Abstract
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant neurodegenerative disease characterized by a progressive cerebellar syndrome, which can be isolated or associated with extracerebellar signs. It has been shown that patients affected by SCA2 present also cognitive impairments and psychiatric symptoms.
The cerebellum is known to modulate cortical activity and to contribute to distinct functional networks related to higher-level functions beyond motor control. It is therefore conceivable that one or more networks, rather than isolated regions, may be dysfunctional in cerebellar degenerative diseases and that an abnormal connectivity within specific cerebello-cortical regions might explain the widespread deficits typically observed in patients.
In the present study, the network-based statistics (NBS) approach was used to assess differences in functional connectivity between specific cerebellar and cerebral “nodes” in SCA2 patients. Altered inter-nodal connectivity was found between more posterior regions in the cerebellum and regions in the cerebral cortex clearly related to cognition and emotion. Furthermore, more anterior cerebellar lobules showed altered inter-nodal connectivity with motor and somatosensory cerebral regions. The present data suggest that in SCA2 a cerebellar dysfunction affects long-distance cerebral regions and that the clinical symptoms may be specifically related with connectivity changes between motor and non-motor cerebello-cortical nodes.
Keywords: Cerebellum, Cerebral cortex, Resting-state fMRI, Functional connectivity, Nodes
Highlights
-
•
A cerebellar dysfunction affects long-distance cerebral regions in SCA2 patients.
-
•
Connectivity changes affect sensorimotor and cognitive cerebello-cortical nodes.
-
•
Cerebellar symptoms may be related to altered cerebello-cerebral connectivity.
1. Introduction
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant neurodegenerative disease involving the cerebellum. Neuropathological studies confirmed a pattern of grey matter (GM) loss to affect the cerebellar vermis and hemispheres with sparing of the vermian lobules I, II (lingula) and X (nodulus) and of the hemispheric lobules I, II (lingula) and Crus II (Della Nave et al., 2008a) as well as a diffuse damage of the brainstem and cerebellar white matter (WM) (Durr et al., 1995, Gilman et al., 1996, Estrada et al., 1999).
In addition to typical motor deficits (Takahashi et al., 2010), the presence of cognitive impairments in subjects with degenerative ataxia has long been debated (Fehrenbach et al., 1984). Recently, the cognitive performances of SCA2 patients have been exhaustively investigated showing that the patients may present with impairment in several cognitive and emotional domains (Klinke et al., 2010, Sokolovsky et al., 2010, D'Agata et al., 2011, Fancellu et al., 2013, Moriarty et al., 2016).
The evidence of motor, cognitive, and emotional impairments in presence of cerebellar damage has been linked to alterations of cerebro-cerebellar networks (Broich et al., 1987, Clausi et al., 2009, Komaba et al., 2000, Baillieux et al., 2010).
Indeed, the cerebellum has extensive projections to and from cortical regions by means of middle and superior cerebellar peduncles, the main afferent and efferent cerebellar white matter (WM) tracts. These connections are known to be strictly controlateral and to be spatially and functionally organized in distinct parallel loops (Middleton and Strick, 1994, Ramnani, 2006), thus contributing to distinct functional networks (Allen et al., 2005, Habas et al., 2009, De Vico Fallani et al., 2016) clearly related to different functional processes. Within this complex neural system the role of the cerebellum is to integrate multisensory information and then send them back to cerebral cortex (Leggio and Molinari, 2015). More specifically, it has been proposed that the cerebellum modulates the cortical activity (Di Lazzaro et al., 1994, Middleton and Strick, 2000) by detecting predictable sequences and allowing an optimized feedforward control that is necessary to accomplish the different functions successfully (Leggio et al., 2011).
Therefore, it is conceivable that an abnormal connectivity within specific cerebello-cortical circuits might explain the widespread deficits typically observed in SCA2 patients and that one or more networks, rather than isolated regions, might be dysfunctional.
Consistent with this hypothesis, a reduction of brain size has been reported in patients with SCA2, involving not only cerebellum and brainstem, but also other cortical and subcortical areas, such as frontal regions, primary sensorimotor cortex, temporo-mesial and parahyppocampal regions, substantia nigra, middle striatum, and thalamus (Estrada et al., 1999, Brenneis et al., 2003, Fancellu et al., 2013, Mercadillo et al., 2014). All these regions are known to be reciprocally connected with the cerebellum (Schmahmann, 1991, Schmahmann and Pandya, 1997, Middleton and Strick, 2001), indicating that several targets of cerebellar projections, including both motor and non-motor areas, are affected in patients with SCA2.
We hypothesize that cerebellar dysfunctions affect long- distance regions of the brain and clinical symptoms are related with changes in functional connectivity (FC) within specific cerebello-cortical networks.
The investigation of FC may provide important information to further characterize the neural basis and examine the integrity of cerebellar and cerebral networks in SCA2 patients. Indeed FC allows the relationship between the neuronal activation patterns of anatomically separated brain regions to be described (van den Heuvel and Hulshoff Pol, 2010). Amongst the available methods to investigate the brain functional connectivity, resting-state fMRI (RS-fMRI) has been proven reliable and easy to implement (Biswal et al., 1997) and it is particularly suitable for the study of a complex structure like the cerebellum is, in which the function of each subregion is defined by its connection with specific brain areas (Schmahmann and Pandya, 1997, Middleton and Strick, 2001).
While structural patterns associated with cerebellum and cerebral cortex have been largely investigated in SCA2, the few studies, that addressed FC, have used resting-state fMRI approaches limited to investigate specific structures using regions chosen a priori through seed-based analysis (Hernandez-Castillo et al., 2015a) or meaningful functional networks through independent component analysis (ICA) (Hernandez-Castillo et al., 2015a, Cocozza et al., 2015). Indeed, the analysis of pre-defined seed regions or specific networks only represents a small proportion of the brain, thus they may not be able to provide a complete picture of how the connectome is affected in by SCA2. Taking into account these limits, in the present study we aimed to investigate the whole-brain functional organization associated with cerebellar structural degeneration in SCA2 by applying the whole-brain analysis driven by graph theory, a mathematical approach that describes complex systems as networks (Bullmore and Sporns, 2009, Rubinov and Sporns, 2010). In essence, the brain is represented by a graph, composed by number of regions (nodes) that are functionally connected to each other by the edges. The nodes can be defined anatomically or functionally, and edges can be computed from RS-fMRI data. A graph can be represented mathematically by a matrix (connectivity matrix), with each row/column identifying node, and the corresponding value indicating the edge weight. Connectivity matrices can be compared using appropriate statistical tools. Specifically, the Network Based Statistics (NBS) (Zalensky et al., 2010; Han et al., 2013), is a validated statistical method to deal with the multiple comparisons problem when analyzing connectivity matrices (or graphs). NBS can be used to identify connections and sub-networks associated with an experimental effect or showing a between-group difference. (Zalensky et al., 2010, Wen and Hsieh, 2016).
2. Materials and methods
2.1. Subjects
Nine patients with SCA2 [F/M = 6/3; mean age ± SD = 47.6 ± 10.2 years], were recruited from the Ataxia Lab of Foundation Santa Lucia Hospital. Both in-patients (admitted for rehabilitation) and out-patients (followed up at the clinic) were included. At the time of assessment, all patients had > 6 months of illness from the genetically confirmed diagnosis. The absence of any extra-cerebellar lesion was investigated by an expert neuroradiologist and performed by visual inspection of the T2-weighted MRI scans acquired as part of this research study.
All patients underwent a comprehensive neurological examination. They showed a pure cerebellar syndrome. Only CA-2 presented a Babinski sign. Cerebellar motor deficits were assessed using the International Cooperative Ataxia Rating Scale (ICARS, Trouillas et al., 1997). ICARS global score ranges from 0 (absence of any motor deficit) to 100 (presence of motor deficits at the highest degree). Demographic characteristics and total motor scores of the patients are reported in Table 1.
Table 1.
Demographic and clinical characteristics of the patients.
| Case code | Age | Gender | Years of illness | CAG repeats | ICARS total scores |
|---|---|---|---|---|---|
| CA-1 | 42 | F | 1 | 22/39 | 47 |
| CA-2 | 54 | F | 1 | 22/37 | 27 |
| CA-3 | 65 | M | 3 | 22/35 | 27 |
| CA-4 | 42 | M | 1 | 14/47 | 24 |
| CA-5 | 42 | F | 1 | 22/39 | 28 |
| CA-6 | 36 | F | 8 | 22/42 | 37 |
| CA-7 | 62 | F | 4 | 22/37 | 31 |
| CA-8 | 41 | M | 3 | 22/38 | 18 |
| CA-9 | 44 | F | 13 | – | 28 |
ICARS = International Cooperative Ataxia Rating Scale.
A group of 33 healthy subjects (HS) [F/M = 21/12] ranging from 40 to 60 years of age [mean age ± SD = 50.8 ± 8.8 years] with no history of neurological or psychiatric illness were also recruited as control group. A t-test analysis ensured that there was no significant difference in the mean age between the two groups (p = 0.34).
All patients were examined extensively through a neuropsychological protocol, covering all cognitive domains, including: a) verbal long-term memory: 15-Word List (Immediate and Delayed recall) (Carlesimo et al., 1996); b) verbal and visuospatial short-term memory: Digit span and the Corsi Block Tapping task (Monaco et al., 2013); c) executive functions: Phonological Word Fluency (Carlesimo et al., 1996) and Modified Card Sorting Test (Heaton et al., 2000); d) Reasoning: Raven's Coloured Progressive Matrices (Carlesimo et al., 1996); e) constructional praxis: copy of Complex Rey's Figure (Carlesimo et al., 2002); f) language: Naming objects subtest of the BADA (“Batteria per l'Analisi dei Deficit Afasici”, Italian for “Battery for the analysis of aphasic deficits”) (Miceli et al., 1991).
The results of the neuropsychological assessment are reported in Table 2.
Table 2.
Patients' neuropsychological assessment.
| Patients | Tests |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR | DR | PM | WF | fREY | FDS | BDS | FC | BC | WISC |
NamOb (err) | ||
| PErr | TErr | |||||||||||
| CA1 | 45.8 | 10.1 | 28.5 | 33.5 | 33.5 | 5.61 | 4.58 | 3.60 | – | 119 | 108 | 4 |
| CA2 | 46.5 | 10.1 | 29.0 | 19.2 | 26.5 | 5.77 | 2.66 | 4.73 | 4.01 | 108 | 108 | 5 |
| CA3 | 48.7 | 12.5 | 31.0 | 35.2 | 31.5 | 5.60 | 3.50 | 5.57 | 3.77 | 100 | 100 | 3 |
| CA4 | 46.5 | 11.7 | 33.5 | 24.7 | 34.7 | 5.82 | 4.90 | 6.86 | 5.58 | 100 | 100 | 4 |
| CA5 | – | – | 26.0 | 17.3 | 32.2 | 4.47 | 2.37 | 4.43 | 3.58 | 81 | 81 | – |
| CA6 | 45.5 | 8.6 | 30.0 | 23.1 | 30.5 | 5.55 | 3.52 | 3.54 | 3.50 | 138 | 119 | 2 |
| CA7 | 47.3 | 9.7 | 33.0 | 25.5 | 30.0 | 4.13 | 3.19 | 6.15 | 6.01 | 108 | 92 | 1 |
| CA8 | 42.5 | 13.7 | 35.0 | 19.7 | 32.7 | 4.82 | 2.90 | 5.86 | 4.58 | 119 | 108 | 1 |
| CA9 | 44.8 | 10.1 | 27.5 | 36.5 | 27.5 | 5.61 | 4.58 | 4.60 | 4.58 | 119 | 119 | 0 |
| Cut-off | 28.53 | 4.69 | 18.96 | 17.35 | 23.76 | 4.26 | 2.65 | 3.46 | 3.17 | 92 | 92 | ± 2 |
Cut-off values refer to published normative data. Scores below the cut-off are marked in bold.
IR: Rey's 15 mots short term (immediate recall); DR: Rey's 15 mots long term (delayed recall); PM: progressive matrices; WF: word fluency; fREY: Complex Rey's Figure-copy; FDS: forward digit span; BDS: backward digit span; FC: forward Corsi; BC: backwords Corsi; WISC: Wisconsin Card Sorting Test: PErr (perseverative errors), TErr: total errors; NamOb(err): naming objects (errors).
This research study was approved by the Ethics Committee of Santa Lucia Foundation, according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each subject.
2.2. MRI acquisition protocol
All subjects underwent an MRI examination at 3 T (Magnetom Allegra, Siemens, Erlangen, Germany) that included the following acquisitions: 1) dual-echo turbo spin echo [TSE] (TR = 6190 ms, TE = 12/109 ms); 2) fast-FLAIR (TR = 8170 ms, 204TE = 96 ms, TI = 2100 ms); 3) 3D Modified Driven Equilibrium Fourier Transform (MDEFT) scan (TR = 1338 ms, TE = 2.4 ms, Matrix = 256 × 224 × 176, in-plane FOV = 250 × 250 mm2, slice thickness = 1 mm); 3) T2* weighted echo planar imaging (EPI) sensitized to blood oxygenation level dependent imaging (BOLD) contrast (TR: 2080 ms, TE: 30 ms, 32 axial slices parallel to AC-PC line, matrix: 64 × 64, pixel size: 3 × 3 mm2, slice thickness: 2.5 mm, flip angle: 70°) for resting state fMRI. BOLD echo planar images were collected during rest for a 7 min and 20 s period, resulting in a total of 220 volumes. During this acquisition, subjects were instructed to keep their eyes closed, not to think of anything in particular, and not to fall asleep. The TSE scans of patients, acquired as part of this research study, were reviewed by an expert neuroradiologist in order to characterize the brain anatomy and determine the presence of macroscopic structural abnormalities involving extracerebellar structures. For the control group, conventional MRI scans were inspected in order to exclude any pathological conditions according to the inclusion criteria.
2.3. Resting state fMRI data preprocessing
Data were pre-processed using Statistical Parametric Mapping [Wellcome Department of Imaging Neuroscience; SPM8 (http://www.fil.ion.ucl.ac.uk/spm/)], and in-house software implemented in Matlab (The Mathworks Inc., Natick, Massachusetts, USA). For each subject, the first four volumes of the fMRI series were discarded to allow for T1 equilibration effects. The pre-processing steps included correction for head motion, compensation for slice-dependent time shifts, normalization to the EPI template in MNI coordinates provided with SPM8, and smoothing with a 3D Gaussian Kernel with 8 mm3 full-width at half maximum. For each data set the motion parameters estimated during correction were checked to ensure that the maximum absolute shift did not exceed 2 mm and the maximum absolute rotation did not exceed 1.5°. The global temporal drift was removed using a 3rd order polynomial fit and the signal was regressed against the realignment parameters, and the signal averaged over whole brain voxels, to remove other potential sources of bias. Then, all images were filtered by a phase-insensitive band-pass filter (pass band 0.01–0.08 Hz) to reduce the effect of low frequency drift and high frequency physiological noise. Every participant's MDEFT was segmented in SPM in order to estimate the total grey matter (GM) volume. This quantity was compared (using a two-sample t-test) between patients and controls to exclude the presence of macroscopic atrophy in patients.
3. Statistical analysis
3.1. Network based statistics
In order to obtain a connectivity matrix for each participant, we first identified a set of 116 nodes defined by the automated anatomical labelling (AAL) atlas. Each node's mean time course was calculated as the average of the fMRI time series from all voxels within a given region. Correlation matrices were then obtained calculating the correlation between all pairs of nodes' mean signals as described by Serra et al. (2016). In this way, we were able to assess differences in functional connectivity (FC) between specific cerebellar and cerebral “nodes”. The “Networks-based statistics” (NBS) tool developed by Zalensky et al. (2010) was used for statistical comparison. A two-sample t-test was used to compare FC matrices between patients and controls, with 5000 permutations and setting the significant p-value at 0.05 corrected for multiple comparisons by using NBS correction (Zalensky et al., 2010).
4. Results
4.1. Neuropsychological assessment
The neuropsychological assessment revealed the presence of selective and very slight impairments in some patients but did not show clear evidence of general cognitive impairment. Indeed, some patients displayed values below the cut-off in word fluency test and backward digit span (CA5), forward digit span (CA7), and Wisconsin Card Sorting Test (CA6) (see Table 2). These results are consistent with findings that patients who are affected by cerebellar damage do not present with marked cognitive deficits.
4.2. Resting-state fMRI data results
No significant differences were found between total GM volumes of patients [mean ± SD = 655.7 ± 51.5] and controls [mean ± SD = 644.2 ± 49.1] as assessed by the t-test analysis (p = 0.26).
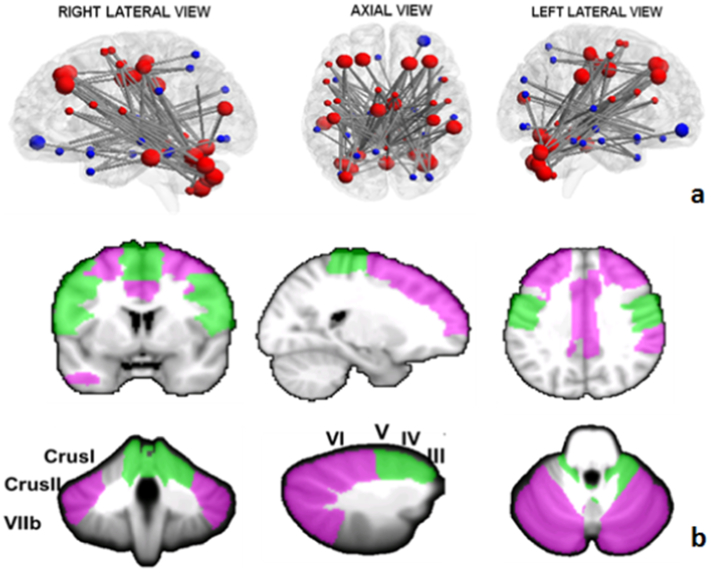
NBS analysis showed altered inter-nodal connectivity between cerebellum and several cerebral regions throughout the whole brain. Overall, 62 nodes and 110 edges showed differences in SCA2 brains compared to control ones while 57 edges and 35 nodes survived after Bonferroni correction for multiple comparisons (FWE = 0.05) (Fig. 1a). According to the cerebellar functional topography, cerebellar nodes in the posterior cerebellum, such as Crus I and Crus II, showed reduced FC with nodes in cortical regions implicated in cognition and emotion, such as superior (SFg) and middle (MFg) frontal gyrus. Similarly, cerebellar nodes in the motor anterior cerebellum, such as lobules III, IV, V, and vermis IV–V, showed reduced FC with nodes in the cortical regions related to motor control, such as precentral (PrG) and postcentral (PcG) gyrus, Rolandic Operculum (RO), supplementary motor area (SMA) (Fig. 1b).
Fig. 1.
a) Network of significantly decreased functional connectivity in SCA2 patients as assessed by NBS analysis (FWE = 0.05). The regions of the cerebello-cortical (red) and cortico-cortical (blue) modules are shown in different colors. Bigger nodes correspond to cerebellar and cortical regions relevant to cognition and emotion; smaller nodes correspond to cerebellar and cortical regions relevant to motor control. The brain network is visualized using the BrainNet Viewer (https://www.nitrc.org/projects/bnv/) (Xia et al., 2013). b) Anatomical representations of cognitive (violet) and motor (green) nodes in the cerebellum and cerebral cortex showing underconnectivity between each other. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Finally, reduced inter-nodal FC was found between cerebellar lobule VI and both cognitive and motor regions in the cerebral cortex, including supramarginal gyrus and supplementary motor area. A similar pattern was found in the vermis lobule VI, showing decreased functional connectivity with regions in the rolandic and frontal operculum as well as the supramarginal area. Detailed results of NBS analysis are reported in Table 3 that shows the cerebello-cortical edges of significant FC decrease in SCA2 patients.
Table 3.
Cerebello-cerebral nodes of functional underconnectivity.
| Pairwise brain regions |
t-Valuesa | ||
|---|---|---|---|
| Cerebellum | Cerebral cortex | ||
| Posterior | R-Crus I | L-temporal inferior | 3.84 |
| L-frontal medial | 4.05 | ||
| L-Crus I | R-frontal superior | 4.38 | |
| R-frontal medial | 5.24 | ||
| L-Crus II | R-frontal superior | 3.93 | |
| R-Frontal medial | 4.24 | ||
| R-CrusII | L-frontal superior | 4.54 | |
| L-frontal medial | 5.38 | ||
| L-temporal inferior | 3.95 | ||
| R-VIIb | L-frontal medial | 5.22 | |
| R-frontal medial | 3.18 | ||
| Intermediate | L-VI | R-supplementary motor | 4.23 |
| L-supplementary motor | 4.29 | ||
| R-cingulum | 3.62 | ||
| L-cingulum | 3.64 | ||
| R-supramarginal | 4.51 | ||
| R-VI | L-supplementary motor | 3.89 | |
| R-supplementary motor | 3.43 | ||
| Anterior | L-III | R-frontal inferior operculum | 3.35 |
| L-frontal inferior operculum | 4.93 | ||
| R-III | L-frontal inferior operculum | 3.61 | |
| L-rolandic operculum | 3.95 | ||
| R-rolandic operculum | 3.43 | ||
| R-IV/V | L-postcentral gyrus | 3.60 | |
| VERMIS | I–II | L-frontal inferior operculum | 3.96 |
| III | L-frontal inferior operculum | 4.86 | |
| R-frontal inferior operculum | 3.57 | ||
| L-frontal inferior,pars triangularis | 4.17 | ||
| R-rolandic operculum | 3.83 | ||
| L-postcentral gyrus | 3.85 | ||
| IV–V | L-precentral gyrus | 4.97 | |
| R-precentral gyrus | 4.79 | ||
| L-frontal inferior operculum | 4.32 | ||
| L-rolandic operculum | 3.42 | ||
| R-rolandic operculum | 4.00 | ||
| L-supplementary motor | 4.09 | ||
| R-supplementary motor | 3.99 | ||
| L-postcentral gyrus | 5.37 | ||
| R-postcentral gyrus | 4.64 | ||
| VI | L-frontal inferior operculum | 3.96 | |
| L-rolandic operculum | 3.69 | ||
| R-rolandic operculum | 3.82 | ||
| R-supramarginal | 3.55 | ||
Difference of functional connectivity into pairwise brain regions in patients with SCA2 compared to controls (p-value < 0.05 in the whole-network comparison using Network Based statistics). R = right; L = left.
t-Values are reported.
5. Discussion
Despite the advancing knowledge of cerebellar functions, the specific role that the cerebellum plays in concert with other brain regions in SCA2 patients remains unclear. RS-fMRI is an ideal method for investigating functional interactions between cerebellum and cerebral cortex in the human brain and it may prove a useful tool for interpreting motor and non-motor impairment driven by the cerebellar damage.
A growing body of studies explored the use of RS-fMRI in functional disconnection in neurological and psychiatric disorders (Greicius and Menon, 2004, Greicius et al., 2007, Rombouts et al., 2005, Rombouts et al., 2009; Liu et al., 2008, Bluhm et al., 2009, Whitfield-Gabrieli et al., 2009). Disrupted functional cerebellar connectivity has been demonstrated in patients with schizophrenia (Liu et al., 2008, Collin et al., 2011), Parkinson's disease (Liu et al., 2013), and major depressive disorder (Ma et al., 2013). Disruption of visual and motor connectivity has also been demonstrated in spinocerebellar ataxia type 7 (SCA 7) supporting the theory that neurodegenerative diseases target specific regions in large-scale networks (Seeley et al., 2007). Further, typical connectivity patterns have been characterized in patients with autosomal dominant spinocerebellar ataxia 17 (SCA17) (Reetz et al., 2012) suggesting that the broad range of symptoms observed in SCA17 patients may primarily reflect the involvement of distinct functional networks affected by the cerebellar atrophy. A disconnection syndrome has been suggested in spinocerebellar ataxia type 1 (SCA 1) by means of intrinsic functional analysis and diffusion tensor imaging (Solodkin et al., 2011).
Accordingly, RS-fMRI studies in healthy subjects provided a detailed mapping of resting state networks of the human cerebellum revealing that distinct networks are associated with each single lobule (Bernard et al., 2012). van den Heuvel and Hulshoff Pol (2010) suggested that there is a more general link between structural and functional connectivity. Indeed, it has been shown that almost all functionally linked regions of the most often reported resting-state networks are structurally interconnected by known white matter tracts (van den Heuvel et al., 2009). This suggests the existence of a general structural core of resting-state networks, supporting the notion of an overall link between structural and functional connectivity on a whole-brain scale (Damoiseaux and Greicius, 2009, Hagmann et al., 2008). These assumptions support the idea that the functional heterogeneity of the cerebellum is reflected in its connectional heterogeneity and give rise to the hypothesis that different cerebello-cortical projections and distinct functional modules can be selectively impaired by cerebellar disorders.
In the present study the pattern of FC alterations between regions in the cerebellum and cerebral cortex has been extensively characterized in SCA2 patients using the NBS approach, that is based on the whole-brain analysis allowing complex systems to be described as networks (Bullmore and Sporns, 2009, Rubinov and Sporns, 2010). We found nodes in the posterior cerebellum to show reduced functional connectivity with nodes in cortical regions related to cognition and emotion and nodes in the anterior cerebellum to show reduced functional connectivity with nodes in the cortical regions related to motor control. This result is, at least in part, in line with a previous FC study in SCA2 patients using a seed-based approach (Hernandez-Castillo et al., 2015a) and showing FC decrease between the right posterior cerebellum and the left superior frontal gyrus, which could impact different cognitive operations such as self-monitoring and verbal/visuospatial working memory (Cao et al., 1998, O'Reilly et al., 2010).
Additionally, we found that in both hemispheres and vermis, lobule VI shows decreased FC with regions related to motor control as well as to cognition and emotion (i.e. supplementary motor area, rolandic opercula, cingulum and supramarginal gyrus). It is worth noting that the right supramarginal gyrus is considered a region strongly implicated in emotional processing related to social judgements and empathy (Silani et al., 2013, Hoffmann et al., 2016). This finding is in line with the evidence suggesting that SCA2 patients are impaired in emotional behaviour (D'Agata et al., 2011, Sokolovsky et al., 2010). The hypothesis is that cerebellar cortical neurodegeneration associated with SCA2 may impact spatially segregated cortical regions that are functionally connected to the cerebellum thus affecting cerebello-cortical functional networks relevant for both motor and non-motor functions. With respect to the latter ones, cumulative evidences have been collected suggesting that connections between posterior cerebellum and cerebral cortex are related to cognitive functions (Stoodley and Schmahmann, 2009, Stoodley and Schmahmann, 2010, Strick et al., 2009, Stoodley et al., 2012). In our SCA2 patients, within the posterior cerebellum the prominent finding was an impaired connectivity towards medial and superior frontal regions. These prefrontal areas have been consistently implicated in different aspects of executive functions using both verbal and visuospatial tasks (Reverberi et al., 2005, Crescentini et al., 2011, Langdon and Warrington, 2000). In spite of this, in the present study only 3 patients presented impaired performances in executive processing and verbal working memory. This datum can be explained by the fact that most standard norms of testing do not detect cognitive impairments in cerebellar cohorts because cerebellar patients' symptoms are present in selective domains and very often, they can be detected only when the patients are compared to matched healthy controls (Tedesco et al., 2011).
An important issue that needs to be discussed is that the pattern of decreased cerebello-cerebral functional connectivity may be at least in part explained by damage of the cortical GM in SCA2. Indeed, cerebellar atrophy has been also reported to reduce GM volume in several supratentorial areas (Brenneis et al., 2003, Della Nave et al., 2008a). Thus, even if in the present study the total GM volume was not significantly different between SCA2 patients and controls, the possibility of local GM loss cannot be ruled out. An alternative explanation for the absence of significant whole brain GM loss in patients might be that the pattern of ponto-cerebellar atrophy associated with SCA2 pathology predominantly entails a WM damage (Della Nave et al., 2008b).
Overall, the observed pattern of inter-nodal underconnectivity is consistent with previous studies using different RS-fMRI approaches (O'Reilly et al., 2010, Bernard et al., 2012) demonstrating in healthy subjects the functional segregation of the cerebellum in sensorimotor and supramodal zones, the former containing overlapping functional connectivity maps for domain-specific motor and somatosensory cortices, the latter for prefrontal and posterior-parietal cortex, and provides important insight into understanding the neural circuit abnormalities in SCA2. In light of the general link between structural and functional connectivity (van den Heuvel et al., 2009) a comprehensive understanding of neural connectivity may require clear evidence as to whether structural connectivity is affected in SCA2. In SCA2 patients microstructural alterations of the cerebellar WM have been reported by Diffusion Tensor Imaging (DTI) studies, showing the prevalent involvement of the main afferent and efferent tracts (i.e. Middle and Superior Cerebellar Peduncle), connecting the cerebellum with both motor and non-motor cortical regions (Mandelli et al., 2007, Della Nave et al., 2008b, Hernandez-Castillo et al., 2015b). Although in the present study structural connectivity has not been specifically investigated, it has to be considered that microstructural abnormalities of the cerebellar WM tracts, typically reported in SCA2 patients, may underlie a deficient structural connectivity that impacts the cerebello-cerebral interplay and results in a lack of functional connectivity.
Cerebellar clusters of significantly reduced functional connectivity have been recently reported by Cocozza et al. (2015) only in the default mode network, executive control network and right fronto-parietal network in patients with SCA2. However, this study used a different resting-state approach (i.e. ICA) limited to investigate connectivity within specific functional networks (Cocozza et al., 2015). In the present study, by using a whole-brain approach, we provide additional evidence that extensive and segregated functional brain changes may occur as the result of the SCA2 degenerative process.
Indeed, cerebello-cerebral functional disconnections are observed in this patient population throughout the brain and they are consistent with the pattern of cerebellar structural alterations mainly involving vermis and cerebellar hemispheres reported by Della Nave and colleagues (2008) In particular, connectivity reduction involved segregated motor and cognitive cerebello-cortical networks with the only exception of lobule VI involvement, not by chance a region in which both motor, cognitive, and emotional functions are localized.
It has to be underlined that we also found Crus II and lobule VII to show a functional disconnection with nodes in superior and middle frontal regions. This evidence is partially inconsistent with previous VBM studies that have shown cerebellar grey matter reduction to spare both Crus II and lobule VII in SCA2 patients (Brenneis et al., 2003, Ying et al., 2006, Della Nave et al., 2008a, Della Nave et al., 2008b). Nevertheless, it has to be considered that a functional coherence between the two cerebellar hemispheres has been widely demonstrated by RS-fMRI studies (Habas et al., 2009, O'Reilly et al., 2010, Buckner et al., 2011). Thus, it is reasonable to hypothesize that the cerebellar regions that are not directly affected by the degenerative process could suffer from the functional release of the affected cerebellar regions and result functionally impaired.
A limitation of this study is that, due to the small number of patients, the cognitive performance has not been directly correlated with functional connectivity alterations observed. Further investigations are needed to support our interpretation with greater patient population.
6. Conclusion
To our knowledge this is the first study using a whole-brain approach to investigate functional organization in SCA2 patients and to detect cerebello-cerebral inter-nodal connectivity changes that can be associated with cerebellar structural abnormalities of SCA2.
Altogether, the present findings show that a cerebellar dysfunction may affect long-distance regions in the cerebral cortex targeted by cerebellar projections and that specific cerebral functional alterations derive from cerebellar structural degeneration typically associated with SCA2 pathology, thus resulting into the multifarious motor, cognitive, and emotional deficits evidenced in patients.
Funding
This work was supported by the Ministry of Education, Universities and Research (MIUR) (Grant Number C26A1329A).
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by the Ministry of Education, Universities and Research (MIUR)- (Grant Number C26A1329AR) to ML, and Ministry of Health (Grant Number RF-2011-02348213) to MM and (Grant Number GR-2013-02354888) to SC.
References
- Allen G., McColl R., Bernard H., Ringe W.K., Fleckenstein J., Cullum C.M. Magnetic Resonance Imaging of cerebellar-prefrontal and cerebellar parietal functional connectivity. NeuroImage. 2005;28:39–48. doi: 10.1016/j.neuroimage.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Baillieux H., De Smet H.J., Dobbeleir A., Paquier P.F., De Deyn P.P., Mariën P. Cognitive and affective disturbances following focal cerebellar damage in adults, a neuropsychological and SPECT study. Cortex. 2010;46(7):869–879. doi: 10.1016/j.cortex.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Bernard J.A., Seidler R.D., Hassevoort K.M., Benson B.L., Welsh R.C., Wiggins J.L., Jaeggi S.M. Resting state functional connectivity networks, a comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 2012;10:6–31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Van Kylen J., Hyde J.S. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Miller J., Lanius R.A., Osuch E.A., Boksman K., Neufeld R.W., Theberge J., Schaefer B., Williamson P.C. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Brenneis C., Bosch S.M., Schocke M., Wenning G.K., Poewe W. Atrophy pattern in SCA2 determined by voxel-based morphometry. Neuroreport. 2003;14:1799–1802.10. doi: 10.1097/00001756-200310060-00008. [DOI] [PubMed] [Google Scholar]
- Broich K., Hartmann A., Biersack H.J., Horn R. Crossed cerebello-cerebral diaschisis in a patient with cerebellar infarction. Neurosci. Lett. 1987;83(1–2):7–12. doi: 10.1016/0304-3940(87)90207-2. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks, graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cao Y., D'Olhaberriague L., Vikingstad E.M., Levine S.R., Welch K.M. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–122. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Buccione I., Fadda L., Graceffa A., Mauri M., Lorusso S., Bevilacqua G., Caltagirone C. Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Riv. Neurol. 2002;12:1–13. [Google Scholar]
- Carlesimo G.A., Caltagirone C., Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- Clausi S., Bozzali M., Leggio M.G., Di Paola M., Hagberg G.E., Caltagirone C., Molinari M. Quantification of gray matter changes in the cerebral cortex after isolated cerebellar damage, a voxel-based morphometry study. Neuroscience. 2009;162(3):827–835. doi: 10.1016/j.neuroscience.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Cocozza S., Saccà F., Cervo A., Marsili A., Russo C.V., Giorgio S.M., De Michele G., Filla A., Brunetti A., Quarantelli M. Modifications of resting state networks in spinocerebellar ataxia type 2. Mov. Disord. 2015;30(10):1382–1390. doi: 10.1002/mds.26284. [DOI] [PubMed] [Google Scholar]
- Collin G., Hulshoff Pol H.E., Haijma S.V., Cahn W., Kahn R.S., van den Heuvel M.P. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psych. 2011;16:2,73. doi: 10.3389/fpsyt.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescentini C., Seyed-Allaei S., de Pisapia N., Jovicich J., Amati D., Shallice T. Mechanisms of rule acquisition and rule following in inductive reasoning. J. Neurosci. 2011;31:7763–7774. doi: 10.1523/JNEUROSCI.4579-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata F., Caroppo P., Baudino B., Caglio M., Croce M., Bergui M., Tamietto M., Mortara P., Orsi L. The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum. 2011;10:600–610. doi: 10.1007/s12311-011-0276-z. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Greicius M.D. Greater than the sum of its parts, a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- De Vico Fallani F., Clausi S., Leggio M., Chavez M., Valencia M., Maglione A.G., Babiloni F., Cincotti F., Mattia D., Molinari M. Interhemispheric connectivity characterizes cortical reorganization in motor-related networks after cerebellar lesions. Cerebellum. 2016:1–18. doi: 10.1007/s12311-016-0811-z. [DOI] [PubMed] [Google Scholar]
- Della Nave R., Ginestroni A., Tessa C., Giannelli M., De Grandis D., Plasmati R., Salvi F., Piacentini S., Mascalchi M. Brain structural damage in spinocerebellar ataxia type 2, a voxel-based morphometry study. Mov. Disord. 2008;23:899–903. doi: 10.1002/mds.21982. [DOI] [PubMed] [Google Scholar]
- Della Nave R., Ginestroni A., Tessa C., Salvatore E., De Grandis D., Plasmati R., Salvi F., De Michele G., Dotti M.T., Piacentini S., Mascalchi M. Brain white matter damage in SCA1 and SCA2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. NeuroImage. 2008;43(1):10–19. doi: 10.1016/j.neuroimage.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Restuccia D., Molinari M., Leggio M.G., Nardone R., Fogli D., Tonali P. Excitability of the motor cortex to magnetic stimulation in patients with cerebellar lesions. J. Neurol. Neurosurg. Psychiatry. 1994;57:108–110. doi: 10.1136/jnnp.57.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr A., Smadja D., Cancel G., Lezin A., Stevanin G., Mikol J. Autosomal dominant cerebellar ataxia type I in Martinique (French West Indies). Clinical and neuropathological analysis of 53 patients from three unrelated SCA2 families. Brain. 1995;118:1573–1581. doi: 10.1093/brain/118.6.1573. [DOI] [PubMed] [Google Scholar]
- Estrada R., Galarraga J., Orozco G., Nodarse A., Auburger G. Spino-cerebellar ataxia 2 (SCA2), morphometric analyses in 11 autopsies. Acta Neuropathol. 1999;97:306–310. doi: 10.1007/s004010050989. [DOI] [PubMed] [Google Scholar]
- Fancellu R., Paridi D., Tomasello C., Panzeri M., Castaldo A., Genitrini S., Soliveri P., Girotti F. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J. Neurol. 2013;260(12):3134–3143. doi: 10.1007/s00415-013-7138-1. [DOI] [PubMed] [Google Scholar]
- Fehrenbach R.A., Wallesch C.W., Claus D. Neuropsychologic findings in Friedreich's ataxia. Arch. Neurol. 1984;41:306–381. doi: 10.1001/archneur.1984.04050150084022. [DOI] [PubMed] [Google Scholar]
- Gilman S., Sima A.A., Junck L., Kluin K.J., Koeppe R.A., Lohman M.E. Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann. Neurol. 1996;39:241–255. doi: 10.1002/ana.410390214. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression, abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C.J., Wedeen V.J., Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7) doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.E., Yoo S.W., Seo S.W., Na D.L., Seong J.K. Cluster-based statistics for brain connectivity in correlation with behavioral measures. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Chelune G.J., Talley J.L., Kay G.G., Curtiss G. In: WCST: Wisconsin Card Sorting Test. Forma completa revisionata. Hardoy M.C., Carta M.G., Hardoy M.J., Cabras P.L., editors. It. O.S. Organizzazioni Speciali; Firenze: 2000. Adattamento italiano a cura di. [Google Scholar]
- Hernandez-Castillo C.R., Galvez V., Mercadillo R.E., Díaz R., Yescas P., Martinez L., Ochoa A., Velazquez-Perez L., Fernandez-Ruiz J. Functional connectivity changes related to cognitive and motor performance in spinocerebellar ataxia type 2. Mov. Disord. 2015;30:1391–1399. doi: 10.1002/mds.26320. [DOI] [PubMed] [Google Scholar]
- Hernandez-Castillo C.R., Galvez V., Mercadillo R., Diaz R., Campos-Romo A., Fernandez-Ruiz J. Extensive white matter alterations and its correlations with ataxia severity in SCA 2 patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F., Koehne S., Steinbeis N., Dziobek I., Singer T. Preserved self-other distinction during empathy in autism is linked to network integrity of right supramarginal gyrus. J. Autism Dev. Disord. 2016;46(2):637–648. doi: 10.1007/s10803-015-2609-0. [DOI] [PubMed] [Google Scholar]
- Klinke I., Minnerop M., Schmitz-Hübsch T., Hendriks M., Klockgether T., Wüllner U., Helmstaedter C. Neuropsychological features of patients with Spinocerebellar Ataxia (SCA) Types 1, 2, 3, and 6. Cerebellum. 2010;9:433–442. doi: 10.1007/s12311-010-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaba Y., Osono E., Kitamura S., Katayama Y. Crossed cerebellocerebral diaschisis in patients with cerebellar stroke. Acta Neurol. Scand. 2000;101(1):8–12. doi: 10.1034/j.1600-0404.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- Langdon D., Warrington E.K. The role of the left hemisphere in verbal and spatial reasoning tasks. Cortex. 2000;36:691–702. doi: 10.1016/s0010-9452(08)70546-x. [DOI] [PubMed] [Google Scholar]
- Leggio M., Molinari M. Cerebellar sequencing, a trick for predicting the future. Cerebellum. 2015;14(1):35–38. doi: 10.1007/s12311-014-0616-x. (Review) [DOI] [PubMed] [Google Scholar]
- Leggio M.G., Chiricozzi F.R., Clausi S., Tedesco A.M., Molinari M. The neuropsychological profile of cerebellar damage, the sequencing hypothesis. Cortex. 2011;47(1):137–144. doi: 10.1016/j.cortex.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Liu H., Edmiston E.K., Fan G., Xu K., Zhao B., Shang X., Wang F. Altered resting-state functional connectivity of the dentate nucleus in Parkinson's disease. Psychiatry Res. 2013;211:64–71. doi: 10.1016/j.pscychresns.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liang M., Zhou Y., He Y., Hao Y., Song M., Yu C., Liu H., Liu Z., Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Ma Q., Zeng L.L., Shen H., Liu L., Hu D. Altered cerebellar-cerebral resting-state functional connectivity reliably identifies major depressive disorder. Brain Res. 2013;1495:86–94. doi: 10.1016/j.brainres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Mandelli M.L., De Simone T., Minati L., Bruzzone M.G., Mariotti C., Fancellu R., Savoiardo M., Grisoli M. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR Am. J. Neuroradiol. 2007;28(10):1996–2000. doi: 10.3174/ajnr.A0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadillo R.E., Galvez V., Dıaz R., Hernàndez-Castillo C.R., Campos-Romo A., Boll M.C., Pasaye E.H., Fernandez-Ruiz J. Parahippocampal graymatter alterations in spinocerebellar ataxia type 2 identified by voxel based morphometry. J. Neurol. Sci. 2014;347:50–58. doi: 10.1016/j.jns.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Miceli G., Laudanna A., Burani C., Capasso R. Ass.ne per lo sviluppo delle ricerche neuropsicologiche. Berdata; Milano: 1991. Batteria per l'analisi dei deficit afasici. [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops, motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. (Review) [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Monaco M., Costa A., Caltagirone C., Carlesimo G.A. Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol. Sci. 2013;34:749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- Moriarty A., Cook A., Hunt H., Adams M.E., Cipolotti L., Giunti P. A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J. Rare Dis. 2016;11(1):82. doi: 10.1186/s13023-016-0447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system, anatomy and function. Nat. Rev. Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Reetz K., Dogan I., Rolfs A., Binkofski F., Schulz J.B., Laird A.R., Fox P.T., Eickhoff S.B. Investigating function and connectivity of morphometric findings—exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17) NeuroImage. 2012;62:1354–1366. doi: 10.1016/j.neuroimage.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi C., Lavaroni A., Gigli G.L., Skrap M., Shallice T. Specific impairments of rule induction in different frontal lobe subgroups. Neuropsychologia. 2005;43:460–472. doi: 10.1016/j.neuropsychologia.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rombouts S.A., Barkhof F., Goekoop R., Stam C.J., Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts S.A., Damoiseaux J.S., Goekoop R., Barkhof F., Scheltens P., Smith S.M., Beckmann C.F. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum. Brain Mapp. 2009;30:256–266. doi: 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity, uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. An emerging concept. The cerebellar contribution to higher function. Arch. Neurol. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. (Review) [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. The cerebrocerebellar system. Int. Rev. Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L., Cercignani M., Bruschini M., Cipolotti L., Mancini M., Silvestri G., Petrucci A., Bucci E., Antonini G., Licchelli L., Spanò B., Giacanelli M., Caltagirone C., Meola G., Bozzali M. “I know that you know that I know”, neural substrates associated with social cognition deficits in DM1 patients. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G., Lamm C., Ruff C.C., Singer T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J. Neurosci. 2013;33(39):15466–15476. doi: 10.1523/JNEUROSCI.1488-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky N., Cook A., Hunt H., Giunti P., Cipolotti L. A preliminary characterization of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav. Neurol. 2010;23:17–29. doi: 10.3233/BEN-2010-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A., Peri E., Chen E.E., Ben-Jacob E., Gomez C.M. Loss of intrinsic organization of cerebellar networks in spinocerebellar ataxia type 1, correlates with disease severity and duration. Cerebellum. 2011;10(2):218–232. doi: 10.1007/s12311-010-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Katada S., Onodera O. Polyglutamine diseases, where does toxicity come from? what is toxicity? where are we going? J. Mol. Cell Biol. 2010;2:180–191. doi: 10.1093/jmcb/mjq005. [DOI] [PubMed] [Google Scholar]
- Tedesco A.M., Chiricozzi F.R., Clausi S., Lupo M., Molinari M., Leggio M.G. The cerebellar cognitive profile. Brain. 2011;134:3672–3686. doi: 10.1093/brain/awr266. [DOI] [PubMed] [Google Scholar]
- Trouillas P., Takayanagi T., Hallett M., Currier R.D., Subramony S.H., Wessel K., Bryer A., Diener H.C., Massaquoi S., Gomez C.M., Coutinho P., Ben Hamida M., Campanella G., Filla A., Schut L., Timann D., Honnorat J., Nighoghossian N., Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J. Neurol. Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network, a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Mandl R.C., Kahn R.S., Hulshoff Pol H.E. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum. Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T., Hsieh S. Network-based analysis reveals functional connectivity related to internet addiction tendency. Front. Hum. Neurosci. 2016;1:10,6. doi: 10.3389/fnhum.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer, a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S.H., Choi S.I., Perlman S.L., Baloh R.W., Zee D.S., Toga A.W. Pontine and cerebellar atrophy correlate with clinical disability in SCA2. Neurology. 2006;66:424–426. doi: 10.1212/01.wnl.0000196464.47508.00. [DOI] [PubMed] [Google Scholar]
- Zalensky A., Fornito A., Bullmore E.T. Network-based statistic, identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]