Abstract
The cumulative effect of repetitive subconcussive collisions on the structural and functional integrity of the brain remains largely unknown. Athletes in collision sports, like football, experience a large number of impacts across a single season of play. The majority of these impacts, however, are generally overlooked, and their long-term consequences remain poorly understood. This study sought to examine the effects of repetitive collisions across a single competitive season in NCAA Football Bowl Subdivision athletes using advanced neuroimaging approaches. Players were evaluated before and after the season using multiple MRI sequences, including T1-weighted imaging, diffusion tensor imaging (DTI), arterial spin labeling (ASL), resting-state functional MRI (rs-fMRI), and susceptibility weighted imaging (SWI). While no significant differences were found between pre- and post-season for DTI metrics or cortical volumes, seed-based analysis of rs-fMRI revealed significant (p < 0.05) changes in functional connections to right isthmus of the cingulate cortex (ICC), left ICC, and left hippocampus. ASL data revealed significant (p < 0.05) increases in global cerebral blood flow (CBF), with a specific regional increase in right postcentral gyrus. SWI data revealed that 44% of the players exhibited outlier rates (p < 0.05) of regional decreases in SWI signal. Of key interest, athletes in whom changes in rs-fMRI, CBF and SWI were observed were more likely to have experienced high G impacts on a daily basis. These findings are indicative of potential pathophysiological changes in brain integrity arising from only a single season of participation in the NCAA Football Bowl Subdivision, even in the absence of clinical symptoms or a diagnosis of concussion. Whether these changes reflect compensatory adaptation to cumulative head impacts or more lasting alteration of brain integrity remains to be further explored.
Keywords: Subconcussive, Repetitive impacts, Football, MRI, Accelerometers
Highlights
-
•
Significant MRI changes were seen after 1 season in asymptomatic football players.
-
•
Changes (post- vs pre-season) were found in measures of CBF, rs-fMRI, and SWI.
-
•
Changes were greater in athletes with larger numbers of high-G impacts (≥ 80G).
1. Introduction
There has been a growing concern over sports-related brain injuries and possible long-term consequences; however, there has been less of a focus on the cumulative effects of repetitive subconcussive impacts. Subconcussive impacts are defined as events similar to those giving rise to a concussion, or mild traumatic brain injury (mTBI), but apparently involving insufficient impact forces or accelerations to produce symptoms associated with mTBI (Shuttleworth-Edwards et al., 2008). Many contact sports, including American football, soccer, rugby, boxing, wrestling, and lacrosse, result in rather high numbers of repetitive head impacts throughout a season and career, with football having been observed to lead possibly to thousands of subconcussive impacts for a single player over the course of one season (Merchant-Borna et al., 2016, Talavage et al., 2014).
The cumulative effects of multiple mild TBI incidents or accumulation of subconcussive impacts remain understudied and poorly understood. Many studies have demonstrated that multiple TBI events lead to greater functional impairments than are associated with only a single TBI (Longhi et al., 2005, Prins et al., 2013), suggesting that longer-term structural changes occur, possibly with each such incident (Giza and Kutcher, 2014). Further, there appear to be various long-term cognitive, motor, and psychiatric deficits associated with repeated injuries (Guskiewicz et al., 2000, Riemann and Guskiewicz, 2000) and likely with greater cumulative exposure to subconcussive impacts (Bailes et al., 2013, Montenigro et al., 2016). Animal and human research have demonstrated that repetitive impacts can cause pathophysiological changes in the brain and central nervous system without the presence of acute behavioral changes (Bauer et al., 2001, Talavage et al., 2014). However, since subconcussive impacts typically go undiagnosed or unmanaged (Baugh et al., 2012, McKee et al., 2009), the means by which accumulation of impacts over the course of a season (or career) can lead to altered neurobiology later in life remains inferred (Broglio et al., 2012). Nonetheless, many recent case studies have demonstrated gross and microscopic pathology changes that are best attributed to repeated mTBI and exposure to subconcussive impacts (McKee et al., 2009, Omalu et al., 2005).
Within American football, studies have shown that specific positions may have a differential risk of concussion and exposure to subconcussive impacts, and, therefore, neurodegenerative diseases (Lehman, 2013). It is thought that “speed” players (quarterback, running back, wide receiver, defensive back, safety, linebacker, etc.) and “non-speed” players (offensive and defensive line) are at differing risks, due to the nature of their positions. “Speed” players often build up momentum prior to tackling or being tackled, whereas “non-speed” players typically engage in hits immediately after the ball is snapped (Broglio et al., 2012, Pellman et al., 2004). However, there is still great debate regarding whether greater risk of mTBI exists for players exposed to the higher-magnitude impacts (i.e., speed players) or those exposed to the repetitive immediate head impacts (i.e., non-speed players). Of interest here, several studies have shown that linemen receive more cumulative impacts (especially at the front of the head) and develop more post-impact symptoms than other positions (Baugh et al., 2015, Crisco et al., 2010).
Several previous studies have found a linkage between the degree (quantified by number or average magnitude) of exposure to repetitive subconcussive impacts and the degree of pathophysiological change in the brains of collision-sport athletes. These changes have been observed in asymptomatic athletes through evaluation of measures derived from: cognitive testing (Breedlove et al., 2012, Talavage et al., 2014, Breedlove et al., 2014, Nauman et al., 2015); task-driven and resting-state brain behavior (Abbas et al., 2015c, Breedlove et al., 2012, Talavage et al., 2014, Robinson et al., 2015); neurovascular coupling (Svaldi et al., 2015, Svaldi et al., 2016); white matter health (Davenport et al., 2014, Mayer et al., 2015, Obler et al., 2010, Chun et al., 2015); and gray matter (Bazarian et al., 2014, Mayer et al., 2015) volume, including hippocampal-specific assessment (Singh et al., 2014). Additionally, the biochemistry of athletes has been observed to vary over the course of exposure to repetitive impacts (Poole et al., 2014, Poole et al., 2015). Further, the differential response to impacts appears to be markedly dependent on previous concussive history (Johnson et al., 2014).
In this multimodal MRI study, the cumulative effects of subconcussive impacts were examined on NCAA Football Bowl Subdivision players over the course of a single intervarsity athletic season. Athletes were evaluated using MRI measures before (pre-) and immediately after (post-) their competition season, and monitored during the majority of activities using helmet-based accelerometers (BodiTrak) to quantify impact exposure. The explicit MRI sequences included T1-weighted imaging, diffusion tensor imaging (DTI), arterial spin labeling (ASL), resting-state functional MRI (rs-fMRI), and susceptibility weighted imaging (SWI). We predicted that greater accumulation of high intensity impacts would jeopardize functional/structural and vascular integrity of the brain, as assessed in the manner we have previously documented in clinically asymptomatic athletes (Slobounov et al., 2010, Talavage et al., 2016, Zhu et al., 2015). Based on prior reports, we also predicted that offensive and defensive linemen would have the greatest number of cumulative impacts (at any threshold level), and that this group would be most likely to evidence neural changes, as observed via MRI. Our specific hypotheses were that a differential sensitivity related to accelerometer measures would be observed for the MRI modalities likely to be associated with cumulative subconcussive exposure over the entire athletic season. Specifically, we expected ASL and SWI abnormalities would be related to rs-fMRI measures and accelerometer measures in clinically asymptomatic athletes studied before and after the football season.
2. Methods
2.1. Subjects
Twenty-three (23) male collegiate student football athletes participated in this study. Twenty (20) players completed both MRI scans: within one week before the athletic season began (before any contact practices began during preseason and the regular season) and within one week after the last game of the season (post-season). All subjects provided informed consent, as approved by the Penn State University Institutional Review Board. Image quality assurance measures resulted in exclusion of data from two subjects, resulting in a pool of 18 subjects (21.6 ± 1.28 years old) for subsequent analyses. Our MRI analyses relied on accurate segmentation of 3D MPRAGE volumetric images based on FreeSurfer (Fischl et al., 2002). One subject failed on segmentation due to severe motion artifacts. The other failed on segmentation due to inappropriate image acquisition. Of these 18 subjects, previous concussion history includes no (n = 10), one (n = 6), or two (n = 2) prior diagnosed injuries. However, none of the athletes were recovering from, or were diagnosed with, a concussion during the period of study, or in the nine months prior to the pre-season evaluation.
2.2. Impact history assessment
Impacts to the head for all 23 participating athletes were monitored using the BodiTrak system from HeadHealth Network. These helmet-based sensors comprise elastic fabric with pressure monitors and impact sensors, and provide estimates of both linear acceleration (in units of G) and location of incident impact. The sensors were to be fitted and placed into the athlete's helmet during practices (53 possible sessions; see Table 1), but were not worn during games. Sensor data were collected and, over the course of the season, counts of accumulated (practice) events were assessed using thresholds of ≥ 25G and ≥ 80G, attempting to quantify the majority, if not all, impacts that are likely to be relevant to brain health (McCuen et al., 2015), as well as the count of hits traditionally expected to be associated with concussion (Broglio et al., 2010).
Table 1.
Accelerometer monitoring totals for football athletes (including position information) participating in this study. Players who missed a portion of the season due to injury are indicated by an asterisk (*). Players whose season ended prematurely due to injury are indicated by a double asterisk (**). Players whose scans were omitted from analysis due to incomplete or artifact-contaminated imaging data are indicated by italics.
| Subject ID | Primary position(s) | Monitored sessions (53 possible) | # Hits exceeding 25G | # Hits exceeding 80G |
|---|---|---|---|---|
| 1 | TE | 27* | 17 | 0 |
| 2 | OL | 42 | 98 | 3 |
| 4 | DL (T) | 37 | 121 | 5 |
| 5 | DL (E) | 38 | 187 | 7 |
| 6 | DB (S) | 45 | 107 | 5 |
| 7 | DL (T) | 47 | 159 | 11 |
| 8 | OL | 47 | 465 | 10 |
| 9 | OL | 26* | 28 | 0 |
| 10 | OL | 49 | 359 | 15 |
| 11 | OL (C/G) | 30 | 226 | 5 |
| 12 | RB | 43 | 123 | 13 |
| 13 | LB/DB (S) | 51 | 126 | 2 |
| 14 | DL (E) | 37 | 159 | 4 |
| 15 | DL (E) | 31 | 102 | 7 |
| 16 | DL (T) | 2** | 11 | 2 |
| 17 | DL | 36 | 101 | 2 |
| 18 | DL (T) | 47 | 76 | 2 |
| 19 | OL | 38 | 184 | 1 |
| 20 | LB | 45 | 152 | 1 |
| 21 | OL (T) | 48 | 308 | 7 |
| 22 | TE | 37 | 218 | 13 |
| 23 | LB | 17** | 95 | 12 |
| 24 | OL | 39 | 317 | 2 |
2.3. MRI acquisition
MRI data were collected on a Siemens 3T Prisma MR scanner (Siemens, Erlangen, Germany) with a 32-channel head coil. Images were obtained for each subject prior to participation (Pre) and after the football season had ended (Post) using the following acquisitions: (1) high-resolution 3D T1-weighted; (2) a resting-state functional MRI (rs-fMRI) acquisition; (3) 3D pulsed arterial spin labeling (ASL); (4) diffusion-weighed images from which diffusion tensors could be computed (DTI); and (5) susceptibility-weighted imaging (SWI).
For structural and volumetric analysis, 176 T1-weighted 1-mm3 isotropic volumetric images (3 min 31 s), with cerebrospinal fluid (CSF) suppressed, were obtained to cover the whole brain with a 3D magnetization prepared rapid acquisition gradient recalled echo (3D MPRAGE) sequence with the following parameters: TE = 1.77 ms, time of inversion (TI) = 850 ms, TR = 1700 ms, flip angle = 9°, matrix size = 320 × 260 × 176, voxel size = 1 mm × 1 mm × 1 mm, receiver bandwidth = 300 Hz/pixel, and parallel acceleration factor = 2.
To study resting-state brain function, a 10-min echo-planar imaging dataset with whole-brain coverage was acquired with the following parameters: 72 contiguous 2-mm axial slices in an interleaved order, time of echo (TE) = 35.8 ms, time of repetition (TR) = 2000 ms, flip angle = 90°, voxel resolution = 2 mm × 2 mm × 2 mm, matrix size = 104 × 104, 300 total volumes acquired. Note that this dataset was acquired while the subject was asked to relax with eyes closed, while remaining awake. Prior to analysis (see below), the first four data points were discarded.
Regional cerebral blood flow data were acquired using 3D ASL with the following parameters (6 min 2 s): 40 axial slices, TE = 15.62 ms, TR = 4600 ms, voxel resolution = 1.5 mm × 1.5 mm × 3 mm, field of view = 192 mm × 192 mm, bolus duration = 700 ms, perfusion mode set at PICORE Q2TIPS, parallel acceleration factor = 2.
Diffusion tensor data were computed from diffusion-weighted images acquired with a spin-echo echo-planar imaging (EPI) sequence (11 min 28 s) with the following parameters: 72 contiguous 2-mm axial slices in an interleaved order, matrix size = 110 × 110, voxel size = 2 mm × 2 mm × 2 mm, TE = 94 ms, TR = 9.8 s, 30 diffusion-weighted volumes (one per gradient direction) with b = 1000 s/mm2, 30 diffusion-weighted volumes with b = 2000 s/mm2, seven volumes with b = 0 and parallel imaging acceleration factor = 2. Only the 30 diffusion-weighted volumes with b = 1000 s/mm2, and the volume with b = 0 that was acquired with these diffusion-weighted volumes were selected to calculate the diffusion metrics.
SWI images were acquired with 3D gradient echo sequence with following parameters (6 min): TE1 = 10.65 ms, TE2 = 19.18 ms, TE3 = 27.71 ms, TR = 36 ms, flip angle = 20°, matrix size = 320 × 320 × 96, voxel size = 0.8 mm × 0.8 mm × 1.6 mm, receiver bandwidth = 1336 Hz/pixel, parallel acceleration factor = 2, saving both magnitude and phase images.
2.4. Cortical and subcortical volumetric analyses
All cortical and subcortical volumes were extracted from the T1-weighted images via the FreeSurfer standard processing pipeline (Fischl et al., 2002). The quality of paracellation results for each subject was confirmed by visual inspection with the original T1 images. Two-tail paired t-tests were applied to assess all regional cortical and subcortical volume differences between Pre and Post measurements. We specifically focused on right and left hippocampal volumes, in which prior studies have observed changes associated with mild traumatic brain injury (Zagorchev et al., 2016).
2.5. Resting-state fMRI analysis
2.5.1. Individual subject pre-processing
The “afni_proc.py” routine in AFNI software (Cox, 1996) was used to generate the scripts to preprocess the rs-fMRI data. For each subject, any signal spikes in the signal intensity time courses were first detected and removed. Data points with excess motion were identified (normalized motion derivative > 0.5 or voxel outliers > 10%) and modeled in analysis. The acquisition timing difference was then corrected across slice locations. The functional images were then aligned to T1-weighted high-resolution volumetric images. With the third functional image as the reference, rigid-body motion correction was applied in three translational and three rotational directions. Note that the measures of motion and the motion derivatives were estimated, and these estimations were later modeled in data analysis. For each subject, spatial blurring with a full width half maximum of 4 mm was used to reduce random noise, and also to reduce effects of both inter-subject anatomical and Talairach transformation variation during group analysis.
At each voxel the “3dDeconvolve” routine in AFNI (Cox, 1996) was used to identify and remove contributions due to motion (reference signals described above), baseline fluctuations, and system-induced signal trends (up to 4th order) from the time-series at each voxel. The mean signal time courses in the CSF and the white matter were also modeled and removed with the “3dDeconvolve” routine. Finally, a band-pass filter with a range of 0.009 Hz–0.08 Hz was applied.
The pre-processed time courses obtained above at all brain voxels were then assessed in subsequent correlation analyses.
2.5.2. Subject-level rs-fMRI network analyses
For each subject, the cortical nodes of the 17 networks derived by (Yeo et al., 2011) from rs-fMRI datasets in 1000 healthy young adults, were identified in the native space using FreeSurfer (Fischl et al., 2002). Correlation analyses of rs-fMRI time courses between the nodes of each network were performed using AFNI (Cox, 1996). The mean correlation was calculated for all node-pairs in each network. We were specifically interested in the default-mode network (DMN) A, which is a subnetwork of the DMN as identified by (Yeo et al., 2011). This DMN subnetwork includes nodes at right and left posterior cingulate cortices (PCC), right and left dorsal prefrontal cortices (PFCd), right and left medial prefrontal cortices (PFCm), right and left inferior parietal lobules (IPL), and right temporal gyrus. The functional connectivity to the PCC within the DMN has previously been observed to change subsequent to concussion (Johnson et al., 2012, Zhu et al., 2015). The correlation analysis between the functional connectivity of the DMN A network and the impact history will be explored, as detailed later.
For group statistical analyses, the correlation coefficients were converted to Z values through Fisher's Z-transformation. Two-tail paired t-tests on were then performed, comparing Pre and Post measurements, to assess whether there was a significant change in the connectivity of each network.
2.5.3. Group-level rs-fMRI seed-based whole-brain analysis
The “3dfim +” routine in AFNI (Cox, 1996) was used to correlate the time course in every voxel of the brain against the space-averaged time course from several seed regions. Seed regions were selected based on recent work documenting alterations following concussion in functional connectivity relative to the right and left isthmi of cingulate cortex (ICC), and the right and left hippocampi (Zhang et al., 2012, Zhu et al., 2015). First, correlation coefficients were converted to Z values through Fisher's Z-transformation to improve the normality of the distribution. These Z values were then warped to the Talairach standard space for inter-subject analysis. Comparison analyses between two sessions were performed on the transformed correlations with the seed regions.
For between-session analysis, an ANOVA was performed on the Z values using a mixed-effect two-factor model. The first factor was session, modeled as a fixed effect. The second factor was subject, was modeled as a random effect. Cluster significance was determined on the ANOVA, described above, corrected for multiple comparisons using Monte Carlo simulation. First, the spatial smoothness of the image data was estimated using “3dFWHMx” in AFNI (Cox, 1996). A cluster analysis was then used to estimate the overall statistical significance with respect to the whole brain (e.g., Ward, 2000). Based on the simulations, a cluster's connectivity was considered significant only if it was within an 832 mm3 cluster in which the voxels were contiguous and each had a voxel-based correlation with the seed corresponding to an uncorrected p ≤ 0.005—achieving an effective pCorrected < 0.0478.
2.6. Cerebral blood flow (CBF) measurements
The CBF map derived from the ASL acquisition for each subject was aligned to the T1-weighted high-resolution volumetric images, based on the 12-parameter affine transformation in AFNI (Cox, 1996). The mean CBF value was extracted from each of the 70 cortical regions based on FreeSurfer segmentation (Fischl et al., 2002). The mean cortical CBF, aggregated across the 70 cortical regions, was also calculated. Two-tail paired t-tests contrasting Pre and Post measurements were applied to the mean cortical CBF and each of the 70 cortical regions. For the region-based analysis, a Bonferroni correction was applied, leading to a significance threshold of p ≤ 0.05/70 = 0.00071 (for a corrected p ≤ 0.05).
2.7. Neuronal fiber integrity evaluation with tract-based spatial statistics (TBSS) on DTI metrics
White matter integrity was assessed using Tract-Based Spatial Statistics (TBSS) in FSL (Smith et al., 2004). All FA images were aligned to the ICBM-DTI-81 white-matter atlas developed by Mori et al. (2008). The FA skeleton mask was created with a threshold of > 0.2 on the FA skeleton. This FA skeleton mask was divided to 48 anatomical regions based on the ICBM-DTI-81 white-matter atlas. From the resultant data, computed measures included fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD).
Two-tail paired t-tests comparing Pre and Post measures of FA, MD, RD, and AD were applied to the overall skeleton and the 48 regions. For the regional analyses, a relatively liberal Bonferroni correction was applied with the diffusion metrics treated separately, leading to a significance threshold of p ≤ 0.001 (for a corrected p ≤ 0.05). A more stringent threshold of p ≤ 0.00026 would be necessary to detect overall significant change of diffusion.
2.8. Exploratory vascular integrity analysis using SWI
SWI data were examined for changes between Pre and Post measurements that might indicate development of leaks or damage to the brain vasculature as a consequence of a season's accumulation of exposure to head impacts. Acquired images underwent a standardized pre-processing pipeline, including head motion correction, brain extraction, noise reduction, Gibbs ringing artifact compensation, and MR field inhomogeneity correction. Images were then aligned into a standard reference space (MNI152 template). Resulting volumes were divided into 55 disjoint regions at a gyrus level (Lancaster et al., 2000). Within-subject intensity differences between Pre and Post (specifically Post–Pre) were computed on a voxel basis in each region, for each subject.
SWI intensity change measures were evaluated on a region level within each subject, and also at a subject level. The statistical significance of the change measure for each region in each subject was evaluated using a Wilcoxon Rank Sum test, comparing the distribution of voxel-wise intensity differences (i.e., Post–Pre) within the region for the test subject against the aggregated distribution of voxel-wise intensity differences within the same region in the remaining 17 subjects. Over the corpus of subjects, 990 comparisons were effected, so application of a Bonferroni correction required a statistical significance threshold of p < 0.00005. Having identified significantly changed regions within each subject, a subject-level analysis was conducted by comparing the number of significant regions within each subject to the expected rate of observation of regions under a Bernoulli trial model, in which each region was treated as independent, with the chance observation rate set to p < 0.05. Declaration of an individual subject as statistically significantly changed required observation of eight or more significantly changed regions, corresponding to the 99.9% confidence interval (i.e., the 95% confidence interval corrected for multiple comparisons across 18 subjects; 0.05/18 = 0.00277).
2.9. Relation of neuroimaging changes to impact exposure
On an exploratory basis, the hypothesis that greater impact exposure measures would be correlated with asymptomatic changes detectable using neuroimaging, two analyses were conducted for each obtained neuroimaging measure. First, the magnitude of the change in the neuroimaging measure was correlated with the impact history for the imaged players. Second, impact history metrics were contrasted across groups athletes formed as follows: (a) groups formed based on athletes whose neuroimaging measures exhibited vs. did not exhibit statistically-significant changes, or (b) when no significant changes were observed, groups formed by contrasting athletes in the upper-half of the neuroimaging measure distribution against athletes in the lower-half of the measure distribution. For both analyses, four quantities associated with an athlete's impact history were considered: (1) total exposure ≥ 25G, (2) total exposure ≥ 80G, (3) average per-practice-session exposure for impacts ≥ 25G, or (4) average per-practice-session exposure for impacts ≥ 80G. When contrasting athletes exhibiting high or low neuroimaging measure changes, the impact history measures between the two groups were assessed using an unpaired t-test, and the variance of these measures (a proxy for the distribution of impact histories) was evaluated using an f-test. Comparisons were effected on global and regional measures derived from the CBF and the functional connectivity of the DMN A network, and on the aggregate analysis of regional measures for the SWI acquisition. Note that subject 16 was omitted from these analyses, due to the limited number of practice sessions in which impacts were recorded (see Table 1).
A Bonferroni correction was effected by analysis type (mean or variance) for each tested impact history metric. For global analyses of either mean or variance metrics, this resulted in a significance threshold of p < 0.0125. For regional analyses, the threshold was dependent on the number of evaluated regions—e.g., 4 mean tests over 70 regions in the CBF data require an individual region significance threshold of p < 0.000179, while 4 variance tests over 48 regions in the DTI data require a threshold of p < 0.000261.
3. Results
3.1. Impact history findings
Accelerometer data collected from the football players (and the number of sessions for which useful data were collected) are shown in Table 1. The raw number of hits meeting/exceeding thresholds of 25G and 80G are reported. Four players missed practice due to non-neurological injuries, with two subsequently returning to play. Based loosely of work by McCuen et al. (2015) who proposed that because moderately low G events are sufficiently common, they are unlikely to contribute to cumulative cellular injury in the brain, we based the lower threshold as 25G. Blows above 80G have historically been interpreted to be “concussive” in nature, however past evaluation of accumulations of events in high school athletes (Breedlove et al., 2012, Cummiskey, 2016) suggest that these blows are neither uncommon, nor inherently linked to diagnosis of a concussion. However, under the hypothesis that cellular injury accumulates with each blow, larger blows would be expected to contribute more (on an individual blow basis) than smaller blows, and be more likely to be associated with the crossing of an injury threshold level (Talavage et al., 2016). As such, the use of the 80G threshold level should be selecting for the “most injurious” blows.
Rates of experience of impacts varied by position and by time within the season. On a per-practice-session basis, each player received an average of 4.35 impacts meeting or exceeding 25G, with an average of 0.15 of these impacts meeting or exceeding 80G. For players at speed positions (n = 6), these numbers were 2.93 impacts ≥ 25G and 0.14 impacts ≥ 80G per session, whereas players at non-speed (n = 17) positions averaged 4.84 impacts ≥ 25G and 0.22 impacts ≥ 80G per session.
Of additional interest, 78% of the impacts meeting or exceeding 80G (and 34.2% of all recorded impacts) took place during the 12 practices (22.6% of all possible sessions) taking place prior to the first game of the season.
3.2. Cortical and subcortical volume findings
No significant changes on the cortical and subcortical regional volumes were found, even at a liberal threshold of p < 0.001, taking into consideration of only partial correction of multiple comparison. For the right hippocampus, the volumes were (Pre) 4644 ± 478 mm3 and (Post) 4715 ± 410 mm3, representing a non-significant increase of 1.5%. For the left hippocampus, the volumes were (Pre) 4782 ± 388 mm3 and (Post) 4664 ± 477 mm3, representing a non-significant decrease of 2.47%.
3.3. Resting-state fMRI findings
3.3.1. Subject-level rs-fMRI network analysis
No significant changes were observed when contrasting Pre and Post average connectivity within each of the 17 networks. Similarly, no significant changes were observed when contrasting the Pre and Post within-network connectivity of the DMN.
3.3.2. Group-level rs-fMRI seed-based analysis
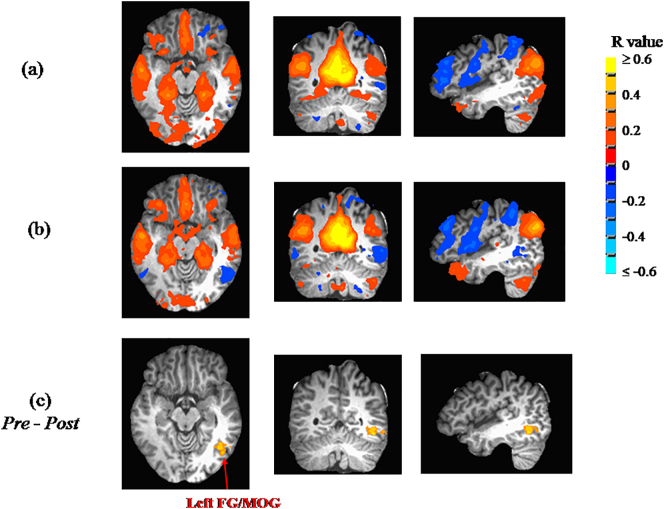
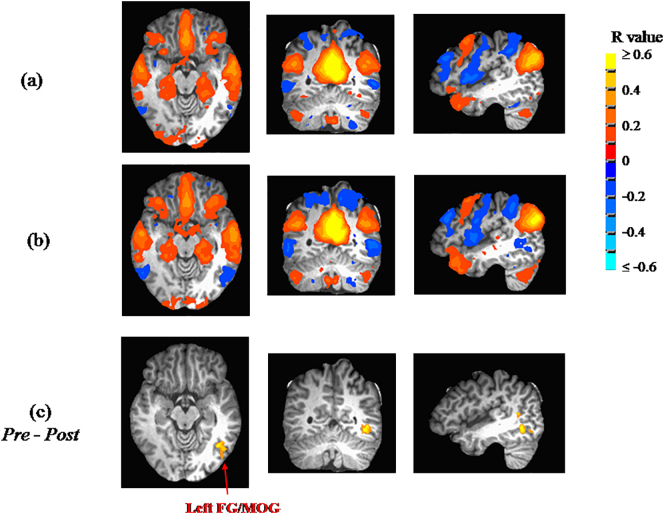
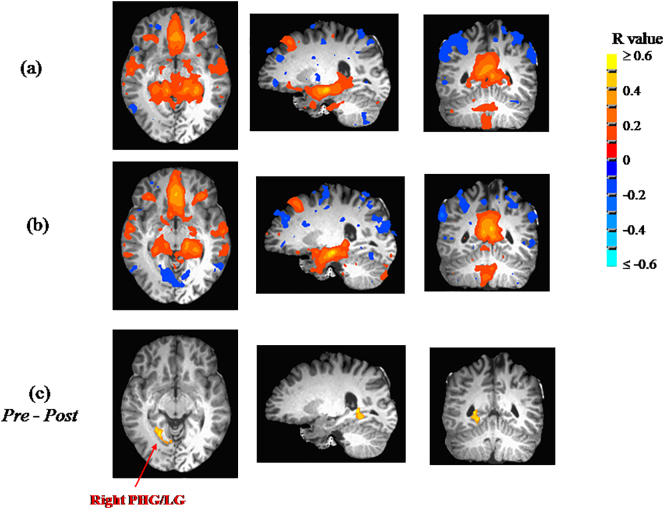
Findings from seed-based whole-brain functional connectivity analyses are summarized in Table 2. Significant changes from Pre to Post in functional connectivity of clusters to seed regions were only observed for three of the four seed regions (right ICC, left ICC, left hippocampus). For right ICC, a statistically-significant change in correlation was observed with the left fusiform gyrus/middle occipital gyrus (left FG/MOG), shifting from a weak positive correlation at the Pre measurement to an anti-correlation at Post (see Fig. 1). For the left ICC, the same structure (i.e., left FG/MOG) exhibited the same statistically-significant change in correlation (from weak positive correlation to anti-correlation; see Fig. 2). For left hippocampus, the same direction of correlation change (from weak positive correlation to anti-correlation) was observed, but now for the right parahippocampal gyurs (right PHG; see Fig. 3). No regions exhibited significant changes in functional correlation to the right hippocampus.
Table 2.
Changes in average correlation between pre-participation (Pre) and post-participation (Post) functional connectivity with selected seed regions.
| Seed region | Significant cluster found | Cluster size (mm3) | Talairach coordinate at cluster centroid | Mean R @ Pre | Mean R @ Post |
|---|---|---|---|---|---|
| Right ICC | Left FG/MOG | 1963 | (L44, P55, I9) | 0.046 | − 0.102 |
| Left ICC | Left FG/MOG | 1666 | (L44, P58, I9) | 0.043 | − 0.108 |
| Left hippocampus | Right PHG/LG | 1554 | (R16, P57, I2) | 0.045 | − 0.111 |
Isthmus of cingulate cortex = ICC, fusiform gyrus = FG, middle occipital gyrus = MOG, PHG = parahippocampal gyrus, LG = lingual gyrus. Talairach coordinate in (R/L, A/P, I/S) format, where R/L = right/left, A/P = anterior/posterior, I/S = inferior/superior.
Fig. 1.
The mean connectivity to the right isthmus of cingulate cortex (ICC) (only showing R ≥ 0.1) are shown at (a) Pre, and (b) Post. (c) Whole-brain ANOVA revealed a significant change (p ≤ 0.048 after whole-brain correction; n = 18) from weak positive correlation prior to participation (Pre) to a post-participation (Post) anti-correlation of the right ICC with the left fusiform gyrus/middle occipital gyrus (FG/MOG).
Fig. 2.
The mean connectivity to the left isthmus of cingulate cortex (ICC) (only showing R ≥ 0.1) are shown at (a) Pre, and (b) Post. (c) Whole-brain ANOVA revealed a significant change (p ≤ 0.048 after whole-brain correction; n = 18) from weak positive correlation prior to participation (Pre) to a post-participation (Post) anti-correlation of the left ICC with the left fusiform gyrus/middle occipital gyrus (FG/MOG).
Fig. 3.
The mean connectivity to the left hippocampus (only showing R ≥ 0.1) are shown at (a) Pre, and (b) Post. (c) Whole-brain ANOVA revealed a significant change (p ≤ 0.048 after whole-brain correction; n = 18) from weak positive correlation prior to participation (Pre) to a post-participation (Post) anti-correlation of the left hippocampus with the right parahippocampal gyrus/lingual gyrus (PHG/LG).
3.4. Cerebral blood flow (CBF) findings
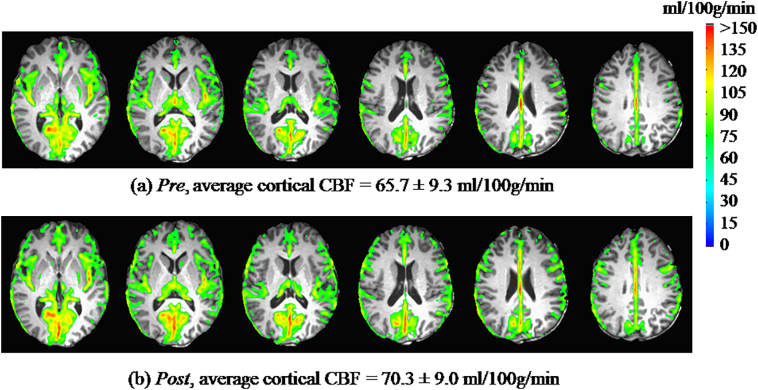
Statistically significant increases in CBF were observed on both a global and regional basis. There was a statistically significant (p = 0.048) global increase of CBF in the cortex from Pre to Post (Fig. 4), shifting from (Pre) 65.7 ± 9.3 ml (blood)/100 g (tissue)/min to (Post) 70.3 ± 9.0 ml (blood)/100 g (tissue)/min. A regionally-specific significant (pBonferroni = 0.0157) increase in CBF was observed for the right postcentral gyrus—Pre: 48.5 ± 10.1 ml (blood)/100 g (tissue)/min; Post: 57.1 ± 9.7 ml (blood)/100 g (tissue)/min.
Fig. 4.
A significant global increase in cerebral blood flow (CBF) was observed when comparing Post to Pre (p = 0.048; n = 18). CBF maps—thresholded for CBF ≥ 70 ml (blood)/100 g (tissue)/min—are shown for (a) Pre, and (b) Post.
3.5. Neuronal fiber integrity evaluation with tract-based spatial statistics (TBSS) on DTI metrics
No significant changes (all p > 0.0097 before correction) were observed when contrasting DTI metrics (FA, MD, AD and RD) at Pre and Post measurements, whether evaluated on the overall FA skeleton or the 48 anatomical regions based on the ICBM-DTI-81 white-matter atlas.
3.6. Exploratory vascular integrity findings
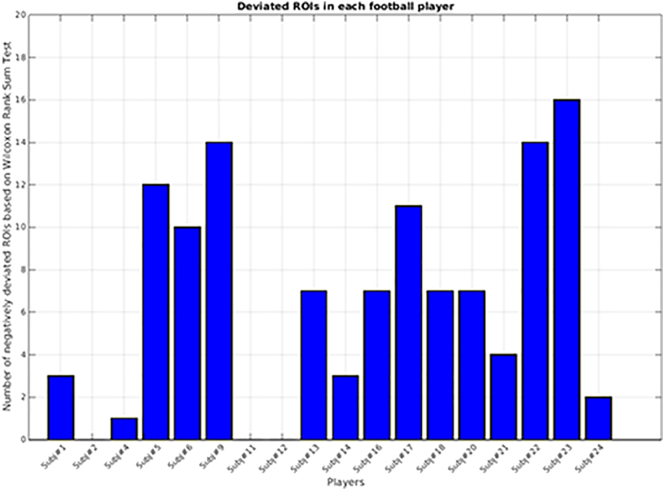
From the Wilcoxon Rank Sum test, 6 of the 18 football players (33%; Fig. 5) exhibited at the post-participation (Post) measurement, relative to the pre-participation (Pre) measurement, outlier (pBonferroni < 0.05) SWI signal decreases (see Fig. 6) in at least 8 (of 55) cortical regions.
Fig. 5.
6 of the 18 athletes for whom SWI data were analyzed exhibited supra-chance numbers of regions (8 of 55 regions; pBonferroni < 0.05) in which the distribution of intensity changes (Post–Pre) for the subject was significantly lower than the distribution of intensity changes in the other 17 subjects (Wilcoxon Rank Sum; pUncorrected < 0.05/990 = 0.00005).
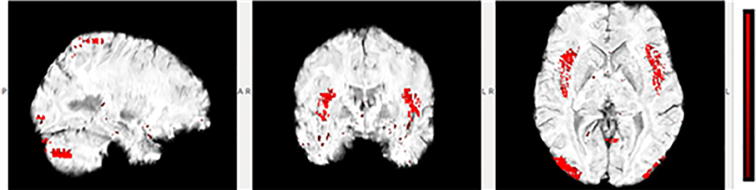
Fig. 6.
Selected slices for Subject 6 (a defensive back) with an overlay highlighting those voxels in which SWI signal decreases were observed at Post, relative to Pre. Highlighted voxels belong to several of the 10 regions in this subject found to be statistically-significantly decreased (see Fig. 5; Wilcoxon Rank Sum test at the pUncorrected < 0.00005 level, corresponding to pBonferroni < 0.05), with the saturation of the color reflecting the change measure in the given voxel.
3.7. Relation of neuroimaging changes to impact exposure
No individual regions exhibited a statistically significant relationship between impact history metrics and measures either of CBF or functional connectivity.
While there were no significant changes observed between the Pre and Post measures of within-network connectivity of the DMN, a statistically-significant (p < 0.002) linkage was observed wherein greater increases in DMN connectivity over the course of the season were found to be associated with athletes who experienced a broader/higher distribution of per-practice-session impacts exceeding ≥ 80G, per the f-test.
On a global basis, the increase observed from Pre to Post in CBF was greater for athletes who experienced a broader/higher distribution of per-practice-session impacts ≥ 80G, per the f-test (p < 0.005).
Athletes who exhibited statistically significant numbers of regions with decreased SWI signal intensity were also found to have experienced a broader/higher distribution of per-practice-session impacts ≥ 80G, per the f-test (p < 0.004).
4. Discussion
Due to the ongoing controversy regarding the effects of participation in high-collision sports, such as American football, on brain functional/structural and vascular integrity, this study contrasted neuroimaging-based measures of brain integrity obtained before and after a single season of NCAA Football Bowl Subdivision competition. Statistically-significant changes were observed (at Post, relative to Pre) in measures derived from multiple MRI modalities: CBF, rs-fMRI, and SWI. Critically, these changes were found to be greater in athletes who were more likely to have an impact history including larger average numbers of high-G impacts (≥ 80G) per monitored practice session. A linkage between exposure measures, particularly a measure previously suspected to be related to concussive injury and neuroimaging, observed changes occurring over the course of a competition season strongly suggests that even asymptomatic athletes are at potential risk of long-lasting changes to brain functional and structural integrity.
4.1. Impact history
The findings that during the preseason practices (a) athletes experienced more impacts on a per-session basis, and (b) were exposed to high-magnitude impacts at a higher-than-average rate, are consistent with multiple observations that most hits are experienced during the beginning of the season (Houck et al., 2016, Kerr et al., 2015).
We also found that an athlete's impact exposure history was markedly dependent on the position played, with non-speed players (offensive and defensive lines) being again found to be at the greatest risk of accumulating large numbers of impacts of all magnitudes (Baugh et al., 2015, Crisco et al., 2010, Talavage et al., 2014).
4.2. Neuroimaging assessment
MRI results derived from contrasting pre- and post-season scans revealed several findings of interest that further support the developing hypothesis that accumulation of subconcussive impacts can affect brain functional and structural integrity. These findings are in contrast to the clinical observation that all of the athletes participating in this study were “normal”—none of the players was diagnosed with a concussion during the season, as documented by medical records. In support of this clinical viewpoint, no change was observed between Pre and Post measurements from measures of gray matter integrity or white matter integrity. Conversely, changes that differ from the athletic training staff's perspective were observed in measurements of whole-brain resting-state functional connectivity, cerebral blood flow, and neurovascular integrity. Each of these measures is evaluated, below.
Gray Matter Integrity (No Change): While there have been reports of statistically-significant changes in gray matter volume (particularly in the area of the hippocampus) in athletes having a history of concussion (Meier et al., 2016) or who have played for an extensive period of time (Koerte et al., 2016), it is not surprising that we did not find changes in regional cortical or subcortical volumes over the brief period of time represented by a single competition season of college football.
4.2.1. White matter integrity (no change)
No changes in DTI metrics were found in the players under study. This is not altogether surprising as there have been conflicting outcomes from multiple previous DTI studies (Hobbs et al., 2016, McAllister et al., 2014, Chun et al., 2015). Further, in a previous combined fMRI/DTI study on a comparable population, no changes in DTI-assessed fractional anisotropy was observed in post-concussed athletes by 30 days post-injury (Zhang et al., 2010). It should be noted, however, that the use of DTI for subjects exposed to cumulative subconcussive impacts is intriguing, because this modality is particularly sensitive to white matter changes and may have the potential to detect diffuse axonal injury that is known to occur in collision sports (Niogi and Mukherjee, 2010, Narayana et al., 2015).
4.2.2. Resting-state-fMRI (change)
Whole-brain functional connectivity analysis revealed significant changes over the course of the competition season in the strength/nature of connections to the right ICC, left ICC, and left hippocampus. In all cases, weak connections (weakly correlated) became more substantially anti-correlated.
Further, while no statistically-significant change between Pre and Post measurements was found in the strength of connections within the DMN, those variations present in the within-network correlations were, in fact, found to be significantly related to the likelihood of exposure of athletes to high-G impacts.
Although the functional connectivity of DMN was found to be altered in previous concussion studies (Slobounov et al., 2011, Johnson et al., 2012, Zhu et al., 2015), we did not observe significant changes within DMN when contrasting Pre and Post findings. This observation is consistent with our previous report that DMN remained intact, despite the multiple high intensity collisions that asymptomatic players experience in a single rugby game (Johnson et al., 2014). Similarly, in the sub-acute phase of concussive injury, connections within DMN were found to remain unaltered whereas the number and strength of functional connections between DMN and other regions in the brain were impaired after concussion (Johnson et al., 2012).
However, the seed-based analyses revealed that some brain regions outside to the key regions of DMN were found to have changed from being weakly correlated regions within the DMN, to being substantially more anti-correlated. While the interpretation of this observation is not straightforward, it is reasonable to interpret this change of connectivity as a sign of recruiting additional neural resources during the post-season period to maintain normal resting brain operations. This hypothesis is consistent with previous findings obtained from longitudinal (within one season) analysis of high school football players by Abbas and colleagues (Abbas et al., 2015a, Abbas et al., 2015b). Their findings included an initial drop in cortical connectivity to the DMN (posterior cingulate cortex seed region) during pre-season practices, followed by a gradual increase during the period of accumulation of blows.
In the high school athletes, connectivity appeared to peak late within, or at the end of, the competition season, with late-season drops in some populations hypothesized to be the result of an increase in practice and game intensity associated with the post-season football tournament. Specifically, the players observing a late-season drop in connectivity had experienced 40% of their impacts over the final 25% of the season. In the present study of collegiate football athletes, there was not a significant late-season increase in the intensity of practices (see Impact History Findings, above) and the post-season measurement was acquired almost immediately after the end of the football season, suggesting that it is likely to be comparable to the later in-season findings in the high school athletes who did not experience a large jump in practice intensity.
4.2.3. Cerebral blood flow (change)
A global increase in CBF was found in the cortex, with the magnitude of this increase greater in those athletes who were more likely to have experienced larger numbers of high-G (≥ 80G) impacts. The increase of global CBF observed after the football season may be an adaptive response of the brain, allowing it better to match CBF with changes in metabolic demand driven by the experience of multiple high-intensity impacts (Velarde et al., 1992).
An increase in metabolic demand would be consistent with prior findings in high school football and soccer athletes wherein decreases in the cerebrovascular response, a possible indicator of elevated resting metabolism was observed following repeated exposure to impacts (Svaldi et al., 2015, Svaldi et al., 2016). It should be noted, however, that in the case of diagnosed concussion injury, it is common for CBF to be reported as decreased (Bartnik-Olson et al., 2014, Maugans et al., 2012, Wang et al., 2015), though these decreases have been observed to be transient, likely fading away within one month of the injury (Meier et al., 2016).
4.2.4. Neurovascular integrity (change)
An arguably generalized decrease in SWI signal intensity was observed for 6 of 18 athletes, with these athletes again demonstrating an impact history suggesting greater exposure to high-G impacts. Given that SWI has previously been demonstrated to be sensitive to the iron in ferritin, hemosiderin, and deoxyhemoglobin (Haacke et al., 2009a, Haacke et al., 2009b), the observation of signal decreases is a marker for likely accumulation of blood outside the vasculature. In the case of vehicular accidents or other hemorrhagic events, the SWI-observed lesions tend to be quite focal and markedly contrast with the tissue around them (e.g., Mittal et al., 2009). Therefore, it is unlikely that large scale vascular damage has occurred in these subjects. Rather, the observed linkage between the impact history and the presence of regions with decreased SWI signal intensity suggests those athletes who experienced a broader range of impacts (i.e., were more likely to experience high-G impacts) have suffered microhemorrhages. It remains to be investigated through longer-term and more longitudinal follow-up if such consequences of exposure to repetitive impacts are persistent or may be repaired by the body.
4.3. Implications and future work
It is increasingly apparent that a link exists between repetitive high intensity impacts in collision-based sports and the neuroimaging detection of short-and long-term consequences, independent of the presence of externally observable symptoms. The neuroimaging-based finding of post-season changes in cerebral blood flow, brain connectivity and the likely presence of microhemorrhages, all of which were found to be associated with greater levels of exposure to repetitive impacts, support our hypothesis that even a single season of NCAA Football Bowl Subdivision participation can induce pathophysiological changes in the brains of clinically asymptomatic athletes reminiscent of those previously documented in concussed athletes. Further, the changes observed in these college athletes are consistent with those previously reported in asymptomatic high school athletes, providing independent support for the argument that symptoms are not a necessary observation for the presence of changes in brain structural/functional or vascular integrity. It yet remains to be explored whether these changes reflect compensatory adaptation to cumulative head impacts over the single football season, more long-lasting or permanent alteration of brain health.
Tracking players at risk for concussive and subconcussive injury throughout their collegiate career and beyond, via longitudinal multi-modal imaging studies, could provide more insight into the long-term consequences of repetitive impacts on physiological changes. The present study has explored a relatively small cohort of collegiate football players; however, findings were consistent with multiple studies in asymptomatic high school athletes, and with studies of concussed collegiate athletes, increasing confidence in their validity. Future studies directed at longitudinal tracking of the development of neuroimaging-detected changes as a function of exposure to impacts, and which incorporate more physiological and possibly genetic features, appear promising as a means to develop prevention strategies for protecting athletes at risk for brain injuries.
The finding of increased resting cerebral blood flow in athletes following a prolonged period of repeated exposure to high magnitude impacts would argue that a relatively static state of increased metabolism has been introduced. This increase in resting CBF is consistent with findings of decreased cerebrovascular reactivity in high school athletes (Svaldi et al., 2015, Svaldi et al., 2016). A decrease in reactivity under hypercapnic conditions could most simply arise from a decreased available dynamic range for blood flow. The finding of increased resting CBF is expected to lead to a reduction in the dynamic range available under fMRI, and would thus be expected to produce smaller BOLD fluctuations. Reduced BOLD fluctuations would then be expected to produce a decrease in the spatial extent of the observed DMN connectivity (assuming the measurement noise floor remains relatively constant across imaging sessions)—an observation previously reported in a variety of non-concussed athletes (Johnson et al., 2014, Abbas et al., 2015c). While a decrease in DMN connectivity was not observed in this work, increases the strength of (anti-)correlation with the DMN were observed for multiple inferior regions of the brain, a finding that is supported by preliminary findings in high school athletes that asymptomatic individuals having previously been diagnosed with a concussion exhibited a greater strength of correlation than did athletes without a history of prior concussion (Abbas et al., 2015c). Overall, this ensemble of findings argues that changes observed via neuroimaging in athletes exposed to repeated subconcussive impacts are most likely to originate at the level of the neurovascular coupling, suggesting either physical disruption at the capillary level (or some level below the resolution of the imaging methodology) or disruption of the signaling networks by which cerebral blood flow is regulated.
5. Conclusion
In a study of clinically asymptomatic collegiate football athletes, statistically-significant MRI changes were observed that are likely a consequence of participation for one season at the NCAA Football Bowl Subdivision level. Specifically, these changes (at Post, relative to Pre) were found in measures derived from multiple MRI modalities: CBF, rs-fMRI, and SWI. Critically, these changes were greater in athletes who were more likely to have an impact history including larger average numbers of high-G impacts (≥ 80G). A linkage between high intensity impacts and neuroimaging-observed changes adds to the growing literature in support of the hypothesis that collision-sport athletes may be at increased risk of long-lasting changes to brain functional and structural integrity. Future work in larger cohorts and involving a broader array of integrated biomarkers will enable more precise identification of athletes who are at risk, and will facilitate development of intervention strategies to permit collision-sport participation with reduced risk.
Acknowledgments
Funding: This project was entirely internally funded by Dr. Slobounov's lab. We would like to express special thanks to Dr. Josef Pfeuffer, Siemens Healthcare, Erlangen for providing us with the advanced 3D ASL WIP package. We would like to thank Penn State football players for their time and efforts participating in this study. We also would like to thank Katie Finelli for recruitment and logistics and Madeleine Scaramuzzo for collecting the accelerometer data.
Contributor Information
Semyon M. Slobounov, Email: sms18@psu.edu.
Alexa Walter, Email: aow5128@psu.edu.
Hans C. Breiter, Email: h-breiter@northwestern.edu.
David C. Zhu, Email: David.Zhu@radiology.msu.edu.
Xiaoxiao Bai, Email: xxb4@psu.edu.
Tim Bream, Email: htb2@psu.edu.
Peter Seidenberg, Email: pseidenberg@hmc.psu.edu.
Xianglun Mao, Email: mao48@purdue.edu.
Brian Johnson, Email: bdj5039@psu.edu.
Thomas M. Talavage, Email: tmt@purdue.edu.
References
- Abbas K., Goni J., Talavage T. 2015. History of Concussion Reduces Brain Resting State Network Efficiency. (Biomedical Engineering Society 2015 Annual Meeting). Tampa, FL, October. (#P-Fr-609) [Google Scholar]
- Abbas K., Shenk T.E., Poole V.N., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M., Robinson M.E. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting state functional magnetic resonance imaging study. Brain Connect. 2015;5:91–101. doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- Abbas K., Shenk T.E., Poole V.N., Robinson M.E., Leverenz L.J., Nauman E.A., Talavage T.M. Effects of repetitive sub-concussive brain injury on the functional connectivity of default mode network in high school football athletes. Dev. Neuropsychol. 2015;40(1):51–56. doi: 10.1080/87565641.2014.990455. [DOI] [PubMed] [Google Scholar]
- Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 2013;119:1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- Bartnik-Olson B.L., Holshouser B., Wang H., Grube M., Tong K., Wong V., Ashwal S. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J. Neurotrauma. 2014;31:1497–1506. doi: 10.1089/neu.2013.3213. [DOI] [PubMed] [Google Scholar]
- Bauer J.A., Thomas T.S., Cauraugh J.H., Kaminski T.W., Hass C.J. Impact forces and neck muscle activity in heading by collegiate female soccer players. J. Sports Sci. 2001;19:171–179. doi: 10.1080/026404101750095312. [DOI] [PubMed] [Google Scholar]
- Baugh C.M., Kiernan P.T., Kroshus E., Daneshvar D.H., Montenigro P.H., McKee A.C., Stern R.A. Frequency of head-impact-related outcomes by position in NCAA division I collegiate football players. J. Neurotrauma. 2015;32(5):314–326. doi: 10.1089/neu.2014.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., Stern R.A. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Zhu T., Zhong J., Janigro D., Rozen E., Roberts A., Javien H., Merchant-Borna K., Abar B., Blackman E.G. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove E., Robinson M., Talavage T., Morigaki K., Yoruk U., O'Keefe K., King J., Leverenz L.J., Gilger J.W., Nauman E.A. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J. Biomech. 2012;45(7):1265–1272. doi: 10.1016/j.jbiomech.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Breedlove K., Breedlove E., Robinson M., Poole V., King J., Rosenberger P., Rasmussen M., Talavage T.M., Leverenz L.J., Nauman E.A. Detecting neurocognitive & neurophysiological changes as a result of subconcussive blows in high school football athletes. Athletic Training and Sports Health Care. 2014;6:119–127. [Google Scholar]
- Broglio S.P., Eckner J.T., Paulson H.L., Kutcher J.S. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 2012;40:138–144. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio S.P., Schnebel B., Sosnoff J.J., Shin S., Feng X., He X., Zimmerman J. The biomechanical properties of concussions in high school football. Med. Sci. Sports Exerc. 2010;42(11):2064–2071. doi: 10.1249/MSS.0b013e3181dd9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun I.Y., Mao X., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M. DTI detection of longitudinal WM abnormalities due to accumulated head impacts. Dev. Neuropsychol. 2015;40(2):85–91. doi: 10.1080/87565641.2015.1020945. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crisco J.J., Fiore R., Beckwith J.G., Chu J.J., Brolinson P.G., Brolinson P.G., Duma S., McAllister T.W., Duhaime A., Greenwal R.M. Frequency and location of head impact exposures in individual collegiate football players. J. Athl. Train. 2010;45:549–559. doi: 10.4085/1062-6050-45.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummiskey B. Purdue University; West Lafayette, Ind, USA: 2016. Characterization and Evaluation of Head Impact Sensors and Varsity Football Helmets [M.S. thesis] p. 2015. [Google Scholar]
- Davenport E.M., Whitlow C.T., Urban J.E., Espeland M.A., Jung Y., Rosenbaum D.A., Gioia G., Powers A.K., Stitzel J.D., Maldjian J.A. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma. 2014;31:1617–1624. doi: 10.1089/neu.2013.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Giza C.G., Kutcher J.S. An introduction to sports concussions. Continuum (Minneap Minn) 2014;20(6):1545–1551. doi: 10.1212/01.CON.0000458975.78766.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M., Weaver N.L., Padua D.A., Garrett W.E. Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 2000;28:643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- Haacke E.M., Makki M., Ge Y., Maheshwari M., Sehgal V., Hu J., Selvan M., Wu Z., Latif Z., Xuan Y., Khan O., Garbern J., Grossman R.I. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J. Magn. Reson. Imaging. 2009;29(3):537–544. doi: 10.1002/jmri.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke E.M., Mittal S., Wu Z., Neelavalli J., Cheng Y.C. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR. 2009;30(1):19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J., Young I., Bailes J. Sports-related concussions: diagnosis, complications, and current management strategies. Neurosurg. Focus. 2016;40(4) doi: 10.3171/2016.1.FOCUS15617. [DOI] [PubMed] [Google Scholar]
- Houck Z., Asken B., Bauer R., Pothast J., Michaudet C., Clugston J. Epidemiology of sport-related concussion in an NCAA division 1 football bowl subdivision sample. AJSM. 2016;44(9):2269–2275. doi: 10.1177/0363546516645070. [DOI] [PubMed] [Google Scholar]
- Johnson B., Neuberger T., Gay M., Hallett M., Slobounov S. Effects of subconcussive head trauma on the default mode network of the brain. J. Neurotrauma. 2014;31:1907.3. doi: 10.1089/neu.2014.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Zhang K., Gay M., Horovitz S., Hallett M., Sebastianelli W., Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage. 2012;59(1):511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr Z.Y., Hayden R., Dompier T.P., Cohen R. Association of equipment worn and concussion injury rates in National Collegiate Athletic Association football practices: 2004–2005 to 2008–2009 academic years. Am. J. Sports Med. 2015;43(5):1134–1141. doi: 10.1177/0363546515570622. [DOI] [PubMed] [Google Scholar]
- Koerte I.K., Hufschmidt J., Muehlmann M., Tripodis Y., Stamm J.M., Pasternak O., Giwerc M.Y., Coleman M.J., Baugh C.M., Fritts N.G., Heinen F., Lin A., Stern R.A., Shenton M.E. Cavum Septi Pellucidi in symptomatic former professional football players. J. Neurotrauma. 2016;33(4):346–353. doi: 10.1089/neu.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Freitas C.S., Rainey L., Kochunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman E.J. Epidemiology of neurodegeneration in American-style professional football players. Alzheimers Res. Ther. 2013;5(34):1–8. doi: 10.1186/alzrt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi L., Saatman K.E., Fujimoto S., Raghupathi R., Meaney D.F., McMillian B.S.A., Conte V., Laurer H.L., Stein S., Stocchetti N., McIntosh T.K. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56(2):364–374. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- Maugans T.A., Farley C., Altaye M., Leach J., Cecil K.M. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129:28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Ling J.M., Dodd A.B., Gasparovic C., Klimaj S.D., Meier T.B. A longitudinal assessment of structural and chemical alterations in mixed martial arts fighters. J. Neurotrauma. 2015;32:1759–1767. doi: 10.1089/neu.2014.3833. [DOI] [PubMed] [Google Scholar]
- McAllister T.W., Ford J.C., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Bolander R.P., Tosteson T.D., Turco J.H., Raman R., Jain S. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82(1):63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuen E., Svaldi D., Breedlove K., Kraz N., Cummiskey B., Breedlove E.L., Traver J., Desmond K.F., Hannemann R.E., Zanath E., Guerra A., Leverenz L., Talavage T.M., Nauman E.A. Collegiate womens soccer palyers suffer greate cumulative head impacts than their high school counterparts. J. Biomech. 2015;48(13):3720–3723. doi: 10.1016/j.jbiomech.2015.08.003. [DOI] [PubMed] [Google Scholar]
- McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T.B., Bellgowan P.S., Bergamino M., Ling J.M., Mayer A.R. Thinner cortex in collegiate football players with, but not without, a self-reported history of concussion. J. Neurotrauma. 2016;33(4):330–338. doi: 10.1089/neu.2015.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant-Borna K., Asselin P., Narayan D., Abar B., Jones C.M., Bazarian J.J. Novel method of weighting cumulative helmet impacts improves correlation with brain white matter changes after one football season of sub-concussive head blows. Ann. Biomed. Eng. 2016;44(12):3679–3692. doi: 10.1007/s10439-016-1680-9. [DOI] [PubMed] [Google Scholar]
- Mittal S., Wu Z., Neelavalli J., Haacke E.M. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am. J. Neuroradiol. 2009;30(2):232–252. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenigro P.H., Alosco M.L., Martin B.M., Daneshvar D.H., Mez J., Chaisson C.E., Nowinski C.J., Au R., McKee A.C., Cantu R.C., McClean M.D., Stern R.A., Tripodis Y. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J. Neurotrauma. 2016;34(2):328–340. doi: 10.1089/neu.2016.4413. (2016 Mar 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Oishi K. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana P.A., Yu X., Hasan K.M., Wilde E.A., Levin H.S., Hunter J.V., Miller E.R., Patel V.K., Robertson C.S., McCarthy J.J. Multi-modal MRI of mild traumatic brain injury. Neuroimage Clin. 2015;7:87–97. doi: 10.1016/j.nicl.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauman E.A., Breedlove K.M., Breedlove E.L., Talavage T.M., Robinson M.E., Leverenz L.J. Post-season neurophysiological deficits assessed by ImPACT and fMRI in athletes competing in American football. Dev. Neuropsychol. 2015;40(2):85–91. doi: 10.1080/87565641.2015.1016161. [DOI] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Obler L.K., Rykhlevskai E., Schnyer D., Clark-Colton M.R., Spiro A., Hyun J., Kim D., Goral M., Albert M.L. Bilateral brain regions associated with naming in older adults. Brain Lang. 2010;113:113–123. doi: 10.1016/j.bandl.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu B.I., DeKosky S.T., Minster R.L., Kamboh M.I., Hamilton R.L., Wecht C.H. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(128–134):1524–4040. doi: 10.1227/01.neu.0000163407.92769.ed. (Electronic) [DOI] [PubMed] [Google Scholar]
- Pellman E.J., Powell J.W., Viano D.C., Casson I.R., Tucker A.M., Feuer H., Lovell M., Waeckerle J.F., Robertson D.W. Concussion in professional football: epidemiological features of game injuries and review of the literature – part 3. Neurosurgery. 2004;54:81–96. doi: 10.1227/01.neu.0000097267.54786.54. [DOI] [PubMed] [Google Scholar]
- Poole V.N., Abbas K., Shenk T.E., Breedlove E.L., Breedlove K.M., Robinson M.E., Leverenz L.J., Nauman E.A., Talavage T.M., Dydak U. MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Dev. Neuropsychol. 2014;39(6):459–473. doi: 10.1080/87565641.2014.940619. [DOI] [PubMed] [Google Scholar]
- Poole V.N., Breedlove E.L., Shenk T.E., Abbas K., Robinson M.E., Leverenz L.J., Nauman E.A., Dydak U., Talavage T.M. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev. Neuropsychol. 2015;40(1):12–17. doi: 10.1080/87565641.2014.984810. [DOI] [PubMed] [Google Scholar]
- Prins M.L., Alexander D., Giza C.C., Hovda D.A. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma. 2013;30(30–38):1557–9042. doi: 10.1089/neu.2012.2399. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B.L., Guskiewicz K.M. Effects of mild head injury on postural stability as measured through clinical balance testing. J. Athl. Train. 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- Robinson M.E., Shenk T.E., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M. The role of location of subconcussive head impacts in fMRI brain activation change. Dev. Neuropsychol. 2015;40(2):74–79. doi: 10.1080/87565641.2015.1012204. [DOI] [PubMed] [Google Scholar]
- Shuttleworth-Edwards A.B., Smith I., Radloff S.E. Neurocognitive vulnerability amongst University Rigby players versus noncontact sport controls. J. Clin. Exp. Neuropsychol. 2008;30(8):870–884. doi: 10.1080/13803390701846914. [DOI] [PubMed] [Google Scholar]
- Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D., Bellgowan P.S. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311:1883–1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- Slobounov S., Johnson B., Zhang K., Hallett M., Horovitz S., Sebastianelli W., Gay M. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage. 2011;55(4):1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S., Zhang K., Pennell D., Ray W., Johnson B., Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp. Brain Res. 2010;202(2):341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Svaldi D.O., Joshi C., Robinson M.E., Shenk T.E., Abbas K., Nauman E.A., Leverenz L.J., Talavage T.M. Cerebrovascular reactivity alterations in asymptomatic high school football players. Dev. Neuropsychol. 2015;40(2):80–84. doi: 10.1080/87565641.2014.973959. [DOI] [PubMed] [Google Scholar]
- Svaldi D.O., McCuen E.C., Joshi C., Robinson M.E., Nho Y., Hannemann R., Nauman E.A., Leverenz L.J., Talavage T.M. Cerebrovascular reactivity changes in asymptomatic female athletes attributed to high school soccer participation. Brain Imaging Behav. 2016:1–15. doi: 10.1007/s11682-016-9509-6. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Talavage T.M., Nauman E., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K., Feuer H., Leverenz L.J. Functionally detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma. 2014;31:327–338. doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage T.M., Nauman E.A., Leverenz L.J. The role of medical imaging in the recharacterization of mild traumatic brain injury using youth sports as a laboratory. Front. Neurol. 2016;6:273. doi: 10.3389/fneur.2015.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde F., Fisher D.T., Hovda D.A. Fluid percussion injury induces prolonged changes in cerebral blood flow. J. Neurotrauma. 1992;9(402) [Google Scholar]
- Wang Y., Nelson L.D., LaRoche A.A., Pfaller A.Y., Nencka A.S., Koch K.M., McCrea M.A. Cerebral blood flow alterations in acute sport-related concussion. J. Neurotrauma. 2015;33(13):1227–1236. doi: 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. 2000. Simultaneous Inference for fMRI Data. (Milwaukee) [Google Scholar]
- Yeo B.T., Krienen F.M. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorchev L., Meyer C. Differences in regional brain volumes two months and one year after mild traumatic brain injury. J. Neurotrauma. 2016;33(1):29–34. doi: 10.1089/neu.2014.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Johnson B., Penell D., Ray W., Sebastianelli W., Slobounov S. Are functional deficits in concussed individual's parallel structural alterations in white matter: combined fMRI and DTI study. Exp. Brain Res. 2010;4(1):57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Johnson B., Gay M., Horovitz S.G., Hallett M., Sebastianelli W., Slobounov S. Default mode network in concussed individuals in response to the YMCA physical stress test. J. Neurotrauma. 2012;29(5):756–765. doi: 10.1089/neu.2011.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.C., Covassin T., Nogle S., Doyle S., Russell D., Pearson R.L., Monroe J., Liszewski C.M., DeMarco J.K., Kaufman D.I. A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measure with longitudinal resting-state fMRI over thirty days. J. Neurotrauma. 2015;32(5):327–341. doi: 10.1089/neu.2014.3413. [DOI] [PubMed] [Google Scholar]