Abstract
Loss of skeletal muscle mass is a characteristic feature of various pathologies including cancer, diabetes, and obesity, as well as being a general feature of ageing. However, the processes underlying its pathogenesis are not fully understood and may involve multiple factors. Importantly, there is growing evidence which supports a role for fatty acids and their derived lipid intermediates in the regulation of skeletal muscle mass and function. In this review, we discuss evidence pertaining to those pathways which are involved in the reduction, increase and/or preservation of skeletal muscle mass by such lipids under various pathological conditions, and highlight studies investigating how these processes may be influenced by dietary supplementation as well as genetic and/or pharmacological intervention.
Keywords: Atrophy, Catabolism, Fatty acid, Lipid, mTOR, Obesity, Skeletal muscle
Introduction
The maintenance of skeletal muscle mass and integrity is crucial for proper functioning of the musculoskeletal system as well as efficient nutrient uptake and storage. Under normal physiological conditions, a network of interconnected signals serves to co‐ordinate muscle protein synthesis and proteolysis. However, any impairment of these signalling processes can contribute to a loss of muscle mass, or atrophy, which is a feature associated with various pathologies including cancer (termed cachexia), heart disease and obesity, as well as ageing (termed sarcopenia) (see Figure 1).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Moreover, injuries such as severe burns can induce a series of proinflammatory stress responses which have also been linked to muscle wasting post‐injury.13 Consequently, reduced skeletal muscle mass can severely weaken the musculoskeletal system and hamper locomotion, as well as contribute to the development of impaired glucose and lipid homeostasis, particularly in the obese state.
Figure 1.
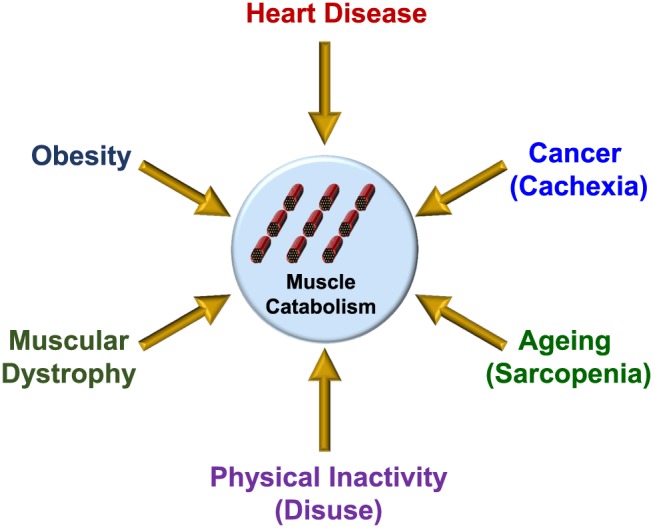
Disorders and conditions which are associated with reduced muscle mass and/or function. Schematic diagram illustrating various pathologies and/or conditions which are associated with increased muscle catabolism coinciding with reduced skeletal muscle mass and/or function.
Conversely, muscle mass can be increased either through hypertrophy, which is characterized by an expansion in the size of pre‐existing myofibres, or through the process of hyperplasia, which involves an increase in the number of cells or fibres.14, 15, 16, 17 Indeed, several model systems have been used to study such growth responses in myogenic progenitor (satellite) cells and/or differentiated myotubes including, for example, the murine C2C12 muscle cell line (myoblasts originally cultured from thigh muscle of C3H mice), rat L6 muscle cells (a skeletal muscle cell line established from thigh muscle of newborn rats), or primary myogenic cells derived from purified muscle fibres.18, 19 Notably, muscle hypertrophy can be induced by multiple anabolic stimuli—among the most studied of which include insulin and insulin‐like growth factor 1 (IGF‐1).20 Indeed, signalling triggered by growth factors such as IGF‐1 act to positively regulate muscle growth, driven at least in part through the induction of protein synthesis.21, 22, 23 Mechanistically, activation of the IGF‐1/IGF‐1 receptor signalling axis leads to the insulin receptor substrate 1 (IRS1)‐dependent recruitment of PI3‐kinase and subsequent activation of protein kinase B (PKB) (also known as Akt) through the generation of phosphatidylintosiol‐3,4,5 trisphosphate (PIP3) by PI3‐kinase. Active Akt then promotes the activation of mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) by phosphorylating and inhibiting its upstream repressor TSC2.24 This then results in the mTORC1‐dependent phosphorylation of p70‐S6 kinase 1 (S6K) and eIF4E‐binding protein (4E‐BP), leading to increased protein synthesis. An alternative downstream target of Akt is glycogen synthase kinase 3β (GSK3β) which becomes phosphorylated and inhibited by active Akt. Repression of GSK3 acts to relieve its inhibition of the initiation factor eIF2B, leading to increased protein synthesis.25 In addition, Akt also phosphorylates and inhibits the Forkhead box O (FOXO) family of transcription factors, thereby repressing their transcriptional activation of the E3 ubiquitin ligases Muscle Atrophy Fbox (MAFbx) (also known as atrogin‐1) and Muscle Ring Finger 1 (MuRF1) which function to promote ubiquitination and subsequent proteasomal degradation of target substrates.9 Pro‐inflammatory cytokines such as tumour necrosis factor alpha (TNF‐α) can also act to induce atrophic genes such as MuRF1 and atrogin‐1/MAFbx by activating the nuclear factor‐kappa B (NF‐kB) family of transcription factors.26 Therefore, a number of distinct signalling pathways have been implicated in controlling skeletal muscle hypertrophy and atrophy. Notably, diet‐induced obesity or short‐term high fat feeding has been shown to promote or augment muscle atrophy and catabolism, as evidenced by reduced muscle mass and muscle fibre size, in association with up‐regulated expression of atrophic factors (i.e. atrogin‐1/MAFbx and MuRF1) and increased rate of proteolysis.10, 27 Allied to this, fat infiltration into muscle has been associated with reduced muscle strength.28 Herein, we discuss the evidence which supports a role for the involvement of fatty acids and derived lipid metabolites in the regulation of skeletal muscle mass and function through their ability to modulate muscle cell growth, proliferation, and/or differentiation. Furthermore, we explore potential mechanisms that may be involved in the control of muscle hypertrophy/atrophy by such lipids.
Fatty acid modulation of skeletal muscle mass and function
Evidence from several studies suggests that saturated and unsaturated fatty acids may act to differentially regulate skeletal muscle mass and function. For example, exposure of C2C12 myotubes to palmitate (C16:0), the most abundant circulating saturated fatty acid, has been shown to decrease myotube diameter and suppress insulin signalling.29 In accord with this, palmitate provision in muscle cells has been reported to induce the expression of pro‐atrophic genes such as atrogin‐1/MAFbx, concomitant with increased nuclear localization of its transcriptional regulator FoxO3.30 In contrast, application of docosahexaenoic acid (DHA), an omega‐3 polyunsaturated fatty acid (PUFA), did not alter myotube morphology when applied alone and was shown to counter‐modulate palmitate‐induced atrophy in C2C12 myotubes.29 Consistent with this, a separate study reported the amelioration of palmitate induced protein degradation in C2C12 myotubes following co‐treatment with DHA.30 Notably, this coincided with the ability of DHA to mitigate enhanced nuclear FoxO3 localization and atrogin‐1/MAFbx gene expression in response to palmitate provision.30
In accord with these findings in cultured muscle cells, several in vivo studies have also reported the ability of unsaturated fatty acids to convey beneficial responses which act to prevent muscle wasting and/or atrophy. For example, feeding mice bearing the colon‐26 adenocarcinoma, an animal model of cancer cachexia, with a diet supplemented with conjugated linoleic acid, was shown to preserve gastrocnemius muscle mass.7 Notably, this protective effect coincided with a reduction in skeletal muscle TNF‐α receptor expression suggesting that the PUFA may act to prevent muscle wasting, at least in part, by reducing the catabolic actions of the cytokine TNF‐α.7, 31 In a separate study, dietary supplementation with eicosapentaenoic acid (EPA; C20:5(n‐3)) attenuated protein degradation in gastrocnemius muscle of mice bearing the cachexia‐inducing MAC16 tumour.4 EPA treatment has also been reported to prevent arthritis‐induced reductions in gastrocnemius muscle weight in rats following administration of Freund's adjuvant, concomitant with the normalization of atrogin‐1/MAFbx and MuRF1 gene expression.32 Moreover, dystrophic hamsters fed a diet enriched in the PUFA α‐linolenic acid (ALA) (C18:3(n‐6)) exhibited improvements in muscle morphology and function, including enlarged myofibres.33 In accord with these findings, omega‐3 and omega‐6 PUFAs have also been shown to increase phosphorylation of p70S6K1 at Thr389, indicative of its increased activity, during myogenic differentiation of L6 myocytes.34 Together, these studies support the notion that unsaturated fatty acids can provide protection against muscle wasting in response to various pathological conditions. Furthermore, these findings highlight the distinct responses that saturated and unsaturated fatty acids induce to promote or counter muscle atrophy and protein degradation, respectively.
Potential factors underlying fatty acid regulation of skeletal muscle size and mass
A number of different signalling pathways and/or intermediates have been implicated as potential mediators of muscle wasting and atrophy, which themselves can be regulated in response to fatty acid provision (see Figure 2). For example, palmitate is known to act as a potent repressor of PKB/Akt directed signalling in skeletal muscle, at least in part through its ability to induce the accumulation of toxic lipid intermediates such as ceramide.35, 36 Indeed, such sphingolipids can act by stimulating protein phosphatase 2A (PP2A) or atypical protein kinase C (PKC) (PKCζ) isoforms to inhibit PKB/Akt.37 In accord with this, C2C12 myotube atrophy induced by TNF‐α has been reported to coincide with elevated levels of intracellular ceramide,38 whereas blocking ceramide synthesis has been shown to attenuate TNF‐α induced muscle atrophy in L6 myotubes, as well as protecting mice against tumour induced (via C26 carcinoma implantation) skeletal muscle atrophy in vivo. 38 Notably, these beneficial responses concurred with increased protein synthesis and decreased proteolysis, concomitant with reduced expression of the atrogin‐1/MAFbx gene via suppressed Foxo3 function, as well as increased abundance of key mediators of protein synthesis including S6K1 and PKB/Akt.38 Moreover, exogenous provision of ceramide in L6 muscle cells has been reported to reduce protein levels of the myogenic transcription factor myogenin via inhibition of phospholipase D, whilst inhibition of ceramide synthesis enhanced myogenin expression and accelerated myotube formation.39 A study by Turpin and colleagues also demonstrated increased muscle ceramide content following acute (5 h) intralipid® infusion, which coincided with the activation of pro‐apoptotic signalling as demonstrated by increased caspase‐3 activity in gastrocnemius muscle.40 However, the role of ceramide in promoting this lipid‐driven increase in muscle apoptosis was not investigated, for example by co‐administration of inhibitors of ceramide synthesis. Alternatively, elevated levels of ceramide associated with hyperlipidaemia may also act to suppress protein synthesis by inducing the expression and/or activity of key repressors of mTORC1‐S6K signalling such as Regulated in Development and DNA Damage 1 (REDD1).41, 42 Notably, it should also be highlighted that the ganglioside GM3 (trisialotetrahexosylganglioside), a sialic acid‐containing glycosphingolipid derived from ceramide, has also been implicated as a negative regulator of skeletal muscle growth and/or differentiation, concomitant with its reported ability to impair insulin action by impairing insulin receptor function.43, 44, 45, 46 Moreover, another ceramide derived lipid, ceramide‐1‐phosphate, has also been shown to stimulate C2C12 myoblast proliferation through a mechanism involving the activation of Akt, mTOR, and ERK1/2.47 Indeed, further work utilizing mice deficient for GM3 synthase, the enzyme responsible for the synthesizing GM3, may shed more light regarding the role of this ganglioside in the control of skeletal muscle mass, for example in response to obesity and/or aging.
Figure 2.
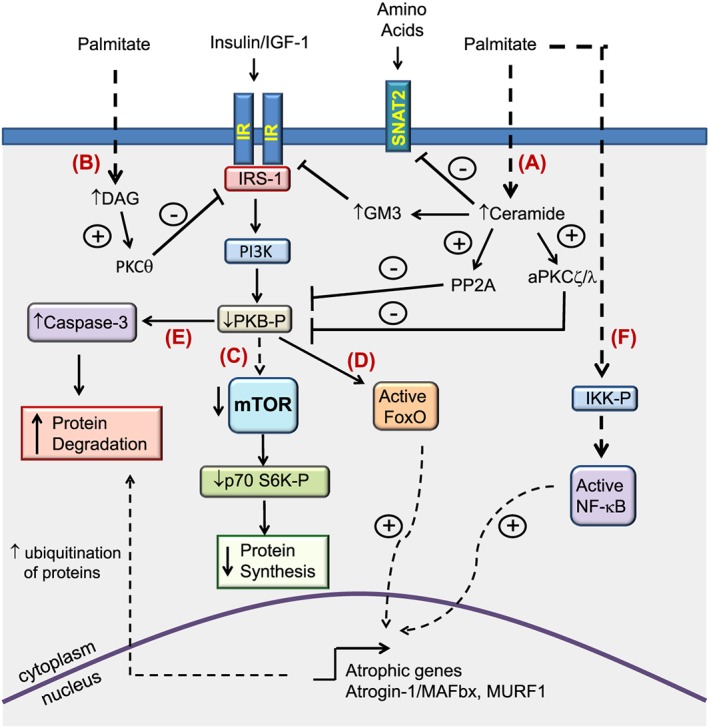
Summary of pathways mediating muscle atrophy by saturated fatty acids. Exposure of muscle cells to saturated fatty acids such as palmitate (C16:0) results in the intracellular accumulation of toxic lipid intermediates such as ceramide and diacylglycerol. (A) Increased ceramide levels can lead to the inhibition of protein kinase B/Akt through activation of atypical protein kinase C(ξ/λ) isoforms and/or protein phosphatase 2A. Moreover, ceramide acts as a precursor for the synthesis of the glycosphingolipid GM3 which has been shown to impair insulin receptor function. In addition, ceramide may also act to modulate nutrient uptake, for example by repressing the expression of the neutral amino transporter SNAT2 thereby reducing cellular amino acid supply. (B) Diacylglycerol‐induced stimulation of protein kinase Cθ has been shown to promote serine phosphorylation of IRS‐1, resulting in its impaired function. The resulting inhibition of protein kinase B/Akt in turn can lead to the repression of protein synthesis through suppression of mechanistic target of rapamycin (mTOR)/p70‐S6 kinase 1‐dependent signalling (C), the activation of Forkhead box O (FoxO) transcription factors and induction of their target atrophic genes (D), and/or the activation of caspase‐dependent proteolysis (E). In addition, stimulation of pro‐inflammatory signalling by long chain saturated fatty acids can lead to the nuclear factor‐kappa B‐dependent upregulation of atrophic genes (F).
In addition to sphingolipids, diacylglycerols (DAGs) are an alternative class of lipid which can be generated in response to fatty acid provision. Notably, increased levels of DAG have been associated with the development of insulin resistance.35, 48 Moreover, increased muscle DAG levels have been detected following lipid infusion in mice, concomitant with increased caspase‐3 activity in gastrocnemius muscle.40 Although little is known regarding the role of DAGs in the regulation of skeletal muscle mass, ex‐vivo mechanical activation of DAG kinaseζ (DGKζ), an enzyme which catalyzes the conversion of DAG to phosphatidic acid (PA), has been reported to promote increased mTOR dependent signalling and associated hypertrophy in isolated mouse extensor digitorum longus (EDL) muscle, concomitant with the reported ability of PA to bind and directly activate mTOR.49, 50 In accord with this, cardiac‐specific overexpression of DGKζ has also been shown to ameliorate myocardial atrophy in streptozotocin‐induced diabetic mice.51 Therefore, these findings suggest that activation and/or overexpression of DGKζ may provide a means of stimulating protein synthetic rates and hypertrophic responses, and thereby ameliorating losses in muscle mass, either through reducing cellular levels of DAG and/or increasing PA‐induced activation of mTOR signalling. Importantly, future work may involve investigating the potential beneficial effects of overexpressing of DGKζ in muscle as a means of countering age and/or diet induced muscle atrophy. In addition, animal models which exhibit elevated levels of DAG in skeletal muscle, including mice which are deficient for hormone‐sensitive lipase (HSL),52 may also be useful for elucidating the role of DAG in skeletal muscle atrophy.
Another important consideration relates to the possibility that distinct DAG species may impact differently on pathways that are involved in regulating muscle mass, for example as determined by the composition of the fatty acyl groups which become esterified at either the sn‐1,2, sn‐1,3, or the sn‐2,3 positions of the glycerol backbone of DAG.53, 54 Indeed, previous work by our group has demonstrated that treatment of rat L6 myotubes with palmitate leads to significant increases in the cellular levels of certain DAG species, as well as total cellular DAG content.55 Furthermore, co‐treatment with the monounsaturated fatty acid (MUFA) palmitoleate (C16:1) was shown to selectively suppress palmitate‐induced increases in the levels of DAG species containing C18:0 and C20:0 saturated fatty acyl moieties, coinciding with the MUFA's anti‐inflammatory action.55 Although not determined in this study, distinct stereoisomers of DAG may also differentially regulate muscle anabolic/catabolic signalling. To support this notion, sn‐1,2 DAG stereoisomers (in comparison to sn‐1,3 isomers) have been reported to be more potent at activating signalling pathways linked to insulin resistance, including the activation of PKC.56 Together, these studies provide emerging evidence that certain DAG molecules/isomers may play a more prominent role in the development of muscle atrophy, for example by promoting insulin resistance and/or increasing pro‐inflammatory drive. However, further work will be required to determine which of these DAG molecules, if any, are responsible for conveying muscle wasting actions. In an attempt to address this, future studies may involve treating cultured muscle cells with different DAG molecules/stereoisomers in order to determine their effects on myogenesis and/or muscle atrophy. Alternatively, further work may also incorporate detailed lipidomic analysis of various intramuscular DAG species in tissue isolated from animal models of muscle wasting, as well as monitoring potential changes in their abundance following interventions that are known to increase muscle mass (e.g. PUFA dietary provision or increased physical activity). Indeed, if such studies were to reveal a key role for DAG accumulation in the development of skeletal muscle atrophy, subsequent work may then involve determining the origin of such DAG species, for example by inhibiting the activity of enzymes implicated in DAG formation during triacylglycerol (TAG) synthesis (e.g. glycerol phosphate transferase (GPAT), acylglycerolphosphate acyltransferase (AGPAT), and lipin), or by altering the activity of enzymes implicated in TAG and/or DAG hydrolysis (e.g. adipose triglyceride lipase (ATGL) or HSL). To this end, previous work by Badin and co‐workers reported elevated ATGL protein abundance in skeletal muscle of type 2 diabetic individuals vs. lean control subjects, as well as reduced muscle HSL expression in obese individuals.57 In addition, the authors of the same study further demonstrated that overexpressing ATGL or inhibiting HSL activity in human primary myotubes resulted in the accumulation of cellular DAG and an associated impairment in insulin signalling. However, whether these changes in DAG levels are linked to muscle atrophy was not determined in this study.
As well as modulating PKB/Akt and/or mTORC1 directed signalling, fatty acids and/or their derived lipids may further contribute to muscle wasting by modulating nutrient (amino acid) transport and/or associated signalling. For example, previous work by our own group and others has demonstrated the ability of ceramide to down‐regulate the expression and/or activity of key nutrient transporters, including the neutral amino acid transporter SNAT2 (SLC38A2).58, 59 By doing so, fatty acids acting through such lipid intermediates may act to impair amino acid uptake, thereby contributing to a loss in muscle mass. Interestingly, in a separate study by our group, incubation of rat L6 myotubes with linoleic acid (C18:2) was shown to restrain adaptive upregulation of SNAT2 expression and activity in response to amino acid starvation.60 Notably, this fatty acid induced reduction in System A transport activity was mediated through increased ubiquitination and proteasomal degradation of SNAT2 protein.60 Conversely, in a separate study by Li and co‐workers, the mRNA expression of the amino acid transceptors LAT1 (an L‐type amino acid transporter) and SNAT2 were reported to be up‐regulated in the longissimus dorsi of pigs fed dietary n‐6 and n‐3 PUFAs.61 Hence, it is possible that fatty acids and/or their derived lipids may function to modulate adaptive strategies which are used by tissues such as skeletal muscle, in order to maximize or minimize nutrient uptake during conditions of fasting or nutrient deprivation.
Role of unsaturated fatty acids in maintaining skeletal muscle size and mass
Importantly, current literature describes evidence to suggest that unsaturated fatty acids may act to counter pro‐atrophic mediators, including those triggered following exposure to saturated fatty acids. For example, MUFAs and PUFAs have been reported to prevent palmitate‐induced reductions in insulin sensitivity as well as conveying anti‐inflammatory effects in skeletal muscle cells.55, 62, 63 Indeed, NF‐kB dependent transcriptional regulation has been implicated in promoting disuse muscle atrophy in rat soleus muscle by increasing FoxO mediated activation of the MuRF1 promoter.64 Moreover, a recent study demonstrated that diminished anabolic signalling in skeletal muscle of aged mice coincided with the accumulation of intramuscular ceramide and DAG, as well as increased TNF‐α mRNA abundance.65 Interestingly, feeding fish oil to weaning piglets, which resulted in the enrichment of EPA, DHA, and total omega‐3 PUFA content within gastrocnemius muscle, coincided with a reduction in muscle TNF‐α levels and reduced expression of Toll‐like receptor 4 (TLR4), a target receptor for saturated fatty acids which stimulates pro‐inflammatory signalling in response to its activation.66 Notably, TLR4 stimulation by its ligand lipopolysaccharide has been reported to induce muscle catabolism in C2C12 myotubes through activation of the ubiquitin–proteasome and autophagy–lysosome pathways.67 In addition, DHA treatment of human muscle cells co‐cultured with macrophages has been shown to attenuate macrophage‐induced protein content of Fn14, a positive modulator of MuRF‐1 expression.68, 69 Therefore, based on these findings, it is conceivable that the reported anti‐inflammatory actions of unsaturated fatty acids in skeletal muscle cells may contribute, at least in part, to their ability to preserve muscle mass and/or function.
Notably, these protective actions may be linked to improvements in mitochondrial function, the impairment of which has been suggested to contribute to diet and/or age‐induced muscle atrophy.27, 70, 71 For example, a recent study by Roseno and colleagues reported that a short‐term (3 week) high fat diet augmented denervation muscle atrophy in mice by inducing protein degradation in mitochondria‐rich soleus, but not in glycolytic EDL muscle.27 Notably, 14 day denervation induced a loss in mitochondrial protein content in the soleus but not the EDL, regardless of diet. Therefore, these findings suggest that denervation‐induced loss of mitochondria and high fat diet‐induced impairment of mitochondrial function may combine to promote skeletal muscle atrophy.27 In contrast, an independent study by Tardif and co‐workers demonstrated that aged rats fed an oleate‐enriched diet display marked improvements in insulin sensitivity as well as increased muscle protein synthesis, concomitant with increased expression of genes implicated in stimulating mitochondrial β‐oxidation including peroxisome proliferator‐activated receptor (PPAR)α and PPARβ, as well as CPT‐1β.72, 73 Moreover, C2C12 myotubes treated with the PUFAs linolenic acid and ALA have been shown to exhibit increased activation of AMPK, another key positive regulator of mitochondrial β‐oxidation.74 In addition, DHA has also been reported to inhibit protein degradation in C2C12 myotubes through a PPARγ‐dependent pathway.75 Indeed, enhanced and/or preserved mitochondrial oxidative capacity, as previously reported in response to sole or co‐provision of unsaturated fatty acids, may also help prevent the intramuscular accumulation of lipotoxic intermediates such as ceramide which have been implicated in promoting muscle atrophy.36, 55, 76, 77 Furthermore, it is possible that PUFA supplementation may act to alter muscle contractile and metabolic properties, for example by promoting a shift from fast glycolytic to slow (oxidative) fibre types. To support this idea, previous work has demonstrated that feeding Wistar rats a diet enriched in n‐3 PUFAs results in the upregulation of proteins implicated in the activation of oxidative metabolism (e.g. mitochondrial uncoupling protein 3 and PPARγ coactivator 1‐α (PGC1α)) in EDL muscle (a fast‐type dominant muscle tissue).78 Interestingly, this PUFA‐mediated metabolic shift also coincided with reduced protein levels of the fast‐type MyHC‐2b (myosin heavy chain 2b) isoform in EDL muscle. Therefore, it is conceivable that a PUFA‐mediated shift towards a slow oxidative muscle fibre type may contribute, at least in part, towards beneficial gains in muscle mass and/or metabolic function.
Alternatively, regulation of muscle mass by lipids may also involve modulation of autophagy, a homeostatic mechanism which facilitates the degradation and recycling of proteins and organelles through the lysosomal machinery.79 Notably, increased autophagic degradation has been reported to coincide with muscle atrophy in various conditions and/or pathologies including cancer,80 denervation,81 as well as ageing.80, 82 Moreover, short‐term (3 week) high fat feeding has been shown to increase the abundance of autophagosome markers in denervated soleus of mice.27 In accord with this, Yuzefovych and co‐workers demonstrated increased autophagy in L6 myotubes following palmitate provision.36 Therefore, although a direct link has yet to be established in vivo, it is conceivable that altered protein turnover via autophagy may, at least in part, mediate lipid‐induced alterations in muscle mass.
It should also be highlighted that certain unsaturated fatty acids can alter the proliferation rate of satellite cells which function as myogenic progenitor cells required for muscle growth and regeneration. For example, DHA and EPA have been shown to inhibit proliferation of C2C12 myoblasts as well as satellite cells isolated from turkey muscle.83, 84 Notably, these growth suppressing actions have been linked to reduced levels of cyclin E and CDK2, proteins which play a critical role in cell cycle progression, as well as suppressed activation of ERK1/2, a mitogen‐activated protein kinase implicated in promoting cell growth and division.84, 85 In contrast, feeding dystrophic δ‐sarcoglycan deficient hamsters a diet enriched in ALA (an omega‐3 PUFA) was demonstrated to increase satellite cell proliferation and differentiation in EDL muscle, concomitant with improved muscular histology.33 Notably, these beneficial responses coincided with the ability of ALA to increase the proportion of α‐MHC positive myofibres in skeletal muscle of dystrophic hamsters, along with a reduction in β‐MHC expression, thereby contributing to the preservation of a more physiological α/β MHC ratio. Moreover, in the same study, supplementation of dietary ALA was also shown to prevent the aberrant cytoplasmic accumulation of key membrane proteins in the adductor muscles of dystrophic hamsters including caveolin‐3, a protein involved in regulating cell adhesion and membrane repair, as well as being implicated in the control of muscle differentiation and insulin induced signalling.33, 86, 87 Indeed, given the fact that aberrations in caveolin‐3 function and/or localization have been associated with various skeletal muscle disease phenotypes,88, 89, 90, 91, 92 it is plausible that fatty acids and/or their lipid derivatives may influence satellite cell proliferation and/or muscle differentiation, at least in part, by altering the function and/or subcellular localization of caveolin isoforms, as well as other key structural membrane components.
The effects of fatty acids upon muscle mass and differentiation may also be mediated through a number of derived lipid metabolites. For example, the ability of the PUFA arachidonic acid (C20; 4n‐6) to increase the size, myonuclear content and protein content of C2C12 myotubes has been shown to be mediated through cyclooxygenase‐2 (COX‐2) activity, implying dependency on downstream prostaglandin synthesis.93 In accord with this, arachidonic acid induced growth of C2C12 myocytes was reported to coincide with increased secretion of the eicosanoids PGF(2α) and PGE.2, 93 It is noteworthy that several studies have also documented the positive role that prostaglandins play in promoting early cell surface events, including cell–cell adhesion, which subsequently mediate the fusion of myoblasts into myotubes.94, 95 Indeed, important follow‐up studies may involve determining the exact identity of the molecular targets through which prostaglandins mediate their actions, for example by acting upon G‐protein linked prostanoid receptors (e.g. EP1).94, 96 In contrast, another arachidonic acid derived lipid metabolite known as 2‐arachidonoylglycerol (2‐AG), a key endogenous lipid ligand of the endocannabinoid system, has recently been reported to inhibit differentiation of primary human satellite cells and murine C2C12 myoblasts by targeting the G‐protein coupled cannabinoid receptor 1, and its subsequent inhibition of Kv7.4 channels.97 Therefore, the possible involvement of such lipid intermediates in regulating muscle catabolism in response to certain pathological conditions cannot be excluded.
Lipid modulation of muscle mass and function: a human perspective
It is well established that there is a progressive loss in skeletal muscle mass and its regenerative capacity in response to aging in humans.9, 98, 99 Moreover, increased adiposity as observed in aging has been linked to altered muscle protein synthetic responses in aged individuals.100 Therefore, it is conceivable that changes in lipid levels and/or composition may contribute to muscle atrophy in these conditions. To support this idea, there is evidence to suggest that modifying dietary composition may impact upon muscle mass and/or function in humans. For example, a study by McGlory and co‐workers reported that consumption of fish oil increased the omega‐3 PUFA content of muscle (Vastus lateralis) in healthy male individuals, which coincided with elevated expression of anabolic signalling proteins including mTOR.101 Moreover, inclusion of dietary MUFAs and PUFAs has been reported to reduce the expression of lipogenic genes in skeletal muscle of insulin resistant subjects, concomitant with reduced fractional synthetic rates of intramuscular DAG and triacylglycerols.102 Notably, these beneficial responses may be linked to improvements in insulin sensitivity conveyed by dietary supplementation of omega‐3 PUFAs in humans.103, 104, 105
As well as dietary interventions, resistance training has also been reported to enhance skeletal muscle innervation in obese older adults, as well as downregulating atrophic markers in skeletal muscle of mice in various models of atrophy.106, 107 Moreover, a study by Mikkelsen and colleagues demonstrated significantly increased thigh muscle area in aged individuals which had undergone life‐long endurance (running) exercise, compared with age‐matched untrained counterparts.108 In accord with these findings, physical exercise training was shown to normalize the levels of atrophic modulators TNF‐α, Murf‐1 and atrogin‐1/MAFbx in the myocardium following the induction of heart failure in rats.109 Therefore, increased physical activity may be used as an alternative and/or additional strategy to counteract the deleterious effects of aging and/or obesity upon muscle mass loss, potentially through countering lipid‐induced insulin resistance and/or chronic low grade inflammation in skeletal muscle.108, 110, 111, 112, 113, 114 Intriguingly, it has been previously reported that dietary supplementation with unsaturated fatty acids may also act to improve physical performance and/or enhance the beneficial metabolic effects associated with exercise, particularly in sedentary or untrained individuals.115 To support this idea, low dietary intake of tuna fish oil has been shown to promote resistance to muscle fatigue in rats, concomitant with a selective increase in DHA membrane phospholipid content within gastrocnemius muscle.116, 117 Therefore, a more integrated approach involving modifications to dietary fat consumption as well as increased physical exercise may provide a more effective strategy to alleviate the deleterious effects associated with muscle atrophy.
Conclusions and future perspectives
To conclude, there is growing appreciation that fatty acids and/or their lipid derivatives can play an important role in modulating skeletal muscle mass and function. Collectively, the evidence presented in this review indicates that saturated fatty acids act to convey detrimental effects upon muscle function, for example by impairing or inducing protein synthesis and catabolism, respectively (see Figure 2). In contrast, a number of different unsaturated fatty acids have been shown to counteract many of the pro‐catabolic actions associated with saturated fatty acid provision (Figure 3). However, further work will be required to delineate the pathways and processes underlying fatty acid‐induced muscle atrophy, as well as those mediating improvements in muscle function in response to the provision of unsaturated fatty acids (i.e. increased protein synthesis, reduced atrophy, improved metabolic function) (Figure 3). To this end, strategies aimed at altering intramuscular lipid content and/or composition under those conditions which can promote muscle wasting (e.g. increased obesity, ageing, physical inactivity), for example by suppressing the accumulation of lipid mediators such as ceramides (e.g. through inhibiting de novo ceramide synthesis) or prostaglandins (e.g. by inhibiting COX‐2 activity), may provide useful insight into the role that distinct classes of lipids play in the modulation of muscle mass and function. Importantly, such work is likely to involve the use of relevant animal models or human subjects that would require to take into consideration factors such as genetic background as well as dietary composition and caloric intake. In addition, these studies may also involve determining potential lipid induced alterations to muscle architecture and fibre type composition which can influence muscle strength, as well as monitoring changes in intramuscular signalling and metabolites within specific muscle fibre types. Allied to this, further work exploring the role of fatty acids and lipid intermediates in regulating the proliferation, differentiation and/or function of human muscle derived satellite cells and primary myotubes would need to be performed in order to make appropriate comparisons with data obtained in other experimental models (e.g. C2C12 myotubes), which have been shown to exhibit functional differences (e.g. in their level of maturation).118 Collectively, data obtained from such studies may lead to the development of novel therapeutic strategies to counteract muscle atrophy, and/or improve regenerative capacity following injury or disease.
Figure 3.
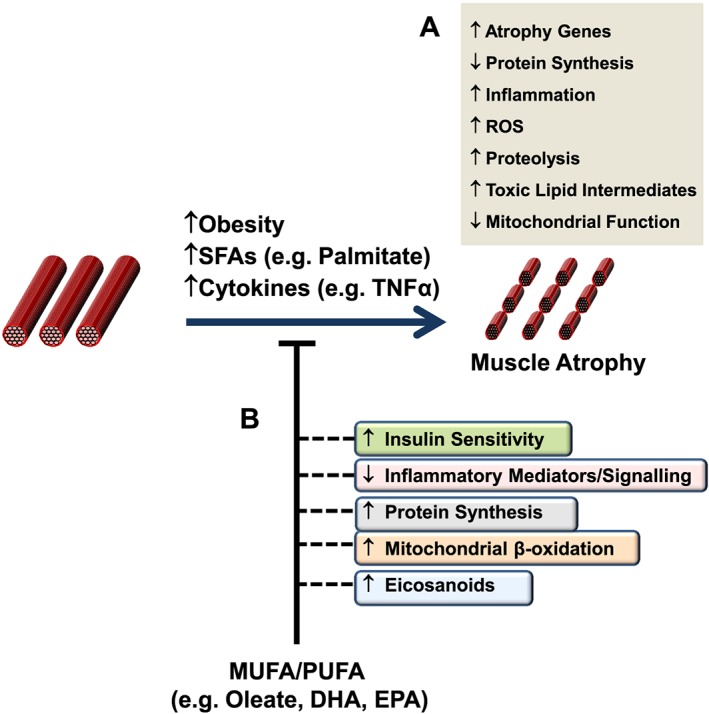
Potential mechanisms by which unsaturated fatty acids may counter obesity and/or fatty acid induced skeletal muscle atrophy. Elevated levels of saturated fatty acids and/or pro‐inflammatory cytokines, for example during obesity, can promote the development of skeletal muscle atrophy through various pathways and processes as indicated (A). Importantly, monounsaturated fatty acids and polyunsaturated fatty acids have been shown to counter these pro‐atrophic actions, which may be mediated through their ability to increase insulin sensitivity and the production of protective eicosanoids, as well as enhancing mitochondrial oxidative capacity and protein synthesis, whilst concomitantly reducing pro‐inflammatory drive (B).
Conflict of interest
The authors declare that there are no conflicts of interest that require to be declared.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.119 We extend our apologies to those whose work has not been cited in this review because of space limitations. Research in the authors' laboratory is supported by funding from BBSRC and Diabetes UK.
Lipina, C. , and Hundal, H. S. (2017) Lipid modulation of skeletal muscle mass and function. Journal of Cachexia, Sarcopenia and Muscle, 8: 190–201. doi: 10.1002/jcsm.12144.
References
- 1. Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long‐term heart failure. Circulation 1990;81:518–27. [DOI] [PubMed] [Google Scholar]
- 2. Vescovo G, Serafini F, Facchin L, Tenderini P, Carraro U, Dalla Libera L, et al. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart (Br Cardiac Soc) 1996;76:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 1997;90:729–38. [DOI] [PubMed] [Google Scholar]
- 4. Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604–9. [PubMed] [Google Scholar]
- 5. Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 2003;5:87–90. [DOI] [PubMed] [Google Scholar]
- 6. Nagai T, Okita K, Yonezawa K, Yamada Y, Hanada A, Ohtsubo M, et al. Comparisons of the skeletal muscle metabolic abnormalities in the arm and leg muscles of patients with chronic heart failure. Circulation J: Off J Jpn Circulation Soc 2004;68:573–9. [DOI] [PubMed] [Google Scholar]
- 7. Graves E, Hitt A, Pariza MW, Cook ME, McCarthy DO. Conjugated linoleic acid preserves gastrocnemius muscle mass in mice bearing the colon‐26 adenocarcinoma. Res Nurs Health 2005;28:48–55. [DOI] [PubMed] [Google Scholar]
- 8. Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1–Akt–mTOR–FoxO pathway. Biogerontology 2013;14:303–23. [DOI] [PubMed] [Google Scholar]
- 9. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin‐1. Am J Physiol Endocrinol Metab 2014;307:E469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le NH, Kim CS, Park T, Park JH, Sung MK, Lee DG, et al. Quercetin protects against obesity‐induced skeletal muscle inflammation and atrophy. Mediators Inflamm 2014;2014:834294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SR, Khamoui AV, Jo E, Park BS, Zourdos MC, Panton LB, et al. Effects of chronic high‐fat feeding on skeletal muscle mass and function in middle‐aged mice. Aging Clin Exp Res 2015;27:403–11. [DOI] [PubMed] [Google Scholar]
- 12. Wagatsuma A, Shiozuka M, Takayama Y, Hoshino T, Mabuchi K, Matsuda R. Effects of ageing on expression of the muscle‐specific E3 ubiquitin ligases and Akt‐dependent regulation of Foxo transcription factors in skeletal muscle. Mol Cell Biochem 2016;412:59–72. [DOI] [PubMed] [Google Scholar]
- 13. Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol 2005;37:1948–61. [DOI] [PubMed] [Google Scholar]
- 14. Kondalenko VF, Sergeev Iu P, Ivanitskaia VV. Electron microscopic study of signs of skeletal muscle fiber hyperplasia in athletes. Arkh Anat Gistol Embriol 1981;80:66–70. [PubMed] [Google Scholar]
- 15. Larsson L, Tesch PA. Motor unit fibre density in extremely hypertrophied skeletal muscles in man. Electrophysiological signs of muscle fibre hyperplasia. Eur J Appl Physiol Occup Physiol 1986;55:130–6. [DOI] [PubMed] [Google Scholar]
- 16. Sjostrom M, Lexell J, Eriksson A, Taylor CC. Evidence of fibre hyperplasia in human skeletal muscles from healthy young men? A left‐right comparison of the fibre number in whole anterior tibialis muscles. Eur J Appl Physiol Occup Physiol 1991;62:301–4. [DOI] [PubMed] [Google Scholar]
- 17. McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol (1985) 1996;81:2004–12. [DOI] [PubMed] [Google Scholar]
- 18. Shinin V, Gayraud‐Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol 2006;8:677–87. [DOI] [PubMed] [Google Scholar]
- 19. Yoon MS, Chen J. PLD regulates myoblast differentiation through the mTOR‐IGF2 pathway. J Cell Sci 2008;121:282–9. [DOI] [PubMed] [Google Scholar]
- 20. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF‐1‐induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 2001;3:1009–13. [DOI] [PubMed] [Google Scholar]
- 21. Dehoux M, Van Beneden R, Pasko N, Lause P, Verniers J, Underwood L, et al. Role of the insulin‐like growth factor I decline in the induction of atrogin‐1/MAFbx during fasting and diabetes. Endocrinology 2004;145:4806–12. [DOI] [PubMed] [Google Scholar]
- 22. Li BG, Hasselgren PO, Fang CH, Warden GD. Insulin‐like growth factor‐I blocks dexamethasone‐induced protein degradation in cultured myotubes by inhibiting multiple proteolytic pathways: 2002 ABA paper. J Burn Care Rehabil 2004;25:112–8. [DOI] [PubMed] [Google Scholar]
- 23. Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF‐I stimulates muscle growth by suppressing protein breakdown and expression of atrophy‐related ubiquitin ligases, atrogin‐1 and MuRF1. Am J Physiol Endocrinol Metab 2004;287:E591–601. [DOI] [PubMed] [Google Scholar]
- 24. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 2002;4:648–57. [DOI] [PubMed] [Google Scholar]
- 25. Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase‐3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett 1998;421:125–30. [DOI] [PubMed] [Google Scholar]
- 26. Pijet B, Pijet M, Litwiniuk A, Gajewska M, Pajak B, Orzechowski A. TNF‐alpha and IFN‐s‐dependent muscle decay is linked to NF‐kappaB‐ and STAT‐1alpha‐stimulated Atrogin1 and MuRF1 genes in C2C12 myotubes. Mediators Inflamm 2013;2013:171437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roseno SL, Davis PR, Bollinger LM, Powell JJ, Witczak CA, Brault JJ. Short‐term, high‐fat diet accelerates disuse atrophy and protein degradation in a muscle‐specific manner in mice. Nutr Metab (Lond) 2015;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 29. Bryner RW, Woodworth‐Hobbs ME, Williamson DL, Alway SE. Docosahexaenoic Acid protects muscle cells from palmitate‐induced atrophy. ISRN Obes 2012;2012:647348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodworth‐Hobbs ME, Hudson MB, Rahnert JA, Zheng B, Franch HA, Price SR. Docosahexaenoic acid prevents palmitate‐induced activation of proteolytic systems in C2C12 myotubes. J Nutr Biochem 2014;25:868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen‐Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol 2006;35:689–96. [DOI] [PubMed] [Google Scholar]
- 32. Castillero E, Martin AI, Lopez‐Menduina M, Villanua MA, Lopez‐Calderon A. Eicosapentaenoic acid attenuates arthritis‐induced muscle wasting acting on atrogin‐1 and on myogenic regulatory factors. Am J Physiol Regul Integr Comp Physiol 2009;297:R1322–31. [DOI] [PubMed] [Google Scholar]
- 33. Fiaccavento R, Carotenuto F, Vecchini A, Binaglia L, Forte G, Capucci E, et al. An omega‐3 fatty acid‐enriched diet prevents skeletal muscle lesions in a hamster model of dystrophy. Am J Pathol 2010;177:2176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Briolay A, Jaafar R, Nemoz G, Bessueille L. Myogenic differentiation and lipid‐raft composition of L6 skeletal muscle cells are modulated by PUFAs. Biochim Biophys Acta 2013;1828:602–13. [DOI] [PubMed] [Google Scholar]
- 35. Watson ML, Coghlan M, Hundal HS. Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid‐induced insulin resistance in rat skeletal muscle cells. Biochem J 2009;417:791–801. [DOI] [PubMed] [Google Scholar]
- 36. Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 2010;299:E1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahfouz R, Khoury R, Blachnio‐Zabielska A, Turban S, Loiseau N, Lipina C, et al. Characterising the inhibitory actions of ceramide upon insulin signaling in different skeletal muscle cell models: a mechanistic insight. PLoS One 2014;9:e101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Larichaudy J, Zufferli A, Serra F, Isidori AM, Naro F, Dessalle K, et al. TNF‐alpha‐ and tumor‐induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet Muscle 2012;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mebarek S, Komati H, Naro F, Zeiller C, Alvisi M, Lagarde M, et al. Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J Cell Sci 2007;120:407–16. [DOI] [PubMed] [Google Scholar]
- 40. Turpin SM, Ryall JG, Southgate R, Darby I, Hevener AL, Febbraio MA, et al. Examination of ‘lipotoxicity’ in skeletal muscle of high‐fat fed and ob/ob mice. J Physiol 2009;587:1593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williamson DL, Dungan CM, Jadhav KS. High lipid concentrations regulate skeletal muscle REDD1. FASEB J 2013;27:Supplement 942.1. [Google Scholar]
- 42. Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Nutrient‐induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1. J Nutr 2015;145:708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leskawa KC, Erwin RE, Buse PE, Hogan EL. Glycosphingolipid biosynthesis during myogenesis of rat L6 cells in vitro. Mol Cell Biochem 1988;83:47–54. [DOI] [PubMed] [Google Scholar]
- 44. Anastasia L, Papini N, Colazzo F, Palazzolo G, Tringali C, Dileo L, et al. NEU3 sialidase strictly modulates GM3 levels in skeletal myoblasts C2C12 thus favoring their differentiation and protecting them from apoptosis. J Biol Chem 2008;283:36265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Papini N, Anastasia L, Tringali C, Dileo L, Carubelli I, Sampaolesi M, et al. MmNEU3 sialidase over‐expression in C2C12 myoblasts delays differentiation and induces hypertrophic myotube formation. J Cell Biochem 2012;113:2967–78. [DOI] [PubMed] [Google Scholar]
- 46. Lipina C, Hundal HS. Ganglioside GM3 as a gatekeeper of obesity‐associated insulin resistance: evidence and mechanisms. FEBS Lett 2015;589:3221–7. [DOI] [PubMed] [Google Scholar]
- 47. Gangoiti P, Bernacchioni C, Donati C, Cencetti F, Ouro A, Gomez‐Munoz A, et al. Ceramide 1‐phosphate stimulates proliferation of C2C12 myoblasts. Biochimie 2012;94:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, et al. Role of diacylglycerol activation of PKCtheta in lipid‐induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A 2014;111:9597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, et al. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem 2014;289:1551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shad BJ, Smeuninx B, Atherton PJ, Breen L. The mechanistic and ergogenic effects of phosphatidic acid in skeletal muscle. Appl Physiol Nutr Metab 2015;40:1233–41. [DOI] [PubMed] [Google Scholar]
- 51. Bilim O, Takeishi Y, Kitahara T, Arimoto T, Niizeki T, Sasaki T, et al. Diacylglycerol kinase zeta inhibits myocardial atrophy and restores cardiac dysfunction in streptozotocin‐induced diabetes mellitus. Cardiovasc Diabetol 2008;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, et al. Hormone‐sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 2002;277:4806–15. [DOI] [PubMed] [Google Scholar]
- 53. Marignani PA, Epand RM, Sebaldt RJ. Acyl chain dependence of diacylglycerol activation of protein kinase C activity in vitro. Biochem Biophys Res Commun 1996;225:469–73. [DOI] [PubMed] [Google Scholar]
- 54. Eichmann TO, Kumari M, Haas JT, Farese RV Jr, Zimmermann R, Lass A, et al. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone‐sensitive lipase, and diacylglycerol‐O‐acyltransferases. J Biol Chem 2012;287:41446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Macrae K, Stretton C, Lipina C, Blachnio‐Zabielska A, Baranowski M, Gorski J, et al. Defining the role of DAG, mitochondrial function, and lipid deposition in palmitate‐induced proinflammatory signaling and its counter‐modulation by palmitoleate. J Lipid Res 2013;54:2366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rando RR, Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun 1984;122:818–23. [DOI] [PubMed] [Google Scholar]
- 57. Badin PM, Louche K, Mairal A, Liebisch G, Schmitz G, Rustan AC, et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 2011;60:1734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down‐regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 2005;19:461–3. [DOI] [PubMed] [Google Scholar]
- 59. Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci U S A 2008;105:17402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nardi F, Hoffmann TM, Stretton C, Cwiklinski E, Taylor PM, Hundal HS. Proteasomal modulation of cellular SNAT2 (SLC38A2) abundance and function by unsaturated fatty acid availability. J Biol Chem 2015;290:8173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li F, Duan Y, Li Y, Tang Y, Geng M, Oladele OA, et al. Effects of dietary n‐6:n‐3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br J Nutr 2015;113:739–48. [DOI] [PubMed] [Google Scholar]
- 62. Coll T, Eyre E, Rodriguez‐Calvo R, Palomer X, Sanchez RM, Merlos M, et al. Oleate reverses palmitate‐induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 2008;283:11107–16. [DOI] [PubMed] [Google Scholar]
- 63. Salvado L, Coll T, Gomez‐Foix AM, Salmeron E, Barroso E, Palomer X, et al. Oleate prevents saturated‐fatty‐acid‐induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK‐dependent mechanism. Diabetologia 2013;56:1372–82. [DOI] [PubMed] [Google Scholar]
- 64. Wu CL, Cornwell EW, Jackman RW, Kandarian SC. NF‐kappaB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am J Physiol Cell Physiol 2014;306:C762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Physiol Regul Integr Comp Physiol 2016;310:R561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y, Chen F, Odle J, Lin X, Zhu H, Shi H, et al. Fish oil increases muscle protein mass and modulates Akt/FOXO, TLR4, and NOD signaling in weanling piglets after lipopolysaccharide challenge. J Nutr 2013;143:1331–9. [DOI] [PubMed] [Google Scholar]
- 67. Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll‐like receptor 4 mediates lipopolysaccharide‐induced muscle catabolism via coordinate activation of ubiquitin–proteasome and autophagy–lysosome pathways. FASEB J 2011;25:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mittal A, Bhatnagar S, Kumar A, Lach‐Trifilieff E, Wauters S, Li H, et al. The TWEAK‐Fn14 system is a critical regulator of denervation‐induced skeletal muscle atrophy in mice. J Cell Biol 2010;188:833–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Finlin BS, Varma V, Nolen GT, Dube J, Starnes CP, Rasouli N, et al. DHA reduces the atrophy‐associated Fn14 protein in differentiated myotubes during coculture with macrophages. J Nutr Biochem 2012;23:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age‐associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 2010;24:1376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gouspillou G, Sgarioto N, Kapchinsky S, Purves‐Smith F, Norris B, Pion CH, et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 2014;28:1621–33. [DOI] [PubMed] [Google Scholar]
- 72. Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, et al. Peroxisome proliferator‐activated receptor‐alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 2002;51:901–9. [DOI] [PubMed] [Google Scholar]
- 73. Tardif N, Salles J, Landrier JF, Mothe‐Satney I, Guillet C, Boue‐Vaysse C, et al. Oleate‐enriched diet improves insulin sensitivity and restores muscle protein synthesis in old rats. Clin Nutr 2011;30:799–806. [DOI] [PubMed] [Google Scholar]
- 74. Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, et al. The stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J Physiol Pharmacol: Off J Korean Phys Soc Korean Soc Pharm 2014;18:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang Y, Lin QW, Zheng PP, Zhang JS, Huang FR. DHA inhibits protein degradation more efficiently than EPA by regulating the PPARgamma/NFkappaB pathway in C2C12 myotubes. BioMed Res Int 2013;2013:318981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lanza IR, Blachnio‐Zabielska A, Johnson ML, Schimke JM, Jakaitis DR, Lebrasseur NK, et al. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high‐fat diet. Am J Physiol Endocrinol Metab 2013;304:E1391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lim JH, Gerhart‐Hines Z, Dominy JE, Lee Y, Kim S, Tabata M, et al. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A‐dependent activation of SIRT1‐PGC1alpha complex. Nutr Metab 2013;288:7117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mizunoya W, Iwamoto Y, Shirouchi B, Sato M, Komiya Y, Razin FR, et al. Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle. PLoS One 2013;8:e80152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011;147:728–41. [DOI] [PubMed] [Google Scholar]
- 80. Penna F, Costamagna D, Pin F, Camperi A, Fanzani A, Chiarpotto EM, et al. Autophagic degradation contributes to muscle wasting in cancer cachexia. Am J Pathol 2013;182:1367–78. [DOI] [PubMed] [Google Scholar]
- 81. O'Leary MF, Vainshtein A, Carter HN, Zhang Y, Hood DA. Denervation‐induced mitochondrial dysfunction and autophagy in skeletal muscle of apoptosis‐deficient animals. Am J Physiol Cell Physiol 2012;303:C447–54. [DOI] [PubMed] [Google Scholar]
- 82. Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC‐1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 2009;106:20405–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83. McFarland DC, Velleman SG, Pesall JE, Coy CS. Effect of lipids on avian satellite cell proliferation, differentiation and heparan sulfate proteoglycan expression. Comp Biochem Physiol A Mol Integr Physiol 2011;159:188–95. [DOI] [PubMed] [Google Scholar]
- 84. Peng Y, Zheng Y, Zhang Y, Zhao J, Chang F, Lu T, et al. Different effects of omega‐3 fatty acids on the cell cycle in C2C12 myoblast proliferation. Mol Cell Biochem 2012;367:165–73. [DOI] [PubMed] [Google Scholar]
- 85. Schevzov G, Kee AJ, Wang B, Sequeira VB, Hook J, Coombes JD, et al. Regulation of cell proliferation by ERK and signal‐dependent nuclear translocation of ERK is dependent on Tm5NM1‐containing actin filaments. Mol Biol Cell 2015;26:2475–90, doi:10.1091/mbc.E14-10-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parton RG, Way M, Zorzi N, Stang E. Caveolin‐3 associates with developing T‐tubules during muscle differentiation. J Cell Biol 1997;136:137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, et al. Insulin resistance in skeletal muscles of caveolin‐3‐null mice. Proc Natl Acad Sci U S A 2004;101:12670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin‐3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD‐1C). Retention of LGMD‐1C caveolin‐3 mutants within the golgi complex. J Biol Chem 1999;274:25632–41. [DOI] [PubMed] [Google Scholar]
- 89. Minetti C, Bado M, Broda P, Sotgia F, Bruno C, Galbiati F, et al. Impairment of caveolae formation and T‐system disorganization in human muscular dystrophy with caveolin‐3 deficiency. Am J Pathol 2002;160:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M, et al. Caveolin‐1/3 double‐knockout mice are viable, but lack both muscle and non‐muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol 2002;160:2207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, et al. Caveolin‐3 knockout mice show increased adiposity and whole body insulin resistance, with ligand‐induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol 2005;288:C1317–31. [DOI] [PubMed] [Google Scholar]
- 92. Gazzerro E, Sotgia F, Bruno C, Lisanti MP, Minetti C. Caveolinopathies: from the biology of caveolin‐3 to human diseases. Eur J Hum Genet: EJHG 2010;18:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Markworth JF, Cameron‐Smith D. Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX‐2‐dependent pathway. Am J Physiol Cell Physiol 2013;304:C56–67. [DOI] [PubMed] [Google Scholar]
- 94. Hausman RE, Velleman SG. Prostaglandin E1 receptors on chick embryo myoblasts. Biochem Biophys Res Commun 1981;103:213–8. [DOI] [PubMed] [Google Scholar]
- 95. Santini MT, Indovina PL, Hausman RE. Prostaglandin dependence of membrane order changes during myogenesis in vitro. Biochim Biophys Acta 1988;938:489–92. [DOI] [PubMed] [Google Scholar]
- 96. Hausman RE, elGendy H, Craft F. Requirement for G protein activity at a specific time during embryonic chick myogenesis. Cell Differ Dev: The Official J Int Soc Dev Biol 1990;29:13–20. [DOI] [PubMed] [Google Scholar]
- 97. Iannotti FA, Silvestri C, Mazzarella E, Martella A, Calvigioni D, Piscitelli F, et al. The endocannabinoid 2‐AG controls skeletal muscle cell differentiation via CB1 receptor‐dependent inhibition of Kv7 channels. Proc Natl Acad Sci U S A 2014;111:E2472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med 2015;21:854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Petersen KF, Morino K, Alves TC, Kibbey RG, Dufour S, Sono S, et al. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc Natl Acad Sci U S A 2015;112:11330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Murton AJ, Marimuthu K, Mallinson JE, Selby AL, Smith K, Rennie MJ, et al. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes 2015;64:3160–71. [DOI] [PubMed] [Google Scholar]
- 101. McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JR, et al. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids 2014;90:199–206. [DOI] [PubMed] [Google Scholar]
- 102. Jans A, van Hees AM, Gjelstad IM, Sparks LM, Tierney AC, Riserus U, et al. Impact of dietary fat quantity and quality on skeletal muscle fatty acid metabolism in subjects with the metabolic syndrome. Metabolism 2012;61:1554–65. [DOI] [PubMed] [Google Scholar]
- 103. Rasic‐Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Sobajic S, Djuric I, et al. Effects of N‐3 PUFAs supplementation on insulin resistance and inflammatory biomarkers in hemodialysis patients. Ren Fail 2007;29:321–9. [DOI] [PubMed] [Google Scholar]
- 104. Dangardt F, Chen Y, Gronowitz E, Dahlgren J, Friberg P, Strandvik B. High physiological omega‐3 fatty acid supplementation affects muscle fatty acid composition and glucose and insulin homeostasis in obese adolescents. J Nutr Metab 2012;2012:395757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jimenez‐Gomez Y, Cruz‐Teno C, Rangel‐Zuniga OA, Peinado JR, Perez‐Martinez P, Delgado‐Lista J, et al. Effect of dietary fat modification on subcutaneous white adipose tissue insulin sensitivity in patients with metabolic syndrome. Mol Nutr Food Res 2014;58:2177–88. [DOI] [PubMed] [Google Scholar]
- 106. Al‐Nassan S, Fujita N, Kondo H, Murakami S, Fujino H. Chronic exercise training down‐regulates TNF‐alpha and atrogin‐1/MAFbx in mouse gastrocnemius muscle atrophy induced by hindlimb unloading. Acta Histochem et Cytochem 2012;45:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci 2015;pii:glv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mikkelsen UR, Couppe C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, et al. Life‐long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev 2013;134:531–40. [DOI] [PubMed] [Google Scholar]
- 109. Adams V, Linke A, Gielen S, Erbs S, Hambrecht R, Schuler G. Modulation of Murf‐1 and MAFbx expression in the myocardium by physical exercise training. Eur J Cardiovasc Prev Rehabil: Official J European Soc Cardiol, Working Groups on Epidemiol Prev Cardiac Rehabil Exercise Physiol 2008;15:293–9. [DOI] [PubMed] [Google Scholar]
- 110. O'Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, et al. Exercise training increases insulin‐stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 2006;49:2983–92. [DOI] [PubMed] [Google Scholar]
- 111. Hawley JA, Lessard SJ. Exercise training‐induced improvements in insulin action. Acta Physiol (Oxf) 2008;192:127–35. [DOI] [PubMed] [Google Scholar]
- 112. Kim JS, Yi HK. Intermittent bout exercise training down‐regulates age‐associated inflammation in skeletal muscles. Exp Gerontol 2015;doi:10.1016/j.exger.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 113. Bucci M, Huovinen V, Guzzardi MA, Koskinen S, Raiko JR, Lipponen H, et al. Resistance training improves skeletal muscle insulin sensitivity in elderly offspring of overweight and obese mothers. Diabetologia 2016;59:77–86. [DOI] [PubMed] [Google Scholar]
- 114. Fukushima Y, Kurose S, Shinno H, Cao Thu H, Takao N, Tsutsumi H, et al. Importance of lean muscle maintenance to improve insulin resistance by body weight reduction in female patients with obesity. Diabetes Metab J 2016;40:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kawabata F, Neya M, Hamazaki K, Watanabe Y, Kobayashi S, Tsuji T. Supplementation with eicosapentaenoic acid‐rich fish oil improves exercise economy and reduces perceived exertion during submaximal steady‐state exercise in normal healthy untrained men. Biosci Biotechnol Biochem 2014;78:2081–8. [DOI] [PubMed] [Google Scholar]
- 116. Henry R, Peoples GE, McLennan PL. Muscle fatigue resistance in the rat hindlimb in vivo from low dietary intakes of tuna fish oil that selectively increase phospholipid n‐3 docosahexaenoic acid according to muscle fibre type. Br J Nutr 2015;114:873–84. [DOI] [PubMed] [Google Scholar]
- 117. Peoples GE, McLennan PL. Long‐chain n‐3 DHA reduces the extent of skeletal muscle fatigue in the rat in vivo hindlimb model. Br J Nutr 2014;111:996–1003. [DOI] [PubMed] [Google Scholar]
- 118. Langelaan ML, Boonen KJ, Rosaria‐Chak KY, van der Schaft DW, Post MJ, Baaijens FP. Advanced maturation by electrical stimulation: differences in response between C2C12 and primary muscle progenitor cells. J Tissue Eng Regen Med 2011;5:529–39. [DOI] [PubMed] [Google Scholar]
- 119. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


