Abstract
Background
Adequate muscle fibre perfusion is critical for the maintenance of muscle mass; it is essential in the rapid delivery of oxygen, nutrients and growth factors to the muscle, stimulating muscle fibre growth. Muscle fibre capillarization is known to decrease substantially with advancing age. However, whether (relative) low muscle fibre capillarization negatively impacts the muscle hypertrophic response following resistance exercise training in older adults is unknown.
Methods
Twenty‐two healthy older men (71 ± 1 years) performed 24 weeks of progressive resistance type exercise training. To assess the change in muscle fibre characteristics, percutaneous biopsies from the vastus lateralis muscle were taken before and following 12 and 24 weeks of the intervention programme. A comparison was made between participants who had a relatively low type II muscle fibre capillary‐to‐fibre perimeter exchange index (CFPE; LOW group) and high type II muscle fibre CFPE (HIGH group) at baseline. Type I and type II muscle fibre size, satellite cell, capillary content and distance between satellite cells to the nearest capillary were determined by immunohistochemistry.
Results
Overall, type II muscle fibre size (from 5150 ± 234 to 6719 ± 446 µm2, P < 0.05) and satellite cell content (from 0.058 ± 0.006 to 0.090 ± 0.010 satellite cells per muscle fibre, P < 0.05) had increased significantly in response to 24 weeks of resistance exercise training. However, these improvements where mainly driven by differences in baseline type II muscle fibre capillarization, whereas muscle fibre size (from 5170 ± 390 to 7133 ± 314 µm2, P < 0.05) and satellite cell content (from 0.059 ± 0.009 to 0.102 ± 0.017 satellite cells per muscle fibre, P < 0.05) increased significantly in the HIGH group, no significant changes were observed in LOW group following exercise training. No significant changes in type I and type II muscle fibre capillarization were observed in response to 12 and 24 weeks of resistance exercise training in both the LOW and HIGH group.
Conclusions
Type II muscle fibre capillarization at baseline may be a critical factor for allowing muscle fibre hypertrophy to occur during prolonged resistance exercise training in older men.
Keywords: Capillary, Exercise training, Elderly, Fibre growth, Skeletal muscle
Introduction
The progressive loss of skeletal muscle mass and strength is a hallmark of ageing and is referred to as sarcopenia. The impact of sarcopenia on health and well‐being is broad and includes impaired function, increased morbidity and increased incidence of institutionalization, reduced quality of life and even death.1, 2, 3, 4, 5 It has been hypothesized that with ageing, skeletal muscle tissue becomes less sensitive to the main anabolic stimuli, i.e. nutrition and physical activity.6, 7, 8, 9 This reduced muscle protein synthetic response is also referred to as ‘anabolic resistance’ and may be a key factor in the development of sarcopenia.10, 11 Although the underlying mechanisms of age‐related anabolic resistance is not fully understood, an emerging body of literature suggests that impairment in muscle fibre perfusion may play a key role.8, 12, 13
Adequate muscle tissue perfusion is critical in muscle mass maintenance and growth, as it is essential in the delivery of oxygen, nutrients and growth factors to the muscle, stimulating muscle protein synthesis. However, arterial blood flow can be reduced in the lower extremities under both post‐absorptive and post‐prandial conditions in older individuals by 20–30%.12, 14, 15, 16 This age‐related reduction in muscle blood flow appears to be independent of muscle mass and related to chronic vasoconstriction, lower O2 demand and/or decreased endothelial wall function.17, 18 Furthermore, the structure of the microvascular bed is known to change substantially with advancing age, particularly as it relates to type II muscle fibre capillarization that decreases with age.19, 20 The observed reduction in muscle fibre capillarization will likely translate to a decrease in overall perfusion of the muscle tissue and increase in the diffusion distance of circulating anabolic signals released from the capillaries to specific target cells in muscle tissue. For example, we19 as well as others21 have shown that a spatial relationship exists between muscle fibre capillaries and muscle stem cells, also known as satellite cells.21 As satellite cells are the main (or only) source of additional myonuclei, they are considered to be important in repair, remodelling and growth of skeletal muscle.22, 23 Activated and differentiating satellite cells are located closer to its nearest capillary as compared with quiescent satellite cells.19, 21 We have recently shown that type II muscle fibre satellite cells are located at a greater distance to its nearest capillary in older as compared with young adults.19 A decline in capillary density and, as such, greater distance between satellite cell and capillaries in older adults may be an important factor in the impaired recovery of skeletal muscle following exercise.24, 25, 26, 27
Resistance exercise training is an effective strategy to increase skeletal muscle mass and strength in healthy and frail older adults.28, 29, 30, 31, 32 As such, resistance exercise training is now the most commonly recommended exercise modality to combat the loss of muscle mass with age. Recent studies have shown that resistance exercise training can correct the age‐related reduction in whole leg blood flow following feeding or feeding combined with exercise.8, 12 However, discrepant results have been reported on the efficacy of resistance exercise training in the restoration of muscle fibre capillary density in healthy older adults. Whereas some do,33, 34 other studies do not35 report an increase in capillary density in response to resistance exercise training in older adults. In addition, whether capillary density is an important factor in the muscle fibre, hypertrophic response following prolonged exercise training in older adults is unknown. Therefore, in the current study, we determined whether whole‐body resistance exercise training is an effective strategy to increase muscle fibre capillarization in healthy older men. In addition, we hypothesized that muscle fibre capillarization at baseline is a critical factor in the skeletal muscle hypertrophic response during prolonged exercise training in older adults.
Methods
Subjects
Twenty‐two healthy older men (age: 71 ± 1 years, height: 1.76 ± 0.01 m, BMI: 27.4 ± 0.6 kg · m−2) were recruited to participate in a 24 week progressive resistance exercise training programme. During an initial screening visit, medical history was evaluated and a resting electrocardiogram and submaximal electrocardiogram were performed prior to inclusion. Participants were excluded in case of (silent) cardiac, peripheral vascular disease or orthopaedic or any other condition that would preclude successful participation in the exercise programme. Furthermore, an oral glucose tolerance test was performed to exclude type 2 diabetes patients from participation. All participants had not participated in any structured exercise training programme in the past 5 years and were all living independently. All participants were informed on the nature and possible risks of the experimental procedures before their written informed consent were obtained. This study was approved by the Medical Ethics Committee of Maastricht University Medical Centre and complied with the guidelines set out in the Declaration of Helsinki. This study was part of a greater project investigating the impact of prolonged resistance exercise training on skeletal muscle mass/strength, and metabolic health in older adults.28, 36, 37 Participants were selected based upon the availability of a muscle biopsy sample on all time points during the intervention programme. To assess the impact of baseline muscle fibre capillarization on the muscle fibre hypertrophic response after resistance exercise training, participants were retrospectively divided into two equal groups (n = 11 per group) based on type II muscle fibre capillarization (corrected for capillary sharing factor and muscle fibre perimeter, also known as capillary‐to‐fibre perimeter exchange (CFPE) index) at baseline. This resulted in a group with a relatively low (LOW; CFPE: 4.4 ± 0.2 capillaries · 1000 µm−1) and high (HIGH; CFPE 6.1 ± 0.4 capillaries · 1000 µm−1) type II muscle fibre CFPE index.
Exercise intervention programme
Supervised resistance exercise was performed three times a week for a 24 week period. Training consisted of a 5 min warm‐up on a cycle ergometer, followed by four sets on both leg press and leg extension machines (Technogym, Rotterdam, the Netherlands). In addition, three sets were performed on the chest press and horizontal row, and (alternating) vertical lat pull‐down and abdominal crunches, or biceps curl and triceps extension. During the first 4 weeks of the training period, the workload was gradually increased from 60% (10–15 repetitions) of 1RM to 80% of 1RM (8–10 repetitions). Starting at week 5, four sets of eight repetitions were performed at 75–80% of 1RM on leg press and leg extension. For the upper body exercises, two sets were increased to three sets starting in week 5. Resting periods of 1.5 and 3 min were allowed between sets and exercises, respectively. Each session ended with a 5 min cool down period on the cycle ergometer. Workload was increased when more than eight repetitions could be performed in three out of four sets. In addition, workload intensity was adjusted based on 1RM tests (performed at weeks 4, 8, 12, 16 and 20).
Muscle biopsy sampling
Three days prior to the start and following 12 and 24 weeks of the intervention (4 days after the final strength test), muscle biopsies were taken from the right leg of each participant in the morning after an overnight fast. After local anaesthesia was induced in the skin, percutaneous needle biopsy samples (50–80 mg) were collected from the vastus lateralis muscle, approximately 15 cm above the patella. Any visible non‐muscle tissue was removed immediately, and biopsy samples were embedded in Tissue‐Tek (Sakura Finetek, Zoeterwoude, the Netherlands), frozen in liquid nitrogen‐cooled isopentane and stored at −80°C until further analyses.
Immunohistochemistry
Frozen muscle biopsies were cut into 5 µm thick cryosections using a cryostat at −20 C and thaw mounted on uncoated pre‐cleaned glass slides. Samples from baseline and after 12 and 24 weeks of resistance exercise training were mounted together on the same glass slide. Samples were stained with antibodies against Pax7 (1:1, cell supernatant from cells obtained from the DSHB, USA), myosin heavy chain type I (clone A4.951 (slow isoform), neat; DSHB, USA), laminin (1:1000, Abcam ab11575, Abcam, Cambridge, MA, USA), CD31 (ab28364 1:30, Abcam, Cambridge, MA, USA). For immunofluorescent detection, secondary antibodies used were as follows: Pax7 (goat anti‐mouse biotin 1:200, streptavidin 594 1:500, Invitrogen, Molecular Probes, Carlsbad, CA, USA); myosin heavy chain type I (clone A4.591) (goat anti‐mouse Alexa Fluor 488, 1:500, Invitrogen); laminin (goat anti‐rabbit Alexa Fluor 488, 1:500, Invitrogen); CD31 (goat anti‐rabbit Alexa Fluor 647, 1:500, Invitrogen, Molecular Probes, Carlsbad, CA, USA). Nuclei were labelled with DAPI (4′,6‐diamidino‐2‐phenylindole) (1:20000, Sigma‐Aldrich, Oakville, ON, Canada), prior to cover slipping slides with fluorescent mounting media (DAKO, Burlington, ON, Canada). Histochemical methods were adapted from previous published methods.19
Slides were viewed with the Nikon Eclipse Ti Microscope (Nikon Instruments Inc., USA), equipped with a high‐resolution Photometrics CoolSNAP HQ2 fluorescent camera (Nikon Instruments, Melville, NY, USA). Images were captured and analysed using the Nikon NIS Elements AR 3.2 software (Nikon Instruments Inc., USA). All images were obtained with the 20× objective, and at least 200 muscle fibres/subject/time point were included in the analyses for satellite cell content, capillary content, fibre size, and fibre perimeter. Fibre circularity was calculated (4π · fibre size)/(perimeter)2. Whereas no difference over time was observed in the LOW group, type II muscle fibre circularity was significantly higher at 12 and 24 weeks of resistance exercise training compared with baseline in the HIGH group (see Table S1). The quantification of muscle fibre capillaries was performed on ≥ 50 muscle fibres/subject/time point. Based on previous work,33 quantification was made of (i) capillary contacts (CC), (ii) the capillary‐to‐fibre ratio (C/Fi) and (iii) capillary‐to‐fibre perimeter exchange (CFPE) index. The SC‐to‐capillary distance measurements were performed on all satellite cells that were adjoined by other fibres. The measurement was taken by identifying a satellite cell (i.e., Pax7+ co‐localized with DAPI, beneath the basal lamina, identified by laminin) and tracing the perimeter of the muscle fibre of which it was associated to, down to the nearest capillary. If two capillaries were situated within visually similar distances, both distances would be traced and the lesser of the two would be recorded (See Figure 1 for representative images of the staining). The areas selected for analysis were free of ‘freeze fracture’ artefact, and care was taken such that longitudinal fibres were not used in the analysis.
Figure 1.

Representation of fibre type‐specific analyses of skeletal muscle satellite cell content, capillarization and distance measurement between satellite cell to its nearest capillary in healthy older men. (A) MHCI (green) + Laminin (green) + Dapi (blue) + Pax7 (red) + CD31 (purple) staining. (B) Pax7 only. (C) Dapi only. (D) Pax7 + Dapi. (E) MHCI + Dapi. (F) MHCI + Pax7 + CD31 (yellow line indicates the measurement of satellite cell distance to its nearest capillary). (G) Dapi + Pax7 + CD31. Arrows point at the satellite cells.
Statistical analyses
Data are expressed as means ± SEM. Exercise training induced changes were analysed using repeated measures ANOVA with time (baseline, 12 weeks, 24 weeks) and fibre type (Type I vs. Type II) as within‐subject factors. In the event of significant time x fibre type interactions, type I and type II muscle fibres were analysed separately. Additional repeated measures ANOVA analyses were performed with time (baseline, 12 weeks, 24 weeks) and fibre type (Type I vs. Type II) as within‐subject factors and group (LOW vs. HIGH) as the between‐subject factor. In case of significant time x fibre type x group or time x group interaction LOW and HIGH groups were analysed separately. Bonferroni correction was applied to correct for multiple testing. Significance was set at P < 0.05. All calculations were performed using SPSS version 21.0 (Chicago, IL).
Results
Muscle fibre size and type distribution
Overall
At baseline, muscle fibre size was significantly smaller in type II compared with type I muscle fibres (Table 1, P < 0.01). We observed a significant time x fibre type interaction (P < 0.05), as such, type I and type II muscle fibre size were analysed separately. Whereas no change in type I muscle fibre size was observed, type II muscle fibre size increased significantly in response to 24 weeks of resistance exercise training (P < 0.05, Table 1). Percentage of type I and II muscle fibres did not change as a result of exercise training (Table 1).
Table 1.
Muscle fibre size and type distribution
| Baseline | 12 weeks | 24 weeks | |
|---|---|---|---|
| Muscle fibre size (µm2) | |||
| Type I | 5832 ± 246 | 6118 ± 302 | 6267 ± 310 |
| Type II | 5150 ± 234a | 5776 ± 407 | 6719 ± 446b |
| Fibre type % | |||
| Type I | 55 ± 4 | 56 ± 3 | 53 ± 3 |
| Type II | 45 ± 4 | 44 ± 3 | 47 ± 3 |
Values are means ± SEM.
significantly different compared with Type I (P < 0.05).
significantly different compared with baseline and 12 weeks (P < 0.05).
LOW vs. HIGH group
No significant difference in type I and type II muscle fibre size was observed between the LOW and HIGH group at baseline. As we observed a significant time x group interaction (P < 0.05), the LOW and HIGH group were analysed separately. In the LOW group, no significant change in type I and type II muscle fibre size was observed in response to exercise training (Main effect of time, P = 0.142; Figure 2A and B). In the HIGH group, type I and type II muscle fibres were analysed separately as there was a time x fibre type interaction (P < 0.05). Whereas type I muscle fibre size tended (Main effect of time, P = 0.058) to increase, we observed a significant increase in type II muscle fibre size in the HIGH group after 12 and 24 weeks of resistance exercise training (Main effect of time, P < 0.01; Figure 2A and B). Interestingly, the percentage of type I muscle fibres was significantly lower in the LOW vs. the HIGH group (45 ± 4 vs. 64 ± 5% type I muscle fibres, respectively; Main effect of group, P < 0.001), which remained unchanged in both the LOW and HIGH group during the intervention (Figure 3A and B).
Figure 2.

Muscle fibre size in type I (A) and type II (B) muscle fibres. LOW: relatively low baseline type II muscle fibre capillary‐to‐fibre perimeter exchange (CFPE) index (n = 11). HIGH: relatively high baseline type II muscle fibre CFPE index (n = 11). Data represent mean ± SEM. Asterisk (*) denotes significantly different compared with pre (P < 0.05).
Figure 3.

Muscle fibre type distribution expressed as proportion of (A) and cross‐sectional area (CSA) occupied by (B) type I muscle fibres. LOW: relatively low baseline type II muscle fibre capillary‐to‐fibre perimeter exchange (CFPE) index (n = 11). HIGH: relatively high baseline type II muscle fibre CFPE index (n = 11). Data represent mean ± SEM. Number sign (#) denotes significantly different data compared with HIGH group (P < 0.05).
Muscle fibre capillaries
Overall
The number of type II muscle fibre CC was significantly lower than in type I muscle fibres before the start of exercise training (Table 2, P < 0.01). In addition, the C/Fi and CFPE index were significantly lower in type II compared with type I muscle fibres (Table 2, P < 0.01). No changes in type I and type II muscle CC, C/Fi and CFPE index were observed in response to 12 and 24 weeks of resistance type exercise training.
Table 2.
Muscle fibre capillarization and satellite cell content
| Baseline | 12 weeks | 24 weeks | |
|---|---|---|---|
| CC | |||
| Type I | 3.08 ± 0.08 | 2.95 ± 0.11 | 2.91 ± 0.16 |
| Type II | 2.62 ± 0.18a | 2.56 ± 0.14a | 2.67 ± 0.22a |
| C/Fi | |||
| Type I | 2.14 ± 0.10 | 2.07 ± 0.10 | 2.12 ± 0.14 |
| Type II | 1.49 ± 0.06a | 1.45 ± 0.06a | 1.66 ± 0.11a |
| CFPE (capillaries · 1000 µm−1) | |||
| Type I | 7.04 ± 0.30 | 6.84 ± 0.26 | 6.84 ± 0.39 |
| Type II | 5.21 ± 0.29a | 4.77 ± 0.15a | 5.25 ± 0.23a |
| Satellite cell content (per muscle fibre) | |||
| Type I | 0.077 ± 0.006 | 0.084 ± 0.008 | 0.089 ± 0.009b |
| Type II | 0.058 ± 0.006a | 0.082 ± 0.011 | 0.090 ± 0.010b |
| Satellite cell distance to nearest capillary (µm) | |||
| Type I | 17.6 ± 1.1 | 18.8 ± 1.2 | 18.2 ± 1.0 |
| Type II | 24.4 ± 1.9a | 25.6 ± 1.8a | 24.4 ± 1.7a |
Values are means ± SEM.
significantly different compared with Type I (P < 0.05).
significantly different compared with baseline (P < 0.05).
CFPE, capillary‐to‐fibre perimeter exchange; C/Fi, Individual muscle fibre capillary‐to‐fibre ratio.
LOW vs. HIGH group
Type I and type II muscle fibres CFPE index and C/Fi were significantly lower in the LOW compared with the HIGH group (P < 0.0001; Figure 4A–D). The number of type II muscle fibre CC was significantly lower in the LOW vs. the HIGH group (P < 0.05; Figure 4E–F). No change in type I and type II muscle fibre CFPE, C/Fi and/or CC was observed in response to 12 and 24 weeks of resistance exercise training in both the LOW and HIGH group (Figure 4A–F).
Figure 4.

Muscle fibre capillarization in type I (A‐C‐E) and type II (B‐D‐E) muscle fibres. CFPE: capillary‐to‐fibre perimeter exchange index. C/Fi: Individual muscle fibre capillary‐to‐fibre ratio. CC: capillary contacts. LOW: relatively low baseline type II muscle fibre CFPE index (n = 11). HIGH: relatively high baseline type II muscle fibre CFPE index (n = 11). Data represent mean ± SEM. Number sign (#) denotes significantly different data compared with HIGH group (P < 0.05).
Muscle satellite cells
Overall
Prior to the intervention, type II muscle fibre satellite cell content was significantly lower compared with type I muscle fibres (P < 0.01; Table 2). In response to 24 weeks of resistance exercise training, satellite cell content increased significantly in both type I and type II muscle fibres (P < 0.05; Table 2). At baseline, satellite cell distance to its nearest capillary was significantly greater in type II compared with type I muscle fibres (P < 0.001; Table 2). Type I and type II muscle fibre satellite cell distance to nearest capillary did not change in response to 12 and 24 weeks of resistance exercise training (Table 2).
LOW vs. HIGH group
At baseline, type I and type II muscle fibre satellite cell content was not different between the LOW and HIGH group. As a significant time x group interaction (P < 0.05) was observed, we assessed the change over time in the LOW and HIGH group separately. In the LOW group, no significant change in type I and type II muscle fibre satellite cell content was observed in response to the intervention (Figure 5A and B). In contrast, we observed a main effect of time in type I and type II muscle fibre satellite cell content in the HIGH group (P < 0.05). Post hoc pairwise comparisons showed that type II muscle fibre satellite cell content tended to be higher at 12 (P = 0.058) and 24 weeks (P = 0.085) after resistance exercise training compared with baseline in the HIGH group (Figure 5A and B). Type I and type II muscle fibre satellite cell distance to nearest capillary was not different between the LOW and HIGH group. In addition, no changes were observed in type I and type II satellite cell distance to nearest capillary in response 12 and 24 weeks of exercise training in both groups (Figure 5C and D).
Figure 5.
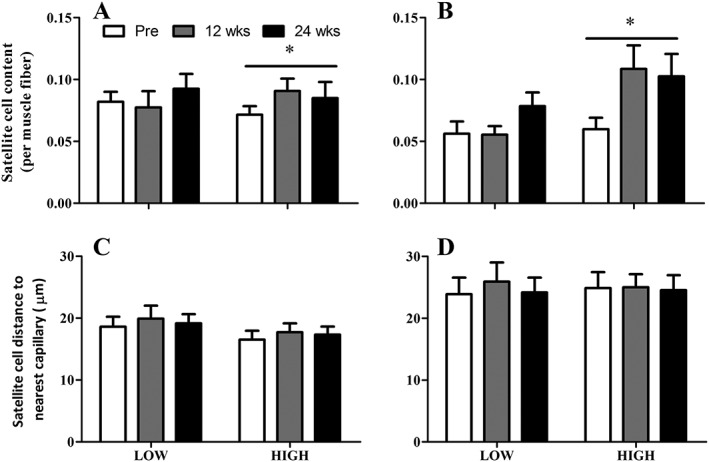
Muscle satellite cell content and distance to nearest capillary in type I (A and C) and type II (B and D) muscle fibres. LOW: relatively low baseline type II muscle fibre capillary‐to‐fibre perimeter exchange (CFPE) index (n = 11). HIGH: relatively high baseline type II muscle fibre CFPE index (n = 11). Data represent mean ± SEM. Asterisk (*) denotes significant effect of time (P < 0.05).
Discussion
In this study, we report that prolonged whole‐body resistance exercise training induced muscle fibre hypertrophy is not accompanied by an increase in capillary density in older men. Interestingly, however, we observed that the increase in type II muscle fibre size following 24 weeks of resistance type exercise training was mainly driven by individuals who had a higher muscle fibre capillarization at baseline.
Resistance exercise training is a known stimulus for inducing muscle fibre hypertrophy in both young and older adults.39, 40 In this study, we report an increase in type II muscle fibre size in response to 12 (increase of 18 ± 6%) and 24 weeks (increase of 35 ± 7%) of resistance exercise training in healthy older men (Table 1). These results are comparable with previous resistance exercise training studies in older adults.28, 30, 32, 36, 41, 42 Nevertheless, previous studies have shown that exercise training induced muscle fibre growth is considerably lower in healthy older when compared with young adults.41, 42 Adequate muscle fibre perfusion is critical for the delivery of oxygen, nutrients and growth factors necessary to facilitate muscle fibre growth during post‐exercise recovery. As such, it has been hypothesized that the age‐related decline in muscle fibre perfusion is a key factor in the blunted anabolic response to exercise training in older adults.8, 12, 13, 17, 20, 43, 44 Cross‐sectional studies have shown that leg blood flow is better preserved in resistance exercise trained older adults as compared with sedentary older adults.45 In addition, prolonged resistance exercise training is known to increase basal leg blood flow in both young46 and older adults.47 However, the actual perfusion of muscle tissue relies on the muscle fibre capillary scaffolding between individual muscle fibres. Mixed results have been reported on the increase in muscle fibre capillary density following prolonged resistance exercise training in older adults. Whereas some34, 48 reported a significant increase in muscle fibre capillary density following prolonged resistance exercise training in older adults, others do not.35 In this study, we report no change in muscle fibre capillarization in response to 12 and 24 weeks of resistance exercise training in older men. These discrepancies may, in part, be explained by the differences in the exercise training protocols employed. Previous studies showing an increase in muscle fibre capillary density34, 48 have generally used exercise training protocols that target only the lower limbs, whereas in the present study, whole‐body resistance exercise was performed. We specifically chose to examine the response to whole‐body resistance exercise as this is a commonly practised approach in older adults. Nonetheless, this suggests that a certain exercise intensity or volume may be required to elicit an angiogenic response with resistance exercise training in older adults; however, this notion requires further exploration.
Resistance type exercise training specifically targets the growth of type II muscle fibres in both young and older adults. However, the structure of the microvascular bed is known to change substantially with advancing age, particularly as it relates to type II muscle fibre capillarization that decreases with age.19, 20 We hypothesized that type II muscle fibre capillarization at baseline may be a critical factor in the type II muscle fibre hypertrophic response during prolonged resistance exercise training in older men. Therefore, we split participants into two equal groups (n = 11 per group) who had a relatively low (LOW) or high (HIGH) type II muscle fibre CFPE index at baseline and assessed the changes in type I and type II muscle capillary density, fibre type distribution and fibre size over time. Type II muscle fibre capillary density was significantly lower on all capillary parameters (i.e. CC, C/Fi and CFPE) in the LOW compared with the HIGH group, yet, muscle fibre capillarization remained unchanged in response to prolonged exercise training in both groups. Interestingly, participants in the HIGH group had a significantly higher percentage of type I muscle fibres compared with the LOW group (64 ± 5 vs. 45 ± 4%, respectively). Because of their oxidative nature, type I muscle fibres are typically associated with a greater number of capillaries than type II muscle fibres.43 As muscle capillaries are frequently shared between different muscle fibre types, a higher percentage of type I muscle fibres will also likely result in an enhanced perfusion of type II muscle fibres. Interestingly, however, we observed a substantial increase in type II muscle fibre size following 12 and 24 weeks of exercise training in the HIGH group only, there was no significant change in the LOW group. Whereas there is absolutely no change in type II muscle fibre size during the first 12 weeks of exercise training in the LOW group, it does appear to increase slightly at 24 weeks of resistance training. The relatively small number of participants included per group, in this proof‐of‐principle study, may have prohibited us to detect this change statistically. Nevertheless, this study is the first to suggest that capillarization of type II muscle fibres at baseline may be a critical factor in stimulating muscle fibre hypertrophy during prolonged resistance exercise training in older men.
As myonuclei are post‐mitotic, muscle satellite cells play an important role in donating new nuclei to existing fibres to support muscle fibre repair, remodelling and growth.22 Consistent with previous studies, we report a significant increase in type I and type II muscle fibre satellite cell content in response to prolonged resistance exercise training in older men.30, 31, 32, 36, 42 More importantly, we observe that the exercise‐induced increase in muscle fibre size in the HIGH group was accompanied by a substantial increase in satellite cell content, whereas no change was observed in the LOW group. Whether the lack of change in satellite cell content in the LOW group is due to a lower muscle fibre capillarization at baseline or just merely because of the absent muscle fibre growth in response to exercise training requires further investigation. Nonetheless, the spatial structure of the muscle fibre microvascular bed appears to be an important determinant of muscle satellite cell activation.19, 21
Previously, we19 as well as others21 have shown that activated satellite cells are located at a closer proximity to capillaries as compared with quiescent satellite cells. Interestingly, we19 have recently shown that type II muscle fibre associated satellite cells are located at a greater distance to their nearest capillary in older compared with young men. This greater distance between the satellite cell and capillaries in older adults may be an important factor in the impaired satellite cell response during post‐exercise recovery observed in humans.24, 25, 26, 27 However, satellite cell distance to its nearest capillary was not different between groups and did not change over time. This would suggest that the greater distance observed between satellite cells to its nearest capillary in type II compared with type I muscle fibres is not a limiting factor during muscle fibre hypertrophy in response to prolonged exercise training in older men.
Resistance exercise training is currently the most commonly recommended exercise modality to combat the loss of muscle mass with age. The present study, however, clearly shows that performing prolonged whole‐body resistance type exercise training does not increase type II muscle fibre capillary density in older men. In addition, muscle fibre capillarization at baseline appears to be a determining factor in the muscle hypertrophic response following exercise training in older adults. Other exercise modalities, such as continuous aerobic or interval exercise training, have consistently reported increases in muscle fibre capillary density in older adults.20, 48, 49, 50 It would be interesting to examine whether, for example, aerobic pre‐conditioning could alleviate the blunted increase in skeletal muscle mass and strength during traditional resistance exercise training in older adults.41, 42 In addition, future studies are needed to assess whether improved muscle fibre capillarization may also improve satellite cell function in senescent muscle during post‐exercise recovery.
In conclusion, type II muscle fibre capillarization at baseline may be a critical factor for allowing muscle fibre hypertrophy following resistance exercise training to occur in older men.
Conflict of interest
All authors report no conflict of interest.
Supporting information
Table S1. Muscle fiber circularity
Supporting info item
Acknowledgements
The Pax7 hybridoma cells developed by Dr A. Kawakami, the A4.951 developed by Dr H. Blau were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA. This study was approved by the Medical Ethics Committee of Maastricht University Medical Centre, and complied with the guidelines set out in the Declaration of Helsinki. Participants gave their informed written consent prior to their inclusion to the study. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.50
Snijders, T. , Nederveen, J. P. , Joanisse, S. , Leenders, M. , Verdijk, L. B. , van Loon, L. J. C. , and Parise, G. (2017) Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. Journal of Cachexia, Sarcopenia and Muscle, 8: 267–276. doi: 10.1002/jcsm.12137.
References
- 1. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010;39:412–423, doi:10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C. Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif Tissue Int 2015;97:445–452, doi:10.1007/s00223-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413–421. [DOI] [PubMed] [Google Scholar]
- 4. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 5. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012;31:652–658, doi:10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 6. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 2015;10e0140903:doi:10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katsanos CS, Kobayashi H, Sheffield‐Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–1073. [DOI] [PubMed] [Google Scholar]
- 8. Phillips BE, Atherton PJ, Varadhan K, Limb MC, Wilkinson DJ, Sjoberg KA, et al. The effects of resistance exercise training on macro‐ and micro‐circulatory responses to feeding and skeletal muscle protein anabolism in older men. J Physiol 2015;593:2721–2734, doi:10.1113/JP270343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age‐related differences in the dose‐response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–217, doi:10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab = Physiologie appliquee, nutrition et metabolisme 2009;34:377–381, doi:10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 11. Phillips BE, Hill DS, Atherton PJ. Regulation of muscle protein synthesis in humans. Curr Opin Clin Nutr Metab Care 2012;15:58–63, doi:10.1097/MCO.0b013e32834d19bc. [DOI] [PubMed] [Google Scholar]
- 12. Phillips B, Williams J, Atherton P, Smith K, Hildebrandt W, Rankin D, et al. Resistance exercise training improves age‐related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J Appl Physiol 2012;112:347–353, doi:10.1152/japplphysiol.01031.2011. [DOI] [PubMed] [Google Scholar]
- 13. Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, et al. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial‐dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 2010;95:3848–3857, doi:10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 2006;290:H272–H278, doi:10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 15. Skilton MR, Lai NT, Griffiths KA, Molyneaux LM, Yue DK, Sullivan DR, et al. Meal‐related increases in vascular reactivity are impaired in older and diabetic adults: insights into roles of aging and insulin in vascular flow. Am J Physiol Heart Circ Physiol 2005;288:H1404–H1410, doi:10.1152/ajpheart.00484.2004. [DOI] [PubMed] [Google Scholar]
- 16. Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999;100:164–170. [DOI] [PubMed] [Google Scholar]
- 17. Dinenno FA, Seals DR, DeSouza CA, Tanaka H. Age‐related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 2001;531:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong‐Poi H, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006;290:E1191–E1197, doi:10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 19. Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, et al. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 2016;doi:10.1002/jcsm12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol 1995;78:2033–2038. [DOI] [PubMed] [Google Scholar]
- 21. Christov C, Chretien F, Abou‐Khalil R, Bassez G, Vallet G, Authier FJ, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 2007;18:1397–1409, doi:10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol 2015;6:283, doi:10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mauro A. Satellite cell of skeletal muscle fibres. J Biophys Biochem Cytol 1961;9:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 2006;33:242–253, doi:10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- 25. McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA, Parise G. Elevated SOCS3 and altered IL‐6 signaling is associated with age‐related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 2013;304:C717–C728, doi:10.1152/ajpcell.00305.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age‐related human muscle stem cell dysfunction. FASEB J : official publication of the Fed Am Soc Exp Biol 2012;26:2509–2521, doi:10.1096/fj.11-198663. [DOI] [PubMed] [Google Scholar]
- 27. Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, et al. The skeletal muscle satellite cell response to a single bout of resistance‐type exercise is delayed with aging in men. Age 2014;36:9699, doi:10.1007/s11357-014-9699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Churchward‐Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LC, et al. There Are No nonresponders to resistance‐type exercise training in older Men and women. J Am Med Dir Assoc 2015;doi:10.1016/j.jamda.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 29. Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, et al. Protein supplementation increases muscle mass gain during prolonged resistance‐type exercise training in frail elderly people: a randomized, double‐blind, placebo‐controlled trial. J Am Med Dir Assoc 2012;13:713–719, doi:10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 30. Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fibre type‐specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 2009;64:332–339, doi:10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age 2014;36:545–547, doi:10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, et al. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve 2008;38:1147–1154, doi:10.1002/mus.21054. [DOI] [PubMed] [Google Scholar]
- 33. Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflugers Archiv : European journal of physiology 1997;433:238–244. [DOI] [PubMed] [Google Scholar]
- 34. Frontera WR, Meredith CN, O'Reilly KP, Evans WJ. Strength training and determinants of VO2max in older men. J Appl Physiol 1990;68:329–333. [DOI] [PubMed] [Google Scholar]
- 35. Hagerman FC, Walsh SJ, Staron RS, Hikida RS, Gilders RM, Murray TF, et al. Effects of high‐intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci 2000;55:B336–B346. [DOI] [PubMed] [Google Scholar]
- 36. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance‐type exercise training. J Gerontol A Biol Sci Med Sci 2013;68:769–779, doi:10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 37. Leenders M, Verdijk LB, Van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig WK, et al. Protein supplementation during resistance‐type exercise training in the elderly. Med Sci Sports Exerc 2013;45:542–552, doi:10.1249/MSS.0b013e318272fcdb. [DOI] [PubMed] [Google Scholar]
- 38. Van Abbema R, De Greef M, Craje C, Krijnen W, Hobbelen H, Van Der Schans C. What type, or combination of exercise can improve preferred gait speed in older adults? A meta‐analysis BMC geriatrics 2015;15:72, doi:10.1186/s12877-015-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance‐type exercise training: a meta‐analysis. Am J Clin Nutr 2012;96:1454–1464, doi:10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 40. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofibre hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 2006;101:531–544, doi:10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 41. Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofibre growth among resistance‐trained young and older men and women. Am J Physiol Endocrinol Metab 2006;291:E937–E946, doi:10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 42. Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol 2014;116:998–1005, doi:10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 43. Toda N. Age‐related changes in endothelial function and blood flow regulation. Pharmacol Ther 2012;133:159–176, doi:10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 44. Miyachi M, Tanaka H, Kawano H, Okajima M, Tabata I. Lack of age‐related decreases in basal whole leg blood flow in resistance‐trained men. J Appl Physiol 2005;99:1384–1390, doi:10.1152/japplphysiol.00061.2005. [DOI] [PubMed] [Google Scholar]
- 45. Tanimoto M, Kawano H, Gando Y, Sanada K, Yamamoto K, Ishii N, et al. Low‐intensity resistance training with slow movement and tonic force generation increases basal limb blood flow. Clin Physiol Funct Imaging 2009;29:128–135, doi:10.1111/j.1475-097X.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 46. Anton MM, Cortez‐Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol 2006;101:1351–1355, doi:10.1152/japplphysiol.00497.2006. [DOI] [PubMed] [Google Scholar]
- 47. Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol 1997;82:1305–1310. [DOI] [PubMed] [Google Scholar]
- 48. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Skeletal muscle adaptations to endurance training in 60‐ to 70‐yr‐old men and women. J Appl Physiol 1992;72:1780–1786. [DOI] [PubMed] [Google Scholar]
- 49. Robbins JL, Duscha BD, Bensimhon DR, Wasserman K, Hansen JE, Houmard JA, et al. A sex‐specific relationship between capillary density and anaerobic threshold. J Appl Physiol 2009;106:1181–1186, doi:10.1152/japplphysiol.90947.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Muscle fiber circularity
Supporting info item


