point that provides maximum chi‐square or provides minimum p‐value is chosen to be the point for dichotomizing the continuous variable.
Kaplan–Meier methods were used to compare PFS and OS between these groups of high vs. low SMD and skeletal muscle index. Survival was then compared by log‐rank test and multivariate Cox proportional hazards modelling using sex and R‐IPI score as covariates. ORR was compared by chi‐squared analysis employing a two‐sided p‐value. Statistical analysis was done using Statistical Analysis System (SAS, version 9.3 from SAS Institute Incorporated, Cary, North Carolina).
Results
Patients
Between 2004 and 2009, 232 patients with DLBCL were treated with chemoimmunotherapy. Of those, 224 were eligible for review, with eight excluded because of either ineligible pre‐treatment CT images (6) or no images available for review (2). There were 124 males and 100 females. Median age at diagnosis was 62, with a range of 21 to 88 years. While median stage at diagnosis was III, the majority of patients presented with stage IV disease (46%). Median R‐IPI score was 3 with all patients receiving R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Patient characteristics are summarized in Table 1. For the entire cohort, 5 year PFS and OS were 69 and 72%, respectively.
Table 1.
Patients demographics and response to chemoimmunotherapy divided by skeletal muscle density (SMD) and low (≤25 kg/m2) or high (>25 kg/m2) body mass index (BMI)
| Demographic | All (224) | Low SMD (116) | High SMD (108) | Low vs. High SMD p‐value | ||
|---|---|---|---|---|---|---|
| Low BMI (43) | High BMI (73) | Low BMI (38) | High BMI (70) | |||
| Age at diagnosis, median (years) | 62 (21–88) | 65 | 69 | 49 | 56 | <0.0001 |
| Sex, # | 0.02 | |||||
| Male | 125 (56%) | 19 (44%) | 34 (47%) | 25 (66%) | 47 (59%) | |
| Female | 99 (44%) | 24 (56%) | 39 (53%) | 13 (34%) | 23 (41%) | |
| Body weight, median (kg) | 75.7 | 61.7 | 83.3 | 61.8 | 78.8 | <0.0001 |
| Body mass index, median (kg/m2) | 26.8 | 22.5 | 30.1 | 22.2 | 28.0 | <0.0001 |
| Stage, # | 0.24 | |||||
| I | 50 (22%) | 8 (19%) | 13 (18%) | 6 (16%) | 23 (33%) | |
| II | 44 (20%) | 5 (12%) | 13 (18%) | 12 (32%) | 14 (20%) | |
| III | 27 (12%) | 4 (9%) | 13 (18%) | 3 (8%) | 7 (10%) | |
| IV | 103 (46%) | 26 (60%) | 34 (47%) | 17 (45%) | 26 (33%) | |
| R‐IPI, # | 0.0001 | |||||
| 0 | 18 (8%) | 0 | 2 (3%) | 8 (21%) | 8 (11%) | |
| 1 | 44 (20%) | 5 (12%) | 12 (16%) | 9 (24%) | 18 (26%) | |
| 2 | 48 (21%) | 6 (14%) | 14 (19%) | 5 (13%) | 23 (33%) | |
| 3 | 58 (26%) | 18 (42%) | 21 (29%) | 7 (18%) | 12 (17%) | |
| 4 | 40 (18%) | 8 (19%) | 15 (21%) | 9 (24%) | 8 (11%) | |
| 5 | 16 (7%) | 6 (14%) | 9 (12%) | 0 | 1 (1%) | |
| Chemoimmunotherapy cycles received, median | 6 | 6 | 6 | 6 | 6 | |
| Best response to chemoimmunotherapy, # | 0.04 | |||||
| Complete response | 168 (75%) | 30 (70%) | 46 (63%) | 31 (82%) | 61 (87%) | 0.0007 |
| Partial response | 41 (18%) | 9 (21%) | 21 (29%) | 7 (18%) | 4 (6%) | |
| Stable disease | 2 (0.1%) | 1 (2%) | 0 | 0 | 1 (1%) | |
| Progressive disease | 13 (6%) | 3 (7%) | 6 (8%) | 0 | 4 (6%) | |
| Required at least 1 cycle delayed, # | 90 (40%) | 17 (40%) | 30 (41%) | 18 (47%) | 25 (36%) | 0.78 |
| CT measures | ||||||
| Median skeletal muscle area (cm2) | 130.7 | 109.1 | 126.0 | 127.6 | 157.6 | <0.0001 |
| Median skeletal muscle index (SMI, cm2/m2) | 47.7 | 40.4 | 46.5 | 45.0 | 54.3 | <0.0001 |
| SMD (HU) | 34.1 | 32.9 | 25.0 | 45.5 | 38.9 | <0.0001 |
Skeletal muscle density
Median SMD for the entire group was 34.1 HU. Cut‐point analysis revealed a substantial difference in survival with SMD at 40 and 33 HU for non‐overweight and overweight patients, respectively. These definitions are nearly identical to the 41 and 33 HU for non‐overweight and overweight solid tumour patients, respectively.1 Using one definition over the other changed the designation of only one patient between high or low SMD and did not meaningfully change outcomes—therefore, previously validated solid tumour SMD definitions were used for survival comparisons. Median PFS was not reached in either high or low SMD group, but patients with low SMD had profoundly poorer PFS (Figure 1, HR 2.28, 1.33–3.76, p = 0.002). At 5 years, PFS was 81 vs. 60% in high and low SMD patients, respectively (p = 0.001). Similarly, median OS in the high SMD group was not reached vs. 103.6 months in the low group (Figure 2, HR 3.50, 1.99–6.16, p < 0.0001). Five‐year OS was 86 vs. 58% (p < 0.0001) in high and low SMD patients, respectively.
Figure 1.
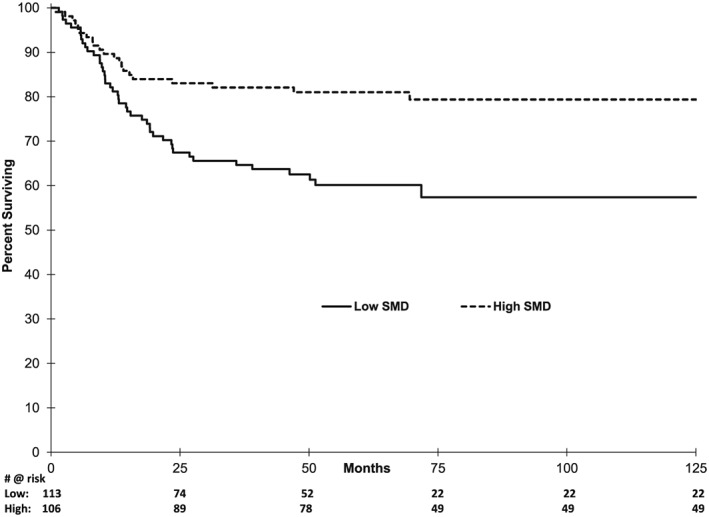
Kaplan–Meier (KM) analysis of progression free survival based on skeletal muscle density (SMD).
Figure 2.
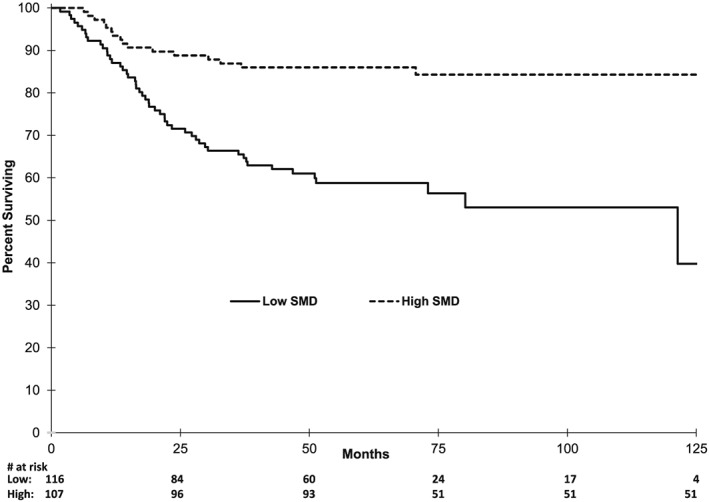
KM analysis of overall survival by SMD.
After adjusting for R‐IPI scores and sex in multivariate Cox proportional hazards regression (Table 2), low SMD retained prognostic significance in OS (HR 2.52, 1.40–4.54, p = 0.002, R 2 = 0.133). Its prognostic ability in PFS trended toward significance (HR 1.56, 0.90–2.71, p = 0.11)
Table 2.
Univariate and multivariate Cox proportional hazards modelling by skeletal muscle density (SMD), Revised International Prognostic Index (R‐IPI), and sex
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | p‐Value | Hazard ratio | 95% CI | p‐Value | |
| PFS | R‐IPI score 1–2 | 1.58 | 0.36–6.92 | 0.54 | 1.29 | 0.29–5.75 | 0.74 |
| R‐IPI score 3–5 | 5.39 | 1.31–22.17 | 0.02 | 4.20 | 0.98–17.88 | 0.05 | |
| Sex, male | 0.87 | 0.54–1.42 | 0.59 | 0.98 | 0.60–1.61 | 0.95 | |
| Low SMD | 2.28 | 1.35–3.83 | 0.002 | 1.56 | 0.90–2.71 | 0.11 | |
| OS | R‐IPI score 1–2 | 3.05 | 0.40–23.08 | 0.28 | 2.18 | 0.28–16.74 | 0.45 |
| R‐IPI score 3–5 | 10.13 | 1.40–73.31 | 0.02 | 6.26 | 0.84–46.4 | 0.07 | |
| Sex, male | 1.00 | 0.62–1.62 | 1 | 1.23 | 0.76–2.02 | 0.40 | |
| Low SMD | 3.45 | 1.96–6.07 | <0.0001 | 2.52 | 1.40–4.54 | 0.002 | |
Skeletal muscle index
There was no significant difference in PFS comparing patients above and below established skeletal muscle index levels set out by either Prado, Martin or Lanic et al. (p = 0.19, 0.17, and 0.23, respectively). OS comparison was also not significant by these three definitions. By cut‐point analysis, the most significant difference was identified at cross‐sectional muscle area of 53.3 cm2/m2 for males and 40.2 cm2/m2 for females. Using these levels, patients with low skeletal muscle index had a trend toward improved PFS (HR 0.65, 0.41–1.05, p = 0.08). This definition of skeletal muscle index was not prognostic of OS (HR 0.77, 0.48–1.25, p = 0.30).
Given previous evidence of poorer survival in sarcopenic elderly patients, a subgroup analysis of elderly in this cohort (as defined by Lanic et al. to be patients > 70 years of age) was done using Lanic et al. parameters and our cut‐points. Having skeletal muscle indices below Lanic et al.'s and our own cut‐points yielded PFS HRs of 0.24 (0.01–0.61, p = 0.003) and 0.19 (0.07–0.50, p = 0.0007), respectively. Similar to PFS, OS for patients with low skeletal muscle indices had HRs of 0.45 (0.21–0.99, p = 0.05) and 0.38 (0.17–0.82, p = 0.01), respectively.
Response to chemoimmunotherapy
In secondary endpoint analysis, ORR were very similar between high and low SMD groups (95.4 vs. 91.4%, p = 0.17). However, there was a strong association between high SMD patients and attaining a complete response (85 vs. 66%, p = 0.0007, Table 1). There was no difference in ORR based on skeletal muscle index.
Discussion
Values for skeletal muscle mass as estimated by skeletal muscle index have been used to describe sex and age‐based outcomes in DLBCL. Sarcopenia, the loss of skeletal muscle, has been associated with poorer elderly patient outcomes.13 Conversely, higher muscle mass is suggested as a reason for increased rituximab clearance and poorer outcomes in males.10, 15 In this study, low SMD appeared to be more prognostic of poor patient survival than skeletal muscle index. Low SMD is also similarly more prognostic than skeletal muscle index in melanoma, renal cell cancer, and follicular lymphoma.6, 7, 8 It is important to note that cut‐point analysis in our study independently established the same SMD parameters defined in solid organ malignancies—providing early validation of its continued use of these cut points in DLBCL.1 While additional software was used to retrospectively determine SMD levels in patients' pre‐treatment CT scans, mean muscle radiation attenuation is data routinely measured by CT scanners. As such, SMD is easily obtainable data that does not require any additional assays or equipment to complement R‐IPI in stratifying DLBCL patient prognosis.
However, the fact that SMD was strongly associated with complete response to rituximab‐based therapy raises questions as to whether it is simply prognostic or also a predictor of treatment response. Perhaps its ability to predict complete response is therefore also related to its PFS prognostic ability. Although multivariate PFS analysis did not quite meet statistical significance, it should be noted that the original R‐IPI was established based on its ability to predict OS.12 Our findings were meant to be exploratory and, therefore, in a retrospective model, cannot determine the answer to the question of whether SMD is a predictive or prognostic marker. Along those lines, further investigation as to whether low SMD can be reversed following chemoimmunotherapy (and whether this change then impacts outcomes) is currently being investigated.
A unifying theory explaining how ectopic fat infiltration of muscle develops is elusive and likely suggests that multiple factors are at play. Although early thinking suggested this phenomenon was part of normal ageing, larger studies now suggest that intermuscular fat development is more of a product of illness and/or inactivity.21, 22 The latter explanation may suggest that low SMD in this study may be a form of objective and quantitative performance status measurement. However, performance status is taken into account in R‐IPI scoring and so, for low SMD to be independently prognostic in a model that already includes performance status would seem surprising. Rather, based on differences in complete response to rituximab‐based therapy, low SMD may suggest a more substantial change in overall patient physiology as a result of DLBCL or cancer as a whole. Ectopic fatty infiltration of skeletal muscles has been observed in other diseases such as severe type 2 diabetes, where its presence is associated with particularly poor disease control and prognosis. This altered muscle physiology in diabetes is associated with elevated serum inflammatory markers such as tumour necrosis alpha, C‐reactive protein, and interleukin‐6.3 Speculating in this way, DLBCL patients with low SMD may have either more severe disease or a specific, pro‐inflammatory, host response that leads to changes in muscle physiology manifesting in both intermuscular fat deposition and poorer clinical outcomes. DLBCL itself is a heterogeneous disease made up of multiple subtypes: where non‐germinal centre disease is thought to behave more poorly than germinal centre type even with rituximab‐containing therapy.23, 24 Given that low SMD is also prognostic of follicular lymphoma outcomes,8 SMD is unlikely to be strictly related to non‐germinal centre DLBCL. Subsequent work is needed to properly characterize the pathways involved in fatty infiltration of muscle and how it may relate to lymphoma.
Lanic et al. sought to extrapolate findings in solid organ malignancies where sarcopenia is associated with poorer outcomes and apply it to elderly DLBCL patients.13 Their review found sarcopenia was more prevalent amongst patients ≥ 70 years old and predicted poorer survival. While sarcopenia is more prevalent in the elderly, the concept of poorer outcomes in sarcopenic patients should apply to the whole DLBCL population. However, our findings suggest that sarcopenic DLBCL patients in fact have a trend toward better PFS. As part of a pre‐planned subgroup analysis similar to the Lanic et al. group, elderly patients (age > 70 years) displayed an even more pronounced improvement in both PFS and OS. These findings would seem to not only contradict Lanic et al.'s findings, but it also speaks against findings in other solid organ tumours.1, 19, 25 An explanation as to why this stands in opposition to prior literature regarding sarcopenia is not currently available. One possible explanation might relate to the unusually high proportion of females in the study of Lanic et al..9 Pfreundschuh et al. 11 theorized that males had an increased clearance because of their higher average weight leading to less rituximab exposure and consequently poorer outcomes.14 It is possible that this concept may apply to this sarcopenic population. As a result of having less skeletal muscle mass, sarcopenic patients may have lower volumes of distribution and are therefore being exposed to relatively higher concentrations of rituximab as compared with similarly sized patients who have normal skeletal muscle mass. Therefore, these patients may have improved outcomes as a consequence of higher rituximab plasma concentrations. Prospective studies are needed to confirm this speculation.
Limitations of this study include inherent bias introduced by a retrospective analysis. Serum inflammatory markers were also not uniformly available across this sample because of its retrospective nature as well. During time points studied, eight cycles of R‐CHOP chemotherapy had not yet been thought to be equivalent to six cycles and was therefore still utilized. While R‐IPI accounts for Eastern Cooperative Oncology Group performance status, more objective performance measures such as walking speed or hand grip were clearly not evaluable retrospectively. Consequently, future directions include incorporating SMD assessment with R‐IPI prospectively. Given a possible link between SMD and inflammation, studies are underway to determine if there is an association between low SMD and serial measurements of inflammatory serum markers. A separate assessment of whether SMD changes with chemoimmunotherapy is also underway.
Disclaimers
None
Sources of support
None
Conflicts of interest
None
Presented in part at the 7th International Conference of the Society on Sarcopenia, Cachexia, and Wasting Disorders, December 9–11, 2013; and at the American Society of Hematology Annual Meeting 2013, December 5–9, 2013.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Saropenia, and Muscle update 2015.26 No funding was provided for this study, and all authors have no conflicts of interest to declare.
Chu, M. P. , Lieffers, J. , Ghosh, S. , Belch, A. , Chua, N. S. , Fontaine, A. , Sangha, R. , Turner, R. A. , Baracos, V. E. , and Sawyer, M. B. (2017) Skeletal muscle density is an independent predictor of diffuse large B‐cell lymphoma outcomes treated with rituximab‐based chemoimmunotherapy. Journal of Cachexia, Sarcopenia and Muscle, 8: 298–304. doi: 10.1002/jcsm.12161.
References
- 1. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol: official J of the Am Society of ClinOncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 2. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care 2010;13:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miljkovic I, Kuipers AL, Kammerer CM, Wang X, Bunker CH, Patrick AL, et al. Markers of inflammation are heritable and associated with subcutaneous and ectopic skeletal muscle adiposity in African ancestry families. Metab Syndr Relat Disord 2011;9:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tardif N, Salles J, Guillet C, Tordjman J, Reggio S, Landrier JF, et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2alpha activation. Aging Cell 2014;13:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579–3585. [DOI] [PubMed] [Google Scholar]
- 7. Antoun S, Lanoy E, Iacovelli R, Albiges‐Sauvin L, Loriot Y, Merad‐Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013. [DOI] [PubMed] [Google Scholar]
- 8. Chu MP, Lieffers J, Ghosh S, Belch AR, Chua NS, Fontaine A, et al. Skeletal muscle radio‐density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. PLoS One 2015;10:e0127589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moormeier JA, Williams SF, Golomb HM. The staging of non‐Hodgkin's lymphomas. Semin Oncol 1990;17:43–50. [PubMed] [Google Scholar]
- 10. Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP‐like chemotherapy with or without rituximab in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: 6‐year results of an open‐label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013–1022. [DOI] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi‐weekly CHOP‐14 with or without rituximab in elderly patients with aggressive CD20+ B‐cell lymphomas: a randomised controlled trial (RICOVER‐60). Lancet Oncol 2008;9:105–116. [DOI] [PubMed] [Google Scholar]
- 12. Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R‐IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Blood 2007;109:1857–1861. [DOI] [PubMed] [Google Scholar]
- 13. Lanic H, Kraut‐Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 2013. [DOI] [PubMed] [Google Scholar]
- 14. Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, et al. The role of sex and weight on rituximab clearance and serum elimination half‐life in elderly patients with DLBCL. Blood 2012;119:3276–3284. [DOI] [PubMed] [Google Scholar]
- 15. Riihijarvi S, Taskinen M, Jerkeman M, Leppa S. Male gender is an adverse prognostic factor in B‐cell lymphoma patients treated with immunochemotherapy. Eur J Haematol 2011;86:124–128. [DOI] [PubMed] [Google Scholar]
- 16. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 17. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied physiology, nutrition, and metabolism = Physiologie appliquee. Nutr metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 18. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 2000;89:104–110. [DOI] [PubMed] [Google Scholar]
- 19. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 20. Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time‐to‐event outcomes. Technical Report Series 2006 June 2006.
- 21. Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol 1985. 2008;105:1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys Ther 2011;91:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature 2000;403:503–511. [DOI] [PubMed] [Google Scholar]
- 24. Nyman H, Adde M, Karjalainen‐Lindsberg ML, Taskinen M, Berglund M, Amini RM, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B‐cell lymphoma patients treated with immunochemotherapy. Blood 2007;109:4930–4935. [DOI] [PubMed] [Google Scholar]
- 25. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin cancer Res: an official J of the Am Association for Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 26. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


