Abstract
The aim of the present study was to evaluate the functions of miR-200c in the regulation of tumor growth and metastasis in renal cancer cells, and to investigate the underlying mechanisms. In this study, miR-200c was up- and downregulated in two renal cancer cell lines, namely ACHN and A498, and the proliferation, colony formation, migration and invasion of the cells were measured. The expression levels of various mRNAs and proteins were then analyzed using reverse transcription-quantitative polymerase chain reaction and western blotting, respectively. It was found that miR-200c suppressed proliferation, migration and invasion of the renal cancer cells and, conversely, the inhibition of endogenous miR-200c resulted in increased cell proliferation and metastasis. Furthermore, a luciferase reporter assay revealed that miR-200c directly targeted the 3′ untranslated regions of the oncogenes B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi-1) and E2F transcription factor 3 (E2F3) mRNAs, reduced the expression of Bmi-1 and E2F3 and regulated the expression of downstream genes, including E-cadherin, N-cadherin, vimentin, p14 and p16. These results indicate a tumor suppressor role for miR-200c in renal cancer cells via the direct targeting of Bmi-1 and E2F3.
Keywords: miR-200c, Bmi-1, E2F3, renal cancer cells
Introduction
MicroRNAs (miRNAs) are a class of non-coding endogenous single-strand RNAs, 20–23 nucleotides (nt) in length, which negatively regulate target gene expression post-transcriptionally to induce degradation of multiple mRNAs, translational repression and gene silencing by pairing to the 3′ untranslated region (3′UTR) of target mRNA (1,2). miRNAs are indicated to be involved in numerous key cellular processes, including cell growth, differentiation and death, and in particular, regulate the initiation, development and progression of human cancers, including tumor growth, apoptosis, invasion and metastasis (3). In malignancy, miRNAs play roles as either oncogenes or tumor suppressor genes (4,5).
Malignant tumor progression is characterized by the process of epithelial-to-mesenchymal transition (EMT), and is considered an essential step in tumor metastasis (6). During the EMT process, epithelial tumor cells are induced to lose epithelial polarity and the epithelial marker E-cadherin, and to gain mesenchymal phenotypes and increased expression of mesenchymal markers vimentin and N-cadherin and to exhibit increased migratory and invasive capabilities and behavior (6,7). B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi-1) and E2F transcription factor 3 (E2F3) are well recognized as oncogenes in cancer initiation and progression (8,9), and are reported to promote EMT by suppressing E-cadherin expression (10), and inhibiting the expression of cell cycle inhibitors p14 and p16 (11,12).
miR-200 is a family of tumor suppressor miRNAs, which includes miR-200a, miR-200b, miR-200c, miR-141 and miR-429, and is markedly involved in tumor initiation and progression (13). Studies have demonstrated that expression of the miR-200 family is significantly reduced in cells undergoing EMT (14–16). As an important member of the miR-200 family and a powerful regulator of EMT, miR-200c is indicated to maintain the epithelial phenotype of tissues by inhibiting the expression of E-cadherin transcription factor ZEB1 in certain types of cancer (16,17). In a recent study, miR-200c was reported to modulate EMT in human renal cell carcinoma (18).
Renal cancer is the most common cancer of the urinary system worldwide, with 63,920 estimated new cases and 13,860 estimated mortalities owing to cancer in 2014 (19), and the incidence is increasing (19–21). Due to the absence of effectiveness and specificity, the options for therapy of metastatic renal cancer remain limited (22); thus, the development of new treatments to treat renal cancer in necessary. In the present study, the effects of miR-200c were investigated by up- and downregulating miR-200c expression in two renal cancer cell lines (ACHN and A498) and the possible mechanisms of action were evaluated.
Materials and methods
Cell lines and culture
Human renal cancer ACHN and A498 cells were purchased from Guangzhou Jennio Biological Technology Co., Ltd. (Guangzhou, China). ACHN cells were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), and A498 cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. The two cell lines were cultured at 37°C under an atmosphere of 5% CO2 in humidified air.
miRNA infection of the cells
The miR-200c and control miRNA (plasmid, pEZX-MR03) and miR-200c inhibitor and control small interfering RNAs (siRNAs) (plasmid, CMV-RFP-U6-miRNA inhibitor-PGK-puromycin) were purchased from GeneCopoeia, Inc. (Guangzhou, China). Infection was performed in accordance with the manufacturer's protocol. Briefly, ACHN and A498 cells were cultured in medium supplemented with 10% FBS for 24 h prior to miRNA infection, and then the viral vectors were added into the medium for infection, respectively. Cells were then cultured in medium containing 1 µg/ml puromycin at 48 h post-transfection.
Bioinformatic analysis
Candidate targets for miR-200c were searched for using TargetScan Human 7.1 (http://www.targetscan.org/), a bioinformatic tool for miRNA target screening.
Luciferase reporter assay
A luciferase reporter assay was performed with Dual-Luciferase Reporter 1000 Assay system (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol. Briefly, after culturing until 70% confluence, HEK293 cells were co-infected with miR-200c/control miRNA and the 3′UTR (wild type or mutant) of Bmi-1 or E2F3 (GeneCopoeia, Inc.). The firefly and Renilla luciferase activities were then analyzed.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA (enriched for miRNAs) was extracted using an E.Z.N.A. miRNA kit (Omega Bio-Tek, Inc., Norcross, GA, USA) according to the manufacturer's instructions. The total RNAs were purified by treatment with gDNA Eraser from a PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). A RT-qPCR assay was performed using a Thermal Cycler Dice Real Time System (TP800; Takara Biotechnology Co., Ltd.), PrimeScript miRNA qPCR Starter kit Ver.2.0 (Takara Biotechnology Co., Ltd.) and SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) according to the manufacturers' instructions. For the miRNA expression assay, two-step RT-qPCR was employed with specific primers for miR-200c and RNU6B (the latter was an internal control) following the manufacturer's protocol. The PCR was carried out at 95°C for 10 sec, followed by 40 cycles of amplification at 95°C for 5 sec and 55°C for 20 sec. For relative mRNA expression analysis, two-step RT-qPCR was employed with specific primers for GAPDH (as internal control), Bmi-1, E2F3, E-cadherin, N-cadherin, vimentin, p14 and p16 following the manufacturer's protocol, and PCR was carried out at 95°C for 30 sec, followed by 40 cycles of amplification at 95°C for 5 sec and 56°C for 30 sec. All results were representative of three independent assays, and the expression levels were expressed according to the 2−ΔΔCq method (23). The designed specific primers are listed in Table I.
Table I.
Sequences of target gene primers for reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence 5′-3′ | Tm (°C) |
|---|---|---|
| RNU6B | F: CTCGCTTCGGCAGCACA | 59.42 |
| R: AACGCTTCACGAATTTGCGT | 55.75 | |
| miR-200c | F: TAATACTGCCGGGTAATGATGG | 58.21 |
| R: TCGTATCCAGTGCAGGGTC | 59.72 | |
| GAPDH | F: TGCACCACCAACTGCTTAG | 60.07 |
| R: AGTAGAGGCAGGGATGATGTTC | 59.72 | |
| Bmi-1 | F: TGGATCGGAAAGTAAACAAAGAC | 56.60 |
| R: TGCATCACAGTCATTGCTGCT | 58.01 | |
| E2F3 | F: TGCCTGACTCAATAGAGAGCCTAC | 61.97 |
| R: TCCCATTGTGGTCTTGGTTGT | 58.01 | |
| E-cadherin | F: GAAAGCGGCTGATACTGACC | 59.85 |
| R: CGTACATGTCAGCCGCTTC | 59.72 | |
| N-cadherin | F: GGTGGAGGAGAAGAAGACCAG | 61.92 |
| R: GGCATCAGGCTCCACAGTG | 61.88 | |
| Vimentin | F: GGGAGAAATTGCAGGAGGAG | 59.85 |
| R: AGGTCAAGACGTGCCAGAGAC | 61.92 | |
| p14 | F: GTTCTTGGTGACCCTCCGGATT | 61.94 |
| R: ATCAGCACGAGGGCCACAG | 61.88 | |
| p16 | F: CCCAACGCACCGAATAGTTAC | 59.97 |
| R: ACGGGTCGGGTGAGAGTG | 61.86 |
Tm, melting temperature; F, forward; R, reverse; miR, microRNA; Bmi-1, B-cell-specific Moloney murine leukemia virus insertion site 1; E2F3, E2F transcription factor 3.
Western blot analysis
ACHN and A498 cells were lysed with RIPA buffer (Beyotime Institute of Biotechnology, Shanghai, China) and total proteins were extracted by centrifuging at 10,000 × g for 10 min at 4°C. The proteins were quantified using an Enhanced BCA Protein Assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Proteins (30 µg/lane) were separated by SDS-PAGE (10% gel) and then transferred to a PVDF membrane (EMD Millipore, Bedford, MA, USA). The blotting membranes were incubated overnight (16 h) at 4°C with anti-Bmi-1 antibody (40 kD; 1:20,000; ab115251; Abcam, Cambridge, UK), anti-E2F3-1 antibody (37 kD; 1:2,000; ab50917; Abcam, Cambridge, UK), anti-E-cadherin antibody (135 kD; 1:1,000; cat. no. 5296; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-N-cadherin antibody (140 kD; 1:1,000; ab18203; Abcam), anti-vimentin antibody (57 kD; 1:2,000; cat. no. 5741; Cell Signaling Technology, Inc.), anti-p14 antibody (14 kD; 1:500; cat. no. 2407; Cell Signaling Technology, Inc.), anti-p16 antibody (16 kD; 1:500; ab118459; Abcam) or anti-β-tubulin antibody (55 kD; 1:50,000; cat. no. 70004; EarthOx Life Sciences, Millbrae, CA, USA; loading control), respectively, and then probed with a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:10,000; E030120; EarthOx Life Sciences) or a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G secondary antibody (1:10,000; E030110; EarthOx Life Sciences) for 1 h at room temperature. The bands were visualized using Luminata Crescendo Western HRP Substrate (WBLUR0500; EMD Millipore) with exposure to X-OMAT BT film (Carestream Health, Rochester, NY, USA). Three replicates were performed.
Cell proliferation assays
Proliferation of ACHN and A498 cells was detected using a CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega Corporation) in accordance with the manufacturer's protocol. Briefly, cells were seeded in a 96-well cell culture cluster (Corning Incorporated, Corning, NY, USA) at a density of 3,000 cells per well with 100 µl culture medium. After 5 days, the culture medium was removed and replaced with an equal volume of medium containing CellTiter 96 AQueous One Solution reagent, and the cells were then incubated at 4°C for 2 h. The absorbance was detected at 490 nm using a 96-well plate reader.
Colony formation assay
A colony formation assay was performed according to a slightly modified method (24). Briefly, cells were seeded into 60-mm plastic dishes (Nest Biotechnology, Hong Kong, China) at a density of 1,000 cells per well, and cultured at 37°C under an atmosphere of 5% CO2 in humidified air (ACHN cells were cultured for 3 weeks and A498 cells were cultured for 2 weeks, respectively). The numbers of colonies were counted after staining with Coomassie Brilliant Blue.
Wound healing (migration) and transwell (invasion) assays
ACHN and A498 cells were seeded into 12-well cell culture plates (3×105 cells per well; Nest Biotechnology). The wound healing assay was performed using a sterile pipette tip to make a scratch through the confluent monolayer when cells reached 100% confluence. Cells were washed three times with PBS and cultured with medium supplemented with 1% FBS. The cell migration was observed after culture for 24 h. The percentage of wound closure was determined with 3 replications, and five randomly chosen fields were calculated for each replicate. For the transwell assay, 3×105 cells in 150 µl serum-free medium supplemented with 1% FBS were placed into the upper chamber of the insert (membrane pore size, 8 µm; Corning) with Matrigel (BD Biosciences, Billerica, MA, USA), and 500 µl medium supplemented with 10% FBS was added to the lower chamber of a 24-well plastic plate. Following 24 h of culture at 37°C, the cells remaining in the upper chamber or on the upper membrane were removed. The number of cells adhering to the lower membrane of the inserts was counted after staining with Crystal Violet Staining Solution (Beyotime Institute of Biotechnology) for 10 min.
Statistical analysis
The cell proliferation assay was performed in five independent experiments and other analyses were repeated in three independent experiments. Results are presented as mean ± standard error of the mean. All data were analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) by one-way analysis of variance, and differences between treatments were assessed using a Fisher's least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-200c suppressed the proliferation, migration and invasion of renal cancer cells
To determine the biological functions of miR-200c, ACHN and A498 cells were stably transduced with miR-200c. RT-qPCR analysis demonstrated that miR-200c was then stably overexpressed (P<0.001; Fig. 1A). A cell proliferation assay was performed to examine the effect of miR-200c on renal cancer cell growth. Ectopic miR-200c induced a significant suppression in cell proliferation in the two renal cancer cell lines (P<0.001; Fig. 2A). A colony formation assay was then performed, and it was found that upregulation of miR-200c reduced colony formation by ACHN cells (P<0.01; Fig. 2B) and A498 cells (P<0.05; Fig. 2B), consistent with the results of the cell proliferation assay. Furthermore, a wound healing assay and transwell assay were performed to evaluate the effect of miR-200c on renal cancer cell metastasis. Ectopic miR-200c significantly decreased the extent of wound healing (% wound closure) in the two cell lines (P<0.001; Fig. 2C). Transwell assays with Matrigel demonstrated that over-expressed miR-200c significantly inhibited the invasive capacity of the two cell lines (P<0.01; Fig. 2D). These results indicated that miR-200c inhibited the cell growth and metastasis of renal cancer cells.
Figure 1.

miR-200c levels in renal cancer cells following miRNA infection. (A) miR-200c was overexpressed by a viral vector; (B) endogenous miR-200c was inhibited by a specific inhibitor. *P<0.05 and ***P<0.001 vs. the control group. miR, microRNA
Figure 2.
Overexpressed miR-200c suppressed renal cancer cell growth and metastasis. (A) Cell proliferation of ACHN and A498 cells. (B) Colony formation and (C) wound healing assay following miRNA infection. (D) Transwell invasion assay of ACHN and A498 cells was performed using Matrigel. *P<0.05, **P<0.01 and ***P<0.001 vs. the control group. miR, microRNA; d, days.
Downregulated endogenous miR-200c increased renal cancer cell growth and metastasis
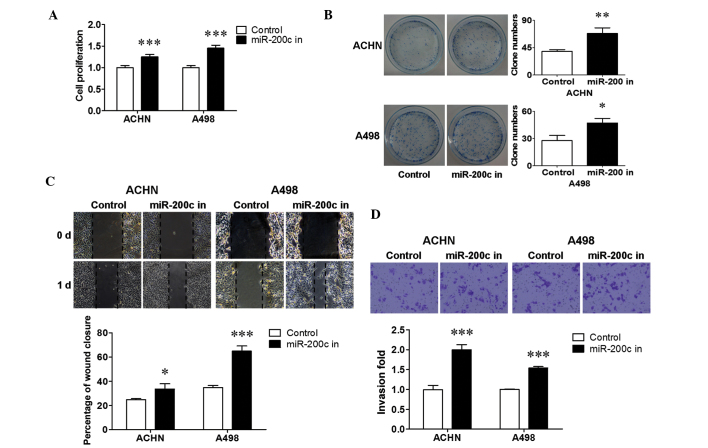
miR-200c was stably inhibited in ACHN and A498 cells to reveal the biological significance of endogenous miR-200c. RT-qPCR demonstrated that miR-200c was stably downregulated in the two cell lines (P<0.05; Fig. 1B). To determine the influence of downregulated miR-200c on renal cancer cell growth, a cell proliferation and colony formation assay was performed. It is shown in Fig. 3 that the inhibition of endogenous miR-200c resulted in an increase in cell proliferation (P<0.001; Fig. 3A), and a promotion in colony formation in ACHN (P<0.01; Fig. 3B) and A498 (P<0.05; Fig. 3B) cells. The changes in renal cancer cell metastasis were then evaluated using a wound healing assay and a transwell assay. The results revealed that the downregulation of endogenous miR-200c significantly stimulated migration (P<0.05 for ACHN cells; P<0.001 for A498 cells; Fig. 3C) and invasion (P<0.001; Fig. 3D) in both cell lines compared with that in the control. Together, these results suggest that downregulated endogenous miR-200c promoted cell growth and metastasis in renal cancer cells.
Figure 3.
Inhibited endogenous miR-200c increased renal cancer cell proliferation and metastasis. (A) Cell growth of renal cancer cells. (B) Colony formation and (C) migration of renal cancer cells in a wound closure assay following miRNA transfection. (D) Transwell invasion assay of ACHN and A498 cells was performed with Matrigel. *P<0.05, **P<0.01 and ***P<0.001 vs. the control group. miR, microRNA; in, inhibitor; d, days
miR-200c regulated cell proliferation and metastasis by directly targeting of oncogenes Bmi-1 and E2F3
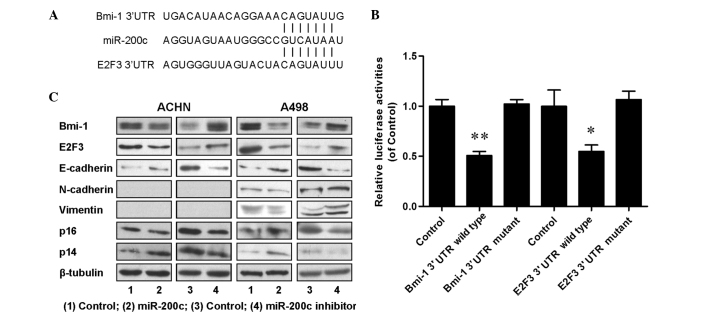
To dissect the molecular mechanism by which miR-200c acts as tumor suppressor in renal cancer cells, candidate targets for miR-200c were searched for using TargetScan, a bioinformatic tool for miRNA target screening. It was found that oncogenes Bmi-1 and E2F3 were putative targets of miR-200c (Fig. 4A). A miR-200c-based luciferase assay in HEK293 cells was then performed to measure if miR-200c directly binds to the 3′UTRs of Bmi-1 and E2F3, and the results demonstrated that miR-200c directly binds to the 3′UTRs of Bmi-1 (P<0.01; Fig. 4B) and E2F3 (P<0.05; Fig. 4B), as significantly decreased luciferase activities were observed for the wild type but not for the mutant 3′UTR. A western blot assay was performed to evaluate the expression levels of Bmi-1 and E2F3, and their downstream proteins including E-cadherin, N-cadherin, vimentin, p14 and p16. The results (Fig. 4C) indicated that the expression of Bmi-1, E2F3 and N-cadherin was downregulated, and the expression of E-cadherin, p14 and p16 was upregulated in miR-200c overexpressing cells; and increased expression levels of Bmi-1, E2F3 and N-cadherin, and decreased expression levels of E-cadherin, p14 and p16 were detected in endogenous miR-200c downregulated cells. An RT-qPCR assay was also performed and similar tendencies were detected for the associated mRNAs (Fig. 5). All the aforementioned data demonstrated that miR-200c regulated renal cancer cell growth and metastasis by directly targeting the 3′UTR of Bmi-1 and E2F3 and inducing changes in the expression levels of Bmi-1 and E2F3 and their downstream genes in cell proliferation and EMT.
Figure 4.
miR-200c acted as tumor suppressor by directly binding to oncogenes Bmi-1 and E2F3. (A) miR-200c and its putative binding sequences in the 3′UTRs of Bmi-1 and E2F3. (B) miR-200c suppressed luciferase activities; (C) Effect of up/downregulated miR-200c on relative protein expression. *P<0.05 and **P<0.01 vs. the control group. miR, microRNA; Bmi-1, B-cell-specific Moloney murine leukemia virus insertion site 1; E2F3, E2F transcription factor 3; 3′UTR, 3′ untranslated region.
Figure 5.
Effect of over- and downexpressed miR-200c on relative mRNA levels. Effects of miR-200c overexpression on relative mRNA levels in (A) ACHN and (B) A498 cells. Effects of downexpressed miR-200c on Bmi-1, E2F3, E-cadherin, N-cadherin, vimentin, p14 and p16 expression in (C) ACHN and (D) A498 cells. *P<0.05, **P<0.01 and **P<0.01 vs. the control group. miR, microRNA; Bmi-1, B-cell-specific Moloney murine leukemia virus insertion site 1; E2F3, E2F transcription factor 3; 3′UTR, 3′ untranslated region.
Discussion
MicroRNAs have been demonstrated to play critical roles as oncogenes or tumor suppressors in human cancers in previous studies (25). miR-200 is a family of tumor suppressor miRNAs consisting of five members significantly involved in the inhibition of EMT (13), and as a member of the miR-200 family, miR-200c was reported to modulate EMT in human renal cell carcinoma in a recent study (18). In the present study, the effects of up- and downregulation of the expression of miR-200c on cell proliferation, migration and invasion in renal cancer cells, and the mechanisms of miR-200c regulation, were investigated. The results demonstrated that ectopic miR-200c significantly inhibited cell growth, migration and invasion, and a reduction of endogenous miR-200c expression recovered cell proliferation and metastasis in renal cancer cells. They also indicated that miR-200c suppressed renal cancer cell growth and metastasis by directly targeting oncogenes Bmi-1 and E2F3, and then modulating the expression of downstream proteins.
The miR-200 family has been demonstrated to be downregulated during tumor progression and acts as a critical suppressor in the regulation of epithelial-to-mesenchymal transition (EMT), cancer stem cell (CSs) self-renewal and differentiation, cell division and apoptosis, and reversal of chemoresistance (13). miR-200c is an important member of the miR-200 family, and downregulation of its expression level has been observed in several tumors of the urinary system (26–30). A recent study of renal cell carcinoma demonstrated that miR-200c was downregulated in renal cell carcinoma, and restoration of miR-200c markedly suppressed the migration and invasion of SN12-PM6 and 786–0 cells (18); these results suggest that miR-200c acted as a tumor suppressor in renal cell carcinoma by modulating EMT. In the present study, the over- and downregulation of miR-200c expression was enforced in two renal cancer cell lines and the results indicated that ectopically expressed miR-200c significantly inhibited the metastatic ability of ACHN and A498 cells, and suppressed expression of endogenous miR-200c promoted the metastatic capacity of the two renal cancer cell lines. These results were consisted with the aforementioned studies, and indicate that miR-200c functions as a tumor suppressor miRNA in renal cancer by inhibiting the EMT process. Yu et al (31) investigated the influence of miR-200c on the proliferation of pancreatic cancer cells and indicated that upregulation of miR-200c could stimulate the proliferation of pancreatic cancer cells. However, the results of the present study are inconsistent with this, as they indicated that miR-200c significantly decreased renal cancer cell proliferation. It is suggested the different results may be due to the difference in organ type.
Bmi-1 plays a key role in cell cycle regulation, cell immortalization and cell senescence as a polycomb gene family member (8,32). It is well recognized that Bmi-1 is frequently upregulated in various human cancers, and plays important roles as an oncogene in cancer initiation and progression (8,33). E2F3 is another oncogene that is overexpressed in bladder and prostate cancers (34,35), and participated in controlling the cell cycle progression and proliferation of cancer cells (9). In the present study, Bmi-1 and E2F3 were predicted as functional targets of miR-200c by TargetScan analysis, and the effects of miR-200c on the 3′UTRs and expression of Bmi-1 and E2F3 were then measured. It was found that miR-200c directly bound to the 3′UTRs of Bmi-1 and E2F3, and decreased the expression levels of Bmi-1 and E2F3 mRNAs and proteins. These results are consistent with the results for cell functions in this study, and suggest that Bmi-1 and E2F3 are oncogenes in renal cancer that are inhibited by miR-200c.
EMT is a critical process driving cancer metastasis, which is characterized by the loss of epithelial marker E-cadherin and a stimulation of the mesenchymal markers vimentin and N-cadherin, followed by an induction of increases in migratory and invasive behavior (7). Bmi-1 is reported to induce EMT by repressing E-cadherin expression (10). In the present study, the expression levels of E-cadherin and N-cadherin were examined, and it was demonstrated that the ectopic expression of miR-200c increased E-cadherin expression and decreased N-cadherin expression, whereas the downregulated expression of endogenous miR-200c decreased E-cadherin expression and increased N-cadherin expression. These results demonstrated that miR-200c regulated Bmi-1 expression and then modulated EMT by stimulating E-cadherin expression and suppression of N-cadherin expression. Furthermore, Bmi-1 is reported as an oncogene, which acts by regulating the expression of cell cycle inhibitors p14 and p16 (11,12,36,37). P14 is reported to prevent the degradation and inactivation of p53 by binding to E3 ubiquitin-protein ligase (38), and p16 is recognized as an inhibitor of cyclin-dependent kinases 4 and 6 (35), which arrests the cell cycle in the G1/S phase (39). In the present study, the expression levels of p14 and p16 were also measured and the results indicated that overexpressed miR-200c increased the expression of p14 and p16, and the downregulation of miR-200c suppressed the expression of p14 and p16. These results suggest that miR-200c regulates Bmi-1 expression and then modulates cell proliferation by increasing the expression of p14 and p16.
In summary, the present study investigated the functions and mechanisms of miR-200c in the regulation of renal cancer cell proliferation and metastasis. The results demonstrated that miR-200c acted as a tumor suppressor by inhibiting cell proliferation, migration and invasion in renal cancer cells. In addition, downregulated endogenous miR-200c recovered renal cancer cell growth and metastasis, and miR-200c possessed the potency to suppress renal cancer cell growth and metastasis via the downregulation of the oncogenes Bmi-1 and E2F3. These findings provide important basic information relevant to renal cancer intervention, prevention and therapy.
Acknowledgements
This study was supported in part by the following grants: Science and Technology Planning Project of Guangdong Province (grant no. 2012B031800221) and The National Natural Science Funds (grant no. 81272833) of China.
References
- 1.Brown M, Suryawanshi H, Hafner M, Farazi TA, Tuschl T. Mammalian miRNA curation through next-generation sequencing. Front Genet. 2013;4:145. doi: 10.3389/fgene.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Pavelic S Kraljevic, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: New perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L, Li J, Song L. Bmi-1, stem cells and cancer. Acta Biochim Biophys Sin (Shanghai) 2009;41:527–534. doi: 10.1093/abbs/gmp040. [DOI] [PubMed] [Google Scholar]
- 9.Olsson AY, Feber A, Edwards S, Te Poele R, Giddings I, Merson S, Cooper CS. Role of E2F3 expression in modulating cellular proliferation rate in human bladder and prostate cancer cells. Oncogene. 2007;26:1028–1037. doi: 10.1038/sj.onc.1209854. [DOI] [PubMed] [Google Scholar]
- 10.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119:3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Yoon SY, Kim CN, Joo JH, Moon SK, Choe IS, Choe YK, Kim JW. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett. 2004;203:217–224. doi: 10.1016/j.canlet.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Silva J, Garcia JM, Peña C, García V, Domínguez G, Suárez D, Camacho FI, Espinosa R, Provencio M, España P, Bonilla F. Implication of polycomb members Bmi-1, Mel-18 and Hpc-2 in the regulation of p16INK4a, p14ARF, h-TERT, and c-Myc expression in primary breast carcinomas. Clin Cancer Res. 2006;12:6929–6936. doi: 10.1158/1078-0432.CCR-06-0788. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10:219–222. doi: 10.4161/cbt.10.3.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 17.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Chen X, Wang R, Xiao P, Xu Z, Chen L, Hang W, Ruan A, Yang H, Zhang X. MicroRNA-200c modulates the epithelial-to-mesenchymal transition in human renal cell carcinoma metastasis. Oncol Rep. 2013;30:643–650. doi: 10.3892/or.2013.2530. [DOI] [PubMed] [Google Scholar]
- 19.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 21.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 22.Stadler WM. Maturing of renal cancer therapeutics. J Clin Oncol. 2014;32:722–724. doi: 10.1200/JCO.2013.54.1748. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Fang F, Chang R, Yang L. MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor β receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology. 2013;58:205–217. doi: 10.1002/hep.26315. [DOI] [PubMed] [Google Scholar]
- 25.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 27.Castro-Vega LJ, Jouravleva K, Liu WY, Martinez C, Gestraud P, Hupé P, Servant N, Albaud B, Gentien D, Gad S, et al. Telomere crisis in kidney epithelial cells promotes the acquisition of a microRNA signature retrieved in aggressive renal cell carcinomas. Carcinogenesis. 2013;34:1173–1180. doi: 10.1093/carcin/bgt029. [DOI] [PubMed] [Google Scholar]
- 28.Nakada C, Matsuura K, Tsukamoto Y, Tanigawa M, Yoshimoto T, Narimatsu T, Nguyen LT, Hijiya N, Uchida T, Sato F, et al. Genome-wide microRNA expression profiling in renal cell carcinoma: Significant down-regulation of miR-141 and miR-200c. J Pathol. 2008;216:418–427. doi: 10.1002/path.2437. [DOI] [PubMed] [Google Scholar]
- 29.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. MiR-200 regulates PDGFDmediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PloS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, Nakata K, Tanaka M. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169. doi: 10.1186/1476-4598-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Jiang X, Li H, Guo L, Jiang W, Lu SH. MiR-203 inhibits the proliferation and self-renewal of esophageal cancer stem-like cells by suppressing stem renewal factor Bmi-1. Stem Cells Dev. 2014;23:576–585. doi: 10.1089/scd.2013.0308. [DOI] [PubMed] [Google Scholar]
- 34.Feber A, Clark J, Goodwin G, Dodson AR, Smith PH, Fletcher A, Edwards S, Flohr P, Falconer A, Roe T, et al. Amplification and overexpression of E2F3 in human bladder cancer. Oncogene. 2004;23:1627–1630. doi: 10.1038/sj.onc.1207274. [DOI] [PubMed] [Google Scholar]
- 35.Foster CS, Falconer A, Dodson AR, Norman AR, Dennis N, Fletcher A, Southgate C, Dowe A, Dearnaley D, Jhavar S, et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene. 2004;23:5871–5879. doi: 10.1038/sj.onc.1207800. [DOI] [PubMed] [Google Scholar]
- 36.Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–536. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 37.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/S0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson K, Landberg G. Subcellular localization, modification and protein complex formation of the cdk-inhibitor p16 in Rb-functional and Rb-inactivated tumor cells. Int J Cancer. 2006;118:1120–1125. doi: 10.1002/ijc.21466. [DOI] [PubMed] [Google Scholar]