Abstract
Curcumin, a specific secondary metabolite of Curcuma species, has potentials for a variety of beneficial health effects. It is nowadays used as a dietary supplement. Everolimus (EVL) is an immunosuppressant indicated for allograft rejection and cancer therapy, but with narrow therapeutic window. EVL is a substrate of P-glycoprotein (P-gp) and cytochrome P450 3A4 (CYP3A4). This study investigated the effect of coadministration of curcumin on the pharmacokinetics of EVL in rats and the underlying mechanisms. EVL (0.5 mg/kg) was orally administered without and with 50 and 100 mg/kg of curcumin, respectively, in rats. Blood samples were collected at specific time points and EVL concentrations in blood were determined by QMS® immunoassay. The underlying mechanisms were evaluated using cell model and recombinant CYP 3A4 isozyme. The results indicated that 50 and 100 mg/kg of curcumin significantly decreased the AUC0-540 of EVL by 70.6% and 71.5%, respectively, and both dosages reduced the Cmax of EVL by 76.7%. Mechanism studies revealed that CYP3A4 was markedly activated by curcumin metabolites, which apparently overrode the inhibition effects of curcumin on P-gp. In conclusion, oral intake of curcumin significantly decreased the bioavailability of EVL, a probe substrate of P-gp/CYP 3A4, mainly through marked activation on CYP 3A4.
Curcumin (Diferuloylmethane), a polyphenol constituent in spice turmeric, is a specific secondary metabolite of Curcuma longa, C. zedoaria, C. aromatica, C. wenyujin and C. kwangsiensis1. Numerous beneficial health effects of curcumin have been reported such as vascular protection2, cancer prevention3 and anti-Alzheimer's disease4, etc. Therefore, curcumin is nowadays used as a dietary supplement. Pharmacokinetic studies of oral curcumin indicated that the major molecules in the systemic circulation were the glucuronides and sulfates of curcumin, whereas only trace of the parent form of curcumin was detected in rats and humans5,6. Besides, demethoxycurcumin and bis-demethoxycurcumin, the phase I metabolites of curcumin, as well as their conjugated metabolites have also been detected in mice7.
Everolimus (EVL), a macrolide immunosuppressant, is usually prescribed with corticosteroids and cyclosporine to prevent kidney, liver or heart transplant rejection in clinical practice8. In recent years, EVL has also been used to treat advanced breast cancer9 and advanced renal cell carcinoma10. The recommended therapeutic range of EVL is 3–8 ng/mL in renal and cardiac transplant patients. The higher acute rejection rate was reported when the trough EVL level was below 3 ng/mL. On the other hand, higher EVL trough level had been associated with increasing incidence of thrombocytopenia11. In addition, pulmonary toxicity is a potentially life - threatening complication of EVL12. Commonly reported adverse effects of EVL included stomatitis, hyperglycemia, rash and fatigue13,14. Most of the adverse effects are dose-related, and dosage reduction or discontinuation of EVL is usually adopted to avoid severe adverse reactions12,13,14.
In regard to the pharmacokinetics of EVL, the oral bioavailability was only 15–16% with peak concentration being reached within 0.5–4.0 h, and the half-life is 18–35 h15. Owing to its narrow therapeutic index, regular blood concentration monitoring of EVL is required to ensure therapeutic efficacy15,16,17. EVL is a substrate of P-glycoprotein (P-gp), a drug-efflux transporter, and primarily metabolized by CYP3A4, an important drug-metabolizing enzyme12. Any potential modulation effects on P-gp or CYP 3A4 may alter the oral bioavailability of EVL, which might affect the efficacy or toxicity of EVL in patients.
Numerous in vitro studies have reported that curcumin inhibited not only P-gp18,19,20, but also CYP3A421,22,23. Based on these in vitro results, coadministration of curcumin should increase the oral bioavailability of substrates of P-gp or CYP 3A4. However, till now no in vivo relevant evidence has been reported in literature. Therefore, this study was set to investigate the effect of coadministration of curcumin on the pharmacokinetics of EVL, a probe substrate of P-gp/CYP 3A4, in rats. Furthermore, the underlying mechanisms of interaction were evaluated using cell model and recombinant CYP 3A4 isozyme.
Results
Effect of curcumin on EVL pharmacokinetics in rats
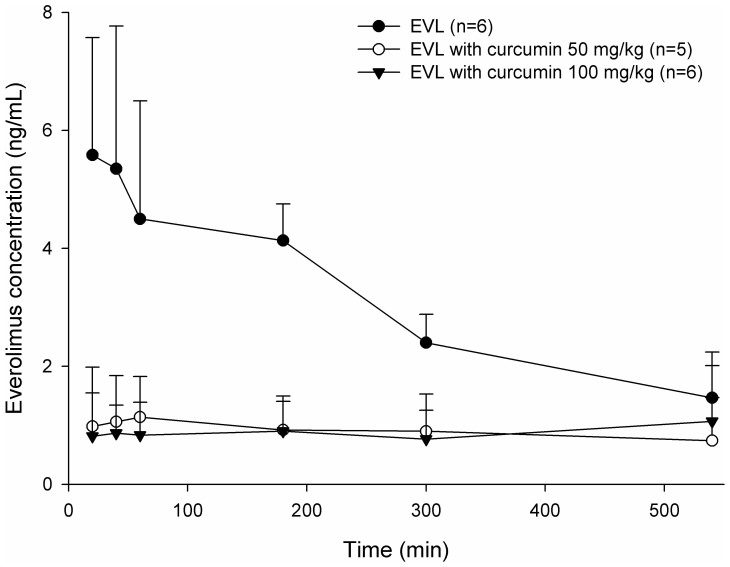
The blood EVL concentration – time profiles after oral administration of EVL alone and coadministrations with 50 or 100 mg/kg of curcumin are shown in Figure 1. The pharmacokinetic parameters of EVL after various treatments are given in Table 1. The results showed that 50 and 100 mg/kg of curcumin both significantly decreased the Cmax of EVL by 76.7%, and reduced the AUC0-540 by 70.6% and 71.5%, respectively. The MRT of EVL was significantly increased by 100 mg/kg of curcumin by 35.3%, but not affected by 50 mg/kg of curcumin.
Figure 1. Mean (±S.D.) blood concentration-time profiles of everolimus (EVL) after oral administration of EVL alone ( , 0.5 mg/kg) and coadministration with 50 mg/kg (
, 0.5 mg/kg) and coadministration with 50 mg/kg ( , n = 5) and 100 mg/kg (
, n = 5) and 100 mg/kg ( ) of curcumin to rats (n = 6).
) of curcumin to rats (n = 6).
Table 1. Pharmacokinetic parameters of EVL in rats after various treatments.
| TreatmentsParameter | EVL alone | EVL + curcumin (50 mg/kg) | EVL+ curcumin (100 mg/kg) |
|---|---|---|---|
| Cmax | 6.0 ± 1.8 | 1.4 ± 0.9 *** (−76.7%) | 1.4 ± 1.2 *** (−76.7%) |
| AUC0−540 | 1637.7 ± 256.8 | 481.8 ± 327.8 *** (−70.6%) | 466.0 ± 330.2 *** (−71.5%) |
| MRT | 207.8 ± 23.8 | 251.2 ± 28.5 | 281.2 ± 37.6 ** (35.3%) |
Data are expressed as the mean ± S.D.
*p <0.05, ** p < 0.01, *** p < 0.001.
Cmax (ng/mL): peak blood concentration.
AUC0−540 (ng·min/mL): area under the blood concentration–time curve from 0 to 540 min.
MRT (min): mean residence time.
Effect of curcumin on P-gp activity
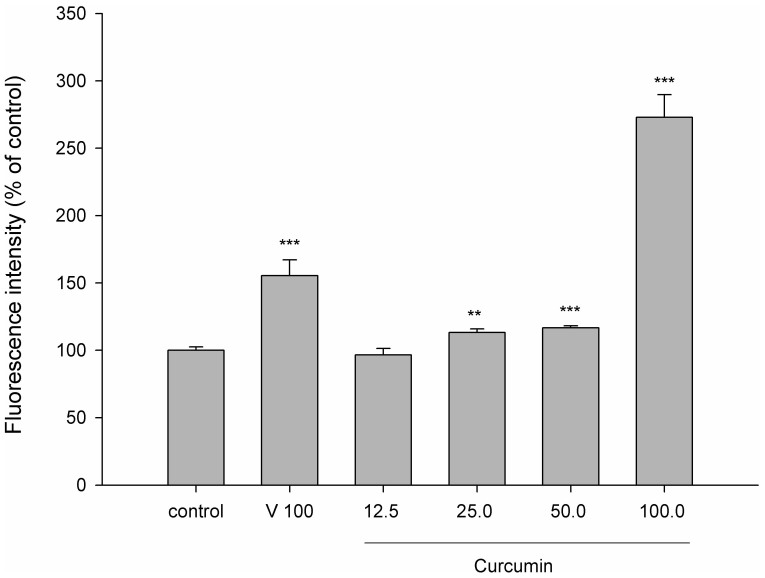
Figure 2 shows the effect of curcumin on the intracellular accumulation of rhodamine 123, a typical substrate of P-gp. The results indicated that 25, 50 and 100 μM of curcumin significantly decreased the efflux function of P-gp by 13.2%, 16.7% and 172.9%, respectively. As a positive control of P-gp inhibitor, verapamil inhibited the intracellular accumulation of rhodamine 123 by 55.5%.
Figure 2. Effect of curcumin (µg/mL) on the intracellular accumulation of rhodamine 123 in LS 180 cells.
C: control, V: verapamil (positive control of P-gp inhibitor, 100 µg/mL). **p < 0.01, *** p < 0.001.
Characterization of curcumin serum metabolites (CSM)
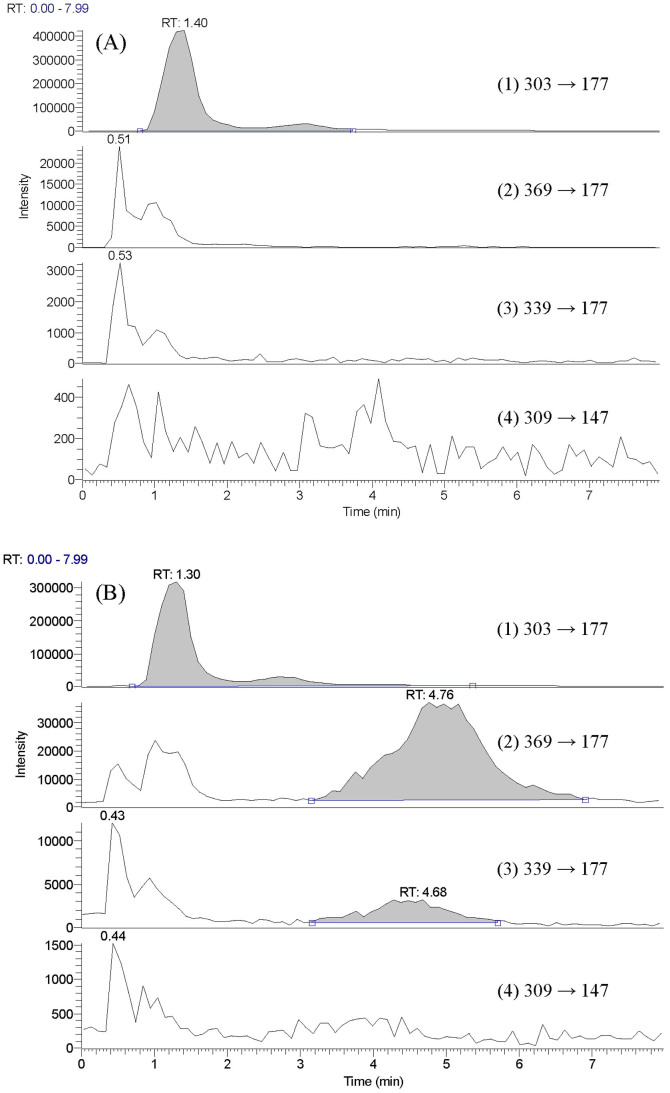
An LC-MS/MS method using selective reaction monitoring (SRM) technique was performed to analyze CSM prior to and after treatment with sulfataser/glucuronidase. The results shown in Figure 3(A) indicated that curcumin, demethoxycurcumin and bis-demethoxycurcumin were not detected before enzymatic hydrolysis of CSM. After treatment with sulfatase/glucuronidase, the peaks of curcumin and demethoxycurcumin emerged, indicating that the glucuronides/sulfates of curcumin and demethoxycurcumin were present in CSM. Apparently, the concentration of curcumin glucuronides/sulfates was higher than that of demethoxycurcumin glucuronides/sulfates.
Figure 3. LC-MS/MS chromatograms of hesperetin (1, internal standard), curcumin (2), demethoxycurcumin (3) and bis-demethoxycurcumin (4) in serum prior to (A) and after hydrolysis with sulfatase/glucuronidase (B).
Effects of curcumin, demethoxycurcumin, bis-demethoxycurcumin and CSM on CYP 3A4 activity
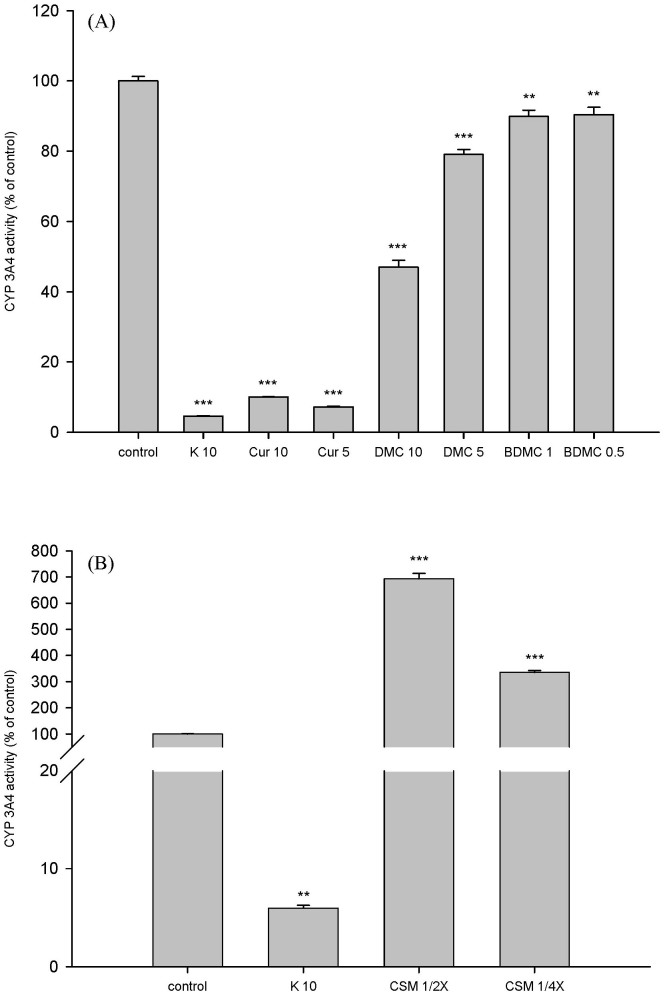
The effects of curcumin, demethoxycurcumin, bis-demethoxycurcumin and CSM on CYP 3A4 activity are shown in Figure 4(A) and 4(B), respectively. Curcumin at 5 and 10 µM significantly inhibited the activity of CYP 3A4 by 93% and 90%, respectively. Demethoxycurcumin at 5 and 10 µM significantly inhibited the activity of CYP 3A4 by 21% and 53%, respectively. Bis-demethoxycurcumin at 0.5 and 1.0 µM significantly inhibited the activity of CYP 3A4 by 10%.
Figure 4. Effects of curcumin (Cur, µM), demethoxycurcumin (DMC, µM), bis-demethoxycurcumin (BDMC, µM) (A) and CSM (1/2- and 1/4-fold serum concentrations) (B) on CYP 3A4 activity.
K: ketoconazole (positive control of CYP 3A4 inhibitor, µM). *p <0.05, **p < 0.01, *** p < 0.001.
On the contrary, CSM at 1/4- and 1/2-fold serum concentrations significantly increased the activity of CYP 3A4 by 235% and 593%, respectively, when compared to those of correspondent concentrations of blank serum specimen. As a positive control of CYP 3A4 inhibitor, ketoconazole significantly decreased CYP 3A4 activity by 95%.
Discussion
In this study, EVL was used as a probe substrate of P-gp/CYP 3A4. The results showing that the Cmax and AUC0-540 were markedly decreased by coadministration of curcumin at both dosages of 50 and 100 mg/kg indicated that the oral bioavailability of EVL in rats was significantly reduced by concurrent intake of curcumin. Observation on the blood profiles of EVL revealed that the absorption of EVL was apparently hampered by curcumin. In regard to the magnitudes of interactions, two dosages of curcumin demonstrated comparable influences, implying that the interaction machinery had been saturated at the lower dosage.
EVL is a substrate of P-gp and CYP 3A412. The absorption of EVL should be highly correlated with the function and expression of P-gp and CYP 3A4. In order to delineate the underlying mechanism of the acute inhibition effect of curcumin on EVL absorption, in vitro and ex-vivo models were employed to investigate the effects of oral curcumin on the activities of P-gp and CYP 3A4, respectively.
In P-gp mediated transport study of rhodamine 123, the result showing that curcumin significantly increased the intracellular accumulation of rhodamine 123 indicated that the efflux activity of P-gp was inhibited by curcumin, which was in good agreement with several previous reports18,19,20, although a different cell model was used in the present study. We thus can infer that this inhibition effect of curcumin on P-gp did not play an important role in the mechanism of decreased absorption of EVL in rats.
For evaluating the in vivo effect of curcumin on CYP 3A4 activity, CSM was prepared from rats receiving curcumin to mimic the virtual molecules interacting with CYP 3A4 in the enterocytes and hepatocytes based on the consideration of metabolic fate of curcumin5,6,7. After characterization by LC-MS/MS method, CSM was found containing curcumin metabolites including the glucuronides/sulfates of curcumin and demethoxycurcumin, whereas the free forms of curcumin, demethoxycurcumin and bis-demethoxycurcumin were not detected. This finding further confirmed that curcumin was rapidly and extensively metabolized after oral intake5,6,7. The results of CYP 3A4 assay showed that 1/2- and 1/4-fold serum concentrations of CSM remarkably increased the activity of CYP 3A4, which could account for the markedly decreased absorption of EVL via enhancing the first pass effect during the early phase. Accordingly, the activation effect of CSM on CYP 3A4 could be attributed to the conjugated metabolites of curcumin and/or demethoxycurcumin. We thus strongly suggest that using the metabolites of curcumin in in vitro studies to investigate the bioactivities was important for understanding the virtual effects and mechanism of curcumin in the in vivo system.
In order to investigate the structure-activity relationship of curcuminoids regarding their modulation on CYP 3A4, three pure compounds including curcumin, demethoxycurcumin and bis-demethoxycurcumin were in parallel evaluated with CSM using this specific method. The results showing that curcumin inhibited CYP 3A4 was in good agreement with previous in vitro studies21,22,23. Like curcumin, demethoxycurcumin and bis-demethoxycurcumin also inhibited the activity of CYP 3A4, but in lesser extent. Apparently, the inhibition effects of curcuminoid free forms on CYP 3A4 were opposite to the activation effect by CSM. In fact, free forms of these curcuminoids might have no chance to interact with CYPs located in the enterocytes and hepatocytes judged from their rapid metabolism by conjugation reactions5,6,7.
Taken together, our mechanism studies showed that curcumin inhibited P-gp, whereas CSM markedly activated CYP 3A4. Based on the effect of oral corcumin on the pharmacokinetics of EVL in rats, it clearly implied that the activation effect on CYP 3A4 by CSM had overwhelmed the inhibition effect on P-gp by curcumin, which resulted in a net effect of decreased absorption of EVL.
CYP 3A4 is an important human metabolizing enzyme present in intestine and liver. Clinically, more than 50% of drugs are metabolized by CYP3A4, including proton pump inhibitors (esomeprazole, omeprazole), antihyperlipidemic (atorvastatin, simvastatin), anti-HIV agents (indinavir, ritonavir), anti-infection agents (erythromycin, ketoconazole,), immunosuppressants (cyclosporine, tacrolimus), anti-hypertensive agents (amlodipine, felodipine), anticonvulsants (carbamazepine), anti-depressants (quetiapine, sertraline) and anti-cancer agents (paclitaxel, vinblastine), etc24. Moreover, it has been well recognized that CYP 3A4 was involved in numerous clinical life-threatening drug-drug interactions, such as ketoconazole-terfenadine25 and simvastatin-cisapride26. In addition, grapefruit-felodipine interaction was arisen from inhibition on intestinal CYP 3A427. On the contrary, several food - drug interactions such as mulberry-cyclosporine28, resveratrol-cyclosporine29 and soymilk-cyclosporine30, which resulted in decreased blood levels of cyclosporine, were stem from activation on CYP 3A4.
Given CSM is a strong activator of CYP 3A4, curcumin would be a promising chemoprevention agent for numerous xenobiotics. If a CYP 3A4 substrate drug is taken concomitantly with curcumin, we assumed that the efficacy of this medication might be ameliorated owing to greatly enhanced metabolism, even it was a P-gp substrate like EVL. On the other hand, for a drugs which is a P-gp substrate but not metabolized by CYP 3A4, such as digoxin and talinolol, the blood levels might be elevated by coadministered curcumin due to P-gp inhibition. Therefore, it is suggested that curcumin and curcumin-containing dietary supplements are not recommended for chronic patients using medications regularly. In conclusion, oral intake of curcumin significantly decreased the absorption of EVL mainly through marked activation on CYP 3A4 by curcumin metabolites.
Methods
Chemicals and reagents
Everolimus (Certican®, 0.5 mg/tab) was kindly provided by Novartis (Taiwan) Co. Ltd. Curcumin (purity 94%), demethoxycurcumin (purity 98%), hesperetin (purity 95%), rhodamine 123, sodium dodecyl sulfate (SDS), Triton X-100, verapamil and sulfatase (type H-1 from Helix pomatia) were purchased from Sigma (St. Louis, MO, USA). bis-demethoxycurcumin (purity 99%) was obtained from ChromaDex (Irvine, CA, USA). Dulbecco's Modified Eagle Medium (DMEM), trypsin/EDTA, nonessential amino acid, Hank's Buffered Salt Solution (HBSS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and Vivid® CYP450 screening kits were obtained from Invitrogen (Grand Island, NY, USA). 3-(4′,5′-Dimethylthiazol-2′-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Alfa Aesar (Lancaster, UK).Methanol was LC grade and was purchased from Echo (Miaoli, Taiwan). Milli-Q plus water (Millipore, Bedford, MA, USA) was used for all preparations.
Animals and drug administration
Male Sprague-Dawley rats were supplied by National Laboratory Animal Center (Taipei, Taiwan) and kept in the Animal Center of China Medical University (Taichung, Taiwan). The animal study was under “The Guidebook for the Care and Use of Laboratory Animals (2002)” published by the Chinese Society of Animal Science, Taiwan. The protocol was approved by the Animal Management Committee, China Medical University (Permit Number: CMU-102-144-N). The narcotization was performed under isoflurane, and all efforts were made to minimize suffering. A total of 17 rats weighing 280–350 g (n = 5–6 in each group) were fasted for 12 h before drug administration.
Certican® was ground into fine powders and solubilized with PEG 400 to afford 0.5 mg/mL of EVL. In a parallel design, a dose of 0.5 mg/1.0 mL/kg of EVL was orally given to rats via gastric gavages without and with 50/1.0 mL/kg or 100 mg/2.0 mL/kg of curcumin, which was also solubilized with PEG 400 to afford concentrations of 25 and 50 mg/mL.
Determination of blood EVL concentration
The blood EVL concentration was measured by a QMS® Everolimus Immunoassay kit supplied by Thermo Fisher Scientific (Fremont, CA, USA), which had been reported comparable with LC-MS method31. The assay was calibrated for concentration range from 1.5 to 20 ng/mL. The lower limit of quantitation (LLOQ) of this assay is 1.3 ng/mL.
Cell line and culture conditions
LS-180, human colon adenocarcinoma cell line, was purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in DMEM medium with 10% fetal bovine serum (Biological Industries Ltd., Kibbutz Beit Haemek, Israel), 0.1 mM nonessential amino acid, 100 units/mL of penicillin, 100 μg/mL of streptomycin and 292 μg/mL of glutamine. Cells were grown at 37°C in a humidified incubator containing 5% CO2. The medium was replaced every two days and cells were subcultured when 80 to 90% confluency was reached.
Cell viability assay
The effects of curcumin, verapamil and DMSO on viability of LS 180 were evaluated by MTT assay32. Cells were seeded into a 96-well plate. After overnight incubation, the tested agents were added and incubated for 24 h. MTT (5 mg/mL) was added into each well and incubated for 4 h. In this period, MTT was turned to formazan crystal by live cells. SDS solution (10%) was added to liquefy the purple crystal and the optical density was detected at 570 nm by a microplate reader (BioTex, Highland Park, Winooski, VT, U.S.A.).
Effect of curcumin on P-gp activity
The effects of curcumin on P-gp - mediated transport of rhodamine 123 were evaluated by following previous studies with some modification33,34,35. LS-180 cells (1×105) were cultured in 96-well plate. The medium was removed and washed with ice-cold PBS after overnight incubation. One hundred microliter of rhodamine 123 in HBSS (10 μM) was put into each well and incubated at 37°C. After 1-h incubation, the supernatant was removed, and cells were washed twice with ice-cold PBS. Curcumin, verapamil (as a positive control of P-gp inhibitor) and DMSO were added into correspondent wells and incubated at 37°C. After 4-h incubation, the medium was removed and the cells were washed twice with ice-cold PBS again. Then, 0.1% Triton X-100 (100 μL) was added to lyse the cells, and the fluorescence was measured with excitation at 485 nm and emission at 528 nm.
In order to quantitate the content of protein in each well, 10 μL of cell lysate was added to 200 μL of diluted protein assay reagent (Bio-Rad, Hercules, CA, U.S.A.) and the optical density was measured at 570 nm. The relative intracellular accumulation of rhodamine 123 was calculated by comparing with that of control after protein correction.
Preparation of curcumin serum metabolite (CSM)
Based on a previous study reporting the biological fate of curcumin5,6,7, CSM of rats was prepared to mimic the molecular forms which interacted with CYP 3A4 in the enterocytes. After overnight fasting, six rats were orally administered curcumin (100 mg/kg) and blood was collected via cardiopuncture at 30 min after dosing. The blood was centrifuged to obtain serum.
Characterization of CSM
A portion of serum was characterized prior to and after hydrolysis with sulfatase/glucuronidase following a previous method using LC-MS/MS with some modifications31. Briefly, 100 μL serum sample was mixed with 50 μL pH 5 acetate buffer or sulfatase (type H-1 from Helix pomatia, containing 1000 units/mL of sulfatase and 39,861 units/ml of β-glucuronidase), 50 μL ascorbic acid (200 mg/mL) and incubated at 37 °C for 30 min. After hydrolysis, the serum sample was partitioned with 200 μL ethyl acetate (containing 2.0 μg/mL hesperetin as internal standard). The ethyl acetate layer was evaporated under N2 to dryness and reconstituted with an appropriate volume of mobile phase prior to LC-MS/MS analysis.
The HPLC system was equipped with Accela 1250 pump and auto-sampler (Thermo Fisher Scientific Inc. U.S.A.). Chromatographic separation of analytes was achieved using a Thermo Hypersil GOLD C18 analytical column (50 mm × 2.1 mm, 1.9 μm) with a prefilter. The mobile phase consisted of 0.01% formic acid (A) and acetonitrile containing 0.01% formic acid (B) (A:B = 60:40), and eluted isocratically for 8 min. The flow rate was 0.2 mL/min. The column effluent was detected by H-ESI (heated-electrospray ionization) -II probe with Quantum Access MAX triple stage quadrupole (TSQ) mass spectrometer (Thermo Fisher Scientific Inc. U.S.A.). Nitrogen was used as sheath gas at 40 arbitrary units and auxiliary gas at 10 arbitrary units. The collision energy was set at 10 V, spray voltage at 4700 V, capillary temperature at 325°C, vaporizer temperature at 300°C and tube lens offset at 114 V. The following mass transitions were used for selected reaction monitoring analysis (SRM): curcumin (369/177), demethoxycurcumin (339/177), bis-demethoxycurcumin (309/147) and the internal standard hesperetin (303/177). The ESI-MS spectra were recorded in positive ion mode.
Effects of curcumin, demethoxycurcumin, bis-demethoxycurcumin and CSM on CYP3A4 activity
Vivid® CYP450 screening kit was used for evaluating the effects of curcumin, demethoxycurcumin, bis-demethoxycurcumin and CSM on the activity of CYP3A4. All the procedures were performed according the manual provided by Invitrogen. Briefly, after incubating CYP450 recombinant BACULOSOMES®, glucose-6-phosphate and glucose-6-phosphate dehydrogenase with CSM (1/2- and 1/4- fold serum concentrations) in 96-well black plate at room temperature for 20 min, a specific CYP3A4 substrate (Vivid® BOMR) and NADP+ were added, and incubated at room temperature for another 30 min. At the end of incubation, ketoconazole was added to stop the reaction and the fluorescence was measured with excitation at 530 nm and emission at 590 nm.
Data analysis
The peak blood concentration (Cmax) was obtained from experimental observation. The pharmacokinetic parameters of EVL were analyzed by noncompartment model of the program WinNonlin® (version 1.1 SCI software, Statistical Consulting, Inc., Apex, NC). The area under the blood concentration - time curve (AUC0-t) was calculated using trapezoidal rule to the last point. One way ANOVA with Scheffe’s test was used for statistical comparison. Statistical significance level was set at p < 0.05 as significant.
Acknowledgments
The work was in part supported by the Ministry of Science and Technology, Taipei, Taiwan, R.O.C. (MOST 103-2320-B-039-025, NSC102-2320-B-039-014-MY2 and NSC102-2320-B-039-008); and China Medical University, Taichung, Taiwan, R.O.C. (CMU103-105, CMU102-TC-03).
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.-W.H. contributed clinical opinion and study design. C.-Y.H., S.-Y.Y., C.-P.Y. and Y.-H.P. performed experimental work, data analysis and formulation of the article. P.-D.L.C. and Y.-C.H. made contributions to conception, study design and revision of the manuscript.
References
- Chihiro T., Natsuki N., Fumiyuki H. & Katsuko K. Comparison of anti-inflammatory activities of six Curcuma Rhizomes: a possible curcuminoid-independent pathway mediated by Curcuma phaeocaulis extract. Evid Based Complement Alternat Med 3, 255–260 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakmareong S. et al. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with l-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol 383, 519–529 (2011). [DOI] [PubMed] [Google Scholar]
- Odot J. et al. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int J Cancer 111, 381–387 (2004). [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M., Borrelli L. A., Rozkalne A., Hyman B. T. & Bacskai B. J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem 102, 1095–1104 (2007). [DOI] [PubMed] [Google Scholar]
- Li J. et al. A rapid and simple HPLC method for the determination of curcumin in rat plasma: assay development, validation and application to a pharmacokinetic study of curcumin liposome. Biomed Chromatog 23, 1201–1207 (2009). [DOI] [PubMed] [Google Scholar]
- Vareed S. K. et al. Pharmacokinetics of curcumin conjugate metabolites in healthy subjects. Cancer Epidemiol Biomarkers Prev 17, 1411–1417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. et al. Enhancement of curcumin oral absorption and pharmacolinetics of curcuminoids and curcurmin metabolites in mice. Cancer Chemother Pharmacol 69, 679–689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurk-Turner C., Manitpisitkul W. & Cooper M. A comprehensive review of everolimus clinical reports: a new mammalian target of rapamycin inhibitor. Transplantation 94, 659–668 (2012). [DOI] [PubMed] [Google Scholar]
- Baselga J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366, 520–529 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008). [DOI] [PubMed] [Google Scholar]
- Mabasa V. H. & Ensom M. H. The role of therapeutic monitoring of everolimus in solid organ transplantation. Ther Drug Monit 27, 666–676 (2005). [DOI] [PubMed] [Google Scholar]
- Zaza G. et al. Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin Dev Immunol 2013, 403280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Sousa R., Santana I. A., Testa L., de Melo Gagliato D. & Mano M. S. Biological therapies in breast cancer: common toxicities and management strategies. Breast 22, 1009–1018 (2013). [DOI] [PubMed] [Google Scholar]
- Junpaparp P., Sharma B., Samiappan A., Rhee J. H. & Young K. R. Everolimus-induced severe pulmonary toxicity with diffuse alveolar hemorrhage. Ann Am Thorac Soc 10, 727–729 (2013). [DOI] [PubMed] [Google Scholar]
- Kirchner G. I., Meier-Wiedenbach I. & Manns M. P. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet 43, 83–95 (2004). [DOI] [PubMed] [Google Scholar]
- Bohra R. et al. Everolimus and sirolimus in combination with cyclosporine have different effects on renal metabolism in the rat. PLoS One 7, e48063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchaud C. & Marquet P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: part II. Clin Pharmacokinet 48, 489–516 (2009). [DOI] [PubMed] [Google Scholar]
- Anuchapreeda S., Leechanachai P., Smith M. M., Ambudkar S. V. & Limtrakul P. N. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol 64, 573–582 (2002). [DOI] [PubMed] [Google Scholar]
- Lu W. D., Qin Y., Yang C. & Li L. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics 68, 694–701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T., Ibe M., Umegaki K., Shinozuka K. & Yamada S. Effects of dietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol Pharm Bull 33, 255–259 (2010). [DOI] [PubMed] [Google Scholar]
- Appiah-Opong R., Commandeur J. N., van Vugt-Lussenburg B. & Vermeulen N. P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology 235, 83–91 (2007). [DOI] [PubMed] [Google Scholar]
- Bamba Y., Yun Y. S., Kunugi A. & Inoue H. Compounds isolated from Curcuma aromatica Salisb. inhibit human P450 enzymes. J Nat Med 65, 583–587 (2011). [DOI] [PubMed] [Google Scholar]
- Hou X. L. et al. Curdione plays an important role in the inhibitory effect of Curcuma aromatica on CYP3A4 in Caco-2 Cells. Evid Based Complement Alternat Med 2011, 913898 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. F. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab 9, 310–322 (2008). [DOI] [PubMed] [Google Scholar]
- Thakrar B. T., Grundschober S. B. & Doessegger L. Detecting signals of drug-drug interactions in a spontaneous reports database. Br J Clin Pharmacol 64, 489–495 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard C. et al. Study of the drug-drug interaction between simvastatin and cisapride in man. Eur J Clin Pharmacol 57, 229–234 (2001). [DOI] [PubMed] [Google Scholar]
- Fujita K. Food-drug interactions via human cytochrome P450 3A (CYP3A). Drug Metabol Drug Interact 20, 195–217 (2004). [DOI] [PubMed] [Google Scholar]
- Hsu P. W. et al. Potential risk of mulberry-drug interaction: modulation on P-glycoprotein and cytochrome P450 3A. J Agric Food Chem 61, 4464–4469 (2013). [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Tsai S. Y., Hou Y. C. & Chao P. D. L. Inductive modulation on P-glycoprotein and cytochrome 3A by resveratrol, a constituent of grapes. Food Chem 133, 683–688 (2012). [Google Scholar]
- Yu C. P. et al. Potential modulation on P-glycoprotein and CYP3A by soymilk and miso: in vivo and ex-vivo studies. Food Chem 149, 25–30 (2014). [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Davis B. & Chow L. Evaluation of QMS Everolimus assay using Hitachi 917analyzer: comparison with liquid chromatography/mass spectrometry. Ther Drug Monit 33, 149–154 (2011). [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65, 55–63 (1983). [DOI] [PubMed] [Google Scholar]
- Jia J. X. & Wasan K. M. Effects of monoglycerides on rhodamine 123 accumulation, estradiol 17 beta-D-glucuronide bidirectional transport and MRP2 protein expression within Caco-2 cells. J Pharm Pharm Sci 11, 45–62 (2008). [DOI] [PubMed] [Google Scholar]
- Lin S. P., Chao P. D., Tsai S. Y., Wang M. J. & Hou Y. C. Citrus grandis peel increases the bioavailability of cyclosporine and tacrolimus, two important immunosuppressants, in rats. J Med Food 14, 1463–1468 (2011). [DOI] [PubMed] [Google Scholar]
- Yu C. P. et al. Quercetin and rutin reduced the bioavailability of cyclosporine from Neoral, an immunosuppressant, through activating P-glycoprotein and CYP 3A4. J Agric Food Chem 59, 4644–4648 (2011). [DOI] [PubMed] [Google Scholar]