Abstract
Prostaglandin E2 (PGE2) has been implicated in cell invasion in hepatocellular carcinoma (HCC), via increased β1-integrin expression and cell migration; however, the mechanism remains unclear. PGE2 exerts its effects via four subtypes of the E prostanoid receptor (EP receptor 1–4). The present study investigated the effect of EP1 receptor activation on β1-integrin expression and cell migration in HCC. Cell migration increased by 60% in cells treated with 17-PT-PGE2 (EP1 agonist), which was suppressed by pretreatment with a β1-integrin polyclonal antibody. PGE2 increased β1-integrin expression by approximately 2-fold. EP1 receptor transfection or treatment with 17-PT-PGE2 mimicked the effect of PGE2 treatment. EP1 siRNA blocked PGE2-mediated β1-integrin expression. 17-PT-PGE2 treatment induced PKC and NF-κB activation; PKC and NF-κB inhibitors suppressed 17-PT-PGE2-mediated β1-integrin expression. FoxC2, a β1-integrin transcription factor, was also upregulated by 17-PT-PGE2. NF-κB inhibitor suppressed 17-PT-PGE2-mediated FoxC2 upregulation. Immunohistochemistry showed p65, FoxC2, EP1 receptor and β1-integrin were all highly expressed in the HCC cases. This study suggested that PGE2 upregulates β1-integrin expression and cell migration in HCC cells by activating the PKC/NF-κB signaling pathway. Targeting PGE2/EP1/PKC/NF-κB/FoxC2/β1-integrin pathway may represent a new therapeutic strategy for the prevention and treatment of this cancer.
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer death in the United States and worldwide, especially in males1,2. Recent cases of HCC are increasing in United States and Canada2. Although a combination of resection and chemotherapy can improve survival, HCC prognosis is still extremely poor, especially in advanced HCC, which is often associated with malignant migration and metastasis3.
Prostaglandin E2 (PGE2), one of most important products of cyclooxygenase-2 (COX-2), has been proposed as an important cellular factor associated with tumor development in many types of cancers4,5,6,7. Previous studies indicated that COX-2 expression was upregulated in many cancer tissues and that exogenous PGE2 increased cancer cell growth, migration and invasion5,6,7,8. In hepatocellular carcinoma, PGE2 was reported to activate Akt and FAK signaling pathways to promote cell proliferation and migration8,9, and to upregulate MMP-2 expression to promote cell invasion10. New targets aimed at cellular COX-2/PGE2 signaling pathways have provided therapeutic strategies for the treatment of metastasis of HCC11.
Integrins are a family of transmembrane cellular receptors that mediate cell-cell and cell-matrix interactions. They are heterodimeric glycoproteins, serve as adhesion receptors for ECM proteins and also transduce biochemical signals into the cell. These receptors are composed of an α and a β subunit. Integrins of the β1-family mainly transduce signals from the extracellular matrix to modulate growth, differentiation, invasion or metastasis12. β1-integrin has been implicated in cell proliferation, adhesion and metastasis in a wide variety of human cancers, including breast, colon and ovary13,14,15,16. In HCC, β1-integrin is necessary for cell migration17 and protects tumor cells from chemotherapy-induced apoptosis18. Recently, β1-integrin was identified as a suitable marker in HCC identification, classification, prevention and treatment19,20.
In Huh-7 cells, PGE2 increased β1-integrin expression and promoted cell adhesion and migration10. However, the exact mechanism remains largely unknown. PGE2 regulates tumor development and progression by combining with E prostanoid receptors (EP receptors) on the surface of the cell membrane21. Our data showed that the EP1 receptor plays a major role in PGE2-mediated β1-integrin expression. The current study suggested that PGE2 regulates β1-integrin expression and cell migration in HCC cells through the EP1 receptor, and the PKC/NF-κB/FoxC2 signaling pathway may be involved in EP1 receptor-mediated β1-integrin upregulation.
Results
The EP1 receptor is involved in PGE2-mediated β1-integrin expression and cell migration in HCC cells
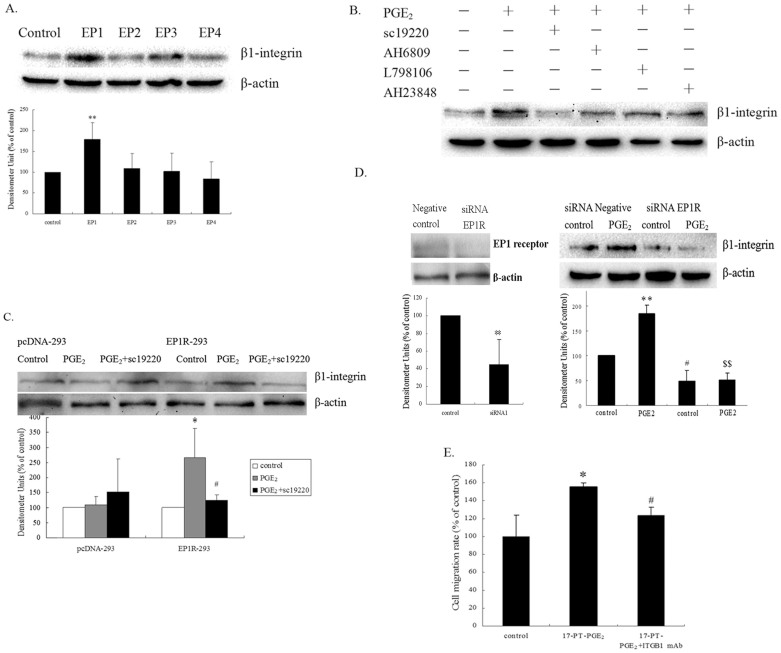
Huh-7 cells were treated with EP1, EP2, EP3 and EP4 receptor agonists. Fig. 1A showed that treatment with butaprost (EP2 agonist), sulprostone (EP3 agonist) and PGE1 alcohol (EP4 agonist), respectively, had little or no effect on β1-integrin expression. By contrast, treatment with 17-PT-PGE2, a specific agonist of EP1 receptor, significantly enhanced β1-integrin expression. Pretreatment with antagonists of EP receptors in Huh-7 cells showed mild effects on PGE2-mediated β1-integrin upregulation, except for treatment with sc-19220, a specific antagonist of the EP1 receptor, which markedly blocked PGE2-mediated β1-integrin upregulation (Fig. 1B).
Figure 1. EP1 receptor activation promoted β1-integrin expression in hepatocellular carcinoma cells.
(A). Effects of EP agonists on β1-integrin expression in Huh-7 cells. Huh-7 cells were exposed to 5 μM EP1 agonist (17-PT-PGE2), EP2 agonist (butaprost), EP3 agonist (sulprostone) and EP4 agonist (PGE1 alcohol) for 24 h, respectively. The cropped gels are used and full-length gels are presented in Supplementary Figure S1 and S2. (B). Effects of EP antagonists on PGE2-mediated β1-integrin expression in Huh-7 cells. Huh-7 cells were pretreated with various EP antagonists for 1 h, followed by PGE2 for 24 h (EP1 antagonist sc19220, EP2 antagonist AH6809 and EP3 antagonist L-798106, EP4 antagonist AH23848). The cropped gels are used and full-length gels are presented in Supplementary Figure S3 and S4. (C). Effects of expression of the EP1 receptor on PGE2-mediated β1-integrin regulation in HEK293 cells. HEK293 cells (3 × 105 cells) were transfected with EP1R-pcDNA3 plasmid or empty pcDNA3 plasmid as a control. After transfection, cells expressing the EP1 receptor were selected by G418. EP1 receptor-transfected HEK293 cells were exposed to PGE2 for 24 h, with or without sc19220 pre-treatment. Results are presented as the mean ± SD from three different experiments. *P < 0.05, compared to control cells; #P < 0.05, compared with PGE2-treated cells. (D). RNA interference targeting the EP1 receptor suppressed PGE2-mediated β1-integrin upregulation in Huh-7 cells. Huh-7 cells were transfected with an EP1R-siRNA. After 72 h, the cells were exposed to PGE2 for 24 h. The cropped gels are used and full-length gels are presented in Supplementary Figure S5 and S6. Results are shown as the mean ± SD from three different experiments. ** indicates a significant difference at P < 0.01 compared with the cells without PGE2 treatment; # indicates a significant difference at P < 0.05 compared with the siRNA negative control cells. $$ indicates a significant difference at P < 0.01 compared with the siRNA negative control cells after PGE2 treatment. (E). Effect of anti-β1-integrin antibody on 17-PT-PGE2-mediated cell migration in Huh-7 cells. The cell migration assay was performed in a12-well transwell. Huh-7 cells were pretreated with an anti-β1-integrin antibody for 30 min, followed by stimulation with PGE2. The in vitro migration activity was measured after 24 h. Results are presented as the mean ± SD from three different experiments. *P < 0.05, compared with control cells; #P < 0.05, compared with 17-PT-PGE2 –treated group. The gels have been run under the same experimental conditions.
To corroborate the role of the EP1 receptor in the induction of β1-integrin expression, HEK293 cells were transfected with the EP1R-pcDNA3. Fig. 1C showed that expression of the EP1 receptor did not alter the basal expression level of the β1-integrin protein. However, β1-integrin expression was significantly upregulated in the EP1R-transfected cells (compared with the control cells) when treated with PGE2. The PGE2-induced β1-integrin expression was diminished by the addition of sc-19220 in EP1R-transfected cells.
To further study the specific role of EP1 in β1-integrin expression, Huh-7 cells were transfected with an EP1R siRNA. As shown in Fig. 1D, depletion of the EP1 receptor greatly reduced the basal level of β1-integrin protein. PGE2 induced β1-integrin expression was completely blocked in the EP1R siRNA-transfected cells.
To demonstrate if β1-integrin was involved in EP1 receptor-mediated cell migration in HCC cells, Huh-7 cells were pretreated with a β1-integrin polyclonal antibody (AB1952P) for 30 min, followed by the incubation with 17-PT-PGE2. Cell migration was increased by 60% when treated with 17-PT-PGE2. The pretreatment by the β1-integrin polyclonal antibody (3 μg/ml) significantly inhibited 17-PT-PGE2-mediated cell migration (Fig. 1E). These data indicated that β1-integrin plays an important role in EP1 receptor-mediated cell migration in HCC cells, and PGE2 increased β1-integrin expression and promoted cell migration via the EP1 receptor in Huh-7 cells.
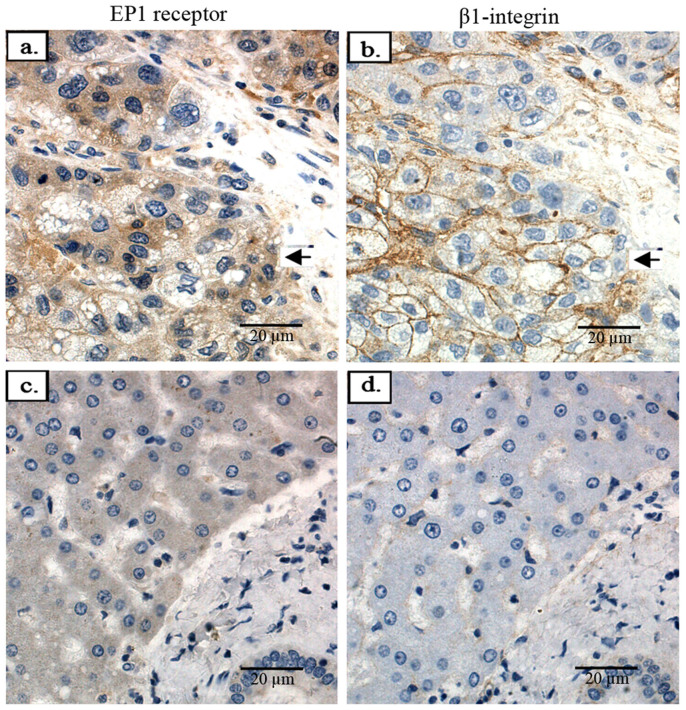
We went on to detect the correlationship between the expression of the EP1 receptor and β1-integrin in liver cancer tissues. By immunohistochemistry, all 24 samples showed positive EP1 receptor expression in the cytoplasm and membrane. Of the 20 HCC cases tested, β1-integrin expression was mainly found in the membrane of the cancer cells. The samples expressing higher levels of EP1 receptor also displayed higher levels of β1-integrin expression; the normal liver tissue samples with lower levels of EP1 receptor showed lower or even negative expression of β1-integrin (Fig. 2).
Figure 2. The expression of EP1 receptor and β1-integrin in liver cancer tissues.
(a). Representative immunohistochemical images of human hepatocellular carcinoma tissue stained with the anti-human EP1 receptor antibody. (b). Representative immunohistochemical images of human hepatocellular carcinoma tissue stained with the anti-human β1-integrin antibody. (c). Representative immunohistochemical images of normal human liver tissue stained with the anti- EP1 receptor antibody. (d). Representative immunohistochemical images of normal human liver tissue stained with the anti-β1-integrin antibody. (Magnification: ×400).
PKC is involved in EP1 receptor-mediated β1-integrin expression
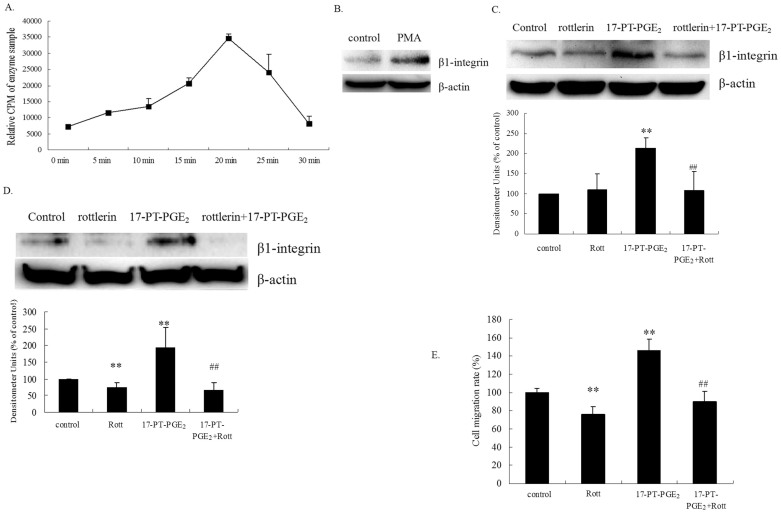
The relationship between PKC activation and β1-integrin expression was examined in the present study. PKC activity in response to 17-PT-PGE2 treatment was measured in Huh-7 cells. Treatment of Huh-7 cells with 17-PT-PGE2 for 15 min resulted in an approximately 2-fold increase in PKC activity, reaching a maximal response (approximately 6-fold) after 20 min of treatment (Fig. 3A). PKC activator phorbol-12-myristate-13-acetate (PMA) markedly increased β1-integrin expression in Huh-7 cells (Fig. 3B). Pre-treatment of cells with the PKC inhibitor rottlerin significantly reduced the 17-PT-PGE2-mediated β1-integrin expression (Fig. 3C). Similarly, pre-treatment of cells with rottlerin diminished 17-PT-PGE2-increased β1-integrin expression in EP1R-transfected HEK293 cells (Fig. 3D). In addition, rottlerin inhibited the EP1 receptor-mediated cell migration (Fig. 3E) in Huh-7 cells.
Figure 3. PKC is involved in EP1 receptor-mediated β1-integrin upregulation in hepatocellular carcinoma cells.
(A). PKC activity assay. Huh-7 cells were treated with 5 μM 17-PT-PGE2 for 0, 5, 10, 15, 20, 25 or 30 min. Equal amounts of total proteins (30 μg) were added to microcentrifuge tubes and assayed for PKC levels using a direct human PKC enzyme activity assay kit. (B). Effect of a PKC activator on β1-integrin expression in Huh-7 cells. Huh-7 cells were treated with 100 nM PMA for 24 h. Total protein was isolated and visualized with an anti-β1-integrin antibody. Levels of β-actin served as a loading control. (C). Effect of a PKC inhibitor on 17-PT-PGE2-mediated β1-integrin expression in Huh-7 cells. Huh-7 cells were treated with 17-PT-PGE2 for 24 h, with or without pre-treatment of 5 μM rottlerin for 1 h. Total protein was isolated and visualized with an anti-β1-integrin antibody. Levels of β-actin served as a loading control. The cropped gels are used and full-length gels are presented in Supplementary Figure S7. Results are presented as the mean ± SD from three different experiments. **P < 0.01, compared with control cells; ##P < 0.01, compared with 17-PT-PGE2 –treated group. (D). Effect of a PKC inhibitor on 17-PT-PGE2-mediated β1-integrin expression in EP1 receptor-expressed HEK293 cells. Stable EP1 receptor-expressed HEK293 cells were treated with 17-PT-PGE2 for 24 h, with or without pre-treatment of rottlerin for 1 h. Total protein was isolated and visualized with an anti-β1-integrin antibody. Levels of β-actin served as a loading control. Results are presented as the mean ± SD from three different experiments. **P < 0.01, compared with control cells; ##P < 0.01, compared with 17-PT-PGE2 –treated group. (E). Effect of a PKC inhibitor on 17-PT-PGE2-mediated cell migration in Huh-7 cells. The cell migration assay was performed in a12-well transwell plate. Huh-7 cells were treated with 17-PT-PGE2 for 12 h, with or without pre-treatment of 5 μM rottlerin for 1 h. Cells on the lower surface were stained with 0.1% crystal violet, solubilized with acetic acid solution and quantified by measuring their absorbance at 570 nm. Results are presented as the mean ± SD from three different experiments. **P < 0.01, compared with control cells; ##P < 0.01, compared with 17-PT-PGE2-treated cells. The gels have been run under the same experimental conditions.
NF-κB is involved in EP1 receptor-mediated β1-integrin expression
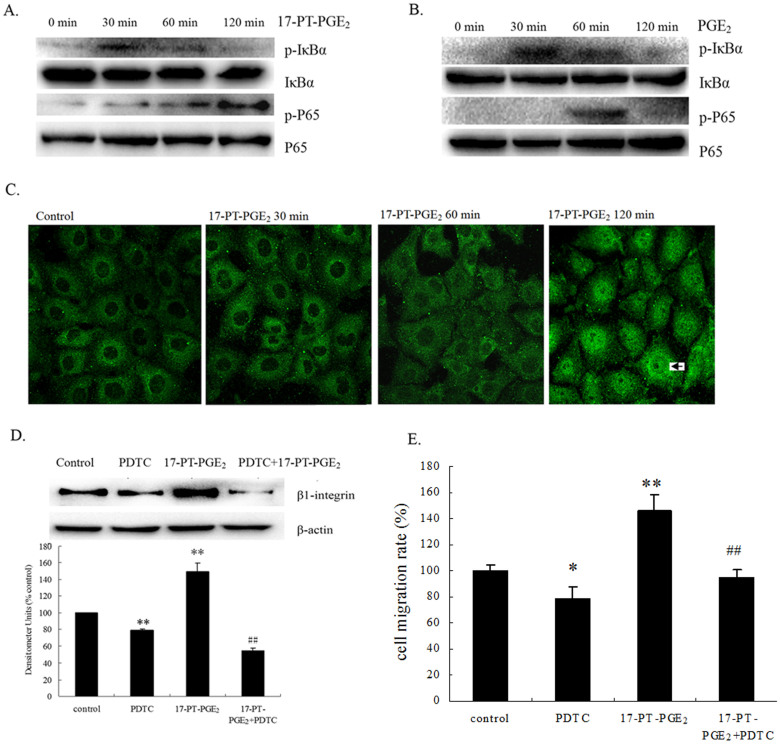
To examine whether NF-κB activation is involved in EP1-induced β1-integrin expression in HCC cells, we detected the phosphorylation of p65 and its upstream molecules regarding EP1 receptor activation. Huh-7 cells were exposed to 17-PT-PGE2 for different periods of time. As shown in Fig. 4A, an increase in p65 phosphorylation at the Ser536 site was detected after 17-PT-PGE2 treatment, reaching its peak 120 min after treatment. For the upstream molecules of p65, IκB-α phosphorylation at the Ser32/36 site was upregulated 30 min after 17-PT-PGE2 treatment. A similar response was found in EP1R-transfected HEK293 cells: in Fig. 4B, the increase in IκB-α and p65 phosphorylation were detected after PGE2 treatment. Furthermore, p65 translocation was detected by immunofluorescence. As shown in Fig. 4C, Huh-7 cells were exposed to 17-PT-PGE2 treatment for different periods of time. Normally, p65 was located in the cytoplasm; after 17-PT-PGE2 treatment for 120 min, activated p65 was translocated into the nuclei. To clarify the role of the NF-κB pathway in EP1 receptor-mediated β1-integrin expression in HCC cells, an NF-κB inhibitor, Ammonium pyrrolidinedithiocarbamate (PDTC), was added to Huh-7 cells. Pretreatment with PDTC inhibited the 17-PT-PGE2-mediated β1-integrin expression (Fig. 4D) and cell migration (Fig. 4E) in Huh-7 cells.
Figure 4. NF-κB is involved in EP1 receptor-mediated β1-integrin upregulation in hepatocellular carcinoma cells.
(A). Effects of 17-PT-PGE2 on NF-κB and IκB phosphorylation in Huh-7 cells. Huh-7 cells were treated with 5 μM 17-P-T-PGE2 for 0, 30, 60, 120 min. Equal amounts of total proteins were separated by SDS-PAGE. Relative levels of phosphorylated and total IκBα or p65 were determined using specific antibodies. The cropped gels are used and full-length gels are presented in Supplementary Figure S8 and S9. (B). Effects of PGE2 on NF-κB and IκB phosphorylation in EP1 receptor-expressed HEK293 cells. EP1 receptor-expressed HEK293 cells were treated with PGE2 for 0, 30, 60, 120 min. (C). Effects of 17-PT-PGE2 on NF-κB translocation in Huh-7 cells. Huh-7 cells were treated with 5 μM 17-PT-PGE2 for 0, 30, 60, 120 min; the cells were then fixed with ice-cold methanol. The p65 protein was detected by immunofluorescence and activated p65 was translocated into the nuclie (arrow). All pictures were taken at 400× magnification. (D). Effect of NF-κB inhibitor on 17-PT-PGE2-mediated β1-integrin expression in Huh-7 cells. Huh-7 cells were treated with 17-PT-PGE2 for 24 h, with or without pre-treatment of PDTC for 24 h. Total protein was isolated and visualized with an anti-β1-integrin antibody. Levels of β-actin served as a loading control. The cropped gels are used and full-length gels are presented in Supplementary Figure S10 and S11. Densitometric quantitation of the above blots is shown. Results are presented as the mean ± SD from three different experiments. **P < 0.01, compared with control cells; ##P < 0.01, compared with 17-PT-PGE2-treated cells. (E). Effect of NF-κB inhibitor on 17-PT-PGE2-mediated cell migration in Huh-7 cells. The cell migration assay was performed in a 12-well transwell plate. Huh-7 cells were treated with 17-PT-PGE2 for 12 h, with or without pre-treatment of 10 μM PDTC for 24 h. Results are presented as the mean ± SD from three different experiments. **P < 0.01, compared with control cells; ##P < 0.01, compared with 17-PT-PGE2-treated cells. The gels have been run under the same experimental conditions.
FoxC2 is involved in the NF-κB signal pathway in EP1 receptor-mediated β1-integrin expression
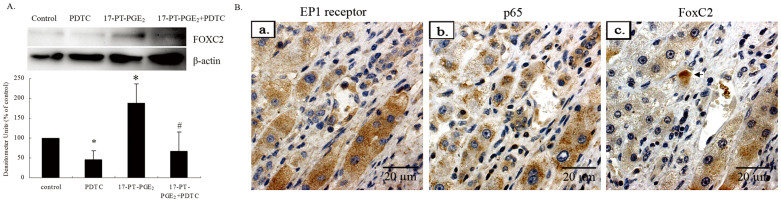
To investigate the direct involvement of the NF-κB pathway in the induction of β1-integrin expression, we analyzed the sequences of human integrin β1 promoter in detail. However, the 2.0-kb fragment upstream of the start codon does not contain any NF-κB transcription factor-binding elements (http://genome.ucsc.edu/cgi-bin/hgTracks). Thus, NF-κB regulation of β1-integrin expression may not act by direct binding to its promotor. There must be another trasnscription factor that binds to the β1-integrin promotor directly to upregulate its expression. Transcripitional regulatory elements are located in chr10:33,239,813–33,330,260. In addition, we identified many Fox-binding elements (FBEs) in the promoter of β1-integrin. (http://genome.ucsc.edu/cgi-bin/hgGateway). Recently, the forkhead transcription factor FoxC2 was reported to upregulate β1-integrin expression by directly binding FBEs in the integrin β1 promoter22. We detected the role of FoxC2 in the EP1 receptor/NF-κB pathway in Huh-7 cells. 17-PT-PGE2 treatment increased FoxC2 expression significantly, while PDTC inhibited 17-PT-PGE2-mediated FoxC2 upregulation completely (Fig. 5A). Thus, the EP1 receptor/NF-κB signal pathway may upregulate β1-integrin expression by promoting FoxC2 expression.
Figure 5. FoxC2 is involved in EP1 receptor/NF-κB-mediated β1-integrin upregulation in hepatocellular carcinoma.
(A). Effect of NF-κB in EP1 receptor-mediated FoxC2 upregulation in hepatocellular carcinoma cells. Huh-7 cells were treated with 17-PT-PGE2 for 24 h, with or without pre-treatment of PDTC for 24 h. Total protein was isolated and visualized with an anti-FoxC2 antibody. Levels of β-actin served as a loading control. The cropped gels are used and full-length gels are presented in Supplementary Figure S12. Densitometric quantitation of the above blots is shown. Results are presented as the mean ± SD from three different experiments. *P < 0.05, compared with control cells; #P < 0.05, compared with 17-PT-PGE2-treated cells. The gels have been run under the same experimental conditions. (B). Co-expression of EP1 receptor, p65 and FoxC2 in liver cancer tissues. (a). Representative immunohistochemical images of human hepatocellular carcinoma tissue stained with the anti-human EP1 receptor antibody. (b). Representative immunohistochemical images of hepatocellular carcinoma tissue stained with the anti-p65 antibody. (c). Representative immunohistochemical images of hepatocellular carcinoma tissue stained with the anti-FoxC2 antibody. (Magnification: ×400).
To observe the effects of FoxC2 and NF-κB on EP1 receptor-mediated β1-integrin expression in HCC tissues, the sections were incubated with anti-p65 and FoxC2 antibodies. By immunohistochemistry, all 20 HCC cases showed positive p65 expression, mainly in the cytoplasm. FoxC2 was expressed mainly in the cytoplasm and some in nuclei (arrow). Serial sections showed highly expression of both p65, FoxC2 and EP1 receptor (Fig. 5B).
Discussion
COX-2-mediated production of PGE2 is involved in cell growth and metastasis of various cancers4,8,23,24,25. Integrins are a family of cell surface receptors for extracellular matrix (ECM) proteins26,27. Among them, β1-integrin-mediated attachment to the ECM results in an activation of protein tyrosine kinases that protect cells from chemotherapy-induced apoptosis28. β1-integrin was highly expressed in liver cancer tissue and mice model20,29. PGE2 improves β1-integrin expression in many cells30,31,32,33. However, the mechanism of PGE2-mediated β1-integrin expression remained unknown in HCC cells.
PGE2 exerts its effects by coupling to four subtypes of the EP receptor21,34. Among the four receptor types, the EP1 receptor was shown to play an important role in the development of many cancer types: EP1 receptor activated PKC/c-Src pathway in primary cultured rat osteoblasts35. The data from our previous studies showed that the EP1 receptor upregulated survivin expression and FAK phosphorylation to promote cell growth and migration in HCC cells36,37. PGE2 promoted human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the EGFR, ERK, CREB and Akt38,39,40. However, until now, little was known about the association between the EP1 receptor and the integrin family.
In the current study, EP1 receptor activation enhanced β1-integrin expression in Huh-7 cells. RNA interference of the EP1 receptor inhibited PGE2-mediated β1-integrin upregulation. Pretreatment with a β1-integrin antibody inhibited 17-PT-PGE2-mediated cell migration. Furthermore, immunohistochemical staining of human HCC tissues showed that EP1 receptor and β1-integrin are both highly expressed in liver cancer tissues. These data suggested that EP1 receptor may promote cell migration by increasing β1-integrin expression in HCC cells.
The PKC family was first identified as intracellular receptors for tumor promoting phorbol esters41. Recently, activation of PKC is thought to play a central role in the regulation of cellular responsiveness to external stimuli41,42. PKC is associated with the development of HCC. For example: PKC expression is significantly correlated with tumor size and the tumor/node/metastasis (TNM) stage43; Reduction of PKC expression by RNA interference or selective inhibitors greatly decreased cell proliferation, migration and invasion44. Our previous results indicated that PKC is involved in EP1 receptor-mediated cell adhesion and migration in HCC cells37. The present data showed that PKC activities were enhanced after 17-PT-PGE2 treatment. The involvement of PKC in EP1 receptor-mediated β1-integrin expression was further confirmed using PMA. In addition, pre-treatment with the rottlerin diminished the 17-PT-PGE2-mediated β1-integrin expression and cell migration.
Transcription factors of the nuclear factor κB (NF-κB)/Rel family play a pivotal role in the inflammatory response and neoplastic development45,46. There are five family members in mammals: RelA (p65), c-Rel, RelB, NF-κB1 (p105/p50) and NF-κB2 (p100/p52). The RelA/p65 activating signaling pathway is a critical regulator for cell growth, differentiation, and tumorigenic transformation45. Indeed, p65 is constitutively activated at Ser536 in cancer cells, and is then translocated from the cytosol into the nucleus to regulate gene expression47,48. NF-κB activation is necessary for the cell migration and invasion49,50. Recently, NF-κB-p65 was found to be involved in progression and development of HCC51,52.
IκB proteins were phosphorylated at Ser32 and Ser36, releasing NF-κB to enter the nucleus where it regulates gene expression45,53. IκB phosphorylation is mediated by a high molecular weight signalsome complex comprising two IκB kinases (IKKα and IKKβ). IKK induces IκBα phosphorylation and degradation, NF-κB nuclear translocation and NF-κB DNA binding activity54.
PKC isoforms play a key role in mediating the NF-κB signal pathway. PKCθ is essential for TCR-initiated NF-kB activation; PKCθ activates NF-κB through the selective induction of IKK55,56,57. Our data show that PKC is involved in EP1 receptor-mediated β1-integrin upregulation; therefore, we hypothesize that NF-κB is also involved in this process. Our study showed that EP1 activation stimulated IκBα phosphorylation, followed by p65 activation and translocation from the cytosol into the nucleus. The NF-κB inhibitor PDTC diminished the 17-PT-PGE2-mediated β1-integrin upregulation and cell migration.
The involvement of NF-κB signal pathway in EP1 receptor-mediated β1-integrin expression suggested the presence of an NF-κB response element in the promotor of β1-integrin; however, we did not find one. Thus, other transcription factor(s) must bind to the β1-integrin promotor directly and be regulated the by NF-κB signal pathway. Interestingly, there are many FBEs in the promotor of β1-integrin in liver cells. The forkhead transcription factor Foxc2 enhances the expression of β1-integrin in osteoblast cells by direct binding to a FBE in its promoter22.
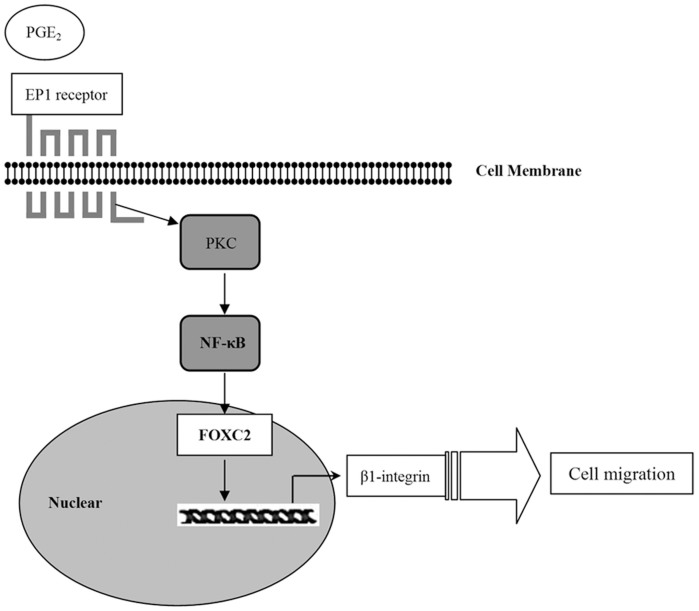
FoxC2 is a member of the family of winged helix/forkhead transcription factors. It is strongly expressed in the developing embryo and is required for various developmental processes58. In particular, FoxC2 is highly expressed in breast cancer, esophageal cancer and colon cancer, and increase the metastatic potential59. NF-κB upregulates FoxC2 expression60; therefore, we investigated the role of FoxC2 in EP1 receptor/NF-κB-mediated β1-integrin expression. Little is known about the association between the EP receptors and FoxC2. Our results showed that EP1 receptor activation increased FoxC2 expression and PDTC pretreatment suppressed 17-PT-PGE2–mediated FoxC2 upregulation. Furthermore, immunohistochemistry showed EP1 receptor, p65, and FoxC2 were all highly expressed in HCC tissues. These data suggested that FoxC2 is also involved in EP1 receptor/NF-κB-mediated β1-integrin expression (Fig. 6).
Figure 6. Proposed mechanisms for PGE2/EP1 receptor-mediated hepatocellular carcinoma cell migration.
Our data showed that the EP1 receptor played a key role in PGE2-mediated hepatocellular carcinoma cell migration. EP1 receptor may upregulate β1-integrin expression to improve cell migration. PKC/NF-κB/FOXC2 signaling pathways were involved in EP1 receptor-mediated β1-integrin expression.
We demonstrated that the PGE2 can upregulate β1-integrin expression via the EP1 receptor to promote HCC cell migration. PKC and NF-κB signaling pathways are involved in EP1 receptor-mediated β1-integrin expression. Our findings provide important new information regarding the putative role of the EP1 receptor in β1-integrin expression in HCC cells and suggest that targeting the PGE2/EP1/PKC/NF-κB/FoxC2/β1-integrin signal pathway may represent a new therapeutic strategy for the prevention and treatment of this malignant disease.
Methods
Materials
The human HCC cell line, Huh-7, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Dulbecco's modified Eagle's medium (DMEM) and Lipofectamine™ 2000 were from Invitrogen (Carlsbad, CA, USA). PGE2, 17-phenyl trinor-PGE2 (17-PT-PGE2), Butaprost, Sulprostone, PGE1 alcohol, sc19220, AH6809 and AH23848 were from Cayman Chemical Co (Ann Arbor, MI, USA). Ammonium pyrrolidinedithiocarbamate (PDTC) and L-798106 were from Sigma-Aldrich (St. Louis, MO, USA). Rottlerin (#557370) and phorbol-12-myristate-13-acetate (PMA, #524400) were obtained from Merck (Darmstadt, Germany). The protein assay was from Bio-Rad (Hercules, CA, USA). Electrochemiluminescence (ECL) reagents were from Amersham Biosciences (Piscataway, NJ, USA). The transwell unit (12-well) was from Costar Corning (USA). The PKC assay kit was from Millipore (Billerica, MA, USA). [γ-32P]ATP (#BLU002A) was from Perkin-Elmer (Waltham, MA, USA). G418 sulfate was from Amresco (Solon, OH, USA). The following were commercially obtained antibodies: the anti-EP1 receptor antibody was obtained from Cayman Chemical Co. (Ann Arbor, MI); the anti-β1-integrin antibodies were obtained from BD Bioscience (#610467, Becton Dickinson, USA) and Chemicon International (#AB1952P, USA); the anti-phosphorylated IκBα antibody (#9246s) and anti-phosphorylated p65 antibody at Ser536 (#3036s) were obtained from Cell Signaling Technology (Danvers, MA, USA); the anti-IκBα antibody (#ab7217) was obtained from Abcam plc (Cambridge, UK); the anti-p65 antibody (#sc-372) and the FITC-labeled secondary antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); the anti-β-actin antibody was obtained from Sigma Chemical Co. (St. Louis, MO). EnVisionTM + single reagents (Mouse, Rabbit) were from DAKO (K4000, K4002, Glostrup, Denmark).
Cell lines and culture
Huh-7 cell line and HEK293 cells were cultured in DMEM with 10% fetal calf serum, 100 IU/ml penicillin and 100 g/ml streptomycin at 37°C with 5% CO2.
Patients and specimens
Primary surgical specimens were obtained from 20 patients (aged from 36 to 61; average is 48) who were clinically diagnosed for HCC, from the first affiliated hospital with Nanjing Medical University between Jan. 2013 and June 2013. Samples 5, 8, 18 have intrahepatic metastasis. None of them had distant metastasis. All of them were approached for participation in the project. There were four surgical specimens of normal liver tissue. All the specimens were collected following approval from the Human Ethics Committee of Nanjing Medical University. The Work conforms to the provisions of the Declaration of Helsinki in 1995 (as revised in Tokyo 2004). All the written informed consent from the donors were obtained for use of these samples in research. Resected specimens were fixed with 10% paraformaldehyde and embedded in paraffin blocks.
Immunohistochemical staining
Sections (4 μm) of 20 tumor blocks were used for immunohistochemical analysis. The slides were placed in boiling citric acid buffer (10 mM sodium citrate and citric acid) for 10 minutes. Sections were treated with primary antibodies β1-integrin (#610467, BD Bioscience), EP1 receptor, p65, and FoxC2, applied at a 1:100 or 1:200 dilution and incubated overnight at 4°C. Bound antibody was detected using EnVision polymer technology. After a complete wash in PBS, the slides were developed in freshly prepared diaminobenzedine solution (DAB) for 8 min, and then counterstained with hematoxylin. PBS substituted for the primary antibody as a negative control. The sections were photographed by LeiCa microscopy and Image analyse system. 4 low power views (400×) were randomly selected from each samples in a blind manner.
Cell migration assays
Cell migration assays were performed in 12-well transwell units. Before the experiment, the lower surfaces of the membranes were coated with gelatin (1%). Huh-7 cells (5 × 104) were added to the upper chamber. Pharmacological agents were added at the indicated times. After incubation at 37°C for 12 h, the cells were fixed with ethanol and then stained with 0.1% crystal violet. After washing with PBS, the cells were removed from the upper surface of the membrane by wiping with moist cotton swabs. Cells migrated to the lower surface of the membrane were solubilized with 300 μl of 10% acetic acid and quantified by measuring the absorbance at 570 nm.
PKC measurements
Cells were treated with pharmacological agents at 37°C for various times, as indicated in the experiments. The cells were collected into lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, 100 μg/ml PMSF and aprotinin), and then cleared by centrifugation at 12,000 × g for 15 min at 4°C. PKC levels were assayed using a direct human PKC enzyme activity assay kit, according to the manufacturer's instructions. Briefly, 10 μl of substrate cocktail, PKA inhibitor cocktail, Assay Dilution Buffer II (ADBII), lipid activator and diluted [γ-32P]-ATP mixture were added to a microcentrifuge tube and then the mixture was incubated for 10 min at 30°C with constant agitation.
A 25-μl aliquot from each sample was transferred onto the center of a P81 phosphocellulose paper. The assay squares were washed with 0.75% phosphoric acid three times, followed by one wash with acetone. The assay squares were transferred to vials with a scintillation cocktail and read in a scintillation counter. The counts per minute (cpm) of the enzyme samples were compared to those of the control samples containing no enzyme.
Plasmid transfections
The pcDNA3-based plasmid encoding the human EP1 receptor (EP1R-pcDNA3) was a generous gift of Kathy McCusker in 2007 (Merck Frosst Centre for Therapeutic Research, Canada). HEK293 cells (2 × 105) were seeded in 6-well culture plates and transfected with the EP1R-pcDNA3 plasmid or pcDNA3 empty vector control (2 μg) using Lipofectamine 2000TM (5 μl). The efficiency of transfection was assayed by flow cytometry. The G418 antibiotic was used to select for HEK293 cells stably expressing the EP1 receptor.
Immunofluorescence assay
Huh-7 cells (2 × 105) were cultured in 6-well plates for 24 h, followed by 17-PT-PGE2 treatment for various period of time. The cells were fixed with ice-cold methanol, permeabilized with 0.1% Triton X-100 in PBS, and incubated at 4 °C overnight with anti-p65 receptor antibody (1:100 dilution). Antibody binding was localized using an FITC-labeled secondary antibody. The cells were visualized using a Confocal Microscope (400×).
RNA interference
The siRNAs targeting the EP1 receptor (EP1R-siRNA) (ID: s194727) were obtained from Ambion. The sequence of the siRNAs used was ACUUCUAAGCACAACCAGAtt (sense sequence). Huh-7 cells (4 × 104) were plated in 12-well plates for 24 h, resulting in a 30–50% confluent cell monolayer. The cells were then transfected with the EP1R-siRNA, or a non-silencing 21-nucleotide irrelevant RNA duplex as a negative control, using LipofectamineTM 2000. After 72 h, depletion of the EP1 receptor was confirmed by western blotting, and the cells were subsequently used for further experiments.
Western blotting
Cells were treated with pharmacological agents at 37°C for various times, as indicated in the experiments. The cells were collected into lysis buffer and then cleared by centrifugation at 12,000 × g for 15 min at 4°C. Equal amounts of total proteins (40 μg) were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. The membranes were probed with the appropriate antibodies at 4°C overnight. The immunoreactivity was detected by ECL and analyzed using Image lab 4.0 analysis software from Bio-Rad.
Statistical analysis
All data are presented as means ± SD. P-values were calculated using the Student's t-test for unpaired samples with MS Excel software. The results were considered significantly different at P < 0.05.
Supplementary Material
Full length gel in figure 1,3,4,5
Acknowledgments
We thank Dr Kathy Mccusker (Merck Frosst Centre for Therapeutic Research, Canada) for providing the pcDNA3 plasmid construct encoding the EP1 receptor. This study was supported in part by the National Natural Science Foundation of China (81101496, 81172003), Research Fund for the Doctoral Program of Higher Education of China (20113234120009) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
The authors declare no competing financial interests.
Author Contributions X.B. participated to the design of the study, to performed and interpreted W.B. analysis on EP1/PKC/NF-κB/FoxC2/β1-integrin pathway and drafted the manuscript. J.W. participated to the collection and interpreted W.B. analysis on EP1/NF-κB/β1-integrin pathway. Y.G. and J.P. performed and interpreted immunohistochemical analyses of EP1 receptor, β1-integrin, p65 and FoxC2. Q.Y. helped to the design of the study, collected and analysed data. M.Z. and H.L. collected samples and carried out immunohistochemical analyses of EP1 receptor and p65 in tumour tissue. L.Z. and J.M. coordinated and interpreted molecular studies and participated in drafting the manuscript. F.S. helped to the design and coordination of the study, and to draft the manuscript. W.S. and Y.W. helped to draft the manuscript. J.L. conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- El-Serag H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 142, 1264–1273 e1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervello M. et al. Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget 3, 236–260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H. & Oshima M. The role of PGE2-associated inflammatory responses in gastric cancer development. Semin Immunopathol. 35, 139–150 (2012). [DOI] [PubMed] [Google Scholar]
- Li Z. et al. PGE2 promotes renal carcinoma cell invasion through activated RalA. Oncogene 32, 1408–1415 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J. et al. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One 7, e46342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan K. et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 65, 6275–6281 (2005). [DOI] [PubMed] [Google Scholar]
- Leng J. et al. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through AKT activation: evidence for AKT inhibition in celecoxib-induced apoptosis. Hepatology 38, 756–768 (2003). [DOI] [PubMed] [Google Scholar]
- Bai X. M. et al. Focal adhesion kinase: important to prostaglandin E2-mediated adhesion, migration and invasion in hepatocellular carcinoma cells. Oncol Rep. 21, 129–136 (2009). [PubMed] [Google Scholar]
- Mayoral R. et al. Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis 26, 753–761 (2005). [DOI] [PubMed] [Google Scholar]
- Wu T. Cyclooxygenase-2 in hepatocellular carcinoma. Cancer Treatment Reviews 32, 28–44 (2006). [DOI] [PubMed] [Google Scholar]
- Schuppan D. & Ocker M. Integrin-mediated control of cell growth. Hepatology 38, 289–291 (2003). [DOI] [PubMed] [Google Scholar]
- Yao E. S. et al. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 67, 659–664 (2007). [DOI] [PubMed] [Google Scholar]
- Nikkola J. et al. Integrin chains beta1 and alphav as prognostic factors in human metastatic melanoma. Melanoma Res. 14, 29–37 (2004). [DOI] [PubMed] [Google Scholar]
- Muller-Klingspor V. et al. Prognostic value of beta1-integrin (= CD29) in serous adenocarcinomas of the ovary. Anticancer Res. 21, 2185–2188 (2001). [PubMed] [Google Scholar]
- Seales E. C. et al. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 65, 4645–4652 (2005). [DOI] [PubMed] [Google Scholar]
- Carloni V. et al. The integrin, alpha6beta1, is necessary for the matrix-dependent activation of FAK and MAP kinase and the migration of human hepatocarcinoma cells. Hepatology 34, 42–49 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Beta 1-integrin protects hepatoma cells from chemotherapy induced apoptosis via a mitogen-activated protein kinase dependent pathway. Cancer 95, 896–906 (2002). [DOI] [PubMed] [Google Scholar]
- Fujimoto A. et al. Identification of cell surface antigen expression in canine hepatocellular carcinoma cell lines. J Vet Med Sci. 75, 831–835 (2013). [DOI] [PubMed] [Google Scholar]
- Lai K. K. et al. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet 7, e1002147 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boie Y. et al. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 340, 227–241 (1997). [DOI] [PubMed] [Google Scholar]
- Park S. J. et al. The forkhead transcription factor Foxc2 promotes osteoblastogenesis via up-regulation of integrin beta1 expression. Bone 49, 428–438 (2011). [DOI] [PubMed] [Google Scholar]
- Abrahao A. C. et al. A role for COX2-derived PGE2 and PGE2-receptor subtypes in head and neck squamous carcinoma cell proliferation. Oral Oncol. 46, 880–887 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T., Gupta S. & Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 191, 125–135 (2003). [DOI] [PubMed] [Google Scholar]
- Zhao Q. T. et al. Potential involvement of the cyclooxygenase-2 pathway in hepatocellular carcinoma-associated angiogenesis. Life Sci. 80, 484–492 (2007). [DOI] [PubMed] [Google Scholar]
- Cary L. A. & Guan J. L. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 4, D102–113 (1999). [DOI] [PubMed] [Google Scholar]
- Missan D. S. & DiPersio M. Integrin control of tumor invasion. Crit Rev Eukaryot Gene Expr. 22, 309–324 (2012). [DOI] [PubMed] [Google Scholar]
- Sethi T. et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 5, 662–668 (1999). [DOI] [PubMed] [Google Scholar]
- Ozaki I. et al. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut 43, 837–842 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierro E. et al. In vitro regulation of beta1 and beta3 integrin subunits in endometrial epithelial cells from normal endometrium. Am J Reprod Immunol. 49, 373–376 (2003). [DOI] [PubMed] [Google Scholar]
- Liu J. F. et al. Cyclooxygenase-2 enhances alpha2beta1 integrin expression and cell migration via EP1 dependent signaling pathway in human chondrosarcoma cells. Mol Cancer. 9, 43–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto M. et al. Enhancement of activated beta1-integrin expression by prostaglandin E2 via EP receptors in isolated human coronary arterial endothelial cells: implication for the treatment of Kawasaki disease. Inflamm Res. 58, 224–228 (2009). [DOI] [PubMed] [Google Scholar]
- Yazawa K. et al. Selective inhibition of cyclooxygenase-2 inhibits colon cancer cell adhesion to extracellular matrix by decreased expression of beta1 integrin. Cancer Sci. 96, 93–99 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y. & Narumiya S. Prostaglandin E receptors. J Biol Chem. 282, 11613–11617 (2007). [DOI] [PubMed] [Google Scholar]
- Tang C. H., Yang R. S. & Fu W. M. Prostaglandin E2 stimulates fibronectin expression through EP1 receptor, phospholipase C, protein kinase Calpha, and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem. 280, 22907–22916 (2005). [DOI] [PubMed] [Google Scholar]
- Bai X. M. et al. Prostaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cells. Life Sci. 86, 214–223 (2010). [DOI] [PubMed] [Google Scholar]
- Bai X. et al. Prostaglandin E(2) receptor EP1-mediated phosphorylation of focal adhesion kinase enhances cell adhesion and migration in hepatocellular carcinoma cells. Int J Oncol. 42, 1833–1841 (2013). [DOI] [PubMed] [Google Scholar]
- Han C. & Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 280, 24053–24063 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Prostaglandin E2 enhances mitogen-activated protein kinase/Erk pathway in human cholangiocarcinoma cells: involvement of EP1 receptor, calcium and EGF receptors signaling. Mol Cell Biochem. 305, 19–26 (2007). [DOI] [PubMed] [Google Scholar]
- Sun B. et al. Prostaglandin E2 receptor EP1 phosphorylate CREB and mediates MMP2 expression in human cholangiocarcinoma cells. Mol Cell Biochem. 378, 195–203 (2013). [DOI] [PubMed] [Google Scholar]
- Bosco R. et al. Fine tuning of protein kinase C (PKC) isoforms in cancer: shortening the distance from the laboratory to the bedside. Mini Rev Med Chem. 11, 185–199 (2011). [DOI] [PubMed] [Google Scholar]
- Rosse C. et al. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 11, 103–112 (2010). [DOI] [PubMed] [Google Scholar]
- Wu T. T. et al. Overexpression of protein kinase C alpha mRNA in human hepatocellular carcinoma: a potential marker of disease prognosis. Clin Chim Acta. 382, 54–58 (2007). [DOI] [PubMed] [Google Scholar]
- Wu T. T. et al. Reduction of PKC alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J Cell Biochem. 103, 9–20 (2008). [DOI] [PubMed] [Google Scholar]
- Chen F., Beezhold K. & Castranova V. Tumor promoting or tumor suppressing of NF-kappa B, a matter of cell context dependency. Int Rev Immunol. 27, 183–204 (2008). [DOI] [PubMed] [Google Scholar]
- Sen R. & Smale S. T. Selectivity of the NF-{kappa}B response. Cold Spring Harb Perspect Biol. 2, a000257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Sanda T. & Asamitsu K. NF-kappa B signaling and carcinogenesis. Curr Pharm Des. 13, 447–462 (2007). [DOI] [PubMed] [Google Scholar]
- Cheng C. Y. et al. IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br J Pharmacol. 160, 1595–1610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. et al. Effects of simvastatin and rosuvastatin on RAS protein, matrix metalloproteinases and NF-kappaB in lung cancer and in normal pulmonary tissues. Cell Prolif. 46, 172–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q. et al. Overexpression of response gene to complement 32 (RGC32) promotes cell invasion and induces epithelial-mesenchymal transition in lung cancer cells via the NF-kappaB signaling pathway. Tumour Biol. 34, 2995–3002 (2013). [DOI] [PubMed] [Google Scholar]
- Li W. et al. Constitutive activation of nuclear factor-kappa B (NF-kB) signaling pathway in fibrolamellar hepatocellular carcinoma. Int J Clin Exp Pathol. 3, 238–243 (2010). [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. Interleukin 23 promotes hepatocellular carcinoma metastasis via NF-kappa B induced matrix metalloproteinase 9 expression. PLoS One. 7, e46264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melisi D. & Chiao P. J. NF-kappa B as a target for cancer therapy. Expert Opin Ther Targets. 11, 133–144 (2007). [DOI] [PubMed] [Google Scholar]
- Li X. et al. IKKalpha, IKKbeta, and NEMO/IKKgamma are each required for the NF-kappa B-mediated inflammatory response program. J Biol Chem. 277, 45129–45140 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalitakul S. et al. Vaspin prevents TNF-alpha-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-kappaB and PKCtheta activation in cultured rat vascular smooth muscle cells. Pharmacol Res. 64, 493–500 (2011). [DOI] [PubMed] [Google Scholar]
- Sun Z. et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404, 402–407 (2000). [DOI] [PubMed] [Google Scholar]
- Lin X. et al. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 20, 2933–2940 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson P. & Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 250, 1–23 (2002). [DOI] [PubMed] [Google Scholar]
- Kume T. The Role of FoxC2 Transcription Factor in Tumor Angiogenesis. J Oncol. 2012, 204593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. H. et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 32, 431–443 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full length gel in figure 1,3,4,5