Abstract
Background
The aim of this study is to assess the prevalence of sarcopenia and investigate the associations between sarcopenia and long‐term mortality and readmission in a population of elderly inpatients in acute care wards.
Methods
We conducted a prospective observational study in the acute care wards of a teaching hospital in western China. The muscle mass was estimated according to a previously validated anthropometric equation. Handgrip strength was measured with a handheld dynamometer, and physical performance was measured via a 4 m walking test. Sarcopenia was defined according to the recommended diagnostic algorithm of the Asia Working Group for Sarcopenia. The survival status and readmission information were obtained via telephone interviews at 12, 24, and 36 months during the 3 year follow‐up period following the baseline investigation.
Results
Two hundred and eighty‐eight participants (mean age: 81.1 ± 6.6 years) were included. Forty‐nine participants (17.0%) were identified as having sarcopenia. This condition was similar in men and women (16.9% vs. 17.5%, respectively, P = 0.915). During the 3 year follow‐up period, 49 men (22.7%) and 9 women (16.4%) died (P = 0.307). The mortality of sarcopenic participants was significantly increased compared with non‐sarcopenic participants (40.8% vs. 17.1%, respectively, P < 0.001). After adjusting for age, sex and other confounders, sarcopenia was an independent predictor of 3 year mortality (adjusted hazard ratio: 2.49; 95% confidential interval: 1.25–4.95) and readmission (adjusted hazard ratio: 1.81; 95% confidential interval: 1.17–2.80).
Conclusions
Sarcopenia, which is evaluated by a combination of anthropometric measures, gait speed, and handgrip strength, is valuable to predict hospital readmission and long‐term mortality in elderly patients in acute care wards.
Keywords: Sarcopenia, Survival, Mortality, Readmission, Acute care
Introduction
Sarcopenia is defined as an age‐related loss of muscle mass, strength, and muscle function, and is currently considered a new geriatric syndrome.1 According to a recent systematic review, the prevalence of sarcopenia was approximately 1–29% in community‐dwelling populations, 14–33% in long‐term care populations, and 10% in a hospitalized population, depending on the characteristics of the study populations and the diagnostic methodology.2
Previous studies have indicated that sarcopenia was significantly associated with repeated falls, fractures, functional decline, disability, hospital and nursing home admissions, poor quality of life, and death. However, most previous studies that have focused on the effect of sarcopenia on mortality have been conducted in community‐dwelling elderly adults3 or nursing home residents.4
Evidence regarding the effect of sarcopenia on survival in hospitalized populations is relatively limited. Recently, Vetrano et al.5 recruited 770 elderly patients in acute care wards, measured the muscle mass via bioelectrical impedance analysis (BIA), and defined sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) criteria. The results indicated that sarcopenia was a significant predictor of 1 year mortality (hazard ratio [HR]: 1.59; 95% confidential interval [CI]: 1.10–2.41). Using similar methods, Cerri et al.6 applied the data from 103 acutely ill elderly patients with malnutrition or at risk of malnutrition and demonstrated that sarcopenia was associated with 3 month mortality (HR was not reported). In addition, Gariballa et al.7 estimated the muscle mass with the mid‐arm muscle circumference (MAMC) and used the EWGSOP criteria to define sarcopenia; they demonstrated that non‐sarcopenia was a protective factor for 6 month mortality in 432 hospitalized patients (HR: 0.45; 95% CI: 0.21–0.97). These evidences indicate that sarcopenia is an important prognostic factor to predict mortality in elderly patients in acute care hospitals, regardless of how it is measured.
However, it remains unclear whether sarcopenia is a predictor of long‐term mortality and readmission in hospitalized elderly patients. In addition, there are limited data that address sarcopenia and its adverse effects on clinical outcomes in non‐Caucasian elderly inpatients. We therefore conducted this study to (i) assess the prevalence of sarcopenia in a population of elderly inpatients in acute care wards and (ii) investigate the associations between sarcopenia and long‐term (3 years) mortality and readmission.
Methods
This study was conducted in the acute care wards of the Center of Gerontology and Geriatrics, West China Hospital of Sichuan University, which is located in Sichuan province, China. The study protocol was approved by the Research Ethics Committee of Sichuan University. All participants (or their legal proxies) signed a written informed consent.
Study population
All subjects aged 60 years and older who were living in the acute care ward of the Center of Gerontology and Geriatrics of West China Hospital between February and August, 2012, were considered eligible for this study. Subjects, who could not communicate with the interviewers or perform the gait speed (GS) test as a result of severe diseases, were excluded from the study. Subjects with cachexia as defined by the Society on Sarcopenia, Cachexia, and Wasting Disorders8 were also excluded. Three hundred and thirteen elderly subjects agreed to participate in this study.
Data collection
The trained interviewers collected the main data from all study subjects using questionnaires via face‐to‐face interviews within 48 hours of admission. The anthropometric measurements were also performed by trained technicians. All interviewers and technicians were trained using investigation manuals, multimedia materials, and simulated patients and were required to pass a training test prior to the formal investigation.
Assessment of sarcopenia
In this study, we adopted the recommended diagnostic algorithm of the Asia Working Group for Sarcopenia (AWGS).9 According to the AWGS recommendation, individuals with a low muscle mass plus either or both a low handgrip strength (HS) and a low gait speed were considered to have sarcopenia; in contrast, the individuals without a low muscle mass, low HS, and low GS were classified as ‘no sarcopenia’.
Muscle mass assessment
Body weight and height were measured using a wall‐mounted stadiometer and a digital floor scale to the nearest 0.1 cm and 0.1 kg, respectively. The body mass index (BMI) was calculated using the weight in kilograms divided by the square of the height in metres (kg/m2). We also measured the calf circumference (CC) with the subject placed in the supine position, with the left knee raised and the calf placed at a right angle to the thigh, using a millimetre‐graded tape to the nearest 0.1 cm.
The muscle mass was estimated by the appendicular skeletal muscle mass (ASM) using a previously validated equation in a Chinese population10:
The body weight, height, and age were measured in kilograms, centimetres, and years, respectively. For sex, the value 1 represented men, and the value 2 represented women.10 Using dual‐energy X‐ray absorptiometry (DEXA) as the gold standard,10 the adjusted R2 of the equation model was 0.90, and the Standard Error of Estimate was 1.63 kg.
After estimating the ASM values, the muscle mass index (SMI) was calculated using the ASM divided by the square of the height in metres (SMI = ASM/height2). In accordance with previous studies,11, 12, 13 the cut‐off of the SMI used in this study was based on the 20% lowest percentile of the study population; therefore, a low muscle mass was classified as an SMI less than 6.70 kg/m2 in men and less than 4.75 kg/m2 in women.
Handgrip strength measurement
The HS was measured using a handheld dynamometer based on strain gauge sensors (EH101, Xiangshan Inc., Guangdong, China) to the nearest 0.1 kg. Both hands were tested with the subject seated; the elbow was flexed at a 110° angle, the wrist was placed in a neutral position, and the interphalangeal joint of the index finger was positioned at a 90° angle. Three readings were obtained for each hand, and the highest value in either hand was used for the analyses. Using the cut‐off points from the AWGS consensus, low HS referred to <26 kg for men and <18 kg for women.9
Gait speed measurement
The GS was evaluated by measuring the participants' usual gait (in m/s) in a 4 m course. The participants were required to walk a 4 m course at their usual gait, with the use of walkers and canes if necessary.14 Using the cut‐off points from the AWGS consensus, a low GS was defined as less than 0.8 m/s9.
Nutritional and cognitive status
We assessed the participants' nutritional status using the revised Mini Nutritional Assessment short‐form.15 A score between 8 and 11 indicates that the subjects are ‘at risk of malnutrition’, whereas a score of ≤7 indicates that the subjects suffer from malnutrition. In addition, prealbumin was measured as a nutrition marker.
We also assessed the cognitive status using the Chinese version of the Mini‐Mental Status Examination.16 Cognitive impairment was defined as a score of Mini‐Mental Status Examination score ≤17 for illiterates, ≤20 for primary school graduates, and ≤24 for high school graduates or individuals with higher education.17
Covariates
The following covariates were collected from the hospital information systems and the face‐to‐face interviews: age, sex, education level (illiterates, primary school graduates, and high school graduates or above), physical activity in the previous 6 months (≥30 min/day or not), smoking status (current smoker or not), alcohol consumption status (current drinker or not), polypharmacy (defined as the concomitant use of five or more medications,18 yes or no), and comorbidities (hypertension, diabetes, ischemic heart disease, respiratory disease, central nervous system disease, gastrointestinal disease, liver disease, chronic kidney disease, osteoarthritis, tumour, acute infection, and falls in the previous 12 months). Depression was evaluated using the Chinese version of the 30‐item Geriatric Depression Scale; a score of ≥11 suggests depression.19 Haemoglobin was also tested for each participant.
Survival status and readmission information
The survival status and readmission information for all participants were obtained via telephone interviews at 12, 24, and 36 months during the 3 year period following the baseline investigation. The survival status was also confirmed using the Sichuan Province Death Registry. For the participants who died during the follow‐up, the period from the first investigation to the date of death was recorded; for the individuals who did not die during the study follow‐up, the period from the first investigation to the end of the last follow‐up was recorded. For the individuals who were readmitted to hospitals several times during the follow‐up, the first readmission time was recorded.
Statistical analyses
All statistical analyses were performed with SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). The categorical data were presented as absolute numbers and percentages (%) of the total. The continuous data were presented as the mean ± standard deviation (SD) if they were normally distributed; otherwise, they were presented as the median ± interquartile range. To compare the differences between groups, we used the Pearson chi‐squared test for categorical data, a one‐way ANOVA for continuous data with a normal distribution; and the Mann–Whitney U test for continuous data with an abnormal distribution. A P‐value less than 0.05 was considered statistically significant.
The time to death was calculated from the date of the baseline investigation to the date of death. Similarly, the time to readmission was calculated from the date of the baseline investigation to the date of the first readmission after discharge. The crude and adjusted HR and 95% CI for mortality and readmission by sarcopenia were estimated using Cox proportional‐hazard models. We adjusted age and sex, as well as other confounders which were significantly different between the sarcopenic group and the non‐sarcopenic group. As a result, Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, and hypertension. Model 3 was adjusted for age, sex, hypertension, nutrition status, BMI, and CC. In these models, the age, BMI, and CC were treated as continuous data, whereas the other variables were treated as categorical data. In addition, Kaplan–Meier curves of survival and readmission were analysed following an adjustment for age and sex to determine the impact of sarcopenia on mortality and readmission, respectively. The differences between the curves were assessed using the log‐rank test.
Results
Description of the study population
Of the 313 participants who agreed to participate in this study, 15 individuals were excluded because of severe disease and 10 individuals were excluded because of cachexia. The remaining 288 participants were included in the baseline analyses. Seventeen participants were lost during follow‐up, which led to a final sample of 271 participants. The mean age of the study participants was 81.1 ± 6.6 years, and 63 participants (21.9%) were women. According to the suggested algorithm of the AWGS, 49 subjects (17.0%) were identified as having sarcopenia (Figure 1). The prevalence of sarcopenia was similar in men and women (16.9% vs. 17.5%, respectively, P = 0.915).
Figure 1.
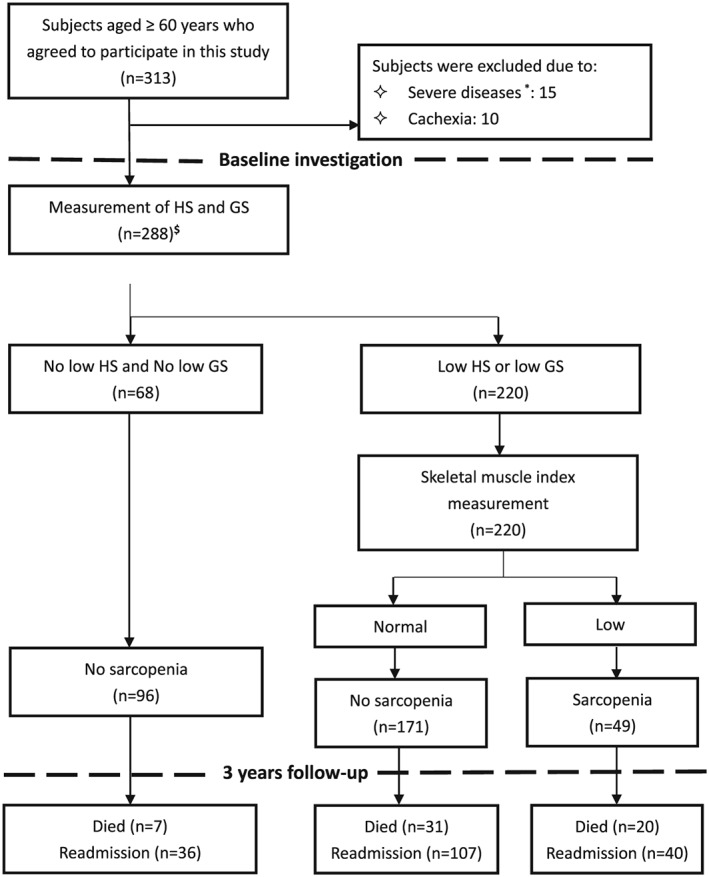
Study profile using the diagnostic algorithm recommended by the Asian Working Group for Sarcopenia (AWGS). GS: gait speed; HS: handgrip strength. * Only those who could not communicate with the interviewers or perform the gait speed test due to severe diseases were excluded. Among the 15 excluded patients, 4 suffered from tumor of any type. $ Among the 288 included patients, 45 suffered from tumor of any type. However, none of these patients with tumor received tumor‐directed therapy (e.g. chemotherapy, immunotherapy, or radiation) during the duration of hospital stay at the baseline investigation.
The baseline characteristics of the study population are presented in Table 1. The subjects with sarcopenia were older compared with the subjects without sarcopenia (mean age 83.7 vs. 80.5, respectively, P < 0.002). Compared with the subjects without sarcopenia, the sarcopenic subjects were more likely to be ‘at risk of malnutrition’, have malnutrition, and exhibit a lower BMI and CC. The common comorbidities (with the exception of hypertension) were similar between the sarcopenic and non‐sarcopenic subjects. In addition, the sarcopenic subjects appeared to have lower haemoglobin and prealbumin; however, the difference was not statistically significant.
Table 1.
Baseline characteristics of participants with or without sarcopenia
| Characteristic | No sarcopenia (n = 239) | Sarcopenia (n = 49) | P |
|---|---|---|---|
| Age (years) | 80.5 ± 6.6 | 83.7 ± 5.9 | 0.002 |
| Men | 187 (78.2) | 38 (77.6) | 0.915 |
| Current smokers | 21 (8.8) | 7 (14.3) | 0.484 |
| Current alcohol drinkers | 29 (12.1) | 4 (8.2) | 0.427 |
| Physical activity ≥30 min/day | 111 (46.0) | 25 (51.0) | 0.523 |
| Comorbidities | |||
| Hypertension | 151 (63.2) | 23 (46.9) | 0.034 |
| Ischemic heart disease | 88 (36.8) | 14 (28.6) | 0.271 |
| Respiratory disease | 63 (26.4) | 17 (34.7) | 0.235 |
| Acute infection | 51 (21.3) | 14 (28.6) | 0.270 |
| Liver disease | 20 (8.4) | 6 (12.2) | 0.388 |
| CKD | 34 (14.2) | 6 (12.2) | 0.715 |
| CNS disease | 12 (5.0) | 3 (6.1) | 0.752 |
| Diabetes | 77 (32.2) | 9 (18.4) | 0.054 |
| Osteoarthritis | 64 (26.8) | 11 (22.4) | 0.529 |
| Tumour of any type | 38 (15.9) | 7 (14.3) | 0.777 |
| Falls in the previous 12 months | 33 (13.8) | 7 (14.3) | 0.930 |
| GI disease | 20 (8.4) | 4 (8.2) | 0.926 |
| Depression | 55 (23.0) | 15 (30.6) | 0.259 |
| Cognitive impairment | 79 (33.1) | 21 (42.9) | 0.189 |
| Polypharmacy | 113 (47.3) | 17 (34.7) | 0.107 |
| Nutrition status | |||
| At risk of malnutrition | 96 (40.2) | 30 (61.2) | <0.001 |
| Malnutrition | 19 (7.9) | 10 (20.4) | |
| BMI (kg/m2) | 23.3 ± 3.3 | 19.0 ± 2.5 | <0.001 |
| CC (cm) | 33.0 ± 4.0 | 30.7 ± 4.2 | <0.001 |
| Gait speed (m/s) | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.212 |
| Handgrip strength (kg) | 21.5 ± 8.7 | 17.5 ± 8.0 | 0.004 |
| SMI (kg/m2) | 7.8 ± 1.0 | 6.7 ± 1.0 | <0.001 |
| Haematological parameters | |||
| Haemoglobin, g/l | 123.9 ± 24.5 | 117.3 ± 21.2 | 0.078 |
| Prealbumin, mg/l | 200.9 ± 55.1 | 189.8 ± 55.3 | 0.459 |
Data are presented as the number (per cent) for the following variables: women, current smokers, current alcohol drinkers, physical activity, specific comorbidities, polypharmacy, and nutrition status. For the other variables, the mean ± SD are used.
One‐way ANOVA was used for the continuous variables, and the Pearson chi‐squared test was used for the categorical variables. During testing, P < 0.05 was considered statistically significant.
BMI, body mass index; CC, calf circumference; CKD, chronic kidney disease; CNS, central nervous system; GI, gastrointestinal; SMI, skeletal muscle index.
Association between sarcopenia and 3‐year mortality
During the 3 year follow‐up, 49 men (22.7%) and 9 women (16.4%) died (P = 0.307). The mortality of the sarcopenic subjects was significantly increased compared with the non‐sarcopenic subjects (40.8% vs. 17.1%, respectively, P < 0.001). As indicated in Table 2, sarcopenia was an independent predictor of 3 year mortality (HR: 2.67, 95% CI: 1.55–4.60) in the unadjusted model. Following an adjustment for the potential confounders, the association between sarcopenia and 3 year mortality remained significant (Table 2). In the full adjusted model, the sarcopenic subjects exhibited an increased 3 year mortality compared with the non‐sarcopenic subjects (HR: 2.49; 95% CI: 1.25–4.95).
Table 2.
Association between sarcopenia and mortality (3 year follow‐up) according to Cox regression models adjusted for potential confounders
| Unadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Sarcopenia | 2.67 (1.55–4.60) | 2.26 (1.29–3.95) | 2.33 (1.32–4.12) | 2.49 (1.25–4.95) |
| Age | 1.63 (0.80–3.32) | 1.66 (0.81–3.39) | 1.42 (0.69–2.92) | |
| Sex (men) | 1.07 (1.02–1.12) | 1.07 (1.02–1.11) | 1.07 (1.02–1.12) | |
| Hypertension | 1.18 (0.69–2.02) | 1.04 (0.59–1.86) | ||
| Malnutrition | 2.09 (1.09–4.02) | |||
| At risk of malnutrition | 1.90 (0.69–5.28) | |||
| BMI | 1.09 (0.99–1.18) | |||
| CC | 1.02 (0.94–1.09) |
Data are presented as hazard ratios (95% confidential intervals). Model 1: adjusted for age and sex. Model 2: adjusted for age, sex and hypertension. Model 3: adjusted for age, sex, hypertension, nutrition status, BMI, and CC.
BMI, body mass index; CC, calf circumference.
The effect of sarcopenia on the 3 year mortality was tested by comparing the Kaplan–Meier survival curves for mortality. The survival curves of the subjects with or without sarcopenia are presented in Figure 2. The survival curves were significantly different in the log‐rank test (P < 0.001).
Figure 2.
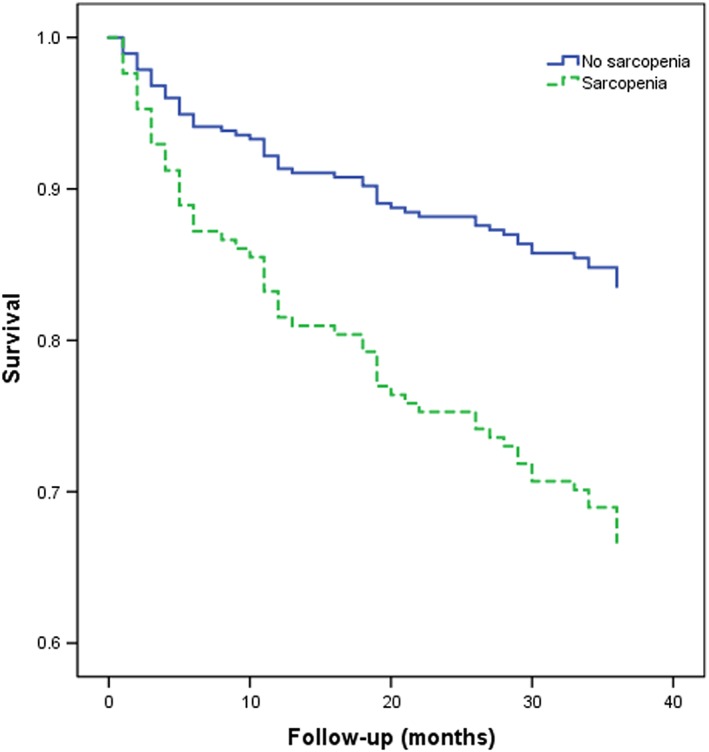
Survival curves of the study population according to sarcopenia at baseline. Survival curves significantly differed in the log‐rank test (P < 0.001).
In addition, malnutrition was also independently associated with mortality (HR: 2.09; 95% CI: 1.09–4.02).
Association between sarcopenia and readmission
During the 3 year follow‐up, 150 men (69.4%) and 33 women (60.0%) were readmitted to the hospital at least one time (P = 0.182). The subjects with sarcopenia were more likely to be readmitted to hospital compared with the subjects without sarcopenia (71.0% vs. 56.3%, respectively, P = 0.027). As presented in Table 3, sarcopenia was an independent predictor for readmission (HR: 1.82; 95% CI 1.28–2.59) in the unadjusted model. Following an adjustment for age, sex, hypertension, nutrition status, BMI, and CC, the sarcopenic subjects remained at an increased risk of readmission compared with the subjects without sarcopenia (HR: 1.81; 95% CI: 1.17–2.80).
Table 3.
Association between sarcopenia and readmission (3 year follow‐up) according to Cox regression models adjusted for potential confounders
| Unadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Sarcopenia | 1.82 (1.28–2.59) | 1.67 (1.16–2.39) | 1.67 (1.16–2.41) | 1.81 (1.17–2.80) |
| Age | 1.37 (0.94–2.00) | 1.37 (0.94–2.01) | 1.45 (0.97–2.17) | |
| Sex (men) | 1.03 (1.01–1.06) | 1.03 (1.01–1.06) | 1.03 (1.01–1.05) | |
| Hypertension | 1.01 (0.74–1.36) | 1.01 (0.73–1.38) | ||
| Malnutrition | 1.02 (0.72–1.44) | |||
| At risk of malnutrition | 1.00 (0.59–1.70) | |||
| BMI | 1.03 (0.98‐1.08) | |||
| CC | 0.98 (0.94‐1.02) |
Data are presented as hazard ratios (95% confidential intervals). Model 1: adjusted for age and sex. Model 2: adjusted for age, sex and hypertension. Model 3: adjusted for age, sex, hypertension, nutrition status, BMI, and CC.
BMI, body mass index; CC, calf circumference.
Kaplan–Meier curves for readmission were also applied to assess the effect of sarcopenia on readmission (Figure 3). The survival curves were significantly different in the log‐rank test (P = 0.019).
Figure 3.
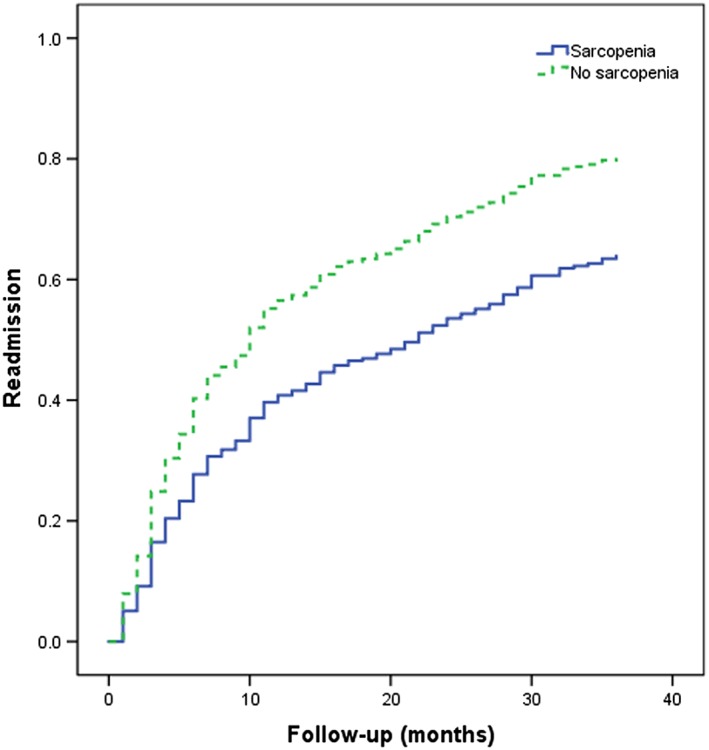
Kaplan–Meier curves for readmission according to sarcopenia at baseline. These curves significantly differed in the log‐rank test (P = 0.019).
Discussion
To the best of our knowledge, this study is the first investigation to address the associations between sarcopenia and long‐term mortality and readmission in a longitudinal study of Chinese hospitalized elderly patients. This study indicates that sarcopenia is common (17.0%) in Chinese elderly patients in acute geriatric wards. Sarcopenia, which is defined by a low muscle mass estimated by anthropometric measurements, a low HS, and a low GS, is associated with long‐term mortality and readmission, independent of age, sex, and other potential confounders.
Numerous studies have addressed the prevalence of sarcopenia in various populations; however, relatively limited evidence is available regarding the prevalence of sarcopenia in acute care geriatric settings. Rossi and colleagues20 applied the diagnostic criteria of the EWGSOP and estimated the muscle mass via BIA. They reported that one of four elderly patients had sarcopenia in acute care departments. Vetrano et al.5 applied similar diagnostic methods and determined that 28% of patients in acute care wards were sarcopenic. However, using the EWGSOP criteria and estimating the muscle mass via the MAMC, Gariballa et al.7 identified only 10% of participants as having sarcopenia among geriatric inpatients. This study applied the AWGS criteria and measured the muscle mass with an anthropometric equation. We identified 17.0% of participants with sarcopenia. It is difficult to compare the results from these different studies because of the various measurement methods, diagnostic criteria, and study populations.
Furthermore, this study demonstrated that sarcopenia may also represent a prognostic factor for readmission during the 3 year follow‐up. This finding was consistent with a previous study.7 In their manuscript, Gariballa et al.7 reported that the risk of readmission during the 6 month follow‐up was significantly decreased in patients without sarcopenia compared with patients with sarcopenia. Additional prospective studies are necessary to confirm this finding in different study populations.
Both malnutrition and sarcopenia are common in elderly patients, especially in acute hospitalized elderly patients.21 In this study, we demonstrated that malnutrition was independently associated with mortality. This finding was consistent with previous studies.22 For example, Cederholm et al. reported that mortality after hospitalization was significantly increased in patients with malnutrition compared with well‐nourished patients.23 Previous studies have also indicated that malnutrition was associated with hospital readmission23, 24; however, we failed to identify this association in our study population.
There are several limitations in this study. First, we estimated the muscle mass with an anthropometric equation, which has previously been validated in Chinese individuals, rather than BIA or DEXA as recommended by the EWGSOP25 and AWGS.9 Anthropometric measures are considered poor markers of muscle mass.25 However, DEXA is expensive and has a risk of X‐ray exposure. BIA is rarely available in hospitals in mainland China, and the cut‐off points of BIA for defining low muscle mass in Chinese elderly individuals have not been established.26, 27 Furthermore, common conditions in hospitalized patients (e.g., fluid and electrolytes imbalance) may lead to erroneous results for BIA.28 Therefore, DEXA and BIA were not appropriate for our study population. More importantly, recent studies that assessed sarcopenia and mortality in elderly individuals used the same concepts of muscle strength and GS; however, the muscle mass was measured in difference ways (BIA,4 CC,29 and MAMC,30 respectively). Regardless of these differences, similar HR values of sarcopenia for mortality were identified. This finding implies that the different measurement techniques for estimating the muscle mass (including anthropometric measures) are all valid for predicting mortality, regardless of the gold standard of DEXA. Furthermore, in a recent study, Alexandre et al.13 demonstrated that the use of anthropometric equations to estimate the muscle mass combined with the handgrip and GS may comprise an important alternative to DEXA to improve the diagnosis of sarcopenia and reduce costs. This study confirmed the conclusion of Alexandre's study in a Chinese elderly inpatient population.
Second, although we adjusted for many regular confounders in this study, unmeasured factors (e.g. some geriatric syndromes, activity of daily living, and interventions) may bias the study results. Third, we did not measure the participants' functional independence, which may serve as an important confounder. Fourth, we excluded patients who could not perform the walking test as well as those with cachexia. This may result in an underestimation of sarcopenia. Fifth, we did not apply the Charlson Comorbidity Index31 to assess chronic comorbidities in the study population. Using this standard index of comorbidity may benefit for comparisons between studies. Finally, similar to all cohort studies, selective survival prior to entry in the cohort should be considered.
Conclusion
Sarcopenia, which is evaluated by a combination of anthropometric measures, GS, and HS, is valuable in the prediction of hospital readmission and long‐term mortality in Chinese elderly patients in acute care wards. Additionally, prospective studies with large sample size are warranted to assess the value of different diagnostic methods (BIA, DEXA, and anthropometric measures) in the prediction of adverse outcomes in different clinical settings.
Conflicts of interest
None declared.
Acknowledgements
We thank all individuals who worked as volunteers in this study for their support. This work was supported by the National Department Public Benefit Research Foundation by the Ministry of Health P. R. China [no. 201002011] and the Key Technologies Research and Development Program by the Science & Technology Department of Sichuan Province [no. 2015GZ0344]. The sponsor had no role in the design, methods, data collection, analysis or preparation of this manuscript. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.32
Yang, M. , Hu, X. , Wang, H. , Zhang, L. , Hao, Q. , and Dong, B. (2017) Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. Journal of Cachexia, Sarcopenia and Muscle, 8: 251–258. doi: 10.1002/jcsm.12163.
References
- 1. Cruz‐Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care 2010;13:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age & Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247–252. [DOI] [PubMed] [Google Scholar]
- 4. Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–126. [DOI] [PubMed] [Google Scholar]
- 5. Vetrano DL, Landi F, Volpato S, Corsonello A, Meloni E, Bernabei R, et al. Association of sarcopenia with short‐ and long‐term mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol A Biol Sci Med Sci 2014;69:1154–1161. [DOI] [PubMed] [Google Scholar]
- 6. Cerri AP, Bellelli G, Mazzone A, Pittella F, Landi F, Zambon A, et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clin Nutr (Edinburgh, Scotland) 2015;34:745–751. [DOI] [PubMed] [Google Scholar]
- 7. Gariballa S, Alessa A. Sarcopenia: Prevalence and prognostic significance in hospitalized patients. Clin Nutr 2013;32:772–776. [DOI] [PubMed] [Google Scholar]
- 8. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: A new definition. Clin Nutr (Edinburgh, Scotland) 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 9. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: Consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 10. Wen X, Wang M, Jiang CM, Zhang YM. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr 2011;20:551–556. [PubMed] [Google Scholar]
- 11. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 12. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 13. Alexandre Tda S, Duarte YA, Santos JL, Wong R, Lebrao ML. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging 2014;18:751–756. [DOI] [PubMed] [Google Scholar]
- 14. Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, et al. Cognitive function, gait speed decline, and comorbidities: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62:844–850. [DOI] [PubMed] [Google Scholar]
- 15. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short‐form (MNA‐SF): A practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782–788. [DOI] [PubMed] [Google Scholar]
- 16. Liu HC, Lin KN, Teng EL, Wang SJ, Fuh JL, Guo NW, et al. Prevalence and subtypes of dementia in Taiwan: A community survey of 5297 individuals. J Am Geriatr Soc 1995;43:144–149. [DOI] [PubMed] [Google Scholar]
- 17. Cui GH, Yao YH, Xu RF, Tang HD, Jiang GX, Wang Y, et al. Cognitive impairment using education‐based cutoff points for CMMSE scores in elderly Chinese people of agricultural and rural Shanghai China. Acta Neurol Scand 2011;124:361–367. [DOI] [PubMed] [Google Scholar]
- 18. Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–995. [DOI] [PubMed] [Google Scholar]
- 19. Chan AC. Clinical validation of the Geriatric Depression Scale (GDS): Chinese version. J Aging Health 1996;8:238–253. [DOI] [PubMed] [Google Scholar]
- 20. Rossi AP, Fantin F, Micciolo R, Bertocchi M, Bertassello P, Zanandrea V, et al. Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc 2014;15:303.e7‐e12. [DOI] [PubMed] [Google Scholar]
- 21. Vandewoude MF, Alish CJ, Sauer AC, Hegazi RA. Malnutrition‐sarcopenia syndrome: Is this the future of nutrition screening and assessment for older adults? J Aging Res 2012;2012:651570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hebuterne X. Malnutrition in the elderly: From sarcopenia to cachexia. Nutr Clin Metab 2003;17:24–35. [Google Scholar]
- 23. Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3‐year mortality. Clin Nutr (Edinburgh, Scotland) 2012;31:345–50. [DOI] [PubMed] [Google Scholar]
- 24. Ulltang M, Vivanti AP, Murray E. Malnutrition prevalence in a medical assessment and planning unit and its association with hospital readmission. Aust Health Rev 2013;37:636–641. [DOI] [PubMed] [Google Scholar]
- 25. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng P, Wu S, Han Y, Liu J, Zhang Y, Zhang E, et al. Differences in body composition and physical functions associated with sarcopenia in Chinese elderly: Reference values and prevalence. Arch Gerontol Geriatr 2015;60:118–123. [DOI] [PubMed] [Google Scholar]
- 27. Wen X, Wang M, Jiang CM, Zhang YM. Are current definitions of sarcopenia applicable of older Chinese adults? J Nutr Health Aging 2011;15:847–851. [DOI] [PubMed] [Google Scholar]
- 28. Bosaeus I, Wilcox G, Rothenberg E, Strauss BJ. Skeletal muscle mass in hospitalized elderly patients: comparison of measurements by single‐frequency BIA and DXA. Clin Nutr (Edinburgh, Scotland) 2014;33:426–431. [DOI] [PubMed] [Google Scholar]
- 29. Arango‐Lopera VE, Arroyo P, Gutierrez‐Robledo LM, Perez‐Zepeda MU, Cesari M. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 2013;17:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landi F, Cruz‐Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013;42:203–209. [DOI] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 32. von Haehling S, Morley JE, Coats ASJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


