Abstract
Millions of people are treated with anthelmintics to control soil-transmitted helminth infections; yet, drug distribution in the plasma and gastrointestinal tract compartments and the pathway of drug uptake into gastrointestinal nematodes responsible for the pharmacological effect are unknown. We assessed the distribution and uptake of albendazole, albendazole sulfoxide, albendazole sulfone in the hookworm Heligmosomoides polygyrus in vitro and in vivo as well as the distribution and uptake of albendazole, mebendazole, and oxantel pamoate in the whipworm Trichuris muris in vitro and in vivo. Oral and intraperitoneal treatments (100 mg/kg) were studied. Drug quantities in helminths and host compartments (stomach, the contents and mucosa of the small and large intestine, and the plasma) were determined using HPLC-UV/vis and anthelmintic activities were recorded using phenotypic readout. The influence of 1-aminobenzotriazole (ABT), an irreversible and unspecific cytochrome P450 inhibitor, on albendazole disposition in mice harboring H. polygyrus was evaluated. In vivo, albendazole was found in quantities up to 10 nmol per ten H. polygyrus and up to 31 nmol per ten T. muris. ABT did not change the levels of albendazole or its metabolites in the plasma of mice harboring H. polygyrus or in H. polygyrus, whereas drug levels in the gastrointestinal tract of host mice doubled. Mebendazole and oxantel pamoate quantities per ten T. muris were as high as 21 nmol and 34 nmol, respectively. Albendazole revealed a very dynamic distribution and high rate of metabolism, hence, H. polygyrus and T. muris are exposed to albendazole and both metabolites via multiple pathways. Diffusion through the cuticle seems to be the crucial pathway of oxantel pamoate uptake into T. muris, and likely also for mebendazole. No relationship between concentrations measured in helminths and concentrations in plasma, intestinal content and mucosa of mice, or drug efficacy was noted for any of the drugs studied.
Keywords: Albendazole, Mebendazole, Oxantel pamoate, Soil-transmitted helminths, Drug entry, Drug uptake
Graphical abstract

Highlights
-
•
No correlation was found between drug amounts in helminths versus plasma or gastric content.
-
•
Metabolism and disposition of albendazole in H. polygyrus was not influenced by a CYP-inhibitor.
-
•
Oxantel pamoate enters T. muris likely from the gastrointestinal tract.
-
•
Albendazole revealed a dynamic distribution and high rate of metabolism regardless of mode of administration.
1. Introduction
Soil-transmitted helminth infections, including Trichuris trichiura, Ascaris lumbricoides, and the hookworms (Necator americanus and Ancylostoma ceylanicum) are a major human health burden in rural, poverty driven settings in the tropics and subtropics (Hotez et al., 2008). The drugs of choice to treat soil-transmitted helminth infections are albendazole and mebendazole, using a single oral dose in the framework of preventive chemotherapy programs (World Health Organisation, 2006). Albendazole shows good efficacy against hookworm infections (cure rates of 72%) when used at a single oral dosage. However, both albendazole and mebendazole show only low to moderate efficacy against trichuriasis (cure rates below 36%) (Keiser and Utzinger, 2008).
Oxantel pamoate was recently shown to be a useful addition to the current drug armamentarium given its high trichuricidal activity (Speich et al., 2015, Keiser et al., 2013).
After oral administration, albendazole is absorbed in low amounts (about 20–30% in mice and rats) in the small intestine, and then quickly metabolized to albendazole sulfoxide (an active metabolite) followed by further metabolism to albendazole sulfone (an inactive metabolite). Therefore, in plasma and urine the parent compound can only be found in trace amounts. The main enzymes responsible for these oxidations are the flavin-containing monooxygenase, cytochrome P450 (CYP) 3A4 and to a smaller extent CYP1A2 (Dayan, 2003, Rawden et al., 2000). In sheep and rats, albendazole sulfoxide is transported across the small intestinal wall from the blood stream into the small intestine, likely due to an active efflux (Merino et al., 2003). The bioavailability of mebendazole is also low and it is extensively metabolized in the liver to inactive molecules (Dayan, 2003). Reduction of mebendazole by the carbonyl reductase was suggested to be the main metabolic pathway (Nishimuta et al., 2013). With regard to oxantel pamoate, very little information is available. In a recent study in rats, we could not quantify any oxantel pamoate in the plasma of rats following oral administration of 100 mg/kg oxantel pamoate per bodyweight (Cowan et al., 2016).
From a pharmacological point of view, gastrointestinal nematodes are interesting target organisms. H. polygyrus is a lumen-dwelling nematode in the anterior duodenum of the mouse host, likely feeding on the epithelial cells of the host rather than the host’s ingesta or blood directly (Bansemir and Sukhdeo, 1994). T. muris' anterior part is anchored in the epithelial cells of the intestinal wall, feeding on the host's mucosa, the largest portion of their body lays freely in the intestinal tract (Tilney et al., 2005). Anthelmintics can therefore hypothetically enter the worms via the epithelial cells, thus from the blood stream via the cytoplasm, or from the gastrointestinal tract via cuticular penetration. It is currently not known how anthelmintics reach their target. It has been suggested that drugs enter intestinal nematodes via the cuticle (Alvarez et al., 2007). This was for example observed for the standard anthelmintic ivermectin in lambs infected with Haemonchus contortus residing in the abomasum, but feeding on blood. In more detail, lambs treated with ivermectin intraruminally showed higher ivermectin levels in H. contortus than lambs treated subcutaneously that had higher plasma levels (Lloberas et al., 2012). Also fenbendazole enters Trichuris suis in host pigs via the transcuticular pathway. On the other hand, fenbendazoles major metabolites, oxfendazole and fenbendazole sulfone, were suggested to enter via the blood-enterocyte interface. The latter assumption was made because the concentration of the metabolites found in the worms correlated best with plasma concentrations, but less with their concentrations of the parent compound observed in the plasma or the gastrointestinal tract (Hansen et al., 2014).
The goal of this study was to investigate the pathway of drug uptake into two yet uninvestigated gastrointestinal nematodes. Our findings might help to clarify whether the differences in albendazole's efficacy against hookworm and Trichuris correlate with the extent of drug uptake. We studied the mode of drug entry of albendazole and its metabolites albendazole sulfoxide and albendazole sulfone into the hookworm Heligmosomoides polygyrus (also referred to as H. bakeri), and albendazole, mebendazole, and oxantel pamoate into the whipworm T. muris in vitro and in vivo. For that purpose we studied the influence of drug concentration and time of drug exposure on drug accumulation in the parasites both in vitro and in vivo. For in vivo studies, we additionally measured drug concentrations in compartments that might influence drug uptake into the helminths, i.e. plasma, and contents and mucosa of the gastrointestinal tract. Complementary in vitro and in vivo tests on H. polygyrus were performed with 1-aminobenzotriazole (ABT), an unselective and irreversible CYP-inhibitor, to assess the involvement of CYPs in albendazole's metabolism in host and parasite.
Finally, we assessed drug activities of albendazole sulfoxide as well as albendazole plus ABT in vitro and in vivo to clarify the role of the active albendazole metabolite and CYP inhibition.
A better understanding about the pharmacokinetics in the host-parasite system will improve the knowledge of existing treatments and shed light on characteristics needed for new drugs.
2. Materials and methods
2.1. Animals and parasites
All animal experiments were authorized by the Canton Basel-Stadt (license number 2070). Four-week-old female NMRI mice were ordered from Charles Rivers (Sulzfeld, Germany). After a one-week adjustment period to the animal facilities, the NMRI mice were infected via oral gavage of 200 or 80 H. polygyrus L3 stage larvae for in vitro or in vivo experiments, respectively. Four-week-old female C57BL/10 mice were purchased from Harlan (Blackthorn, UK). The mice received dexamethasone (Sigma-Aldrich, Buchs, Switzerland) in the drinking water (1 mg/l) to immunosuppress the mice and support worm establishment until one week before treatment. After one week of adjustment, the mice were infected with 200 T. muris eggs. All mice were kept in animal facilities at 22 °C, 50% humidity, with a 12-h light/dark cycle, and water and rodent food (KLIBA NAFAG, Switzerland) ad libitum.
2.2. Drugs and solvents
Albendazole, albendazole sulfoxide, mebendazole, 4-azabenzimidazole, and ABT were purchased from Sigma-Aldrich. Albendazole sulfone was purchased from Witag (Germany) and oxantel pamoate from Megafine Pharma (P) Ltd. (India). Methanol (Sigma-Aldrich) and acetonitrile (Biosolve, Netherlands) were of HPLC-grade. Ammonium formate and formic acid (both Sigma-Aldrich) were used to prepare buffer (25 mM ammonium formate, pH 4.0) for the HPLC measurements.
2.3. In vitro studies
2.3.1. Drug exposure of H. polygyrus for drug entry experiments
Adult H. polygyrus were harvested from infected mice by dissection, from ten days post-infection onwards. RPMI 1640 culture medium (Sigma-Aldrich) was supplemented with 500 U/ml penicillin, 500 μg/ml streptomycin (LuBioScience, Switzerland), and 2.5 μg/ml amphotericin B (Sigma-Aldrich). The worms were maintained at 37 °C and 5% CO2. Albendazole, albendazole sulfoxide and albendazole sulfone were prepared to 10 mM stock solutions in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and diluted in culture medium to 5, 10, 25, 50, 75, and 100 μM to a final volume of 2 ml. Ten worms per well were added and the assay was incubated for 24 h. Furthermore, H. polygyrus were exposed to 50 μM albendazole for 1, 2, 4, 8, 16, and 24 h. The effect of ABT on H. polygyrus' metabolism of the drugs was assessed by incubating worms at 50 μM ABT for 16 h and then adding 50 μM albendazole for an additional incubation period of 8 h. All experiments were performed in duplicates and repeated once.
2.3.2. Drug activity of albendazole, albendazole sulfoxide and albendazole sulfone
For the determination of the activity of albendazole, albendazole sulfoxide and albendazole sulfone, four H. polygyrus per drug were incubated in 2 ml at 200 μM for 72 h. The drug effect was determined using a phenotypic readout, evaluating parasite motility, transparency, and morphology on a scale between three (normal phenotype) and zero (highly impaired phenotype; dead parasite) (Tritten et al., 2012). The phenotypes of parasites exposed to drugs were compared to control worms that had been incubated in DMSO of equivalent volume as the drug-containing wells. The experiments were performed in duplicate and repeated once.
2.3.3. Drug exposure of T. muris to albendazole, mebendazole and oxantel pamoate
Adult T. muris were harvested from infected mice by dissection, 40 days post-infection onwards. The culture medium consisted of RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B. The worms were kept at 37 °C and 5% CO2. T. muris were exposed to 5, 10, 25, 50, 75, and 100 μM albendazole, mebendazole and oxantel pamoate and evaluated 24 h later. In addition, worms incubated in 2 ml of 25 μM oxantel pamoate were evaluated 1, 2, 4, 8, 16, and 24 h post-incubation. The assays were done in duplicate and repeated on a different day.
2.3.4. Washing the helminths after drug exposure
To remove drug from the helminth cuticle after in vitro drug incubations, the worms were briefly dried with a paper towel, washed briefly (<30 s) in 35 ml 0.9% sodium chloride (NaCl) in water solution, dried again with a paper towel and placed into a 1.5 ml conical Eppendorf tube. To confirm completeness of the cleaning process, samples of the NaCl solution after washing the worms (three samples of each treatment and concentration) were collected for measurements. In addition, the medium in which the helminths had been incubated was collected and filtered. The collected media samples and washing solutions were stored at −20 °C until usage. Worm samples were frozen with liquid nitrogen to weaken the cuticle and then stored at −20 °C until usage.
2.4. In vivo studies
2.4.1. Treatment of infected mice for drug distribution and activity studies
Albendazole, albendazole sulfoxide, and ABT were suspended in 7% Tween 80, 3% ethanol, and 90% water (v/v/v). H. polygyrus-infected mice were treated orally with 100 mg/kg albendazole (n = 16), 100 mg/kg albendazole with a pre-treatment of 50 mg/kg ABT (applied 16 h in advance (Boily et al., 2015)) (n = 20), or with 100 mg/kg albendazole sulfoxide (n = 20). The same treatments were also applied into the peritoneal cavity to groups of four mice. The control group consisted of four mice that remained untreated.
Mice harboring the T. muris infection were treated by oral gavage with 100 mg/kg albendazole, 100 mg/kg mebendazole, or 100 mg/kg oxantel pamoate to groups of twelve mice per treatment arm. These treatments were also applied intraperitoneally to groups of three mice at 100 mg/kg for albendazole, 100 mg/kg mebendazole, or 50 mg/kg oxantel pamoate. Three mice remained untreated to serve as control group.
2.4.2. Sampling of the compartments and helminths
H. polygyrus- and T. muris-infected and treated mice were euthanized 2, 5, or 8 h post-treatment, in groups of four or three, respectively, at each time point. These time points were chosen due to an estimated passage time through the gastrointestinal tract in mice of 6–8 h (Padmanabhan et al., 2013). The groups that had received a peritoneal application were euthanized 2 h post-treatment. The blood was collected via heart puncture and the plasma was obtained using heparinized tubes (Sarstedt, Switzerland) and centrifugation (10 min at 16’000 g). The stomach, small intestine and large intestine (cecum till rectum) were cut open longitudinally and the small intestine was cut into 6 cm long sections. The intestines of mice that had received drug via intraperitoneal application were carefully dried with a paper towel, washed in 300 ml NaCl solution (while clamping both ends of the gastrointestinal tract and holding above water), and dried again prior to the sectioning and sampling. Samples of ten H. polygyrus or T. muris were removed from the small or large intestine, respectively, and washed two times in 35 ml of fresh NaCl solution in 50-ml tubes. The worms were dried on a paper towel and placed into a 1.5 ml conical Eppendorf tube. The entire content of each gastrointestinal unit (stomach, small intestine, and large intestine) was individually collected. The walls of the small and large intestine were dabbed with a paper towel to remove remaining debris and were washed in 50-ml tubes filled with 35 ml of NaCl solution. The washed gastrointestinal wall pieces were dried with a paper towel to remove excess water, then, the mucosa was scraped off using a scalpel. The samples were stored at −20 °C until usage.
2.4.3. Drug activity of albendazole sulfoxide and albendazole after ABT pre-treatment against H. polygyrus
Six days after the treatment of H. polygyrus-infected mice, mice (n = 4 per treatment group) were euthanized. The worms were counted and the count was compared to the worm burden of four untreated control mice to determine the worm burden reduction (WBR) (Tritten et al., 2012).
2.5. Determination of drug concentrations in parasites and mice
2.5.1. Processing of helminth samples
In order to weaken the integrity of the cuticle, the worms were thawed and re-frozen with liquid nitrogen for 2 min. Fifty μl water and 4-azabenzimidazole in methanol (serving as internal standard at 75 ng/75 μl in the sample for injection) were added to the thawed samples and the worms were ground using a plastic pistil fitting the 1.5 ml conical tubes (HuberLab AG, Switzerland). The drugs were extracted from the worm suspension by adding 200 μl of pure acetic acid, centrifugation (10 min at 16’000 g), transferring the supernatant into a new tube and evaporating the solvent at 65 °C using a SpeedVac SPD 111V concentrator (Thermo Fisher Scientific, Germany). The extraction step was repeated once. The dried drug samples were reconstituted with 75 μl of 10% (v/v) acetonitrile in ammonium formate aqueous buffer.
Three samples of both helminth species, all untreated, were processed and checked for background peaks at the retention times of all five analytes, plus the internal standard.
2.5.2. HPLC apparatus and parameters
An Agilent Series 1100 (Agilent Technologies, Inc.) was used for HPLC analyses as described elsewhere (Cowan et al., 2016). The separations were conducted at a flow rate of 1 ml/min, using two pumps, a microvacuum degasser, an autosampler (10 °C), and a column heater (25 °C). A reversed phase Kinetex® XB-C18 column (4.5 × 150 mm, 2.6 μm; Phenomenex, USA) was used for all separations and a UV–vis detector measured the absorption at a wavelength of 300 nm. The injection volume was 50 μl for all samples.
2.5.3. Quantification of drugs in helminth samples, incubation media, and NaCl solution used for washing
The calibration curves for albendazole (2.5, 1.25, 0.63, 0.31, 0.16, and 0.01 μg/ml), albendazole sulfoxide (2.5, 1.25, 0.63, 0.31, 0.16, and 0.01 μg/ml), and albendazole sulfone (5.0, 2.5, 1.25, 0.63, 0.31, and 0.16 μg/ml) were prepared in 10% (v/v) acetonitrile in aqueous ammonium formate buffer. The calibration curves for mebendazole (2.5, 1.25, 0.63, 0.31, 0.16, 0.08 μg/ml) and oxantel pamoate (2.5, 1.25, 0.63, 0.31, 0.16, 0.08 μg/ml) were prepared separately, also using 10% acetonitrile in ammonium formate buffer. The internal standard 4-azabendazimidazole, was added to all concentrations of the calibration curves to a concentration of 1 μg/ml, the solutions were measured using the HPLC parameters described earlier (Cowan et al., 2016).
The method of quantification was validated for sensitivity at the retention time of the analytes and the internal standard, the linearity of the analytes' calibration curves, and the accuracy and precision (inter- and intraday). These parameters were determined using quality control samples of high, intermediate, and low concentrations of the respective calibration curve, and at the lower limit of quantification (LLOQ). All parameters were calculated from three repeats per sample, and at three different days for interday validation.
The collected culture media of the drug incubations were filtered through a filter of 0.2 μm mesh width before injection into the HPLC. The NaCl wash solutions, that had been used to wash the worms of the in vitro drug entry experiments, were injected directly.
2.5.4. Quantification of albendazole, albendazole sulfoxide and albendazole sulfone in plasma and other compartments
Albendazole analysis. A stock solution of 10 mg/ml albendazole in DMSO was diluted to 192 μg/ml albendazole in acetonitrile. Then, a serial dilution in 50% (v/v) acetonitrile in aqueous ammonium formate buffer was prepared (192, 96, 48, 24, 12, and 6 μg/ml albendazole) and used to spike blank plasma (Sprague Dawely rat plasma; DUNN Labortechnik, Germany), resulting in 100 μl spiked plasma samples (9.6, 4.8, 2.4, 1.2, 0.6, and 0.3 μg/ml albendazole) containing less than 3% (v/v) organic solvent..
The spiked plasma samples were precipitated using 300 μl methanol containing internal standard (1.5 μg/ml mebendazole). The suspension was mixed for 30 s, centrifuged at 16’000 g for 10 min and the supernatant was transferred to a new tube and dried at 45 °C using a SpeedVac concentrator. Then, the pellet was resuspended with 75 μl of 50% (v/v) acetonitrile in aqueous ammonium formate buffer. For the chromatography of albendazole, an acetonitrile gradient between 50% and 90% acetonitrile in ammonium formate buffer was used during 5 min, detecting at a wavelength of 300 nm. The method was validated using a high (7.1 μg/ml), intermediate (1.8 μg/ml), and low concentration (0.45 μg/ml) within the calibration curve, and the LLOQ (0.3 μg/ml). Parameters tested were the linearity, sensitivity, selectivity, the accuracy and precision (intra- and interday), the recovery and matrix effect. Six replicates of each quality control were used for the validation.
Albendazole sulfoxide and sulfone analysis. Plasma samples of 100 μl obtained from the mice experiments at selected time points were precipitated using 300 μl methanol containing 10 μg/ml 4-azabenzimidazole, mixed for 10 min, the supernatant was moved to a new tube, dried in the SpeedVac concentrator, and the pellets resulting were resuspended in 75 μl of 10% (v/v) aqueous ammonium formate (Cowan et al., 2016).
Samples of the gastrointestinal tract (stomach, small intestinal and large intestinal contents, and the small intestinal and large intestinal mucosa) were diluted with blank Sprague Dawely plasma to provide a similar biological matrix for extraction and similar drug quantities as the validated method for plasma samples. The dilution procedure was as follows: The thawed gastrointestinal samples were homogenized using a spatula, samples of 100 mg were taken and prepared to 100 mg gastrointestinal tract sample per 1 ml plasma. The samples were vortex-mixed, ultrasonicated for 30 min at 37 °C and centrifuged at 16’000 g for 10 min. The supernatant of these samples was diluted 2-fold (stomach contents, small and large intestinal mucosas), or 4-fold (small and large intestinal contents) with plasma. Hundred μl of the diluted samples were processed in the same manner as described for the respective methods for plasma samples, starting at the precipitation using 300 μl methanol containing the respective internal standard.
This method of diluting the gastrointestinal tract samples in plasma was assessed for intraday accuracy and precision in the same manner as the validations of the respective methods described above, using quadruplicate determination.
Samples of the gastrointestinal tract after treatment with albendazole or albendazole sulfoxide were analyzed with both methods. Plasma samples were only analyzed for albendazole sulfoxide and sulfone, since albendazole is quickly metabolized to the two metabolites (Dayan, 2003).
2.5.5. Stability of albendazole and albendazole sulfoxide exposed to gastrointestinal contents
The stability of albendazole and albendazole sulfoxide when exposed to the content of the stomach, as well as the small and large intestine was tested. Four untreated NMRI mice were euthanized, the gastrointestinal contents were collected, homogenized, and 100 mg of each content sample was spiked with 5 μg albendazole or albendazole sulfoxide and incubated at 37 °C in a closed tube. After 24 h, plasma ad 1 ml was added and the samples were further processed according to the method described above.
2.5.6. Verification of albendazole, albendazole sulfoxide and albendazole sulfone using mass spectrometry
The identities of albendazole, albendazole sulfoxide, and albendazole sulfone were verified with mass spectrometry. Worm samples from in vitro drug assays after incubation at concentrations of 25 or 50 μM, as well as worms collected from in vivo experiments were assayed. In addition, a sample of each compartment (stomach, small intestinal content and mucosa, and large intestinal content and mucosa) of both parasite models was tested. For the separation using HPLC (Shimadzu, Kyoto, Japan) the chromatographic parameters were applied as describe above. For the MS measurements the compounds were ionized in positive mode and detected by multiple reaction monitoring on an API 3200 (AB Sciex; Framingham, USA) equipped with a turbo ion spray source.
2.6. Data analysis
Microsoft Excel 2010 was used for all calculations. The accuracy of the analytical methods was determined from the average value of replicates, and the precision was expressed as the coefficient of variation (CV), which was calculated as the ratio of the normalized peak area's standard deviation to the mean value. Multiple measurements of test samples were averaged (arithmetic mean) and the standard deviation (SD) was calculated. The presence of correlation was defined as a correlation coefficient (R2) of the linear trend line if R2 ≥ 0.75. The LLOQs for the different sample types (helminths, gastrointestinal tract, and plasma or culture medium) are listed in Table S1 of the supplementary file.
3. Results
3.1. Quality of in vitro and in vivo experiments and the analytical measurements
Analytes in solvent. The calibration curves of all five analytes in solvent were linear (all R2 = 0.999) and the measurements of the quality controls were accurate and precise, revealing coefficient of variation between measurements of ±14% for the higher, middle and lower quality controls, or ±18% for LLOQ (supplementary file Table S2).
Analyte extraction from helminth suspension. Chromatography of processed blank worm samples of H. polygyrus and T. muris showed that the absorption signals (peak area and height) at the retention times of interest were at least five times smaller than the signals (peak area and height) of the LLOQ of the respective analyte. Results of background signals of T. muris versus analyte signals are presented in the supplementary file Table S3.
Helminth washing solution. NaCl solutions which had been used for washing the worms after drug incubations contained acceptable amounts of residual drug: out of 48 washing solutions tested (including both helminths and the respective analytes that had been used for in vitro drug exposure between 5 and 100 μM) 46 (96%) revealed drug levels which were below the LLOQ.
Quantification of albendazole in plasma. The calibration curve of the quantification of albendazole in plasma was linear (R2 = 0.999). The absorption at the retention time showed a 4.3-fold lower peak height than at LLOQ. All quality controls were measured accurately (±6%) and precisely (≤8%) (supplementary file Table S4). The recovery of albendazole from spiked plasma samples was 93% (±1) and the matrix effect was 91% (±1) (supplementary file Table S5).
Stability in gastrointestinal tract content samples. No biotransformation was observed using HPLC when albendazole and albendazole sulfoxide were incubated with blank samples of the gastrointestinal contents for 24 h.
Accuracy and precision in compartment samples. Albendazole sulfoxide, albendazole sulfone, and mebendazole were accurately and precisely quantifiable in gastrointestinal compartment samples (stomach content, small intestinal content and mucosa, and large intestinal content and mucosa) at the higher and middle quality control concentrations. The amount of albendazole and oxantel pamoate in the two lower concentration of quality controls was above the expected values, up to 139% (small intestinal mucosa) and 128% (small intestinal content), respectively. The concentrations in the stomach at the lower end of the calibration curves were overestimated for all analytes (between 123% and 175% of the expected value). The accuracy and precision of the quantification in the processed and spiked gastrointestinal tract samples are shown in Table S6 of the supplementary file.
MS verification of metabolites. The presence of metabolites albendazole, albendazole sulfoxide and albendazole sulfone quantified with the HPLC methods was confirmed with LC-MS/MS for all different of samples used in this study.
3.2. Drug uptake and elimination by H. polygyrus and T. muris in vitro
Uptake and elimination of albendazole by H. polygyrus. After H. polygyrus were incubated with albendazole at concentrations ranging from 5 to 100 μM for 24 h, albendazole (5–55 nmol/10 worms), albendazole sulfoxide (14–110 nmol/10 worms), and albendazole sulfone (2–22 nmol/10 worms) were detected in worms (Table 1). There was no linear correlation between assay concentration and the concentrations found in the parasite. The culture medium after incubation contained on average 0.5 μM (0.3–0.6 μM) albendazole sulfoxide. Albendazole sulfone could not be quantified in the medium.
Table 1.
In vitro drug uptake and elimination after 24 h exposure to albendazole (ABZ), albendazole sulfoxide (ABZSO), and albendazole sulfone (ABZSO2) by H. polygyrus and T. muris, as well as uptake of mebendazole (MBZ) and oxantel pamoate (OxP) by T. muris.
|
H. polygyrus |
T. muris |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Worms [nmol/10 worms] |
Medium [μM]a |
Worms [nmol/10 worms] |
Medium [μM]a |
Worms [nmol/10 worms] |
Worms [nmol/10 worms] |
||||||||||||||
| Conc. of incub. [μM] | ABZ ± SD | ABZSO ± SD | ABZSO2 ± SD | ABZ ± SD | ABZSO ± SD | ABZSO2 ± SD | ABZ ± SD | ABZSO ± SD | ABZSO2 ± SD | ABZ ± SD | ABZSO ± SD | ABZSO2 ± SD | Conc. of incub. [μM] | MBZ ± SD | Conc. of incub. [μM] | OxP ± SD | |||
| ABZ | 5 | 13 ± 6 | 14 ± 19 | 3 ± 3 | ND | 0.4 ± 0.2 | NP | 94 ± 44 | 45 ± 64 | 15 ± 18 | ND | 1.0 ± 0.5 | MBZ | 5 | 482 ± 388 | OxP | 5 | 13 ± 9 | |
| 10 | 16 ± 5 | 51 ± 9 | 5 ± 2 | ND | 0.4 ± 0.3 | NP | 176 ± 83 | 38 ± 35 | 67 ± 34 | ND | 2.0 ± 0.5 | 10 | 626 ± 463 | 10 | 27 ± 9 | ||||
| 25 | 5 ± 8 | 44 ± 14 | 22 ± 31 | ND | 0.5 ± 0.2 | NP | 263 ± 173 | 106 ± 178 | 90 ± 43 | ND | 2.6 ± 0.9 | 25 | 672 ± 251 | 25 | 65 ± 5 | ||||
| 50 | 28 ± 4 | 30 ± 10 | 2 ± 3 | ND | 0.6 ± 0.3 | NP | 551 ± 528 | 259 ± 285 | 103 ± 64 | ND | 3.5 ± 1.1 | 50 | 826 ± 390 | 50 | 155 ± 41 | ||||
| 75 | 55 ± 49 | 99 ± 7 | 13 ± 25 | ND | 0.6 ± 0.3 | NP | 598 ± 336 | 211 ± 224 | 149 ± 64 | ND | 5.2 ± 1.0 | 75 | 910 ± 362 | 75 | 169 ± 24 | ||||
| 100 | 10 ± 21 | 110 ± 70 | 10 ± 12 | ND | 0.3 ± 0.4 | NP | 641 ± 467 | 221 ± 224 | 141 ± 50 | ND | 3.9 ± 0.5 | 100 | 861 ± 503 | 100 | 197 ± 46 | ||||
| ABZSO | 5 | NP | 27 ± 12 | 16 ± 32 | NP | ND | NP | NP | 61 ± 18 | 6 ± 5 | NP | ND | |||||||
| 10 | NP | 12 ± 8 | 0.5 ± 0.0 | NP | ND | NP | NP | 131 ± 79 | 8 ± 6 | NP | ND | ||||||||
| 25 | NP | 31 ± 6 | 5 ± 3 | NP | ND | NP | NP | 282 ± 114 | 3 ± 3 | NP | ND | ||||||||
| 50 | NP | 56 ± 16 | 12 ± 10 | NP | ND | NP | NP | 563 ± 141 | 8 ± 4 | NP | ND | ||||||||
| 75 | NP | 77 ± 15 | 4 ± 4 | NP | ND | NP | NP | 787 ± 252 | 7 ± 5 | NP | ND | ||||||||
| 100 | NP | 111 ± 50 | 15 ± 25 | NP | ND | NP | NP | 833 ± 229 | 10 ± 4 | NP | ND | ||||||||
| ABZSO2 | 5 | NP | 19 ± 10 | 33 ± 10 | NP | 0.2 ± 0.1 | ND | NP | NP | 122 ± 45 | NP | NP | ND | ||||||
| 10 | NP | 19 ± 3 | 51 ± 23 | NP | 0.2 ± 0.1 | ND | NP | NP | 185 ± 55 | NP | NP | ND | |||||||
| 25 | NP | 13 ± 11 | 23 ± 8 | NP | 0.4 ± 0.0 | ND | NP | NP | 421 ± 171 | NP | NP | ND | |||||||
| 50 | NP | 9 ± 5 | 30 ± 20 | NP | 0.3 ± 0.0 | ND | NP | NP | 780 ± 258 | NP | NP | ND | |||||||
| 75 | NP | 16 ± 11 | 31 ± 8 | NP | 0.3 ± 0.0 | ND | NP | NP | 1127 ± 451 | NP | NP | ND | |||||||
| 100 | NP | 16 ± 8 | 33 ± 21 | NP | 0.2 ± 0.0 | ND | NP | NP | 1348 ± 447 | NP | NP | ND | |||||||
NP: No peak detected; ND: Not done; values are averages of n = 4 ± standard deviation (SD).
Parent compounds were quantified but not presented.
Between 1 and 8 h of incubation of H. polygyrus with albendazole at 50 μM, a linear time-dependent accumulation of both albendazole (R2 = 0.95) and albendazole sulfoxide (R2 = 0.92) was measured (supplementary file Fig. S1). Longer incubation than 8 h did not increase drug concentration in the worms, but plateaued at 29 nmol/10 worms for both compounds. The albendazole sulfoxide concentration in the culture medium, on the other hand, was measured at similar concentrations between 1 and 24 h of incubation with an overall average of 0.7 μM (0.6–1 μM).
When H. polygyrus was exposed to albendazole following a pre-incubation with ABT, no differences in the accumulation of albendazole or its metabolites were found in the worms and in the culture medium.
Uptake and elimination of albendazole sulfoxide by H. polygyrus. After incubation of H. polygyrus with albendazole sulfoxide (5–100 μM) for 24 h, between 12 nmol/10 worms and 111 nmol/10 worms were detected in a concentration dependent manner (R2 = 0.86) (Table 1). Albendazole sulfone was also detected, however, at amounts no higher than 16 nmol/10 worms. Neither albendazole nor albendazole sulfone could be quantified in the culture medium.
Uptake and elimination of albendazole sulfone by H. polygyrus. Incubation of H. polygyrus with albendazole sulfone yielded similar quantities regardless of concentration tested (5–100 μM): albendazole sulfone was present at a mean of 33 nmol/10 worms (23–51 nmol/10 worms) and albendazole sulfoxide at 15 nmol/10 worms (9–19 nmol/10 worms) (Table 1). Albendazole sulfoxide was found in the medium on average at 25 μM (19–39 μM).
Uptake and elimination of albendazole by T. muris. After incubation of T. muris with albendazole between 5 and 100 μM for 24 h, albendazole and its metabolites were measured in a concentration-dependent manner after exposure between 5 and 75 μM in culture medium (Table 1). Albendazole was detected at the highest quantities ranging from 94 to 598 nmol/10 worms (R2 = 0.94), followed by albendazole sulfoxide which was measured at concentrations ranging from 45 to 211 nmol/10 worms (R2 = 0.78), and albendazole sulfone at 15–149 nmol/10 worms (R2 = 0.95). The albendazole sulfoxide concentrations in the culture medium (ranging from 1 to 5.2 μM) increased with increasing albendazole concentrations of incubation. Albendazole sulfone was not detected in the culture medium.
Uptake and elimination of albendazole sulfoxide by T. muris. Exposure of T. muris to albendazole sulfoxide resulted in concentrations of 61 (after exposure to 5 μM) to 833 nmol/10 worms (after exposure to 100 μM), which increased linearly at a concentration range from 5 to 75 μM drug in the culture medium (R2 = 0.96) (Table 1). Albendazole sulfone was detected at low and similar amounts in all samples at an average of 7 nmol/10 worms (3–10 nmol/10 worms).
Uptake and elimination of albendazole sulfone by T. muris. Albendazole sulfone was absorbed into T. muris in a concentration-dependent manner (122–1348 nmol/10 worms (R2 = 0.97)) following incubation of 5–100 μM. Albendazole and albendazole sulfoxide were neither detected in the parasite nor the culture medium (Table 1).
Uptake of mebendazole by T. muris. The entry of mebendazole into T. muris slightly increased with escalating drug concentration. The mebendazole amounts measured ranged between 482 and 910 nmol/10 worms following incubation of 5–100 μM for 24 h (Table 1).
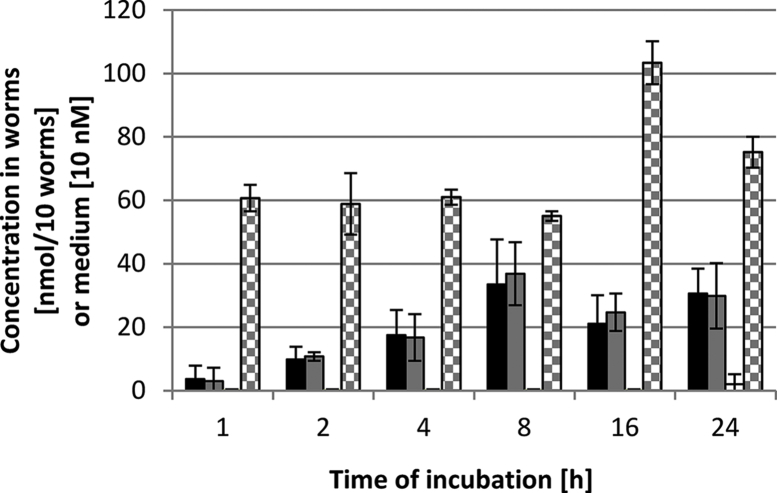
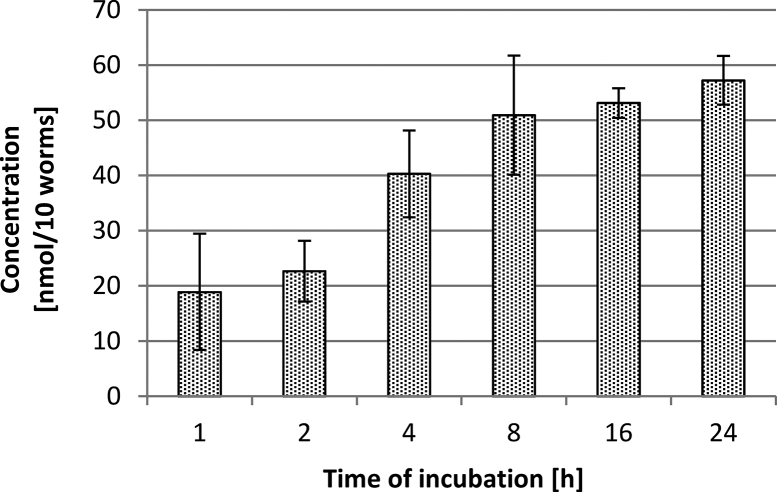
Uptake of oxantel pamoate by T. muris. Oxantel pamoate was detected at levels of 13–197 nmol/10 worms in a concentration-dependent manner (R2 = 0.94) (Table 1). The uptake of oxantel pamoate ceased 8 h after exposure to the drug at 25 μM (supplementary file Fig. S2).
3.3. Drug distribution and biotransformation in infected animals and parasites
3.3.1. Drug distribution in the gastrointestinal tract and plasma of NMRI mice harboring H. polygyrus
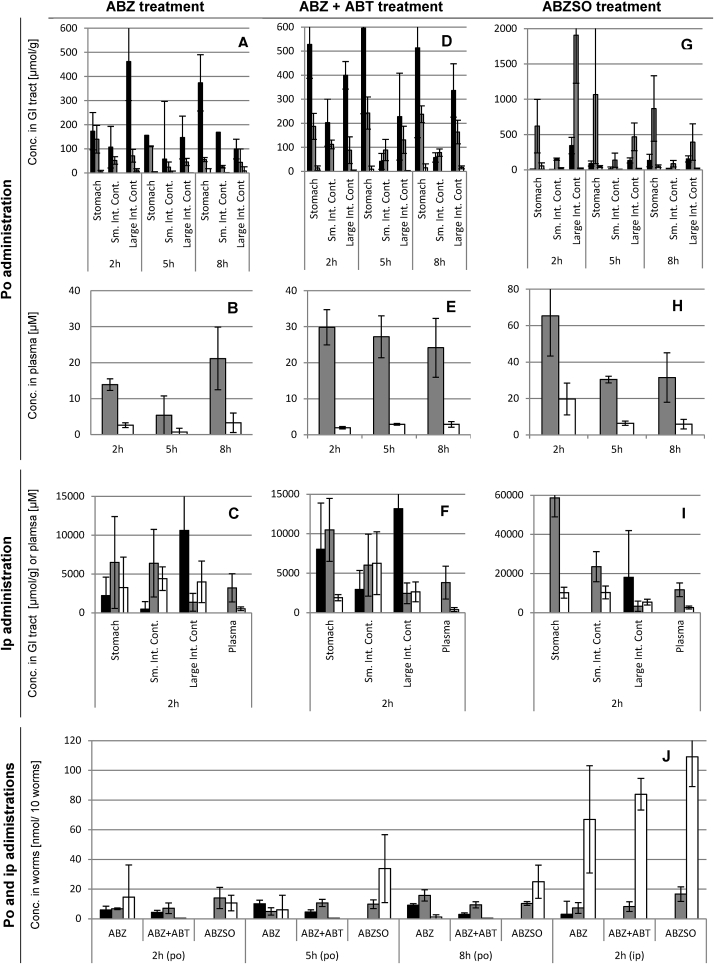
Albendazole treatment. After oral treatment with albendazole, albendazole and albendazole sulfoxide were detected in all gastrointestinal contents (Fig. 1A). This was observed at all sampling times post-treatment (2, 5, and 8 h). In more detail, in the stomach the concentration of albendazole was on average 234 μmol/g (156–373 μmol/g), 170 μmol/g (110–260 μmol/g) for albendazole sulfoxide, and 8 μmol/g (4–16 μmol/g) for albendazole sulfone. In the small intestine, where H. polygyrus resides, albendazole concentrations were on average 111 μmol/g (58–169 μmol/g), albendazole sulfoxide concentrations were on average 51 μmol/g (24–79 μmol/g), and albendazole sulfone was not measured. In the large intestine, the concentrations of albendazole and albendazole sulfoxide decreased over time from 462 μmol/g (349–700 μmol/g) to 99 μmol/g (0–186 μmol/g) and from 71 μmol/g (53–110 μmol/g) to 56 μmol/g (16–112 μmol/g), respectively, whereas the sulfone metabolite was only found in low amounts (below 11 μmol/g). The concentrations in the mucosa were only quantifiable after 8 h for albendazole at 13 μmol/g (0–40 μmol/g) and albendazole sulfoxide at 27 μmol/g (6–47 μmol/g). Plasma concentrations were on average 13.5 μM (5.4–21.2 μM) for albendazole sulfoxide and 2.2 μM (0.7–3.3 μM) for albendazole sulfone (Fig. 1B). A correlation was observed between the concentration of albendazole sulfoxide in plasma and the content of the small intestine (R2 = 0.77). No relation between other compartments and drugs were found.
Fig. 1.
In vivo distribution of albendazole treatments in host and H. polygyrus. Columns of host samples are averages of n = 4 mice and columns of parasite samples are averages of n = 12 measurements (3 samples per mouse). Error bars indicate the standard deviation. ABZ: Albendazole (black columns); ABZSO: albendazole sulfoxide (gray columns); albendazole sulfone (white columns); po: per oral; ip: intraperitoneal; GI: gastrointestinal.
Two hours after intraperitoneal treatment of 100 mg/kg albendazole to NMRI mice elevated concentrations of albendazole (20-fold), albendazole sulfoxide (62-fold), and albendazole sulfone (493-fold) were observed in all gastrointestinal contents (Fig. 1C) compared to oral treatment. The stomach contained albendazole at 2217 μmol/g (0–5259 μmol/g), albendazole sulfoxide at 6486 μmol/g (2725–15225 μmol/g) and sulfone at 3264 μmol/g (0–7832 μmol/g). Albendazole was detected in the small intestinal content at 486 μmol/g (0–1945 μmol/g), albendazole sulfoxide at 6391 μmol/g (905–10367 μmol/g), and albendazole sulfone at 4396 μmol/g (3149–6606 μmol/g). In the large intestinal content, albendazole was detected at 10620 μmol/g (3398–28518 μmol/g), albendazole sulfoxide at 1356 μmol/g (634–3064 μmol/g), and albendazole sulfone at 3988 μmol/g (0–5730 μmol/g). Albendazole was not found in the small intestinal mucosa. Albendazole sulfoxide and sulfone were quantified in the mucosal tissues of the small intestine in concentrations of 1439 μmol/g (433–2148 μmol/g) and 683 μmol/g (627–781 μmol/g), respectively. In the large intestinal mucosa, albendazole concentrations were 416 μmol/g (0–856 μmol/g), albendazole sulfoxide 1060 μmol/g (434–1627 μmol/g), and albendazole sulfone 805 μmol/g (0–1078 μmol/g). In the plasma, albendazole sulfoxide was present at 3222 μM (770–4657 μM) and albendazole sulfone at 527 μM (212–705 μM) (Fig. 1C).
Albendazole with ABT pre-treatment. Oral pre-treatment with ABT followed by albendazole resulted at all time points in higher albendazole levels in the stomach (average of 2.7-fold magnitude) than without ABT (Fig. 1D). In the small intestinal content there was a tendency for a higher albendazole sulfoxide concentration (2.1-fold), and in the large intestinal content, both albendazole and its sulfoxide metabolite were elevated on average 1.9-fold and 2.4-fold between 2 and 8 h post-treatment, respectively compared to albendazole monotherapy. In the mucosa of the small intestine, levels of albendazole were on average 19 μmol/g (0–176 μmol/g) across all time points and of albendazole sulfoxide on average 14 μmol/g (6–26 μmol/g), whereas no albendazole sulfone was present. The albendazole levels in the mucosa of the large intestine were on average 3.8-fold elevated over time compared to albendazole single treatment, and albendazole sulfoxide was 2.8-fold elevated, whereas albendazole sulfone was also not present after this treatment. In the plasma, increased exposure of albendazole sulfoxide (2-fold on average between 2 and 8 h post treatment) was observed (Fig. 1E). Levels of albendazole sulfone in the plasma were not influenced by the ABT pre-treatment, but showed the same concentrations as the albendazole treatment. No correlation between the concentrations of any drugs in any of the compartments could be identified.
Elevated concentrations of albendazole and its metabolites (28-fold, 44-fold and 237-fold increase of albendazole, albendazole sulfoxide, and albendazole sulfone, respectively) were observed in the gastrointestinal tract after parenteral application of albendazole following ABT pre-treatment compared to the oral application of the two drugs (Fig. 1F). Albendazole and its metabolites were slightly elevated (within 2-fold difference) in the mucosa compared to albendazole single treatment applied intraperitoneally.
Albendazole sulfoxide treatment. After the oral application of albendazole sulfoxide to H. polygyrus-infected mice, albendazole and both metabolites were found in the contents of the gastrointestinal tract (Fig. 1G). In all compartments and at all time points albendazole sulfoxide was the predominant entity detected on average of 8-fold higher compared to albendazole and albendazole sulfone. Albendazole was found in the stomach on average 76 μmol/g (7–133 μmol/g), in the small intestine 12 μmol/g (4–17 μmol/g) and in the large intestine at 211 μmol/g (156–344 μmol/g) across all time points. In the mucosa of the small intestine, albendazole, albendazole sulfoxide and sulfone were detected at highest concentrations 5 h after treatment, with 15 μmol/g (0–53 μmol/g), 137 μmol/g (82–289 μmol/g) and 5.7 μmol/g (1–10 μmol/g), respectively. At the other time points tested, albendazole concentrations were below 1 μmol/g, and albendazole sulfoxide and albendazole sulfone concentrations were below 27 μmol/g, 3 μmol/g, respectively. The mucosal tissue of the large intestine contained between 9 and 13 μmol/g albendazole, 16 and 30 μmol/g albendazole sulfoxide, and below 2 μmol/g albendazole sulfoxide between 2 and 8 h post-treatment. Albendazole sulfoxide found in the plasma was increased compared to the albendazole treatment, especially 5 h post-treatment (5.2-fold elevated levels of albendazole sulfoxide) (Fig. 1H). The concentration of albendazole sulfoxide in the plasma correlated with the concentration in the contents of the small intestine (R2 = 0.76) and the large intestine (R2 = 0.78).
When albendazole sulfoxide was given intraperitoneally and samples were collected 2 h post-treatment no albendazole was detected in the stomach, but albendazole sulfoxide at 58655 μmol/g and albendazole sulfone at 10199 μmol/g. In the contents of the small intestine no albendazole was found, but albendazole and albendazole sulfone were present at 23488 μmol/g at and 10341 μmol/g, respectively. The concentrations in the large intestinal contents were 23889 μmol/g for albendazole, 3319 μmol/g for albendazole sulfoxide, and 5542 μmol/g for albendazole sulfone. Overall, the gastrointestinal tract contained on average more albendazole (60-fold), albendazole sulfoxide (86-fold), and albendazole sulfone (553-fold) levels compared to the average value of samples of gastrointestinal contents collected after oral treatment (Fig. 1I). In the small intestinal mucosa no albendazole was detected, but albendazole sulfoxide were present at 6302 μmol/g (3417–7836 μmol/g) and albendazole sulfone at 2307 μmol/g (2050–2610 μmol/g). In the mucosa of the large intestine albendazole was measured at 1446 μmol/g (909–2698 μmol/g), as well as albendazole sulfoxide at 2605 μmol/g (1375–4184 μmol/g) and albendazole sulfone at 2416 μmol/g (2065–2675 μmol/g). In the plasma, albendazole sulfoxide was present at 11726 μM (6667–14477 μM) and albendazole sulfone at 2646 μM (1622–3255 μM).
Uptake of albendazole and its metabolites by H. polygyrus in vivo. In H. polygyrus recovered from mice orally treated with albendazole and albendazole plus ABT, the albendazole amounts were on average 6.2 nmol/10 worms (3–10 nmol/10 worms), whereas albendazole sulfoxide was found in slightly higher amounts with 9.1 nmol/10 worms (5–16 nmol/10 worms) (Fig. 1J). Albendazole sulfone, on the other hand, was only detected in worms of mice that had not been pretreated with ABT (on average 7.3 nmol/10 worms (1–15 nmol/10 worms)). Oral application of albendazole sulfoxide resulted in accumulation of the two albendazole metabolites in the worms. No albendazole was detected. Albendazole sulfoxide was found at amounts of 11.4 nmol/10 worms (9.8–14 nmol/10 worms) and albendazole sulfone at 23.1 nmol/10 worms (11–34 nmol/10 worms). After intraperitoneal application of the three treatments, albendazole sulfoxide was found in similar amounts as the oral applications. However, albendazole sulfone concentrations were increased to an average of 86.4 nmol/10 worms (67–109 nmol/10 worms). Albendazole was only found in one of all H. polygyrus samples (n = 29) recovered of the intraperitoneal treatment. No relationship between the drug concentrations in H. polygyrus and the corresponding concentrations in the plasma, large intestinal content or its mucosa could be observed.
3.3.2. Drug distribution in C57BL/10 mice and T. muris
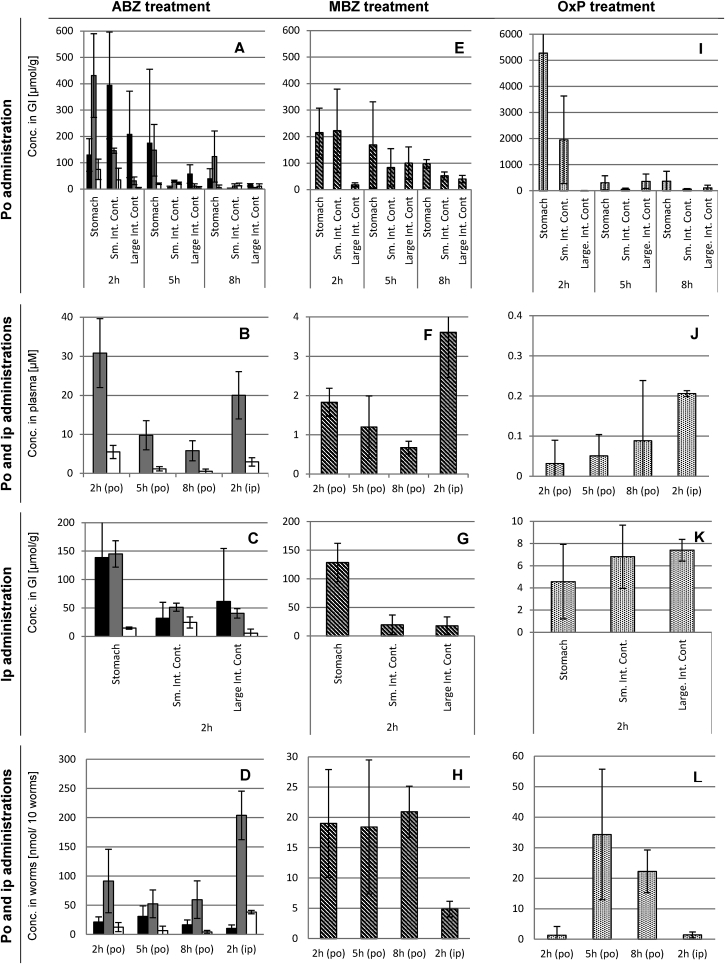
Albendazole treatment. After oral albendazole treatment, albendazole, albendazole sulfoxide and albendazole sulfone were detected in all contents of the gastrointestinal tract, at decreasing concentrations over time and descending order of amount throughout the intestinal tract (Fig. 2A). The highest concentrations between 2 and 8 h post-application were found in the stomach of the animals with average concentrations over time of 114 μmol/g (39–174 μmol/g) for albendazole, 234 μmol/g (145–431 μmol/g) for albendazole sulfoxide, and 34 μmol/g (7–75 μmol/g) for albendazole sulfone. Concentrations in the small intestine dropped from 394 μmol/g (235–622 μmol/g) of albendazole (2 h post-application) to around 5 μmol/g (5 and 8 h post-application), albendazole sulfoxide from 146 μmol/g (138–157 μmol/g) 2 h post-application to 12 μmol/g (3–20 μmol/g) 8 h post-application, and albendazole sulfone from 44 μmol (0.4–85 μmol/g) 2 h post-application to 13 μmol/g (3–22 μmol/g) 8 h post-application. The large intestinal tract, in which T. muris resides, contained 208 μmol/g (97–396 μmol/g), 57 μmol/g (30–97 μmol/g), or 16 μmol/g (11–19 μmol/g) albendazole at 2, 5, or 8 h post-treatment, respectively. The levels of albendazole sulfoxide were lower with 31 μmol/g (17–46 μmol/g), 11 μmol/g (1–17 μmol/g), and 5 μmol/g (4–6 μmol/g) at the given time points. In the mucosa of the small intestine albendazole, albendazole sulfoxide and sulfone were detected 2 h post-treatment at 5 μmol/g (0–12 μmol/g), 25 μmol/g (20–27 μmol/g) and 11 μmol/g (5–16 μmol/g), respectively. Five and eight hours after application, the albendazole, albendazole sulfoxide and sulfone levels were below 4 μmol/g. Also in the mucosa of the large intestine, albendazole was found highest 2 h after treatment at 13 μmol/g (3–19 μmol/g), albendazole sulfone at 22 μmol/g (15–26 μmol/g), and albendazole at 5 μmol/g (2–10 μmol/g). After 5 and 8 h, all concentrations were below 6 μmol/g. Plasma levels of albendazole sulfoxide were highest 2 h post-treatment with 31 μM (24–41 μM) and decreased to 10 μM (7–14 μM) and 6 μM (4–5 μM) at 5 or 8 h post-treatment (Fig. 2B). Albendazole sulfone was measured between 5.5 μM (4–8 μM) 2 h post-treatment to 0.5 μM (0.1–1.2 μM) 8 h post-treatment.
Fig. 2.
In vivo distribution of albendazole, mebendazole and oxantel pamoate in host and T. muris. Columns of host samples are averages of n = 3 mice and columns of parasite samples are averages of n = 9 measurements (3 samples per mouse). Error bars indicate the standard deviation. ABZ: albendazole (black columns); albendazole sulfoxide (gray columns); albendazole sulfone (white columns); MBZ: mebendazole (striped columns); OxP: oxantel pamoate (dotted columns); po: per oral application; ip: intraperitoneal; GI: gastrointestinal.
The distribution of albendazole and its metabolites 2 h after the intraperitoneal application showed similarities to oral treatment of albendazole 5 h post-treatment. All three drugs were present in all parts of the intestinal tract. In the stomach, concentrations of albendazole was 139 μmol/g (77–233 μmol/g), albendazole sulfoxide 145 μmol/g (121–167 μmol/g), and albendazole sulfone 15 μmol/g (13–17 μmol/g). Concentrations of albendazole and albendazole sulfoxide on average 46 μmol/g (32–62 μmol/g) in the small and large intestines (Fig. 2C). Albendazole sulfone was detected at 15 μmol/g in the stomach, 24 μmol/g in the small intestine and 6 μmol/g in the large intestine. In the mucosa of the small intestine, only albendazole sulfoxide was detected at 14 μmol/g (10–21 μmol/g). In the mucosa of the large intestine, albendazole was detected at 8 μmol/g (0–13 μmol/g) and albendazole sulfoxide at 14 μmol/g (12–15 μmol/g). Plasma values were in the same range as after oral application with 20.0 μM (13–24 μM) for albendazole sulfoxide and 2.9 μM (1.7–3.6 μM) for albendazole sulfone (Fig. 2B).
Worms recovered from the treated mice revealed albendazole as well as its metabolites (Fig. 2D). After oral treatment, albendazole sulfoxide was found in highest amounts (52–91 nmol/10 worms), which was on average 3.2-fold higher than albendazole and 11-fold higher than albendazole sulfone. Intraperitoneal treatment resulted in albendazole sulfoxide concentration of 204 nmol/10 worms, which was 20-fold higher than albendazole and 5.3-fold higher than albendazole sulfone. There was no correlation between the drug concentrations measured in T. muris, compared to the plasma or the large intestinal content.
Mebendazole treatment. As shown in Fig. 2E, 2 h after oral treatment with mebendazole, most of the drug was found in the stomach with 215 μmol/g (145–320 μmol/g) and small intestine with 222 μmol/g (110–402 μmol/g), but less in the large intestine with 18 μmol/g (9–25 μmol/g). The concentration in the large intestine was higher 5 h post-treatment with 100 μmol/g (64–171 μmol/g), but again lower 8 h post-treatment with 40 μmol/g (26–55 μmol/g). In the mucosa of the small intestine, 8 μmol/g (5–22 μmol/g) of mebendazole was present after 2 h post-treatment, 6 μmol/g (0.2–14 μmol/g) 5 h post-treatment, and 4 μmol/g (2–6 μmol/g). In the mucosa of the large intestine, 3 μmol/g was found in all mice after 2 h post-treatment, 5 μmol/g (1–11 μmol/g) 5 h post-treatment, and 2 μmol/g (1–3 μmol/g) 8 h post-treatment. Plasma concentrations were low at 0.7–1.8 μM (Fig. 2F).
After intraperitoneal treatment with mebendazole, a similar picture of mebendazole levels was observed in the gastrointestinal tract as after oral dose 8 h post-treatment (Fig. 2G). In the mucosa of the small and the large intestine, 4 μmol/g (1–6 μmol/g) and 1 μmol/g (0–2 μmol/g) was present, respectively. Plasma levels of mebendazole, however, resulted in a slightly higher plasma level compared to the oral treatment with 3.6 μM (2.4–4.7 μM) (Fig. 2F).
After oral treatment with mebendazole, its concentration in T. muris was close to LLOQ, remaining unchanged over time (20 nmol/10 worms) (Fig. 2H). Intraperitoneal application of mebendazole led to less accumulation (5 nmol/10 worms). No correlation between mebendazole concentration in the worms compared to drug found in the plasma or content of the large intestine was observed.
Oxantel pamoate treatment. After oral treatment with oxantel pamoate, large amounts of oxantel pamoate were found in the stomach (5278 μmol/g [467–14373 μmol/g] 2 h post-treatment), which decreased to 302 μmol/g (75–601 μmol/g) and 364 μmol/g (52–792 μmol/g), at 5 and 8 h post-treatment, respectively. In the small intestine 1951 μmol/g (106–3394 μmol/g) were detected 2 h post-treatment, which decreased to 54 μmol/g (17–104 μmol/g) and 61 μmol/g (40–82 μmol/g) 5 and 8 h post-treatment, respectively. The oxantel pamoate concentrations in the large intestine altered over the sampling time points of 2, 5 and 8 h post-treatment from 6 μmol/g (4–6 μmol/g) to 359 μmol/g (48–587 μmol/g) and 110 μmol/g (0–202 μmol/g), respectively (Fig. 2I). The concentration in the small intestinal mucosa was at 31 μmol/g (21–47 μmol/g) 2 h after treatment and decreased to 2 μmol/g (0–5 μmol/g) after 5 and 8 h post-treatment. Oxantel pamoate's plasma levels were below the LLOQ (0.7 μM) at all times (Fig. 2J).
Furthermore, after the application of oxantel pamoate (50 mg/kg) into the peritoneal cavity oxantel pamoate concentrations in the stomach were 5 μmol/g (1–8 μmol/g), in the small intestine 7 μmol/g (4–9 μmol/g) and in the large intestine 7 μmol/g (7–9 μmol/g). In the mucosal tissue of the small intestine, 2 μmol/g (1–3 μmol/g) was found and 17 μmol/g (3–45 μmol/g) were detected in the mucosal tissue of the large intestine (Fig. 2K). The plasma levels were below the LLOQ, nevertheless, a concentration of 0.21 μM (0.20–0.21 μM) was determined (Fig. 2J).
Oral application of oxantel pamoate resulted in accumulation in T. muris only after 5 h (34 nmol/10 worms) and 8 h (22 nmol/10 worms) post-treatment (Fig. 2L). After the intraperitoneal application of oxantel pamoate no compound could be quantified. No correlation between the concentration in T. muris and in the large intestinal content was apparent.
3.4. In vitro and in vivo drug activities
3.4.1. Albendazole, albendazole sulfoxide, and albendazole sulfone activities against H. polygyrus
Albendazole and its two main metabolites showed poor in vitro activity at 200 μM: the drug effect (reduction of parasite viability) was 50% for albendazole, 60% for albendazole sulfoxide, and 40% for albendazole sulfone (Table 2). All parasites were alive at the end of the assay (72 h post-incubation).
Table 2.
In vitro and in vivo activities albendazole (with or without ABT pre-treatment), albendazole sulfoxide, and albendazole sulfone against H. polygyrus, and albendazole, mebendazole and oxantel pamoate against T. muris.
| Drug | Drug effect [%] after drug incubation [μM] of adult H. polygyrus for 72 h | Worm burden reduction [%] after oral drug application [mg/kg] to H. polygyrus-infected mice | Drug effect [%] after drug incubation of adult T. muris at >600 μM or EC50 [μM] after incubation at serial dilution for 72 h | Worm burden reduction [%] after oral drug application of drugs [mg/kg] to T. muris-infected mice |
|---|---|---|---|---|
| Albendazole | 50% [200 μM] | 100% [100 mg/kg]a | <50%b | 42% [300 mg/kg]c |
| Albendazole sulfoxide | 60% [200 μM] | 79% [100 mg/kg] | not done | not done |
| Albendazole sulfone | 40% [200 μM] | not done | not done | not done |
| Albendazole + ABT | not done | 84% [100 mg/kg] | not done | not done |
| Mebendazole | 0% [100 μM]a | 59% [100 mg/kg]a | <50%b | 68% [150 mg/kg]c |
| Oxantel pamoate | not done | not done | 4 μMc | 93% [10 mg/kg]c |
EC50: Concentration of 50% drug efficacy.
Unpublished data.
3.4.2. Efficacy of albendazole sulfoxide and albendazole plus ABT against H. polygyrus
Albendazole sulfoxide (100 mg/kg) revealed a WBR of 79% in H. polygyrus-infected mice, whereas albendazole (100 mg/kg) with an ABT-pretreatment (50 mg/kg) reduced the worm burden by 84% (Table 2).
4. Discussion
Albendazole and mebendazole are widely used drugs for the treatment of soil-transmitted helminth infections (World Health Organisation, 2006). Based on findings with other drug-parasite systems these drugs are likely to enter the parasites from the intestinal lumen via the cuticle (Alvarez et al., 2007, Hansen et al., 2014). However this has yet to be demonstrated for H. polygyrus and T. muris.
This study was conducted to assess the distribution of albendazole, its metabolites albendazole sulfoxide (an active anthelmintic) and albendazole sulfone (the main and inactive metabolite of albendazole sulfoxide) in different compartments of the host and the worm itself to shed light on the compartment responsible for drug accumulation and drug activity against H. polygyrus, and albendazole and mebendazole against T. muris. We also included oxantel pamoate in our studies, because it is a promising drug for the treatment of trichuriasis (Speich et al., 2015). We used two helminth-mouse models: the H. polygyrus-NMRI model, which is a model for hookworm infections (Tritten et al., 2012) and the T. muris-C57BL/10 model, which is an established model for trichuriasis (Keiser et al., 2013). Several procedures used in this study were validated for their quantitative (e.g. washing steps or analytical methods) and qualitative (i.e. verification of metabolite identities using mass spectrometry) reliability.
4.1. Distribution and metabolism of albendazole
Albendazole revealed a very dynamic distribution and high rate of metabolism. In mice, we observed both the parent as well as the metabolites throughout the entire gastrointestinal tract after oral treatment of albendazole as well as albendazole sulfoxide during all time points of sampling. The distribution and pathways of albendazole and its metabolites as observed in our study are depicted in Fig. 3. The oxidative metabolism of albendazole to albendazole sulfoxide and to albendazole sulfone occurred already in the stomach of the mice. Reduction of albendazole sulfoxide to albendazole took also place, however to a smaller extent. These biotransformations had already been demonstrated using artificial cow rumens. In cows, bacterial metabolism is probably responsible for these reactions, since inactivation with heat showed no transformation of albendazole or albendazole sulfoxide (Capece et al., 2001). Our study, in contrast, showed no transformations in isolated stomach content, or in any of the gastrointestinal contents. This suggests that the entire, or a major part of the metabolism, was caused by host gastrointestinal epithelial cells. However, in depth studies would be required, including studies on gastrointestinal microbiota, which are able to metabolize xenobiotics (for instance via oxidation and reduction) (Mikov, 1994), to fully understand the role of the gut flora in albendazole metabolism.
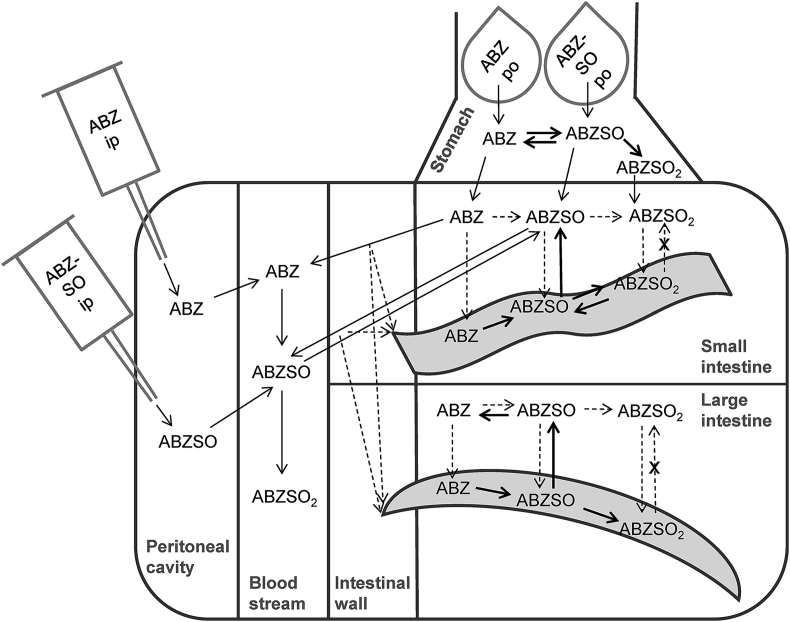
Fig. 3.
Scheme of albendazole (ABZ), albendazole sulfoxide (ABZSO), and albendazole sulfone (ABZSO2) distribution in hosts and H. polygyrus and T. muris. H. polygyrus is depicted wave-shaped and T. muris banana-shaped. Thin continuous arrows indicate known and highly probable pathways of drugs; thick continuous arrows indicate pathways of drugs observed in this study, measured at concentrations above LLOQ; dashed arrows indicate assumed pathways of drugs; dashed crossed arrows indicate probable pathways, but not detected in vitro.
After oral application of albendazole sulfoxide the highest amounts of albendazole were found in the large intestine. Smaller amounts of albendazole were also found in the small intestinal content, but this might have originated from the passage through the stomach, where albendazole was present in slightly higher quantity. The high amounts of albendazole in the large intestine is unlikely to be due to efflux across the intestinal wall, since in the blood stream albendazole is quickly oxidized to its sulfoxide metabolite (Dayan, 2003). Therefore, it is probable that albendazole is either transformed from albendazole sulfoxide by the gut flora, or by the epithelial cells and released back into the gastrointestinal tract.
We also applied the treatments into the peritoneal cavity, a method used to circumvent the gastrointestinal tract to directly enter the blood stream - an alternative to intravenous injection for small animals (Turner et al., 2011). The qualitative distribution was similar to the oral treatments. The presence of albendazole sulfoxide in the gastrointestinal tract after intraperitoneal application of albendazole was not surprising since efflux of albendazole sulfoxide from the blood stream across the small intestinal wall into the gastrointestinal tract has been described for rats and sheep (Merino et al., 2003). After intraperitoneal injection of albendazole sulfoxide, albendazole was only found in the large intestinal content and the mucosal tissue; hence, albendazole seems to be exclusively formed in the large intestine.
Quantitatively speaking, we experienced immense differences in drug exposure between the two nematode models. While the drug exposures in the two models were in the same magnitude after oral treatment of albendazole, NMRI mice showed a 62-fold increase of albendazole sulfoxide exposure, and albendazole sulfone a 500-fold increased exposure after intraperitoneal treatments, whereas C57BL/10 mice showed no such difference. Whether these different exposures between mouse strains are due to differences in absorption into the circulation, efflux into the gastrointestinal tract or metabolic speed has yet to be evaluated.
Strongly elevated concentrations of albendazole and its metabolites were also observed in the mucosal tissue of NMRI mice intraperitoneally treated with albendazole. While the highest concentrations after oral application was at 30 μmol/g drug in mucosal tissue, after the intraperitoneal application the concentrations were above 400 μmol/g. However, sampling mucosal tissue was the most difficult of all compartment samples. Therefore, we evaluated the results for mucosal tissues with reservation. To further investigate the relationship between host mucosal tissue and the parasites and their drug exchange, we suggest experiments in a larger animal, such as the T. suis-pig model, offering larger sample quantities. Furthermore, an omnivorous animal might represent a more accurate model for human-parasite interactions than herbivorous mice.
Also H. polygyrus recovered from intraperitoneally treated mice revealed higher albendazole sulfone concentrations (Fig. 1J) compared to the oral treatments. The accumulation of albendazole sulfone could be a result of absorption from the host (Fig. 1C, F, and I) or a result of H. polygyrus absorbing albendazole or albendazole sulfoxide and metabolizing it to albendazole sulfoxide (Table 1).
Besides the host's metabolism, our in vitro experiments also showed drug biotransformation by the two helminths. Both were able to transform albendazole into the sulfoxide and the sulfone metabolites; H. polygyrus to a higher extent than T. muris. T. muris released albendazole sulfoxide only in very low quantities into the medium in vitro (Table 1), which suggests the albendazole sulfoxide found in the host's large intestine to origin from the host itself. Only H. polygyrus was able to reduce albendazole sulfone to albendazole sulfoxide. However, we did not observe a high rate of biotransformation to reach depletion of the respective parent compounds (data not shown). The ability of nematodes to metabolize absorbed drug and release the products into the environment further complicates the interpretation of in vivo drug uptake into the parasites. Moreover, the higher in vitro drug elimination by H. polygyrus (albendazole-susceptible nematode in vivo) as opposed to the lower elimination by T. muris (albendazole-unsusceptible nematode in vivo) imply that lack of drug activity is not a result of increased drug elimination in the helminth models used.
Interestingly, albendazole sulfone was absorbed more into T. muris than the more lipophilic albendazole and albendazole sulfoxide. This stands in contrast with the common hypothesis of a correlation between compound lipophilicity and drug absorption into helminths (Alvarez et al., 2007). At this point, we have no explanation for this finding.
ABT, an irreversible and CYP-unspecific inhibitor, was incorporated into the experimental design to assess the importance of CYP metabolism in the hookworm model. In the host, treatment of ABT prior to albendazole led to higher amounts of unchanged albendazole in the stomach and in the large intestine. Furthermore, elevated concentrations of albendazole sulfoxide were present the in plasma, especially in the gastrointestinal content. The elevated drug level after ABT pre-treatment are likely to be caused by CYP inhibition. However, since metabolism took place despite ABT pre-treatment, involvement of non-CYP enzymes in albendazole metabolism in the gut or incomplete CYP inactivation by ABT are probable. In contrast to ABT's effect on the distribution in the host, the worms recovered from ABT pre-treated mice prior to albendazole treatment showed no difference in the accumulation of albendazole and its metabolites compared to worms recovered from albendazole only treated mice. This finding is in line with our in vitro studies, where ABT had no effect on the uptake and elimination of albendazole by H. polygyrus. However, CYPs have been described to be involved in benzimidazole metabolism in various helminths (Matoušková et al., 2016), including H. polygyrus (Kerboeuf et al., 1995). Whether CYPs are not involved in albendazole metabolism in H. polygyrus, or whether ABT did not sufficiently inactivate the suitable CYP (Emoto et al., 2005) is not clear at this point. ABT's inhibitory effect in H. polygyrus drug metabolism could be further assessed using other drugs known for CYP metabolism.
The in vitro studies showed that the extent of drug uptake and elimination by the helminths is not always a function of the concentration of drug exposure. It is therefore crucial to test drug uptake and elimination at different concentrations and preferably at pharmacologically relevant concentrations to draw conclusions about a drug's accumulation in the worm.
To sum up, albendazole and its metabolites were highly distributed in the host after oral and intraperitoneal application. Additionally, the drug concentrations in worms recovered from treated mice did not correlate with any compartment of the host nor anthelmintic activity. Therefore, we could not determine the compartment responsible for albendazole and albendazole sulfoxide uptake and efficacy.
4.2. Distribution of mebendazole
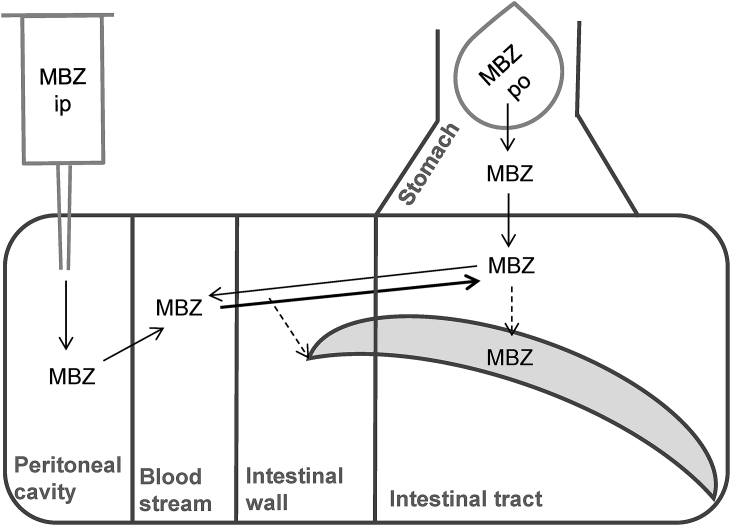
After oral application of mebendazole to T. muris-infected mice, the progression of mebendazole down the enteric route was visible with a continuous exposure of all compartments tested during 8 h. The distribution and pathways of mebendazole are depicted in Fig. 4. Comparable exposures were observed after intraperitoneal application compared to oral application of mebendazole in all compartments, also in the stomach. Efflux from the blood stream into the gastrointestinal tract might be the reason for mebendazole exposure in the gastrointestinal tract. However, to our knowledge, this mechanism has not been described. Mebendazole concentrations found in T. muris recovered from treated mice did not correlate with any compartment tested. Furthermore, in vitro exposure to serial drug dilution showed similar amounts of accumulation for all concentrations. Obviously, in case drug uptake into helminths reaches saturation, comparison between drug concentrations in different compartments of the host with the concentrations in the helminths cannot be used to identify the pathway of drug entry.
Fig. 4.
Scheme of mebendazole (MBZ) distribution in host and T. muris. T. muris is depicted banana-shaped. Thin continuous arrows indicate known and highly probable pathways of mebendazole; thick continuous arrows indicate pathways observed in this study, measured at concentrations above LLOQ; dashed arrows indicate assumed pathways.
Nevertheless, this study demonstrated that the higher efficacy of mebendazole against T. muris compared to albendazole (Table 2) is not a result of better drug absorption because post-treatment both drugs were measured in similar concentrations. In addition, our data hint that levels of mebendazole in the large intestine and cuticular penetration dominate the pathway of drug entry, since concentrations measured in T. muris recovered from treated mice follow rather the pattern of mebendazole found in the large intestine than in the plasma. However, if this pathway is responsible for the anthelmintic activity is not known.
4.3. Distribution of oxantel pamoate
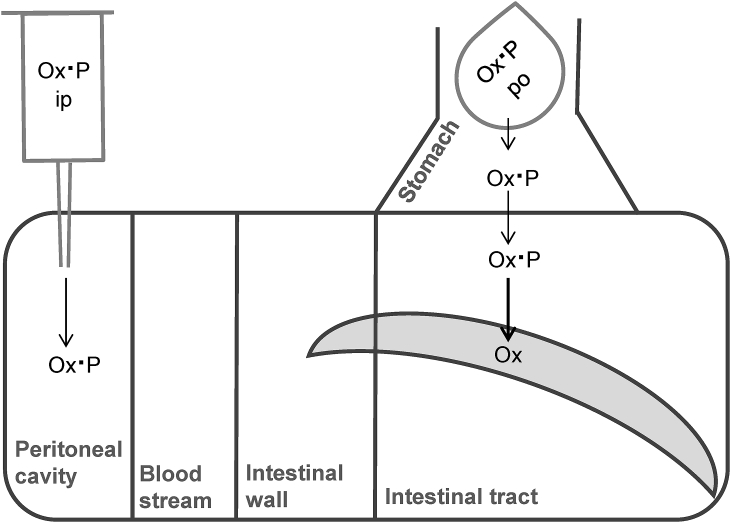
Also for oxantel pamoate a clear progression down the gastrointestinal tract was visible, with a persistent drug exposure during 8 h. The distribution and pathways of oxantel pamoate is depicted in Fig. 5. Three characteristics were noted: First, the stomach contained high amounts of oxantel pamoate at all time points after oral application, especially 2 h after treatment. The generally high values of oxantel pamoate in the gastrointestinal tract confirm that the drug is only little or not at all metabolized. Second, no plasma or mucosa sample contained oxantel pamoate in quantifiable amounts. Third, only very low concentrations of oxantel pamoate could be found in the host's intestinal tract after peritoneal application of the drug and the concentration in the parasites was negligible. These findings confirm that oxantel pamoate is not (or only very little) absorbed or generally distributed across compartments. T. muris recovered from mice treated with oxantel pamoate contained the drug after 5 and 8 h post-treatment (same time points at which oxantel pamoate was present in the large intestine), but contained none 2 h after oral as well as intraperitoneal treatment (sampling time points at which no oxantel pamoate was present in the large intestine). The results indicate that drug can enter T. muris only via gastrointestinal contents and activity is driven by drug levels in the large intestine.
Fig. 5.
Scheme of oxantel pamoate salt (Ox·P) distribution in host and T. muris. T. muris is depicted banana-shaped. Thin continuous arrows indicate known and highly probable pathways of oxantel pamoate; thick continuous arrows indicate pathways observed in this study, measured at concentrations above LLOQ; dashed arrows indicate assumed pathways.
4.4. Albendazole and mebendazole drug activities
In addition to the drug distribution and metabolism studies, we also assessed the in vitro and in vivo activity of albendazole sulfoxide against H. polygyrus. In vitro, H. polygyrus adults were not affected by albendazole sulfoxide; however, also albendazole and mebendazole are known for low activity against adult H. polygyrus in vitro (Table 2). In vivo, despite shorter exposure time we observed high activity of albendazole sulfoxide at a single oral dose of 100 mg/kg, with a WBR of 79%. Moreover, we tested the efficacy of albendazole with a pre-treatment with ABT to assess the influence of CYP inhibition: the worm burden was reduced by 84%, whereas for comparison, albendazole (100 mg/kg) and mebendazole (150 mg/kg) alone resulted in a worm reduction of 100% and 68%, respectively (Table 2). The reason for the differences between the in vitro and in vivo activities of albendazole is not known at the moment and are difficult to explain with the data generated in this study.
5. Conclusion
Our distribution studies showed no link between drug accumulation in the target parasite and drug efficacy. Clearly, drug efficacy is not a matter of how much drug accumulates in the worms, but how much drug interacts with the target. Currently, rodent models are widely used in preclinical pharmacokinetic studies due to their relatively easy handling and low costs (Zhang et al., 2012); however, comparable studies using animal models that are closer to human physiology, i.e. pigs, might be beneficial. More experiments employing “inert” compounds (such as oxantel pamoate, which do almost not leave the compartment of application and seems not to be metabolized) might be beneficial for further studies to exclude bias by ubiquitous presence and drug elimination. Furthermore, autoradiographic techniques (Solon, 2015) or imaging mass spectrometry (Karlsson and Hanrieder, 2016) might be employed to trace drug entry into the worm and distribution within the worm. Comparison of oral and intravenous application of such compounds might be valuable to elucidate the distribution, delivery to the target parasite and the resulting efficacy. However, whether a general pathway can be determined may be disputed.
Funding
This project was supported by the European Research Council (ERC-2013-CoG 614739-A_HERO).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2017.03.005.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1.
Supplementary Figure 2.
References
- Alvarez L.I., Mottier M.L., Lanusse C.E. Drug transfer into target helminth parasites. Trends Parasitol. 2007;23:97–104. doi: 10.1016/j.pt.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Bansemir A.D., Sukhdeo M.V. The food resource of adult Heligmosomoides polygyrus in the small intestine. J. Parasitol. 1994;80:24–28. [PubMed] [Google Scholar]
- Boily M.-O., Chauret N., Laterreur J., Leblond F.A., Boudreau C., Duquet M.-C., Lévesque J.-F., Ste-Marie L., Pichette V. In vitro and in vivo mechanistic studies toward understanding the role of 1-aminobenzotriazole in rat drug-drug interactions. Drug Metab. Dispos. Biol. Fate Chem. 2015;43:1960–1965. doi: 10.1124/dmd.115.066357. [DOI] [PubMed] [Google Scholar]
- Capece B.P., Calsamiglia S., Castells G., Arboix M., Cristòfol C. Effect of ruminal microflora on the biotransformation of netobimin, albendazole, albendazole sulfoxide, and albendazole sulfoxide enantiomers in an artificial rumen. J. Anim. Sci. 2001;79:1288–1294. doi: 10.2527/2001.7951288x. [DOI] [PubMed] [Google Scholar]
- Cowan N., Vargas M., Keiser J. In vitro and in vivo drug interaction study for two lead combinations oxantel pamoate plus albendazole and albendazole plus mebendazole for the treatment of human soil-transmitted helminthiasis. Antimicrob. Agents Chemother. 2016 doi: 10.1128/AAC.01217-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan A.D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141–159. doi: 10.1016/s0001-706x(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Emoto C., Murase S., Sawada Y., Iwasaki K. In vitro inhibitory effect of 1-aminobenzotriazole on drug oxidations in human liver microsomes: a comparison with SKF-525A. Drug Metab. Pharmacokinet. 2005;20:351–357. doi: 10.2133/dmpk.20.351. [DOI] [PubMed] [Google Scholar]
- Hansen T.V.A., Friis C., Nejsum P., Olsen A., Thamsborg S.M. Uptake of benzimidazoles by Trichuris suis in vivo in pigs. Int. J. Parasitol. Drugs Drug Resist. 2014;4:112–117. doi: 10.1016/j.ijpddr.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Hanrieder J. Imaging mass spectrometry in drug development and toxicology. Arch. Toxicol. 2016 doi: 10.1007/s00204-016-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Tritten L., Adelfio R., Vargas M. Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo. Parasit. Vectors. 2012;5:292. doi: 10.1186/1756-3305-5-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Tritten L., Silbereisen A., Speich B., Adelfio R., Vargas M. Activity of oxantel pamoate monotherapy and combination chemotherapy against Trichuris muris and hookworms: revival of an old drug. PLoS Negl. Trop. Dis. 2013;7:e2119. doi: 10.1371/journal.pntd.0002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- Kerboeuf D., Soubieux D., Guilluy R., Brazier J.L., Rivière J.L. In vivo metabolism of aminopyrine by the larvae of the helminth Heligmosomoides polygyrus. Parasitol. Res. 1995;81:302–304. doi: 10.1007/BF00931534. [DOI] [PubMed] [Google Scholar]
- Lloberas M., Alvarez L., Entrocasso C., Virkel G., Lanusse C., Lifschitz A. Measurement of ivermectin concentrations in target worms and host gastrointestinal tissues: influence of the route of administration on the activity against resistant Haemonchus contortus in lambs. Exp. Parasitol. 2012;131:304–309. doi: 10.1016/j.exppara.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Matoušková P., Vokřál I., Lamka J., Skálová L. The role of xenobiotic-metabolizing enzymes in anthelmintic deactivation and resistance in helminths. Trends Parasitol. 2016;32:481–491. doi: 10.1016/j.pt.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Merino G., Molina A.J., García J.L., Pulido M.M., Prieto J.G., Alvarez A.I. Intestinal elimination of albendazole sulfoxide: pharmacokinetic effects of inhibitors. Int. J. Pharm. 2003;263:123–132. doi: 10.1016/s0378-5173(03)00369-7. [DOI] [PubMed] [Google Scholar]
- Mikov M. The metabolism of drugs by the gut flora. Eur. J. Drug Metab. Pharmacokinet. 1994;19:201–207. doi: 10.1007/BF03188922. [DOI] [PubMed] [Google Scholar]
- Nishimuta H., Nakagawa T., Nomura N., Yabuki M. Significance of reductive metabolism in human intestine and quantitative prediction of intestinal first-pass metabolism by cytosolic reductive enzymes. Drug Metab. Dispos. Biol. Fate Chem. 2013;41:1104–1111. doi: 10.1124/dmd.113.051177. [DOI] [PubMed] [Google Scholar]
- Padmanabhan P., Grosse J., Asad A.B.M.A., Radda G.K., Golay X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013;3:60. doi: 10.1186/2191-219X-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawden H.C., Kokwaro G.O., Ward S.A., Edwards G. Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes. Br. J. Clin. Pharmacol. 2000;49:313–322. doi: 10.1046/j.1365-2125.2000.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon E.G. Autoradiography techniques and quantification of drug distribution. Cell Tissue Res. 2015;360:87–107. doi: 10.1007/s00441-014-2093-4. [DOI] [PubMed] [Google Scholar]
- Speich B., Ali S.M., Ame S.M., Bogoch I.I., Alles R., Huwyler J., Albonico M., Hattendorf J., Utzinger J., Keiser J. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial. Lancet Infect. Dis. 2015;15:277–284. doi: 10.1016/S1473-3099(14)71050-3. [DOI] [PubMed] [Google Scholar]
- Tilney L.G., Connelly P.S., Guild G.M., Vranich K.A., Artis D. Adaptation of a nematode parasite to living within the mammalian epithelium. J. Exp. Zool. A Comp. Exp. Biol. 2005;303:927–945. doi: 10.1002/jez.a.214. [DOI] [PubMed] [Google Scholar]
- Tritten L., Nwosu U., Vargas M., Keiser J. In vitro and in vivo efficacy of tribendimidine and its metabolites alone and in combination against the hookworms Heligmosomoides bakeri and Ancylostoma ceylanicum. Acta Trop. 2012;122:101–107. doi: 10.1016/j.actatropica.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. JAALAS. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . 2006. Preventive Chemotherapy in Human Helminthiasis. [Google Scholar]
- Zhang D., Luo G., Ding X., Lu C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B. 2012;2:549–561. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.