Abstract
Background
Muscle dysfunction and sarcopenia have been associated with poor performance status, an increased mortality risk, and greater side effects in oncologic patients. However, little is known about how performance is affected by cancer therapy. We investigated muscle strength in breast cancer patients in different adjuvant treatment settings and also compared it with data from healthy individuals.
Methods
Breast cancer patients (N = 255) from two randomized controlled exercise trials, staged 0–III and aged 54.4 ± 9.4 years, were categorized into four groups according to their treatment status. In a cross‐sectional design, muscle function was assessed bilaterally by isokinetic dynamometry (0°, 60°, 180°/s) as maximal voluntary isometric contraction (MVIC) and maximal isokinetic peak torque (MIPT) in shoulder rotators and knee flexors and extensors. Additionally, muscular fatigue index (FI%) and shoulder flexibility were evaluated. Healthy women (N = 26), aged 53.3 ± 9.8 years, were tested using the same method. Analysis of covariance was used to estimate the impact of different cancer treatments on skeletal muscle function with adjustment for various clinical and socio‐demographic factors.
Results
Consistently, lower muscle strength was measured in shoulder and knee strength in patients after chemotherapy. On average, patients had up to 25% lower strength in lower extremities and 12–16% in upper extremities in MVIC and MIPT during cancer treatment compared with healthy women. No substantial difference between patient groups in shoulder strength, but significantly lower shoulder flexibility in patients with radical mastectomy was measured. Chemotherapy‐treated patients had consistently higher FI%. No serious adverse events were reported.
Conclusions
Breast cancer patients showed markedly impaired muscle strength and joint dysfunctions before and after anticancer treatment. The significant differences between patients and healthy individuals underline the need of exercise therapy as early as possible in order to prevent or counteract the loss of muscle function after curative surgery as well as the consequences of neo‐/adjuvant chemotherapy.
Keywords: Isokinetic, Isometric, Multi‐joint, Muscle function, Chemotherapy
Introduction
Cancer‐related muscle dysfunction is a broad clinical challenge, which is not restricted to palliative or advanced stage patients as it has also been observed in newly diagnosed patients with low tumour burden.1, 2 Many factors can affect skeletal muscle function including age, comorbidities, malnutrition, physical inactivity, tumour‐derived factors, systemic and local cancer treatments, and supportive care medication.3 Observational data indicate that physical activity can reduce breast cancer (BC)‐specific mortality and overall mortality,4 but the role of muscle strength during cancer treatment has been insufficiently investigated. A prospective cohort study, Health, Eating, Activity, and Lifestyle (HEAL) study revealed a high prevalence of sarcopenia and its association with a higher all‐cause mortality hazard ratio of 2.86 in BC survivors.1 Low muscle strength and physical inactivity can be a predictor for persistent fatigue in older, long‐term BC survivors.5 Breat cancer patients undergoing adjuvant chemotherapy (CT) reduce their daily energy expenditure during therapy, which is associated with a loss of muscle mass.6, 7 Furthermore, it was shown that skeletal muscle status is of clinical relevance because it is associated with treatment complications and time‐to‐tumour progression.8 With regard to healthy older individuals, muscle strength, but not mass, was identified as a strong independent predictor of all‐cause mortality.9
Based on the current knowledge of treatment‐related side effects, it can be assumed that muscle function is affected by different cancer treatments. Exercise‐induced adaptations and better muscular performance may attenuate cancer toxicities, which, in turn, could augment cure rate, improve the quality of life for cancer survivors, and maybe even increase long‐term survival.10, 11, 12 Currently, there is insufficient knowledge on influence of cancer treatment on muscle function and strength in patients undergoing BC treatment.13 Since we recently reported that cardiorespiratory performance varies between patient groups defined by cancer treatment,14 we would like to provide an overall picture of the performance status and the different impact of several types of cancer treatment among BC patients by analyzing various muscle strength parameters.
Methods
Population
For this cross‐sectional analysis, baseline data of two randomized controlled exercise trials (RCTs) in BC patients were used, i.e. the BEATE study and the BEST study (ClinicalTrials.gov NCT01106820 and NCT01468766, respectively).15, 16, 17 These two RCTs investigated the effects of 12‐week progressive resistance training in comparison to relaxation training in BC patients undergoing adjuvant CT (BEATE study) or adjuvant radiotherapy (BEST study). Women with histologically confirmed stage 0–III primary BC after lumpectomy or mastectomy were eligible for the studies. Further, inclusion and exclusion criteria and more details of the RCTs are presented elsewhere.15, 16 In a parallel intervention study (INVEST study) with identical surveys, 26 healthy age‐matched control women participated in the same 12‐week progressive resistance training protocol to obtain comparison data.
Both RCTs were conducted with parallel designs at the National Center for Tumor Diseases (NCT) in Heidelberg, Germany. The University of Heidelberg Ethics Committee has approved the trials, and written informed consent was obtained for all procedures from all participants. Based on the different clinically important treatment histories and the healthy subjects, five subgroups were defined: No CT, started (adjuvant) CT, post neo‐adj. CT, post adj. CT, and healthy women. Patients recruited in the BEST study had the baseline strength testing within 14 days before starting radiotherapy. Of these participants, a majority had received surgery only (no CT, n = 105), while some had received CT in the adjuvant (post adj. CT, n = 28) or neo‐adjuvant (post neo‐adj. CT, n = 31) CT setting. Patients enrolled in the BEATE study (started CT, n = 91) performed baseline strength testing at the end of the first or second CT cycle (Figure 1).
Figure 1.
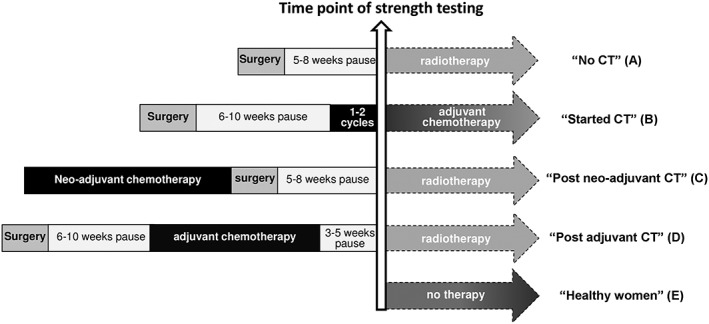
Time point of the strength testing in clinically important treatment groups of breast cancer patients and healthy women.
Medical and patient characteristics
Patient characteristics, treatment and disease modalities, further drug usage, and comorbidities were abstracted from medical records and personal interviews. Overall performance status was determined by the attending oncologist using the Eastern Cooperative Oncology Group (ECOG) performance score system at the time of enrollment. Weight and height were measured at baseline. Exercise behaviour in the year before BC diagnosis was assessed through self‐developed surveys abstracted from the International Physical Activity Questionnaire. Participants were asked about the type, frequency, and duration of exercise (e.g. experiences with resistance training, walking, cycling, and other intentional exercises). Furthermore, patient‐reported outcomes like cancer‐related physical fatigue,18 quality of life,19 and depression20 were assessed by questionnaire.
Assessment of muscle function
Test system
Isokinetic and isometric muscle strength were measured by using IsoMed 2000‐system B‐series version (D&R Ferstl GmbH, Hemau, Germany). The use of isokinetic dynamometer is considered a gold standard method to evaluate strength in cancer patients.2 It has already been shown to be valid and reliable in healthy subjects21 and has been used previously in various cancer populations.22, 23, 24
Muscle function parameters
Maximal isokinetic peak torque (MIPT) was tested for shoulder external and internal rotation and for knee extensors and flexors at the angular velocities of 60 and 180°/s. The range of motion (ROM) for isokinetic knee measurement was limited between the angles from 10° to 90°. The position of the dynamometer for shoulder rotation was tilted at 40° of abduction. The ROM for isokinetic testing was from 10° external rotation to 70° internal rotation. The MIPT for shoulder rotation was calculated for dominant side.
With this device, we also measured the maximal voluntary isometric contraction (MVIC) for shoulder internal rotator in the position of 43° and knee extensor muscles in the position of 35° (0° is straight leg), which sustainably were the strongest angle positions. For BC patients, we calculated MVIC on the operated and non‐operated side. For healthy women, we calculated MVIC from the mean of left and right side.
Muscular fatigue was determined by the calculation of the peak torque decline at 60°/s in knee extensors of the dominant leg. Therefore, we used the muscular fatigue index: FI% = [(peak torque of initial three repetitions−peak torque of final three repetitions)/peak torque of initial three repetitions] × 100, an adapted formula as described by Kannus25 to define the ability of an individual to maintain a level of performance. A high FI% indicates that muscles fatigue quickly. The peak torque of the first repetition overall was markedly lower than that of the second repetition, and it was considered as a first ‘attempt’ for the patient; it was omitted from the calculation of the initial peak torque values.
Additionally, we measured the ROM in the arm elevation with a goniometer in a standardized supine lying position to elicit the flexibility limitations after surgery in both the operated and healthy sides.
Testing protocol
Participants were secured using thigh, pelvic, and torso straps to minimize extraneous body movements. The subjects were permitted to use the handlebars on both sides of the IsoMed 2000 chair (D&R Ferstl GmbH, Hemau, Germany) for additional stability during leg testing, but not for shoulder testing. For the MVIC testing, the participants were instructed to push as hard as possible against the fixed lever arm. Contraction time for MVIC was restricted to 6 s for each position. Each subject performed 10 maximal reciprocal contractions in both angular velocities for MIPT. During testing, both the subject and the instructor were able to see the strength curve on the monitor. Subjects were given verbal encouragement to generate the highest possible strength. Each torque artefact resulting from deceleration, which often exceeds the true peak torque, was removed by using a filter; only gravity‐corrected data were used for analysis.
Data analysis
Clinical and socio‐demographic data were investigated by descriptive analyses for the entire study population. Data were also stratified by the four treatment subgroups and healthy controls. Between‐group differences were assessed using χ 2 or Fisher's exact test for categorical variables and using one‐way analysis of variance for continuous variables. Analyses of covariance (ANCOVA) models were used to test whether the muscle function parameters differed between the four cancer treatment groups and in comparison with healthy individuals. We calculated models adjusted for covariates that seemed biologically reasonable influencing factors. The included covariates are reported in Table 2. Presented here are the parsimonious models including the significant covariates and those that changed the treatment estimate by >10%. Sensitivity analyses with different adjustment sets were performed to investigate the stability of the models.
Table 2.
Maximal voluntary isometric contraction in N × m in different treatment groups and healthy subjects
| Breast cancer treatment groups | |||||||
|---|---|---|---|---|---|---|---|
| Measure/treatment | No CT | Started CT | Post neo‐adj. CT | Post adj. CT | Significant differences between patient groups | Healthy women | Significant differences to healthy women |
| (n = 105) | (n = 91) | (n = 31) | (n = 28) | (n = 26) | |||
| A | B | C | D | E | |||
| Knee extensiona | 126.8 (120.2, 133.5) | 125.4 (118.1, 132.7) | 119.0 (108.1, 129.9) | 122.8 (111.5, 134.1) | n.s. | 138.5 (127.4, 149.5) | n.s. |
| Shoulder internal rotation (op)b | 28.5 (27.3, 29.7) | 29.7 (28.3, 31.0) | 28.3 (26.0, 30.6) | 29.4 (27.0, 31.7) | n.s. | 33.8 (31.4, 36.2)1 | A/E, B/E, C/E |
| Shoulder internal rotation (non‐op)b | 30.1 (28.8, 31.3) | 30.9 (29.5, 32.3) | 28.8 (26.4, 31.1) | 29.1 (26.5, 31.6) | n.s. | 33.8 (31.4, 36.2)c | A/E, B/E, C/E, D/E |
BMI, body mass index; CT, chemotherapy; N × m, Newton metre; op, operated side.
Data presented as adjusted mean with 95% confidence intervals (CI).
Model for knee extension is adjusted for age, BMI (17–<25, 25–30, >30 kg/m2), weight, drugs that influence the muscle tonus, antidepressants, regular cycling, and previous experience in resistance training.
Model for shoulder rotation is adjusted for age, BMI (17–<25, 25–30, ≥30 kg/m2), and previous experience in resistance training.
In healthy women: mean of left and right arm.
The ROM in the shoulder of the operated side was adjusted for the operation type (radical mastectomy and partial mastectomy). All statistics were performed using SAS 9.3 (SAS Institute Inc. NC, USA). The level of significance was set at P < 0.05.
Results
Participant characteristics
Participant characteristics stratified by treatment groups are presented in Table 1. All patients underwent surgical resection with mean (±SD) time‐to‐strength assessment of 65.2 ± 49 days. According to the different treatment settings, there were significant group differences in the timeframe between patients' surgery and the strength assessment (P < 0.001). The longest time period from surgery to muscle strength testing was 180.1 ± 50.5 days in the post adj. CT group. The shortest period was 45.5 ± 12.7 days in the no CT group. Muscle strength testing was 76.4 ± 48.2 days after CT in the post neo‐adj. CT group and 27.6 ± 15.4 days in the post adj. CT group. The group with no CT was older (57.1 ± 8.7 years) than the group post neo‐adj. CT (51.1 ± 9.3 years), started CT group (52.6 ± 9.9 years), and post adj. CT group (54.3 ± 7.9 years). Healthy controls had a mean age of 53.3 ± 9.8 years. There were no significant differences between the treatment groups in weight, height, body mass index (BMI), and in the ECOG status classified by the oncologists. Furthermore, no substantial differences in sport activity pre‐diagnosis were observed between patient groups. The healthy controls had a higher level of moderate physical activity.
Table 1.
Characteristics of the population
| Total | Breast cancer treatment groups | Healthy women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No CT | Started CT | Post neo‐adj. CT | Post adj. CT | P * | P ** | ||||||||||
| Number of women | 281 | 105 | 91 | 31 | 28 | 26 | |||||||||
| Age, mean (SD) | 54.3 | (9.4) | 57.1 | (8.7) | 52.6 | (9.9) | 51.1 | (9.3) | 54.3 | (7.8) | 0.0011 | 53.3 | (9.8) | 0.0027 | |
| Weight, mean (SD) | 72.2 | (13.9) | 73.6 | (13.6) | 71.7 | (14.0) | 71.0 | (11.3) | 74.6 | (18.4) | 0.60 | 67.3 | (11.4) | 0.24 | |
| Height, mean (SD) | 165.9 | (6.6) | 164.9 | (6.9) | 166.3 | (6.7) | 166.1 | (4.8) | 167.3 | (6.9) | 0.26 | 166.9 | (6.7) | 0.33 | |
| BMI, mean (SD) | 26.3 | (5.0) | 27.1 | (5.1) | 25.9 | (4.9) | 25.8 | (4.3) | 26.5 | (5.5) | 0.33 | 24.2 | (4.2) | 0.077 | |
| Menopausal status, n (%) | Pre | 67 | 26% | 19 | 18% | 37 | 41% | 5 | 16% | 6 | 21% | 0.0035 | n.a. | n.a. | |
| Peri | 29 | 11% | 10 | 9% | 8 | 9% | 7 | 23% | 4 | 14% | |||||
| Post | 147 | 58% | 73 | 69% | 39 | 42% | 18 | 58% | 17 | 61% | |||||
| Missing | 12 | 5% | 3 | 4% | 7 | 8% | 1 | 3% | 1 | 4% | |||||
| Days since surgery, mean (SD) | 65.2 | (49.0) | 45.5 | (12.7) | 56.8 | (22.7) | 55.9 | (48.2) | 180.1 | (50.5) | <0.001 | n.a. | n.a. | ||
| Days since CT end, mean (SD) | 55.1 | (44.6) | n.a. | n.a. | 76.4 | (48.2) | 27.6 | (15.4) | <0.001 | n.a. | n.a. | ||||
| Mastectomy, n (%) | Yes | 55 | 22% | 4 | 4% | 33 | 37% | 11 | 36% | 7 | 25% | <0.001 | n.a. | n.a. | |
| Partial mastectomy, n (%) | Yes | 197 | 77% | 101 | 96% | 55 | 60% | 20 | 64% | 21 | 75% | ||||
| Missing | 3 | 1% | 3 | 3% | |||||||||||
| Lymph nodes dissected, n (%) | None | 11 | 4% | 10 | 9.5% | 1 | 1.1% | <0.001 | n.a. | n.a. | |||||
| Sentinel | 147 | 58% | 76 | 72% | 48 | 52% | 14 | 45% | 9 | 32% | |||||
| Axillary | 94 | 37% | 18 | 17% | 40 | 44% | 17 | 55% | 19 | 68% | |||||
| Missing | 3 | 1% | 1 | 1% | 2 | 2% | |||||||||
| Stage, n (%) | 0 | 15 | 6% | 13 | 12% | 2 | 7% | <0.001 | n.a. | n.a. | |||||
| 1 | 118 | 46% | 65 | 62% | 36 | 40% | 10 | 32% | 7 | 25% | |||||
| 2 | 94 | 37% | 26 | 25% | 43 | 47% | 15 | 48% | 10 | 36% | |||||
| 3 | 27 | 11% | 1 | 1% | 11 | 12% | 4 | 13% | 11 | 39% | |||||
| Missing | 1 | <1% | 1 | 1% | |||||||||||
| Taxane, n (%) | Yes | 90 | 35% | n.a. | 32 | 35% | 31 | 100% | 27 | 96% | <0.001 | n.a. | n.a. | ||
| Missing | 1 | <1% | 1 | 1.% | |||||||||||
| Anthracycline, n (%) | Yes | 131 | 51% | n.a. | 77 | 85% | 29 | 96% | 25 | 89% | 0.23 | n.a. | n.a. | ||
| Missing | 3 | 1% | 1 | 1% | 1 | 3% | 1 | 4% | |||||||
| Herceptine treatment, n (%) | Yes | 16 | 6% | 0 | 0% | 4 | 4% | 6 | 19% | 6 | 21% | <0.001 | n.a. | n.a. | |
| Missing | 1 | <1% | 1 | 1% | |||||||||||
| Hormone therapy, n (%) | Yes | 79 | 31% | 55 | 52% | 0 | 0% | 13 | 42% | 11 | 39% | <0.001 | n.a. | n.a. | |
| ECOG, n (%) | 0 | 222 | 87% | 93 | 89% | 79 | 87% | 27 | 87% | 23 | 82% | 0.49 | n.a. | n.a. | |
| 1 | 25 | 10% | 10 | 9% | 6 | 6% | 4 | 13% | 5 | 18% | |||||
| 2 | 1 | <1% | 1 | 1% | |||||||||||
| Missing | 7 | 3% | 1 | 1% | 6 | 6% | |||||||||
| FAQ physical fatigue, mean (SD) | 39.4 | (27.1) | 35.0 | (26.9) | 44.0 | (25.5) | 45.0 | (25.5) | 56.7 | (24.5) | <0.001 | 16.4 | (18.3) | <0.001 | |
| Depression (ADS), mean (SD) | 25.1 | (16.7) | 26.6 | (17.1) | 25.6 | (16.2) | 28.9 | (18.5) | 26.0 | (14.7) | 0.83 | 11.8 | (11.4) | <0.001 | |
| Sports before diagnosis1, n (%) none | 98 | 35% | 46 | 44% | 21 | 23% | 14 | 45% | 9 | 32% | 0.14 | 8 | 31% | 0.0045 | |
| >0–9 MET × h/week | 77 | 27% | 25 | 23% | 24 | 26% | 7 | 23% | 7 | 25% | 14 | 54% | |||
| >9–21 MET × h/week | 55 | 20% | 19 | 18% | 24 | 26% | 6 | 19% | 4 | 14% | 2 | 8% | |||
| >21 MET × h/week | 46 | 16% | 14 | 13% | 21 | 23% | 4 | 13% | 7 | 25% | |||||
| missing | 5 | 2% | 1 | 1% | 1 | 1% | 1 | 4% | 2 | 8% | |||||
| Cyclinga, n (%) | None | 98 | 35% | 38 | 36% | 28 | 31% | 14 | 45% | 10 | 36% | 0.87 | 8 | 31% | 0.26 |
| >0–1 h/week | 85 | 30% | 32 | 31% | 31 | 34% | 9 | 29% | 11 | 39% | 2 | 8% | |||
| >1–3 h/week | 60 | 21% | 22 | 21% | 20 | 22% | 6 | 19% | 3 | 11% | 9 | 34% | |||
| >3 h/week | 31 | 11% | 10 | 9% | 12 | 13% | 2 | 7% | 2 | 7% | 5 | 19% | |||
| Missing | 7 | 3% | 3 | 3% | 2 | 7% | 2 | 8% | |||||||
ADS, German depression scale based on Center for Epidemiological Studies Depression Scale; BMI, body mass index; CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; FAQ, Fatigue Assessment Questionnaire with items for physical fatigue; MET, metabolic equivalent; SD, standard deviation.
Exercise behaviour in the year before breast cancer diagnosis with self‐developed surveys abstracted from the International Physical Activity Questionnaire.
P‐value for one‐way ANOVA for continuous variables and χ 2 or Fisher's exact test for categorical variables only for breast cancer patient groups.
P‐value for one‐way ANOVA for continuous variables and χ 2 or Fisher's exact test for categorical variables for all groups.
Maximal voluntary isometric contraction
The adjusted means and 95% confidence interval (CI) for knee extensors and internal rotators in the operated and non‐operated shoulder for MVIC are presented in Table 2.
Concerning MVIC of knee extensors, the BC treatment groups had impairments of 9–14% in strength in comparison to healthy women, but these differences did not reach statistical significance. There were also no statistically significant differences between the BC patient groups in shoulder MVIC, neither for the operated nor for the non‐operated side. However, healthy women had 12–16% higher MVIC in shoulder internal rotators, which is significantly different in comparison to the BC patient groups.
Maximal isokinetic peak torque
Adjusted means and 95% CI of MIPT in knee and shoulder muscles in two speeds for the treatment groups and healthy women are reported in Table 3.
Table 3.
Maximal isokinetic peak torque in N × m in different treatment groups and healthy subjects
| Breast cancer treatment groups | |||||||
|---|---|---|---|---|---|---|---|
| Measure/treatment | No CT | Started CT | Post neo‐adj. CT | Post adj. CT | Significant differences between patient groups | Healthy women | Significant differences to healthy women |
| (n = 105) | (n = 91) | (n = 31) | (n = 28) | (n = 26) | |||
| A | B | C | D | E | |||
| Knee extensiona in 60°/s | 88.6 (83.2, 94.1) | 92.6 (86.5, 98.7) | 84.0 (76.1, 91.9) | 77.7 (69.9, 85.5) | A/D, B/D | 97.5 (89.5, 105.6) | A/E, C/E, D/E |
| Knee flexiona in 60°/s | 57.2 (53.7, 60.8) | 64.5 (60.6, 68.4) | 53.1 (47.3, 58.9) | 51.9 (45.9, 57.9) | A/B, A/C, B/C, B/D | 69.2 (63.3, 75.1) | A/E, C/E, D/E |
| Knee extensiona in 180°/s | 56.6 (53.3, 59.9) | 58.4 (54.7, 62.1) | 51.5 (46.7, 56.3) | 47.2 (42.5, 51.9) | A/D, B/C, B/D | 63.5 (58.6, 68.3) | C/E, D/E |
| Knee Flexiona in 180°/s | 48.9 (46.2, 51.7) | 53.5 (50.5, 56.5) | 43.2 (38.7, 47.7) | 43.4 (38.8, 48.1) | A/B, B/C, B/D, | 59.6 (55.0, 64.1) | A/E, B/E, C/E, D/E |
| Shoulder internal rotationb in 60°/s | 25.6 (24.6, 26.6) | 26.9 (25.8, 28.0) | 26.0 (24.1, 27.9) | 25.6 (23.7, 27.5) | n.s. | 30.4 (28.4, 32.3) | A/E, B/E, C/E, D/E |
| Shoulder external rotationb in 60°/s | 8.7 (8.0, 9.4) | 8.8 (8.0, 9.5) | 9.2 (7.9, 10.5) | 8.3 (7.0, 9.6) | n.s. | 10.8 (9.4, 12.2) | n.s. |
| Shoulder internal rotationb in 180°/s | 23.7 (22.6, 24.7) | 22.4 (21.4, 23.4) | 22.8 (21.0, 24.6) | 21.7 (19.9, 23.6) | n.s. | 26.3 (24.3, 28.2) | n.s. |
| Shoulder external rotationb in 180°/s | 6.0 (5.4, 6.7) | 5.6 (5.0, 6.2) | 5.7 (4.7, 6.8) | 5.5 (4.4, 6.6) | n.s. | 7.4 (6.3, 8.6) | n.s. |
BMI, body mass index; CT, chemotherapy; MIPT, maximal isokinetic peak torque; N · m, Newton metre.
Data presented as adjusted mean with 95% confidence interval (CI).
Model for knee extension and flexion is adjusted for age, BMI (17–<25, 25–30, ≥30 kg/m2), weight, drugs that influence the muscle tonus, antidepressants, regular cycling, and previous experience in resistance training.
Model for shoulder rotation is adjusted for age, BMI (17–<25, 25–30, ≥30 kg/m2), and previous experience in resistance training.
Breast cancer patient groups had, on average, 5–20% decreased MIPT in knee extensors and a 7–25% decrease in knee flexors compared with healthy women measured with 60°/s (Figure 2). The most impaired groups were those with completed chemotherapies. Isokinetic shoulder internal rotator strength was significantly impaired in all cancer treatment groups when compared with healthy controls. Shoulder internal rotators of the dominant side were 12–16% weaker among the treatment groups in comparison with healthy controls, but within cancer patients, no between‐group differences were found. There were no significant differences in shoulder MIPT between BC patients with regard to the operated side (data not shown).
Figure 2.
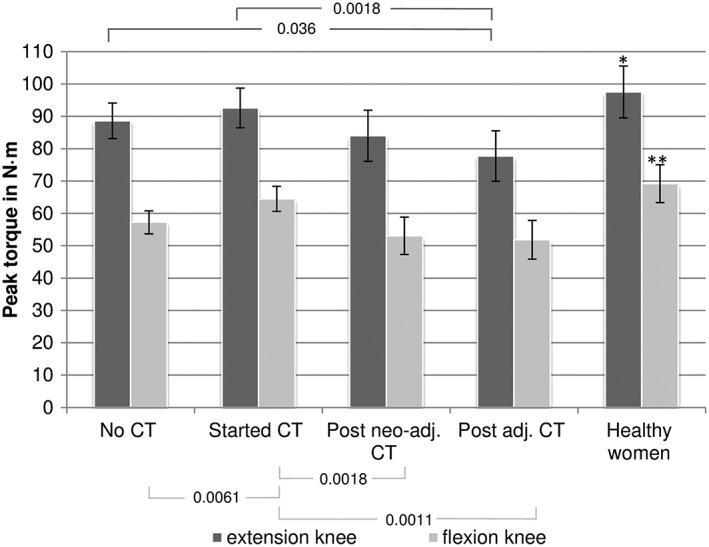
Adjusted means of maximal isokinetic peak torque at 60°/s of extension/flexion knee with 95% confidence intervals. *Significant differences to post neo‐adj. chemotherapy (P = 0.037) and post adj. chemotherapy (P < 0.001); **Significant differences to no chemotherapy (P = 0.0023), post neo‐adj. chemotherapy (P < 0.001), and post adj. chemotherapy (P < 0.001). Models adjusted for age, body mass index (17–<25, 25–30, >30 kg/m2), weight, drugs that influence the muscle tonus, antidepressants, regular cycling, and previous experience in resistance training.
In the ANCOVA model, the covariates that had a significant impact on the strength of lower extremities were cancer treatment, age, BMI, weight, drugs that influence muscle tonus and mood (antidepressants), previous experience in resistance training, and regular cycling (Tables 2 and 3). Other potential confounding factors like orthopaedic dysfunctions, cardiovascular restrictions, cancer‐related physical fatigue (assessed by the Fatigue Assessment Questionnaire), ECOG, and tumour stage showed no significant impact on muscle strength and no confounding on the group effect.
Significant covariates in the model for strength of the operated shoulder were cancer treatment, age, weight, and previous experience in resistance training (Tables 2 and 3). Operation type, number of dissected lymph nodes, pre‐existing injuries in shoulder/arm and time since BC surgery had no significant impact.
Muscular fatigue (FI%)
The greatest fatigue in muscular performance within 10 repetitions could be shown in the post adj. CT group, followed by the groups post neo‐adj. CT, and no CT. All patient groups fatigued faster compared with the healthy individuals, except those patients in the started CT group (Figure 3).
Figure 3.
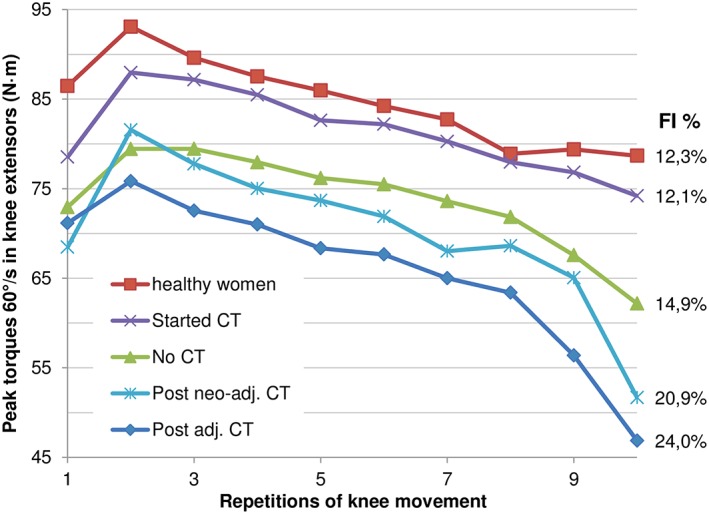
Muscular fatigue over a set and muscular fatigue index (fatigue index = [(peak torque of initial three repetitions−peak torque of final three repetitions)/peak torque of initial three repetitions] × 100) in different treatment groups in knee extensors of the dominant leg. Presented are the unadjusted group means. First repetition omitted from analysis of fatigue index.
Shoulder flexibility
The ANCOVA model showed no significant association with the treatment groups. However, they indicated that the type of surgery and the length of time elapsed since BC surgery was independently influencing factors for shoulder flexibility. The operated side was, on average, 12% less flexible in patients with radical mastectomy compared with partial mastectomy. Furthermore, there was a significant difference in flexibility of the arm elevators in patients <6 weeks post‐surgery (mean of 83°) and those who were tested 6–12 weeks post‐surgery (mean of 90°). No significant differences were identified in patients who were tested >12 weeks post‐surgery (mean of 95°) in comparison with those tested between 6 and 12 weeks post‐surgery.
Discussion
The performed isokinetic and isometric tests were safe and feasible. No adverse events were observed; only sporadic muscle soreness was reported by a few patients. Overall, we observed that BC patients undergoing acute cancer treatment had remarkably impaired strength capacity in both isokinetic and isometric values as well as in muscular fatigue compared with healthy individuals. To our knowledge, this is the first study investigating isometric and isokinetic strength performance in different clinically important BC patient groups, which are at the beginning, or after neo‐adjuvant or adjuvant chemotherapy, or just after surgery. Therefore, our results provide new insights into muscle strength performance of BC patients from several perspectives.
First of all, our findings are predominantly in line with other studies investigating strength performance in cancer patients showing that the muscle status of cancer patients is impaired after treatment. However, most published studies in the field assessed strength performance via handgrip26 or handheld dynamometry,27, 28 with functional tests5 or by using the one repetition maximum method.29, 30 There are also studies using isokinetic testing procedures, but these studies had low sample sizes, and focused on other research questions.23, 31, 32
Regarding the reported performance differences between cancer patients and matched healthy controls, the studies mentioned earlier reported larger differences in strength performance than we observed in our studies. For example, we detected mean differences between 12 and 16% in MVIC for the internal shoulder rotators, whereas a study published by Harrington et al. 28 reported a 26% reduction in a comparable patient group. Lastly, differences with regard to strength testing procedures might be of importance. Handheld dynamometry is known to be a valid and reliable testing procedure, but relatively large measurement errors can occur based on an insufficient standardization of the testing position.33 Computer‐based stationary dynamometry with fixed and therefore highly standardized testing positions will therefore provide more accurate testing values.25
One of the new insights of this study comes along with the isokinetic testing protocol. Because we included two different testing speeds in the protocol, we were able to draw conclusions in relation to muscle fibre activation. Research has shown that at lower angular velocities, muscle fibres I and II can be maximally activated, whereas with increasing speed, less slow twitch fibre (type I) will be recruited.25 With regard to our findings, our results suggest that chemotherapy treatment does not have an impact on fibre activation because the isokinetic strength differences between the CT groups and the non‐CT/started CT groups as well as the control group are comparable in both angular velocities.
New insights could be also reported with regard to the interaction of CT and fatigue resistance of skeletal muscles. We observed that patients having received CT (nearly all treated with anthracycline) had less strength and greater muscular fatigue compared with BC patients without CT or just at the beginning of CT. An explanation for these findings could be an inactivity‐related shift of muscle fibres with a transition to more glycolytic phenotype and a CT‐induced change in mitochondrial capacity of muscle cells.3, 34 This is supported by the observation that CT caused severe reductions in myofibre size, neurogenic alterations, and mitochondria‐related damages in mice as well as in humans.2, 35 Furthermore, it is well known that BC patients reduce their physical activity level during the period of cancer treatment.7 Moreover, our patients reported less activity in the year before. These circumstances potentially lead to a loss of muscle strength, which can be supported by our objective data. In general, individual strength performance in cancer patients may be influenced by various contextual factors. Some of those factors are independent from the cancer setting (e.g. age or motivation of the patient) and some not (e.g. locoregional and systemic therapies and cancer‐related fatigue).33 Receiving chemotherapy might be one of the most important factors as we already reported for cardiorespiratory fitness,14 but the mechanisms and pathways of cancer treatment influences on muscle structure and function are not completely understood. It is supposed that CT causes oxidative stress to normal tissue and directly impacts skeletal muscles and fatigue.34, 36 Other possible pathways could be up‐regulating processes of muscle‐specific enzymes (E3 ubiquitin ligase atrogin1/muscle atrophy F‐box) through the proteasome pathway, the activation of caspase by oxidant‐mediated apoptosis, and the formation of reactive oxygen species in muscle stimulating apoptosis in skeletal myocytes.37 All the factors may result in an increased risk of sarcopenia, which has been already reported by others.1, 8
Sarcopenia is an independent predictor of survival, which is closely related to patients' functional status and potentially to CT toxicity.38, 39 In average, nearly all evaluated BC groups in our study had indications for sarcopenia because of impaired muscle strength and function,40 and those who had received CT in their treatment history are at the highest risk. This might have important clinical implications due to the aforementioned associations with prognostic factors.8, 38, 39 Furthermore, because abnormal loss of muscle strength is associated with loss of autonomy and quality of life, altered functional status, increase of fatigue, and falls,41 the importance of adequate musculoskeletal status should be one of the most important goals during and after anticancer treatment.
This postulation is supported by RCT data suggesting that adding resistance training to standard CT may improve CT completion rate11, 42 as well as BC outcomes.12 Hereby, reduced systemic inflammation43 modulate the insulin pathway,44 favourably affect cell‐mediated immunity,45 and change steroid hormone levels46 are discussed.
Aside from reduced strength capacity, upper‐body mobility restrictions represent a stressful physical limitation in patients undergoing BC surgery.47 We measured a loss of shoulder mobility and decreased shoulder internal rotator strength, resulting in an impaired shoulder function, which may be a result of mastectomy. Impaired shoulder function has been reported in many BC survivors even several years after surgery.48 A recent study by Harrington et al. 28 presented complex analyses using a score (with usage of questionnaires, multiple flexion movements, and MVIC with a handheld dynamometer) for disabilities in shoulder of BC survivors (N = 24) in a similar timeframe after surgery. This study reported significant differences in comparison to healthy controls. We can support the results of this small study, but caution needs to be utilized because different testing procedures were used. Interestingly, the time difference between surgery and testing, type of surgery and pain are considered to have no impact on shoulder strength; only flexibility, which was dependent on the type and time since surgery, was impaired.
Our study has several strengths. We performed stationary isokinetic strength testing, which is the gold standard procedure for functional skeletal muscle assessment. Furthermore, we report data on a very large sample size (n = 255) of early stage BC patients in a well‐defined and clinically relevant time frame. Moreover, we were able to assess many relevant cofactors and include them in adjusted regression models on strength performance in clinically relevant subgroups. Lastly, the current study is the first that reports information about muscular fatigue in relation to different treatment settings, and all patient data could be compared with an age‐matched healthy control group.
This study did include some limitations. The sizes of the groups were unequal because the studies (BEST, BEATE, and INVEST) were not primarily designed for these comparisons. Additionally, the cross‐sectional design limits causal inferences. Furthermore, the healthy women were a convenience sample, and despite matching by age groups, differences need to be interpreted with caution. Nevertheless, the strength performance of our healthy participants was in line with comparably aged healthy women.49 We also found significant age differences between the treatment groups showing a higher age in the non‐CT group. The reason for that is uncertain but might represent the tendency of the treating physician to prescribe CT less frequently to elderly patients due to potential side effects and the fact that response rates vary greatly so that CT is not always beneficial.50 However, the age differences were taken into account by adjusting all models for age. Lastly, due to organizational reasons it was not possible to perform a separate familiarization session on the stationary dynamometer. This may led to an underestimation of strength performance. However, the testing situation was standardized for all participants and therefore comparable for all groups. Furthermore, all participants had a short familiarization time at each testing position immediately before the assessment starts.
In conclusion, our study showed that isometric and isokinetic strength testing appears to be safe in a large cohort of BC patients. We reported about significantly impaired isometric and isokinetic strength capacity with higher muscular fatigue in low extremities and dysfunctions in shoulder mobility in our patients. Overall, receiving CT treatment seems to have the greatest impact on muscular strength.
Based on the findings in different BC subgroups, the prevention of muscle dysfunction should be an important goal during cancer treatment and underlines the importance for the implementation of resistance training regimens during cancer treatment to mitigate or reverse muscle dysfunction. Furthermore, systematic resistance training after BC therapy should be considered to alter consequences of muscle dysfunction in cancer rehabilitation. To further understand the mechanisms of muscular dysfunction in cancer patients, there is a need for the assessment of cellular muscle structure and biomarkers combined with accurate (gold standard) strength testing procedures.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.51
The authors thank the study participants who willingly spent their time to complete the study procedures, the BC centres supporting the recruitment, Dr Jan Oelmann and Dr Andrea Koffka for medical advice and examinations, Petra Armbrust and Dr Tilla Ruf for study coordination and assistance, Werner Diehl for data management, and Michael Paskow for linguistic editing.
The BEST trial was partially funded by the Interdisciplinary Research Funding Program (intramural) of the National Center for Tumor Diseases (NCT), Heidelberg. The training for all three trials (BEST, BEATE, and INVEST) was supported by the Foundation ‘Stiftung Leben mit Krebs’. Dr Joachim Wiskemann was personally supported by the Manfred Lautenschlaeger Foundation.
Klassen, O. , Schmidt, M. E. , Ulrich, C. M. , Schneeweiss, A. , Potthoff, K. , Steindorf, K. , and Wiskemann, J. (2017) Muscle strength in breast cancer patients receiving different treatment regimes. Journal of Cachexia, Sarcopenia and Muscle, 8: 305–316. doi: 10.1002/jcsm.12165.
References
- 1. Villasenor A, Ballard‐Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv : Res Practice 2012;6:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann of Oncol: official J of the European Society for Med Oncol / ESMO 2014;25:947–958. [DOI] [PubMed] [Google Scholar]
- 3. Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol 2012;9:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta‐analysis. Ann of Oncol: official J of the European Society for Med Oncol / ESMO 2014;25:1293–1311. [DOI] [PubMed] [Google Scholar]
- 5. Winters‐Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum 2008;35:815–821. [DOI] [PubMed] [Google Scholar]
- 6. Demark‐Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J of Clin Oncol: official J of the Am Society of Clin Oncol 2001;19:2381–2389. [DOI] [PubMed] [Google Scholar]
- 7. Huy C, Schmidt ME, Vrieling A, Chang‐Claude J, Steindorf K. Physical activity in a German breast cancer patient cohort: one‐year trends and characteristics associated with change in activity level. Eur J Cancer 2012;48:297–304. [DOI] [PubMed] [Google Scholar]
- 8. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin cancer Res: an official J of the Am Association for Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 9. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 10. Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Cancer treatment‐induced alterations in muscular fitness and quality of life: the role of exercise training. Ann Oncol: official J of the European Society for Med Oncol / ESMO 2007;18:1957–1962. [DOI] [PubMed] [Google Scholar]
- 11. Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J of Clin oncol : official J of the Am Society of Clin Oncol 2007;25:4396–4404. [DOI] [PubMed] [Google Scholar]
- 12. Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 2014;46:1744–1751. [DOI] [PubMed] [Google Scholar]
- 13. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta‐analysis. Med Sci Sports Exerc 2013;45:2080–2090. [DOI] [PubMed] [Google Scholar]
- 14. Klassen O, Schmidt ME, Scharhag‐Rosenberger F, Sorkin M, Ulrich CM, Schneeweiss A, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol 2014;53:1356–1365. [DOI] [PubMed] [Google Scholar]
- 15. Potthoff K, Schmidt ME, Wiskemann J, Hof H, Klassen O, Habermann N, et al. Randomized controlled trial to evaluate the effects of progressive resistance training compared to progressive muscle relaxation in breast cancer patients undergoing adjuvant radiotherapy: the BEST study. BMC Cancer 2013;162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt ME, Wiskemann J, Krakowski‐Roosen H, Knicker AJ, Habermann N, Schneeweiss A, et al. Progressive resistance versus relaxation training for breast cancer patients during adjuvant chemotherapy: design and rationale of a randomized controlled trial (BEATE study). Contemp Clin Trials 2013;34:117–125. [DOI] [PubMed] [Google Scholar]
- 17. Steindorf K, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Habermann N, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer‐related fatigue and quality of life. Ann Oncol 2014;25:2237–2243. [DOI] [PubMed] [Google Scholar]
- 18. Glaus A, Mueller S. Measuring fatigue of cancer patients in the German‐speaking region: development of the Fatigue Assessment Questionnaire. Pflege 2001;14:161–170. [DOI] [PubMed] [Google Scholar]
- 19. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 20. Schroevers MJ, Sanderman R, van Sonderen E, Ranchor AV. The evaluation of the Center for Epidemiologic Studies Depression (CES‐D) scale: depressed and positive affect in cancer patients and healthy reference subjects. Qual Life Res 2000;9:1015–1029. [DOI] [PubMed] [Google Scholar]
- 21. Dirnberger J, Wiesinger HP, Kosters A, Muller E. Reproducibility for isometric and isokinetic maximum knee flexion and extension measurements using the IsoMed 2000‐dynamometer. Isokinet Exerc Sci 2012;20:149–153. [Google Scholar]
- 22. Jones LW, Mourtzakis M, Peters KB, Friedman AH, West MJ, Mabe SK, et al. Changes in functional performance measures in adults undergoing chemoradiation for primary malignant glioma: a feasibility study. Oncologist 2010;15:636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kilgour RD, Vigano A, Trutschnigg B, Hornby L, Lucar E, Bacon SL, et al. Cancer‐related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle 2010;1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber MA, Krakowski‐Roosen H, Schroder L, Kinscherf R, Krix M, Kopp‐Schneider A, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer‐related cachexia. Acta Oncol 2009;48:116–124. [DOI] [PubMed] [Google Scholar]
- 25. Kannus P. Isokinetic evaluation of muscular performance: implications for muscle testing and rehabilitation. Int J Sports Med 1994;S11–S18. [DOI] [PubMed] [Google Scholar]
- 26. Kilgour RD, Vigano A, Trutschnigg B, Lucar E, Borod M, Morais JA. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Supp Care in Cancer : official J of the Multinational Association of Supp Care in Cancer 2013;3261–3270. [DOI] [PubMed] [Google Scholar]
- 27. Hummler S, Thomas M, Hoffmann B, Gartner P, Zoz M, Huber G, et al. Physical performance and psychosocial status in lung cancer patients: results from a pilot study. Oncol Res Treat 2014;37:36–41. [DOI] [PubMed] [Google Scholar]
- 28. Harrington S, Padua D, Battaglini C, Michener LA, Giuliani C, Myers J, et al. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. J Cancer Surviv 2011;5:167–174. [DOI] [PubMed] [Google Scholar]
- 29. Galvao DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross‐sectional investigation. Prostate Cancer Prostatic Dis 2009;12:198–203. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz JR, Sui X, Lobelo F, Lee DC, Morrow JR Jr, Jackson AW, et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer epidemiology, biomarkers & prevention : a publication of the Am Association for Cancer Res cosponsored by the Am Society of Preventive Oncol 2009;18:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fong SS, Ng SS, Luk WS, Chung JW, Chung LM, Tsang WW, et al. Shoulder mobility, muscular strength, and quality of life in breast cancer survivors with and without Tai Chi qigong training. Evid‐based complementary and alternat med: eCAM 2013;2013:787169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilcock A, Maddocks M, Lewis M, Howard P, Frisby J, Bell S, et al. Use of a Cybex NORM dynamometer to assess muscle function in patients with thoracic cancer. BMC Palliat Care 2008;7:doi:10.1186/1472-684X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knols RH, Stappaerts KH, Fransen J, Uebelhart D, Aufdemkampe G. Isometric strength measurement for muscle weakness in cancer patients: reproducibility of isometric muscle strength measurements with a hand‐held pull‐gauge dynamometer in cancer patients. Supp Care in Cancer : official J of the Multinational Association of Supportive Care in Cancer 2002;10:430–438. [DOI] [PubMed] [Google Scholar]
- 34. Bonifati DM, Ori C, Rossi CR, Caira S, Fanin M, Angelini C. Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol 2000;46:517–522. [DOI] [PubMed] [Google Scholar]
- 35. Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline‐induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation 2011;124:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilliam LA, St Clair DK. Chemotherapy‐induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal 2011;15:2543–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 2014;49:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 39. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550–557. [DOI] [PubMed] [Google Scholar]
- 40. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beaudart C, Gillain S, Petermans J, Reginster JY, Bruyere O. Sarcopenia: what's new in 2014. Rev Med Liege 2014;69:251–257. [PubMed] [Google Scholar]
- 42. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 2015;doi:10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 43. Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Martin BS, et al. Effect of exercise training on C‐reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun 2005;19:381–388. [DOI] [PubMed] [Google Scholar]
- 44. Ballard‐Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl physiol (Bethesda, Md : 1985) 2005;98:1534–1540. [DOI] [PubMed] [Google Scholar]
- 46. McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, et al. Effect of exercise on serum androgens in postmenopausal women: a 12‐month randomized clinical trial. Cancer epidemiology, biomarkers & prevention : a publication of the Am Association for Cancer Res cosponsored by the Am Society of Preventive Oncol 2004;13:1099–1105. [PubMed] [Google Scholar]
- 47. Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, et al. Upper‐body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer 2012;118:2237–2249. [DOI] [PubMed] [Google Scholar]
- 48. Kootstra JJ, Dijkstra PU, Rietman H, de Vries J, Baas P, Geertzen JH, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat 2013;139:125–134. [DOI] [PubMed] [Google Scholar]
- 49. Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross‐sectional study of muscle strength and mass in 45‐ to 78‐yr‐old men and women. J Appl Physiology (Bethesda, Md : 1985) 1991;71:644–650. [DOI] [PubMed] [Google Scholar]
- 50. Denkert C, Sinn BV, Issa Y, Maria Muller B, Maisch A, Untch M, et al. Prediction of response to neoadjuvant chemotherapy: new biomarker approaches and concepts. Breast Care 2011;6:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


