Abstract
The recently proposed Research Domain Criteria (RDoC) system defines psychopathologies as phenomena of multilevel neurobiological existence and assigns them to 5 behavioural domains characterizing a brain in action. We performed an analysis on this contemporary concept of psychopathologies in respect to a brain phylogeny and biological substrates of psychiatric diseases. We found that the RDoC system uses biological determinism to explain the pathogenesis of distinct psychiatric symptoms and emphasises exploration of endophenotypes but not of complex diseases. Therefore, as a possible framework for experimental studies it allows one to evade a major challenge of translational studies of strict disease-to-model correspondence. The system conforms with the concept of a normality and pathology continuum, therefore, supports basic studies. The units of analysis of the RDoC system appear as a novel matrix for model validation. The general regulation and arousal, positive valence, negative valence, and social interactions behavioural domains of the RDoC system show basic construct, network, and phenomenological homologies between human and experimental animals. The nature and complexity of the cognitive behavioural domain of the RDoC system deserve further clarification. These homologies in the 4 domains justifies the validity, reliably and translatability of animal models appearing as endophenotypes of the negative and positive affect, social interaction and general regulation and arousal systems’ dysfunction.
Keywords: RDoC, Translational research, Animal models, Endophenotypes, Basic studies, Normality and psychopathology continuum
Highlights
-
•
The RDoC system encourages endophenotype-oriented experimental studies in human and animals.
-
•
The system conforms with the normality-pathology continuum concept.
-
•
The RDoC system appears to be a suitable framework for basic research.
-
•
Four RDoC domains show construct and phenomenological homology in human and animals.
-
•
Endophenotype-based models of affective psychopathologies appear most reliable.
1. Nosological entity problem in psychiatry
A fundamental problem of clinical diagnosis in psychiatry is the identification of disease as an objective and unique entity. Emil Kraepelin established the basis for modern nosology of psychiatric diseases (Kraepelin, 1893, Kraepelin, 1896). The contemporary International Statistical Classification of Diseases and Related Health Problems (ICD-10, 1989, http://www.who.int/classifications/icd/en/) and the Diagnostics and Statistics Manual of Mental Disorders (DSM-5, 2013, http://www.dsm5.org/Pages/Default.aspx) imply a clear distinction of nosological entities from normality and from each other. Diagnosis of psychopathologies is mostly based on the presence of deviant behaviour, self-evaluated discontent, behavioural maladaptation and danger posed to the patient or others. The categorical assignment of illnesses is justified by practical needs, such as unification of data across medical institutions, statistics, on default diagnostic efforts and treatment and on official grounds of reimbursement for a diagnostic means and therapeutic interventions by health insurance companies. However, diagnostic complexity and the lack of confidence in the final diagnosis often result in a formal use of contemporary nosological classifications (Maj, 2015).
Nosological entities imply the existence of specific causations. As a result, major efforts have been made to try to identify specific hallmarks of pathogenesis or associated genes using objective measurements in order to gain a high level of diagnostic reliability. Despite this effort, no clear markers have been identified that are indicative for existing psychiatric nosological entities. On a morphological level, volume reduction of cortical areas and the hippocampal formation has been reported for major depression and schizophrenia (Bremner et al., 2000, Meisenzahl et al., 2010, Nelson et al., 1998). Complex changes in molecular factors such as receptors expression, transporters and secondary messengers (Nikolaus et al., 2012, Scarr et al., 2015) contribute to a deterioration in neuroplasticity and synaptogenesis and are seen throughout the brain without clear nosological distinction (Bernardinelli et al., 2014, Kassem et al., 2013). Specificity of genetic signatures will only be possible with a tremendous increase in sample size, which will correspond ironically to lower nosological precision (Hyde et al., 2016, Ripke et al., 2014).
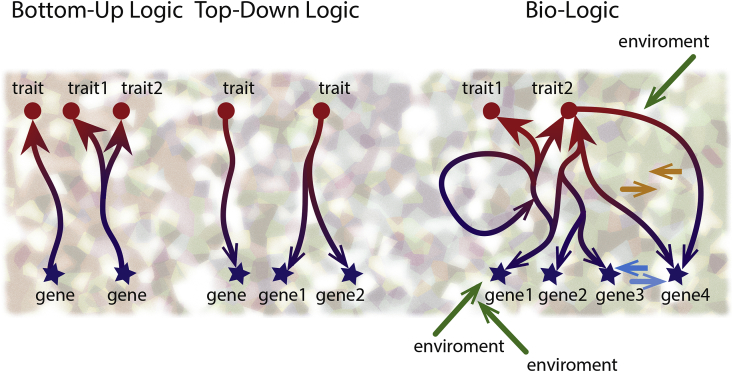
The failure to identify specific markers can be explained as follows: (1) A linear and finite relationship between the cause and outcome is a rare in psychiatric disease (Fig. 1, left). Instead, there are many reciprocal interactions between the biological background, its functional expression and numerous exogenous factors (Fig. 1, right). (2) The theory of allostatic load introduces preceding experience as a factor that may recalibrate the behavioural and physiological reactions and change the adaptation potential (Ellis and Del Giudice, 2014, McEwen, 1998, Nederhof and Schmidt, 2012). Environmental stress is considered as a pathogenetic element of a high importance in psychiatry (De Kloet et al., 2005, McEwen and Stellar, 1993, McEwen, 2008, Millan et al., 2012, Peyrot et al., 2015). (3) The interaction between the genome and the environment has a strong context limiting/permitting component (Notaras et al., 2016). Mechanisms that evolved over millions of years to protect individuals from a life-threatening environment have become maladaptive in respect to contemporary socio-cultural demands (Del Giudice, 2016, Ellis and Del Giudice, 2014, First and Wakefield, 2013). (4) This research approach is problematic as it keeps the nosological entity as the focus. Jaspers (1913) insisted that a nosological entity is a goal for study, but not an objectively existing phenomenon. Thus, any nosological approach to stratify disease should be viewed as a diagnostic strategy rather than approach to research distinct pathogenesis.
Fig. 1.
The bottom-up and top-down model approach in experimental biology. The genotype-outcome interaction implies a dynamic functional network (right), which comprises the bottom-up and top-down directions, the most commonly used approaches to model pathological states (left).
2. Evolutional and neurobiological understanding of psychopathology
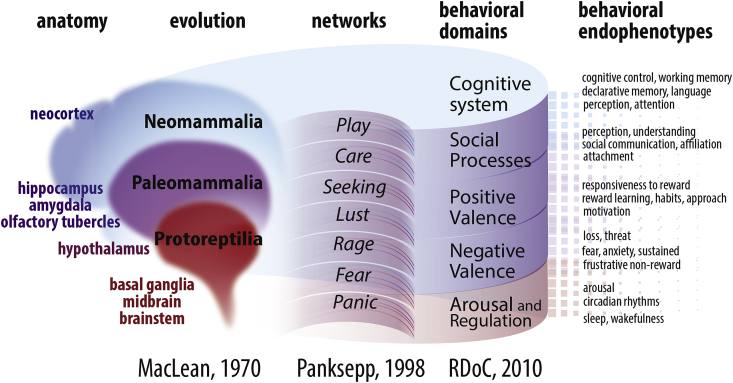
MacLean, 1970, MacLean, 1985, and 1990) first recognized the importance of an evolutionary concept to understand the regulation of mental function and proposed The Triune Brain Model (Fig. 2, left). According to MacLean, the phylogenetically oldest Protoreptilian brain provides instinctive actions, which are the basis of essential behaviours necessary for survival (e.g., exploration, escape behaviour, foraging, feeding, aggression, domination and reproductive behaviour). The Paleomammalian formation comprises parts of the brain that are conventionally assigned to the limbic system (or Papez circuit, Papez, 1937). It is responsible for emotions and motivation and transforms a primary response to more adaptive ones, based on previous experience and instincts. The neocortical Neomammalian formation provides actual declarative knowledge of all incoming sensory information.
Fig. 2.
How The Triune brain and The Affective Neuroscience Scale corresponds to the RDoC system. According to the RDoC system, behavioural endophenotypes attribute 5 main behavioural domains. They are not only distinct but describe personality as an interacting and dynamic individual trait. The domains are objectively different, determined by particular neuronal networks. Separation of the networks is due to a distinction in the functions they serve (The Affective Neuroscience Scale, Panksepp, 1998). This distinction corresponds well with the concept of a gradual evolution of the brain (the triune brain model of MacLean, 1970).
The Triune Brain Model was followed by The Affective Neuroscience Scale system (Davis and Panksepp, 2011, Panksepp, 1998, Panksepp, 2005). According to Panksepp's theory, core emotional affects and defensive behaviours are represented by the diencephalic action systems of “Panic”, “Fear”, Rage”, “Lust”, “Seeking”, Care” and “Play” (Fig. 2, middle). Genetically determined neuronal networks are considered as neurobiological substrates of these behaviours (Panksepp, 1998) and appear to be conserved throughout the mammalian evolution (Berridge, 2000, Saudino, 2005, Rutter et al., 1997). Emotions and defence behaviours serve focused and distinguished behavioural goals, and, therefore, can be controlled with specific stimuli and operate relatively independently (e.g., Anderson and Adolphs, 2014). Three reference points were suggested to specify the network, responsible for 1 of the behaviour characteristics selected by Pankseep: (1) similar neuronal circuits maintain coherent functions; (2) artificial stimulation of the particular network (with a pharmacological, electrophysiological, and opto-/chemo-genetic means) generates predicted responses; and (3) changes in the carriers of the network activity (neurotransmitters and other substances with messenger activity) predict the behavioural changes (Panksepp, 1998). A lot of research aims to decipher the circuitries that predominantly control (1) arousal and sleep (Herrera et al., 2016, Kim et al., 2012, Landgraf et al., 2016), (2) fear, (3) anxiety, (4) aversive memories (Bravo-Rivera et al., 2014, Kim et al., 2013, McCullough et al., 2016, Tovote et al., 2015), (5) reward (Kelley, 2004, Kelley and Berridge, 2002, Smith et al., 2011), (6) attention and motivation (Berthet et al., 2016, Carli and Invernizzi, 2014, Kim, 2013), (7) goal-oriented behaviour and habits (Burguière et al., 2015, Chersi et al., 2013, Frank, 2011, Gremel and Costa, 2013, Medendorp et al., 2011) and (8) social functions (Konopka and Roberts, 2016, Kragel et al., 2015, Sladky et al., 2015, Zikopoulos and Barbas, 2013).
The objective partition of the networks is nonetheless neither a physical nor functional segregation. Thus, emotional affect networks are integrated into the limbic system of the brain. In turn, the emotional homeostasis provided by the limbic system defines the efficacy of both the phylogenetically old defence systems and more advanced cognitive functions (de Waal, 2011, Panksepp, 2005, Park et al., 2016, Vermunt et al., 2016). In turn, the refined cortical functions favour better top-down control over the affective behaviours and provide more efficient adaptation strategies (Adhikari et al., 2015, Comte et al., 2016, Parikh et al., 2016, Rajasethupathy et al., 2015). For instance, recent fMRI findings on activity in the prefrontal cortex and anterior/posterior cingulate cortex have shown that these cortical structures exert inhibitory top-down control of emotional responses. This results in suppression of the context-irrelevant behavioural activity (Fair et al., 2008, Jaffard et al., 2008, Spielberg et al., 2015). Although the interaction between cognitive, emotional and defensive functions is well recognized, the principles and exact mechanisms of their integration are only just starting to be explored (Do-Monte et al., 2015, Fan et al., 2015, Riga et al., 2014, Robinson et al., 2014).
Cognitive-emotional imbalance, as reflected by disturbances in the functional crosstalk between the cortex and limbic/defence systems, is seen as the basis of psychiatric diseases as well as psychiatric symptoms in neurodegenerative disorders (reviewed in Ploog, 2003). The pathology-associated alteration in brain activity can be visualised by functional imaging studies. For example, fMRI has revealed that generalised anxiety coincides with stable characteristic changes in functional connectivity (Makovac et al., 2016, Mohlman et al., 2015; also, Lang et al., 2000).
3. The RDoC system as a new framework for categorizing psychopathologies
The Research Domains Criteria (RDoC, http://www.nimh.nih.gov/research-priorities/rdoc/index) framework was introduced by NIMH experts as an alternative categorization system for psychopathological states (Cuthbert, 2014, Cuthbert and Insel, 2010, Insel et al., 2010). At present, 5 behavioural domains form the basis of the classification matrix: (1) positive valence systems, (2) negative valence systems, (3) arousal/regulation systems, (4) systems for social processes and (5) cognitive systems. These 5 domains and their respective constructs (Fig. 2, right) can be used to comprehensively profile behaviour (http://www.nimh.nih.gov/research-priorities/rdoc/rdoc-constructs). The novelty of the RDoC system is that changes at all neurobiological levels, from molecules to networks, are related to the defined behavioural domains. According to the webpage, the proposed units of analysis are: genes, molecules, cells, circuits, physiology, behaviour, self-reports and paradigms. The development of the RDoC diagnostic criteria was supported by the concept of neuronal networks underlying basic behavioural domains and promoted by advances in functional diagnosis and imaging.
The European Roadmap for Mental Health Research project (ROAMER) aimed to stratify diagnosis, monitoring and treatment of psychiatric disorder. It resulted in the formulation of a similar domain structure (http://www.roamer-mh.org/). The pharmacological treatment cluster and the psychiatric somatic comorbidity cluster were introduced in addition to the domains already included in the RDoC (Schumann et al., 2014).
The authors of the RDoC system state: “There is no claim to “understand” or “explain” DSM/ICD disorders in terms of these (domains and subdomains) functions; rather, the aim is more simply to seek an understanding of how these various systems may become dysregulated to various extents and to relate such dysregulation to relevant symptoms.”
The RDoC system has several advantages as a framework for guiding preclinical studies: (1) The RDoC supports endophenotype (symptom)-oriented studies of psychiatric disorders. (2) The behavioural domain principles do not put a strict demarcation line between normality and pathology. The RDoC system offers a biological basis for personality traits complementing The Five-Factor Model of personality (Costa and McCrae, 1992 Tupes and Christal, 1961; also Davis and Panksepp, 2011, Neuman, 2014). Since psychopathology is considered as a margin case of personality type (Routtenberg, 2002), the RDoC framework implies a normality-pathology continuum. This justifies the search for neurobiological correlates of pathology on a basis of conceptual findings in normal/healthy subjects (Karalunas et al., 2014). (4) At a systemic level, behavioural domains appear to be self-consistent complexes. Therefore, each domain may represent a leading pathogenic risk factor. For example, major depression, as a nosological entity, would be seen not only as result of emotion and mood dysregulation (i.e., changes in the negative valence system), but also as a failure in the social interactions and positive valence systems with respective re-set of target for pharmacological interventions. As a consequence, pharmacological modulation of the oxytocin system for depression treatment can be better understood in the perspective of its role in social interaction (Slattery and Neumann, 2010). (5) The same symptoms are present in majority of psychiatric disorders. The appearance, for instance, of a depressive state across a variety of psychiatric diseases supports the idea that all known nosological entities comprise a continuous spectrum. Respectively, the comorbidity seen between major depression and schizophrenia (Samsom and Wong, 2015), bipolar disorder, attention deficit hyperactivity disorder and autism (Kiser et al., 2015) imply that the same pathophysiological processes occur. Emergence of common genetic risk factors for these diseases is in line with such a scenario (Smoller et al., 2013). Therefore, a symptom (or a drug response) instead of a defined psychiatric disease may be the stratifying principle for genetic studies (Gade et al., 2015, Manchia et al., 2013, Ohi et al., 2015, Papassotiropoulos and de Quervain, 2015).
However, despite its clear advantages, the RDoC system also bears a few shortcomings: (1) The RDoC system does not explicitly take into consideration the developmental and dynamical aspect of psychiatric disease, but only acknowledges their importance (Owen, 2014). (2) It does not consider polarity of parameters, either bipolar (e.g., locomotor activity) or unipolar (e.g., anxiety) (Papassotiropoulos and de Quervain, 2015). (3) The RDoC system does not address the fact that gains and losses of function may be determined by different mechanisms. For instance, direct and indirect pathways of basal ganglia circuit exert biphasic effect in controlling locomotor activity (Gerfen et al., 1990, Oldenburg and Sabatini, 2015). (4) Accordingly to phenomenological aspect of disease, a psychopathological state might be appropriately evaluated only in a context of subject-object and subject-subject interactions. As suggested by Nigg (2015), the RDoC framework does not consider the developmental or psychosocial context of psychopathological signs, therefore, failing to assign a symptom to pathology. (5) RDoC implies that all units of analysis, from genes and molecules to behaviour and paradigms, have the same specific stratification power. However, no correlation between diagnostic values of parameters belonging to different units of analysis may be observed. At the same time, such congruence may be the only bedrock of units’ supervenience and a basis to explore their interrelation. Finally, (6) the RDoC system does not intend to understand and explore a hierarchy and rules of interaction between behavioural domains.
4. Neurobiological coordinates for experimental phenotypes and the RDoC system
4.1. Requirements for experimental models of psychopathological states
The deterministic principle in biology represents a powerful way to understand the biological processes that shape both normality and abnormality. In psychiatry, this principle goes back to Griesenger (1984) who put forward the brain as a substrate of psychiatric illnesses. Today, psychiatric disorders are seen as a result of an interaction between intrinsic and environmental factors. Therefore, a model system should be conceived as a combination of the experimental subject and applied test situation.
Today we acknowledge the probabilistic aspects of disease that require a combination of biological and ecological approaches to model pathology. This renders models powerful in terms of understanding epigenetic mechanisms of psychopathologies and prerequisites of resilience vs. susceptibility to disease. This also claims for more complex and labour-intensive models. For instance, social interaction behaviour can now be observed 24 h a day using « PhenoWorld» (Castelhano-Carlos et al., 2014, Hong et al., 2015, Shemesh et al., 2013). Accurate assessment of cognitive function requires a touch screen approach, which is time consuming and apparatus-demanding (Romberg et al., 2013). Contemporary understanding the difference between mechanisms of sustained fear (anxiety) and phasic fear need sophisticated protocols with versatile time and context parameters of conditioning (Daldrup et al., 2015, Grillon and Davis, 1997, Seidenbecher et al., 2016).” The following validity criteria are used to judge the quality of the disease model: correspondence to the clinical appearance of disease (face validity); similarity of the morpho-physiological features of disease (construct validity); predicted response to pharmacological interventions, specifically active in given pathological conditions (predictive validity) (Belzung and Lemoine, 2011, McKinney and Bunney, 1969, Vervliet and Raes, 2013, Willner, 1984, Willner, 1994). Full adherence to these 3 phenotypic validity criteria is rarely achievable in commonly used experimental animals, such as mice and rats. First of all, tests confirming face validity and respective proxy measures are not always species relevant. Thus, the same responses (fear, disgust, affiliation, or pleasure) may have different expression because they drive species-specific activity (Cosmides and Tooby, 2000, Flack and de Waal, 2007). Second, most models bear a limited number of characteristic pathological traits and rarely show typical comorbid traits, which are co-inherited in human disease. Third, models do not possess characteristic dynamics of traits over the lifetime. Forth, the main drawback of experimental models is that they rarely meet the etiological validity criterion, which includes both the ontopathogenesis and species homology (Belzung and Lemoine, 2011). Therefore, specific animals, perceived as models of disease, fail to reveal risk factors, prognostic markers and new therapeutic targets. These problems characterize the crisis of experimental studies in animals (Ahmed, 2010, Stewart and Kalueff, 2015, Willner and Belzung, 2015).
The RDoC system is a platform that aims to improve translatability of studies from animals to humans, since it supports the endophenotype-based comparison of animals and humans on an objective neurobiological basis across all behavioural domains. The idea of using observable and objectively measured behaviour to create animal models of pathology had already been introduced (Geyer and Moghaddam, 2002, Segal and Geyer, 1985). The RDoC system advanced to allow the models to be systematized and grouped, which makes the behavioural endophenotype the primary focus. The RDoC system offers a useful validation matrix, where the units of analysis serve as criteria and, together with the domains, it may be feasible to delineate the areas of homologies between animal and humans. The RDoc system provides a conceptual approach to study of abnormal endophenotypes considering them as a deviation of normality. Therefore, it strongly supports basic/disease focused studies (Bertuzzi and Cleveland, 2015) and justifies using experimental animals to better understand pathological processes.
4.2. Fitting The Affective Neuroscience Scale
We assume that the most reliable comparison between animals and humans can be done within the 4 RDoC domains, which describe basic emotions and defence responses. These domains fully overlap with The Affective Neuroscience Scale (Fig. 2). For instance, the negative valence systems domain includes the “Fear” and “Panic” systems. The positive valence systems domain, in turn, includes the systems responsible for feelings of pleasure and attachment (“Play”, “Care”, “Lust”, and “Seeking”). The arousal and regulatory systems combines “Play”, “Seeking”, and “Rage” clusters. The social processes domain can be defined as a constellation of the “Play” and “Care”. The anthropocentric names given to emotions, arousal and defence responses should be used with precaution in animals, since they are rather nominal or provisional terms. Using these behavioural emotions with respect to their significance to the entire brain and body would be more representative of their respective functions (see a review of emotion definition in de Waal, 20; also, LeDoux, 2014). To prove behavioural and physiological homologies of emotions and defence programs between species, we should be assured that even if they do not share similar immediate expression, they may still induce the same changes or specifically modify evoked responses to (any) other stimuli. Together with the phenomenological and neuronal networks’ homologies, a similarity between humans and animals in physiological mechanisms as well as in common genetic and developmental phenomena of the affective systems (Anderson and Adolphs, 2014, Heilbronner et al., 2016, Mishra and Gazzaley, 2016) support validity and reliability of the experimental models of dysfunction in the emotional, regulatory and defence responses.
4.3. Concerning the cognitive systems domain
The fifth domain of the RDoC system, the cognitive system is the loose accumulation of a variety of cognitive functions (i.e., perception, attention, declarative memory, language, working memory, cognitive control; https://www.nimh.nih.gov/research-priorities/rdoc/constructs/cognitive-systems.shtml). Although the phylogenetic, physiological and clinical data testify a role of the cortical structures in the implementation of cognitive functions (reviewed in Geschwind and Rakic, 2013), so far, neither an exclusive area of the cortex nor a key network were confirmed to be a primary bearer or centre of cognition.
As a “system”, the cognitive systems domain emerges as an epi-formation including all components of the affective systems plus neocortex and paleocortex. The functional connectivity concept (Friston, 2011) spans neurobiological and (meta)system approaches and implies integration of all brain regions in maintaining cognitive performance. At least in preclinical studies using animal models, it appears to be impossible to separate the cognitive systems domain from other behavioural domains (Cromwell and Panksepp, 2011). The lack of specific responses serves as indirect evidence that the cognitive system has no specific morphological and neurobiological representation. Thus, cognition cannot be selectively modulated and pharmacological intervention is often accompanied by changes in other behavioural domains. Cognitive abilities can be accelerated coincidentally with improved attention and vigilance in attention deficit hyperactivity disorder, ADHD (Briars and Todd, 2016, Chan et al., 2016) or suppression of negative symptoms in major depression (Al-Sukhni et al., 2015, Baune and Renger, 2014) or schizophrenia (Goff et al., 2008).
Primary failure in cognitive control may impact emotion processing and represents a major factor of psychopathology for schizophrenia and anxiety disorders (for instance, Mochcovitch et al., 2014, McTeague et al., 2016). In turn, in depression, deterioration in the emotional domains can lead to cognitive disturbances (Darcet et al., 2016, Kim et al., 2016, Porter et al., 2015). For example, dysfunction in working memory and cognitive flexibility are observed without relation to actual mood state, but are most pronounced during episodes of acute mood disturbance (Snyder et al., 2015).
A complex motivation phenomenon is another example of a crosstalk between arousal/emotional state and cognitive/learning performance. Motivation is perceived as a cognitive function in humans, but at the same time it relates to the positive valence or negative valence systems. The midbrain reward system, in particular, the dopaminergic system, significantly contributes to “calculation” of reward values in relation to effort and predicted outcome (Cools et al., 2009, Saddoris et al., 2015, Schultz and Dickinson, 2000). Therefore, specific pharmacological interventions effective in monoaminergic systems (Berridge and Arnsten, 2013, Westbrook and Braver, 2016) may be a way to study and modulate cognitive function.
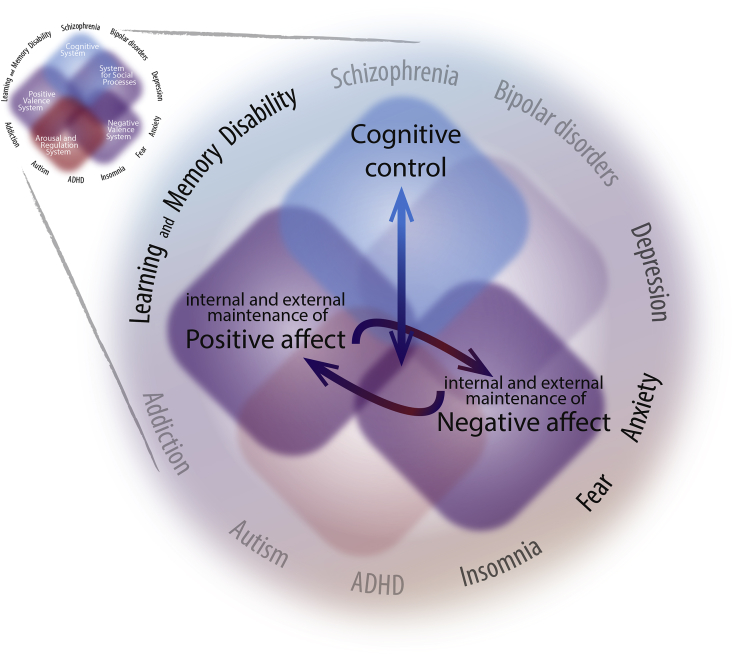
The multidomain assignment might be particularly promising if, for instance, the positive and negative valences are considered as both function and argument of the cognitive system. For instance, effective stress coping is a cognitive system-controlled transition from negative affect to positive affect (Fig. 3). In general, the objective difference between the complexity of interactions in the cognitive and affective systems in small experimental animals and humans provide strong limitations for modelling and studying cognitive disturbances in mice and rats.
Fig. 3.
The “interacting domains” approach. The RDoC paradigm provides the opportunity to create a theoretical framework to decipher interactions between behavioural domains. For instance, the simplest interaction model might comprise 3 domains. Considering a possible hierarchy between domains, it can be predicted that only some integrations will serve healthy brain activity. Thus, negative and positive affect transition is subject to regulation by the cognitive systems domain. Pathology appears as a manifestation of aberrant crosstalk and disturbed hierarchy among the systems. Thus, prevalence of either positive or negative affects feeds back on the cognitive systems.
4.4. Putting animal and human behavioural endophenotypes in a right perspective
Difficulties, which appear when matching animal and human behavioural traits and responses (Nestler and Hyman, 2010), might be resolved in the following ways:
-
1.
A model should be perceived as an endophenotype/symptom-based model and considered only as biological system representing a distinct pathological process, but not a nosological entity.
-
2.
A pathological process must be defined in respect to a particular concept of pathogenesis of psychopathologies. For instance, it might be seen as an imbalance between cortical and subcortical structures or as a state of wrong employment of a generally adaptive process. This will define the interpretation and limit possible misinterpretations acquired due to a deductive analysis (Poldrack, 2006).
-
3.1
Experimental paradigms, which can be used in both animal and human studies and, presumably, deal with the same endophenotypes and readouts, should be first choice (Fonio et al., 2012, Hvoslef-Eide et al., 2015).
-
3.2
Behavioural tests require validation in respect to their face, predictive and construct validities in the given context. Thus, the open field test employed to examine locomotor activity may be a useful tool to discriminate normal adaptive and pathological anxiety (Prut and Belzung, 2003). Novelty-induced grooming is regarded as an example of coordinated activity rather than a pathological endophenotype (Komorowska and Pellis, 2004, Kalueff et al., 2016). Continuous swimming during the forced swim test has an adaptive value if escape is made possible. If not, lasting efforts lead to physical exhaustion, which would increase the probability of negative outcome. The mobility/activity, which can be measured at earlier phases of the forced swim test, may correspond to arousal and high vigilance, whereas the immobility in the later stage may reflect behavioural flexibility (Costa et al., 2013, De Pablo et al., 1989, Molendijk and de Kloet, 2015). Once the meaningfulness of behavioural parameters is validated, a contextual and/or direct analogy between significance of animal and human behavioural traits will indicate the human task equivalent (Czéh et al., 2016).
-
3.3
Evaluation of behavioural responses might be biased due to experimenter's anthropocentrism. For example, a high level of aggression, which would serve as an indicator of pathology in humans (e.g., during acute alcohol intoxication or due to degeneration of cortical structures) plays an adaptive role in mice and is not necessarily accompanied by the development of comorbidities like vulnerability to stress and the development of cardiovascular complications (Brain, 1980, Brain et al., 1993).
-
3.4.
Underestimation of the species-specific social interactions and environmental cues results in artificial conditions of housing and grouping patterns of animals, like single-housing (Arndt et al., 2009, Bartolomucci et al., 2003, Jähkel et al., 2000, van Goethem et al., 2012). Careful analysis and optimization of this and other factors are objective requirements for contemporary experimental designs. Conditions including limited sized lodging, a pathogen-free environment, artificial foraging, limited social and sexual interactions should be carefully considered but not as possible confounding factors, rather as ecological determinants of experimental studies (e.g., Beura et al., 2016). We should use these variables as a source of biological variability of measured parameters (Voelkl and Würbel, 2016).
-
3.5.
Considering the apparent polymodal character of the domains and context-dependent expression of biomarkers, we have to acknowledge that any behavioural task or biomedical measurement can be done appropriately but only on a relative scale. Therefore, an assessment of experimental endophenotypes should be “calibrated” by coupling genetic models (e.g., inbred lines) with the predicted difference in a particular behavioural domain. This is a conceivable way to better standardize and validate experiments. For instance, C57Bl/6 mice show a higher reactivity but a stronger resistance to acute stress than DBA/2 or BALB/c mice (Broadhurst, 1976, Francis et al., 2003, Ibarguen-Vargas et al., 2008). In any test, relevant to assessment of the same domain, we should expect a similar relative difference between reference lines.
-
4.
It should be mandatory to assess the pharmacological responses to prototypic drugs across all behavioural domains. This would protect a model from possible misinterpretations. For example, in our recent studies, we characterized the inbred low trait anxiety behaviour (LAB) mice as a model of a psychotic-like continuum (Yen et al., 2013, Yen et al., 2015). In these LAB mice, we observed endophenotypes that may correspond to the attention deficit hyperactivity disorder, manic psychosis and schizophrenia. However, since amphetamine decreases locomotor activity in these mice, they may be an ADHD model and represent an endophenotype that relates to dysfunction in the regulation/arousal systems domain.
4.5. Matching of pathological symptoms and experimental endophenotypes
Based on the RDoC, the following principles can be applied to better model pathology in animals:
-
•
Assume a model is an endophenotype model (symptom-based);
-
•
Assign the experimental endophenotype to 1 of the 5 RDoC domains;
-
•
Identify corresponding symptoms in humans in the same domain;
-
•
Reveal a spectrum of related clinical symptoms preferentially within a single syndrome;
-
•
Examine whether developmental, dynamic and co-morbidity features of the experimental endophenotype fit to the particular syndrome corresponding to a nosological entity.
-
•
Since disturbance in the cognitive system is a characteristic feature of psychopathology, evaluation of cognitive function (in terms of modality and intensity of changes) should be an ultimate step in proposing the phenotype as a psychiatric disease model.
5. Conclusions
The recently proposed RDoC diagnostic framework is a new strategy of symptom-based classification. It revives and upgrades the idea of characterizing the basic function of brain rather than the typical pathological traits (Schneider, 1959). The RDoC system exploits objective measurements at all levels of neurobiological investigation from molecules to circuits and behaviour and offers a biological basis of diagnosis.
At present, the proposed system is still mechanistic and its nosology-dissecting and aetiology-finding abilities are a matter of debate. However, the reductionism of the RDoC system makes it an important methodological tool. The RDoC system offers a good platform for the integral study of clinical data and for the critical evaluation of existing animal models based on systematic examination of endophenotypes.
To summarise our analysis of the RDoC system, we may conclude that:
-
1.
The RDoC system implies the same mechanisms underlie normal and dysfunctional brain function. The RDoc system also suggests the existence of a psychopathological continuum.
-
2.
In the present state, the RDoC system does not consider dynamical and developmental aspects of a disease, psychosomatic co-morbidities, paradigms of therapeutic interventions nor interactions between domain and components of Units of Analysis.
-
3.
Four of the 5 RDoC domains correspond to The Affective Neuroscience Scale. A homology of the affective systems in humans and animals supports experimental models of the emotion and defence response dysfunction. However, the high translational value of these models is limited by the dissimilarities between developmental programs and psycho-social contexts of human disorders.
-
4.
Nonetheless, the RDoC system offers a valid and instructive framework for basic research allowing one to focus on domains and their constructs and to explore endophenotype-based models. This avoids a major challenge of translational studies, which assume a strict disease-to-model correspondence.
Declaration of conflicting interests
Dr. Kirmeier is a full-time employee in a pharmaceutical company.
The authors declare neither non-financial nor financial conflict of interest.
Acknowledgements
The authors wish to thank Dr. Alec Dick for his critical revision, Mr. Paul Kaplick for his helpful discussions, and Dr. Jessica Keverne for English language copy editing.
Contributor Information
Elmira Anderzhanova, Email: anderzhanova@psych.mpg.de.
Thomas Kirmeier, Email: thomas.kirmeier@hmnc.de.
Carsten T. Wotjak, Email: wotjak@psych.mpg.de.
References
- Adhikari A., Lerner T.N., Finkelstein J., Pak S., Jennings J.H., Davidson T.J., Ferenczi E., Gunaydin L.A., Mirzabekov J.J., Ye L., Kim S.Y., Lei A., Deisseroth K. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179–185. doi: 10.1038/nature15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.H. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci. Biobehav. Rev. 2010;35(2):172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Al-Sukhni M., Maruschak N.A., McIntyre R.S. Vortioxetine: a review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin. Drug Saf. 2015;14(8):1291–1304. doi: 10.1517/14740338.2015.1046836. [DOI] [PubMed] [Google Scholar]
- Anderson D.J., Adolphs R.A. A framework for studying emotions across species. Cell. 2014;157(1):187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt S.S., Laarakker M.C., van Lith H.A., van der Staay F.J., Gieling E., Salomons A.R., van't Klooster J., Ohl F. Individual housing of mice-impact on behaviour and stress responses. Physiol. Behav. 2009;97(3–4):385–393. doi: 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A., Palanza P., Sacerdote P., Ceresini G., Chirieleison A., Panerai A.E., Parmigiani S. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 2003;28(4):540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- Baune B.T., Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression - a systematic review. Psychiatry Res. 2014;219(1):25–50. doi: 10.1016/j.psychres.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Belzung C., Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 2011;1(1):9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardinelli Y., Nikonenko I., Muller D. Structural plasticity: mechanisms and contribution to developmental psychiatric disorders. Front. Neuroanat. 2014;8:123. doi: 10.3389/fnana.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge C.W., Arnsten A.F. Psychostimulants and motivated behavior: arousal and cognition. Neurosci. Biobehav. Rev. 2013;37(9 Pt A):1976–1984. doi: 10.1016/j.neubiorev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Berthet P., Lindahl M., Tully P.J., Hellgren-Kotaleski J., Lansner A. Functional relevance of different basal ganglia pathways investigated in a spiking model with reward dependent plasticity. Front. Neural. Circuits. 2016 doi: 10.3389/fncir.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi S., Cleveland D.W. The curious incident of the translational dog that didn't bark. Trends Cell. Biol. 2015;25(4):187–189. doi: 10.1016/j.tcb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura L.K., Hamilton S.E., Bi K., Schenkel J.M., Odumade O.A., Casey K.A. Nature. 2016;532(7600):512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain P.F. Adaptive aspects of hormonal correlates of attack and defence in laboratory mice: a study in ethobiology. Prog. Brain. Res. 1980;53:391–413. doi: 10.1016/S0079-6123(08)60078-3. [DOI] [PubMed] [Google Scholar]
- Brain P.F., Miras R.L., Berry M.S. Diversity of animal models of aggression: their impact on the putative alcohol/aggression link. J. Stud. Alcohol. Suppl. 1993;11:140–145. doi: 10.15288/jsas.1993.s11.140. [DOI] [PubMed] [Google Scholar]
- Bravo-Rivera C., Roman-Ortiz C., Brignoni-Perez E., Sotres-Bayon F., Quirk G.J. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 2014;34(29):9736–9742. doi: 10.1523/JNEUROSCI.0191-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Narayan M., Anderson E.R., Staib L.H., Miller H.L., Charney D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Briars L., Todd T. A review of pharmacological management of attention-deficit/hyperactivity disorder. J. Pediatr. Pharmacol. Ther. 2016;21(3):192–206. doi: 10.5863/1551-6776-21.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst P.L. The Maudsley reactive and nonreactive strains of rats: a clarification. Behav. Genet. 1976;6(3):363–365. doi: 10.1007/BF01065732. [DOI] [PubMed] [Google Scholar]
- Burguière E., Monteiro P., Mallet L., Feng G., Graybiel A.M. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr. Opin. Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M., Invernizzi R.W. Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Front. Neural Circuits. 2014;8:58. doi: 10.3389/fncir.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelhano-Carlos M., Costa P.S., Russig H., Sousa N. PhenoWorld: a new paradigm to screen rodent behavior. Transl. Psychiatry. 2014;4:e399. doi: 10.1038/tp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E., Fogler J.M., Hammerness P.G. Treatment of attention-deficit/hyperactivity disorder in adolescents: a systematic review. JAMA. 2016;315(18):1997–2008. doi: 10.1001/jama.2016.5453. [DOI] [PubMed] [Google Scholar]
- Chersi F., Mirolli M., Pezzulo G., Baldassarre G. A spiking neuron model of the cortico-basal ganglia circuits for goal-directed and habitual action learning. Neural Netw. 2013;41:212–224. doi: 10.1016/j.neunet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Comte M., Schön D., Coull J.T., Reynaud E., Khalfa S., Belzeaux R., Ibrahim el C., Guedj E., Blin O., Weinberger D.R., Fakra E. Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb. Cortex. 2016;26(1):144–155. doi: 10.1093/cercor/bhu185. [DOI] [PubMed] [Google Scholar]
- Cools R., Frank M.J., Gibbs S.E., Miyakawa A., Jagust W., D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J. Neurosci. 2009;29(5):1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides L., Tooby J. Evolutionary psychology and the emotions. In: Lewis M., Haviland-Jones J.M., editors. Handbook of Emotions. second ed. Guilford; NY: 2000. [Google Scholar]
- Costa P.T., Jr., McCrae R.R. Psychological Assessment Resources, FL; Odessa: 1992. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-factor Inventory (NEO-FFI) manual. [Google Scholar]
- Costa A.P., Vieira C., Bohner L.O., Silva C.F., Santos E.C., De Lima T.C., Lino-de-Oliveira C. A proposal for refining the forced swim test in Swiss mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:150–155. doi: 10.1016/j.pnpbp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Cromwell H.C., Panksepp J. Rethinking the cognitive revolution from a neural perspective: how overuse/misuse of the term 'cognition' and the neglect of affective controls in behavioral neuroscience could be delaying progress in understanding the BrainMind. Neurosci. Biobehav. Rev. 2011;35(9):2026–2035. doi: 10.1016/j.neubiorev.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N. The RDoC framework: continuing commentary. World Psychiatry. 2014;196 doi: 10.1002/wps.20140. NIMH RDoC Workgroup. PMCID: PMC4102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B., Insel T. The data of diagnosis: new approaches to psychiatric classification. Psychiatry. 2010;73(4):311–314. doi: 10.1521/psyc.2010.73.4.311. [DOI] [PubMed] [Google Scholar]
- Czéh B., Fuchs E., Wiborg O., Simon M. Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Daldrup T., Remmes J., Lesting J., Gaburro S., Fendt M., Meuth P., Kloke V., Pape H.C., Seidenbecher T. Expression of freezing and fear-potentiated startle during sustained fear in mice. Genes Brain Behav. 2015;14(3):281–291. doi: 10.1111/gbb.12211. [DOI] [PubMed] [Google Scholar]
- Darcet F., Gardier A.M., Gaillard R., David D.J., Guilloux J.P. Cognitive dysfunction in major depressive disorder. A translational review in animal models of the disease. Pharm. (Basel) 2016;9(1) doi: 10.3390/ph9010009. pii: E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.L., Panksepp J. The brain's emotional foundations of human personality and the affective neuroscience personality scales. Neurosci. Biobehav. Rev. 2011;35(9):1946–1958. doi: 10.1016/j.neubiorev.2011.04.004. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Pablo J.M., Parra A., Segovia S., Guillamón A. Learned immobility explains the behavior of rats in the forced swimming test. Physiol. Behav. 1989;46(2):229–237. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M. What is an animal emotion? Ann. N. Y. Acad. Sci. 2011;1224:191–206. doi: 10.1111/j.1749-6632.2010.05912.x. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. The evolutionary future of psychopathology. Curr. Opin. Psychol. 2016;7:44–50. [Google Scholar]
- Do-Monte F.H., Quiñones-Laracuente K., Quirk G.J. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519(7544):460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Del Giudice M. Beyond allostatic load: rethinking the role of stress in regulating human development. Dev. Psychopathol. 2014;26(1):1–20. doi: 10.1017/S0954579413000849. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Pestke K., Feeser M., Aust S., Pruessner J.C., Böker H., Bajbouj M., Grimm S. Amygdala-hippocampal connectivity changes during acute psychosocial stress: joint effect of early life stress and oxytocin. Neuropsychopharmacology. 2015;40(12):2736–2744. doi: 10.1038/npp.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Wakefield J.C. Diagnostic criteria as dysfunction indicators: bridging the chasm between the definition of mental disorder and diagnostic criteria for specific disorders. Can. J. Psychiatry. 2013;58(12):663–669. doi: 10.1177/070674371305801203. [DOI] [PubMed] [Google Scholar]
- Flack J.C., de Waal F. Context modulates signal meaning in primate communication. Proc. Natl. Acad. Sci. U. S. A. 2007;104(5):1581–1586. doi: 10.1073/pnas.0603565104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonio E., Golani I., Benjamini Y. Measuring behavior of animal models: faults and remedies. Nat. Methods. 2012;9(12):1167–1170. doi: 10.1038/nmeth.2252. [DOI] [PubMed] [Google Scholar]
- Francis D.D., Szegda K., Campbell G., Martin W.D., Insel T.R. Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 2003;6(5):445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Frank M.J. Computational models of motivated action selection in corticostriatal circuits. Curr. Opin. Neurobiol. 2011;21(3):381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Gade K., Malzahn D., Anderson-Schmidt H., Strohmaier J., Meier S., Frank J., Falkai P.G., Rietschel M., Schulze T.G. Functional outcome in major psychiatric disorders and associated clinical and psychosocial variables: a potential cross-diagnostic phenotype for further genetic investigations? World J. Biol. Psychiatry. 2015;16(4):237–248. doi: 10.3109/15622975.2014.995221. [DOI] [PubMed] [Google Scholar]
- Gerfen C.R., Engber T.M., Mahan L.C., Susel Z., Chase T.N., Monsma F.J., Jr., Sibley D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Geschwind D.H., Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80(3):633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M.A., Moghaddam B. Animal models relevant to schizophrenia disorders. In: Davis K.L., Charney D., Coyle J.T., Nemeroff C., editors. Neuropsychopharmacology: the Fifth Generation of Progress. Am. Coll Neuropsychopharmacol. Lippincott; Williams, & Wilkins: 2002. [Google Scholar]
- Goff D.C., Cather C., Gottlieb J.D., Evins A.E., Walsh J., Raeke L., Otto M.W., Schoenfeld D., Green M.F. Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophr. Res. 2008;106(2–3):320–327. doi: 10.1016/j.schres.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel C.M., Costa R.M. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat. Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenger W. Krabbe; Stuttgart: 1984. Pathologie und Therapie der psychischen Krankheiten, für Ärzte und Studierende. [Google Scholar]
- Grillon C., Davis M. Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34(4):451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Heilbronner S.R., Rodriguez-Romaguera J., Quirk G.J., Groenewegen H.J., Haber S.N. Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry. 2016;80(7):509–521. doi: 10.1016/j.biopsych.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C.G., Cadavieco M.C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2016;19(2):290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W., Kennedy A., Burgos-Artizzu X.P., Zelikowsky M., Navonne S.G., Perona P., Anderson D.J. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl. Acad. Sci. U. S. A. 2015;112(38):E5351–E5360. doi: 10.1073/pnas.1515982112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvoslef-Eide M., Mar A.C., Nilsson S.R., Alsiö J., Heath C.J., Saksida L.M., Robbins T.W., Bussey T.J. The NEWMEDS rodent touchscreen test battery for cognition relevant to schizophrenia. Psychopharmacol. Berl. 2015;232(21–22):3853–3872. doi: 10.1007/s00213-015-4007-x. [DOI] [PubMed] [Google Scholar]
- Hyde C.L., Nagle M.W., Tian C., Chen X., Paciga S.A., Wendland J.R., Tung J.Y., Hinds D.A., Perlis R.H., Winslow A.R. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016;48(9):1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarguen-Vargas Y., Surget A., Touma C., Palme R., Belzung C. Multifaceted strain-specific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology. 2008;33(10):1357–1368. doi: 10.1016/j.psyneuen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jaffard M., Longcamp M., Velay J.L., Anton J.L., Roth M., Nazarian B., Boulinguez P. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008;42(3):1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Jähkel M., Rilke O., Koch R., Oehler J. Open field locomotion and neurotransmission in mice evaluated by principal component factor analysis-effects of housing condition, individual activity disposition and psychotropic drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2000;24(1):61–84. doi: 10.1016/s0278-5846(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Jaspers K. Springer; 1913. Allgemeine psychopathologie. Auflage 1. [Google Scholar]
- Kalueff A.V., Stewart A.M., Song C., Berridge K.C., Graybiel A.M., Fentress J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016;17(1):45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Fair D., Musser E.D., Aykes K., Iyer S.P., Nigg J.T. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry. 2014;71(9):1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kassem M.S., Lagopoulos J., Stait-Gardner T., Price W.S., Chohan T.W., Arnold J.C., Hatton S.N., Bennett M.R. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol. Neurobiol. 2013;47(2):645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Kelley A.E. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelley A.E., Berridge K.C. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.I. Neuroscientific model of motivational process. Front. Psychol. 2013;4:98. doi: 10.3389/fpsyg.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A., Latchoumane C., Lee S., Kim G.B., Cheong E., Augustine G.J., Shin H.S. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20673–20678. doi: 10.1073/pnas.1217897109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Adhikari A., Lee S.Y., Marshel J.H., Kim C.K., Mallory C.S., Lo M., Pak S., Mattis J., Lim B.K., Malenka R.C., Warden M.R., Neve R., Tye K.M., Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Nunes P.V., Oliveira K.C., Young L.T., Lafer B. Neuropathological relationship between major depression and dementia: a hypothetical model and review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;67:51–57. doi: 10.1016/j.pnpbp.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Kiser D.P., Rivero O., Lesch K.P. Annual research review: the (epi)genetics of neurodevelopmental disorders in the era of whole-genome sequencing–unveiling the dark matter. J. Child. Psychol. Psychiatry. 2015;56(3):278–295. doi: 10.1111/jcpp.12392. [DOI] [PubMed] [Google Scholar]
- Komorowska J., Pellis S.M. Regulatory mechanisms underlying novelty-induced grooming in the laboratory rat. Behav. Process. 2004;67(2):287–293. doi: 10.1016/j.beproc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Konopka G., Roberts T.F. Insights into the neural and genetic basis of vocal communication. Cell. 2016;164(6):1269–1276. doi: 10.1016/j.cell.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Abel; Leipzig: 1893. Psychiatrie. Auflage 4. [Google Scholar]
- Kraepelin E. Barth; Leipzig: 1896. Psychiatrie. Ein Lehrbuck für Studirende and Ärzte. Auflage 5. [Google Scholar]
- Kragel P.A., Zucker N.L., Covington V.E., LaBar K.S. Developmental trajectories of cortical-subcortical interactions underlying the evaluation of trust in adolescence. Soc. Cogn. Affect. Neurosci. 2015;10(2):240–247. doi: 10.1093/scan/nsu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D., Long J.E., Welsh D.K. Depression-like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. Eur. J. Neurosci. 2016;43(10):1309–1320. doi: 10.1111/ejn.13085. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Davis M., Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J. Affect. Disord. 2000;61(3):137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Coming to terms with fear. Proc. Natl. Acad. Sci. U. S. A. 2014;111(8):2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean P.D. The triune brain, emotion, and scientific bias. In: Schmitt F.O., editor. The Neurosciences: Second Study Program. The Rockefeller University Press; New York: 1970. pp. 336–349. [Google Scholar]
- MacLean P.D. Evolutionary psychiatry and the triune brain. Psychol. Med. 1985;15(2):219–221. doi: 10.1017/s0033291700023485. [DOI] [PubMed] [Google Scholar]
- MacLean P. The triune brain in evolution: role in paleocerebral functions. Springe Science & Business Medir. 1990 doi: 10.1126/science.250.4978.303-a. [DOI] [PubMed] [Google Scholar]
- Maj M. The crisis of confidence in the DSM paradigm and the future of psychiatric diagnosis. Die Psychiatr. 2015;2:67–70. [Google Scholar]
- Makovac E., Watson D.R., Meeten F., Garfinkel S.N., Cercignani M., Critchley H.D., Ottaviani C. Amygdala functional connectivity as a longitudinal biomarker of symptom changes in generalized anxiety. Soc. Cogn. Affect. Neurosci. 2016;11(11):1719–1728. doi: 10.1093/scan/nsw091. pii: nsw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchia M., Adli M., Akula N., Ardau R., Aubry J.M., Backlund L., Banzato C.E., Baune B.T. Assessment of response to lithium maintenance treatment in bipolar disorder: a consortium on lithium genetics (ConLiGen) report. PLoS One. 2013;8(6):e65636. doi: 10.1371/journal.pone.0065636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough K.M., Morrison F.G., Ressler K.J. Bridging the Gap: towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiol. Learn. Mem. 2016;135:27–39. doi: 10.1016/j.nlm.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Int. Med. 1993;153(18):2093–2101. PMID 8379800. [PubMed] [Google Scholar]
- McKinney W.T., Bunney W.E. Animal models of depression. Arch. Gen. Psychiat. 1969;127(2):240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- McTeague L.M., Goodkind M.S., Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp W.P., Buchholz V.N., Van Der Werf J., Leoné F.T. Parietofrontal circuits in goal-oriented behaviour. Eur. J. Neurosci. 2011;33(11):2017–2027. doi: 10.1111/j.1460-9568.2011.07701.x. [DOI] [PubMed] [Google Scholar]
- Meisenzahl E.M., Seifert D., Bottlender R., Teipel S., Zetzsche T., Jäger M., Koutsouleris N., Schmitt G., Scheuerecker J., Burgermeister B., Hampel H., Rupprecht T., Born C., Reiser M., Möller H.J., Frodl T. Differences in hippocampal volume between major depression and schizophrenia: a comparative neuroimaging study. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260(2):127–137. doi: 10.1007/s00406-009-0023-3. [DOI] [PubMed] [Google Scholar]
- Millan M.J., Agid Y., Brüne M., Bullmore E.T., Carter C.S., Clayton N.S., Connor R., Davis S., Deakin B., DeRubeis R.J., Dubois B., Geyer M.A., Goodwin G.M., Gorwood P., Jay T.M., Joëls M., Mansuy I.M., Meyer-Lindenberg A., Murphy D., Rolls E., Saletu B., Spedding M., Sweeney J., Whittington M., Young L.J. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug. Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Mishra J., Gazzaley A. Cross-species approaches to cognitive neuroplasticity research. Neuroimage. 2016;131:4–12. doi: 10.1016/j.neuroimage.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochcovitch M.D., da Rocha Freire R.C., Garcia R.F., Nardi A.E. A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. J. Affect. Disord. 2014;167:336–342. doi: 10.1016/j.jad.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Mohlman J., Eldreth D.A., Price R.B., Staples A.M., Hanson C. Prefrontal-limbic connectivity during worry in older adults with generalized anxiety disorder. Agin. Ment. Health. 2015 doi: 10.1080/13607863.2015.1109058. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Nederhof E., Schmidt M.V. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106(5):691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nelson M.D., Saykin A.J., Flashman L.A., Riordan H.J. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch. Gen. Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13(10):161–169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman Y. Personality from a cognitive-biological perspective. Phys. Life. Rev. 2014;11(4):650–686. doi: 10.1016/j.plrev.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. Editorial: the shape of the nosology to come in developmental psychopathology. J. Child. Psychol. Psychiatry. 2015;56(4):397–399. doi: 10.1111/jcpp.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S., Hautzel H., Heinzel A., Müller H.W. Key players in major and bipolar depression - a retrospective analysis of in vivo imaging studies. Behav. Brain. Res. 2012;232(2):358–390. doi: 10.1016/j.bbr.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Notaras M., Hill R., Gogos J.A., van den Buuse M. BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol. Psychiatry. 2016;21(6):730–732. doi: 10.1038/mp.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K., Hashimoto R., Ikeda M., Yamamori H., Yasuda Y., Fujimoto M., Umeda-Yano S., Fukunaga M., Fujino H., Watanabe Y., Iwase M., Kazui H., Iwata N., Weinberger D.R., Takeda M. Glutamate networks implicate cognitive impairments in schizophrenia: genome-wide association studies of 52 cognitive phenotypes. Schizophr. Bull. 2015;41(4):909–918. doi: 10.1093/schbul/sbu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg I.A., Sabatini B.L. Antagonistic but not symmetric regulation of primary motor cortex by basal ganglia direct and indirect pathways. Neuron. 2015;86(5):1174–1181. doi: 10.1016/j.neuron.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M.J. New approaches to psychiatric diagnostic classification. Neuron. 2014;84(3):564–571. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Oxford University Press; Oxford: 1998. Affective Neuroscience. [Google Scholar]
- Panksepp J. Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005;14(1):30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A., de Quervain D.J. Failed drug discovery in psychiatry: time for human genome-guided solutions. Trends Cogn. Sci. 2015;19(4):183–187. doi: 10.1016/j.tics.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Papez J.W. A proposed mechanism of emotion. Arch. Neur. Psych. 1937;38(4):725–743. [Google Scholar]
- Parikh V., Cole R.D., Patel P.J., Poole R.L., Gould T.J. Cognitive control deficits during mecamylamine-precipitated withdrawal in mice: possible links to frontostriatal BDNF imbalance. Neurobiol. Learn. Mem. 2016;128:110–116. doi: 10.1016/j.nlm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Wood J., Bondi C., Del Arco A., Moghaddam B. Anxiety evokes hypofrontality and disrupts rule-relevant encoding by dorsomedial prefrontal cortex neurons. J. Neurosci. 2016;36(11):3322–3335. doi: 10.1523/JNEUROSCI.4250-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot W.J., Lee S.H., Milaneschi Y., Abdellaoui A., Byrne E.M., Esko T., Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium Corporate Collaborator; Social Science Genetic Association Consortium Corporate Collaborator The association between lower educational attainment and depression owing to shared genetic effects? Results in ∼25 000 subjects. Mol. Psychiatry. 2015;20(6):735–743. doi: 10.1038/mp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploog D.W. The place of the triune brain in psychiatry. Physiol. Behav. 2003;79(3):487–493. doi: 10.1016/s0031-9384(03)00154-9. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Porter R.J., Robinson L.J., Malhi G.S., Gallagher P. The neurocognitive profile of mood disorders - a review of the evidence and methodological issues. Bipolar Disord. Suppl. 2015;2:21–40. doi: 10.1111/bdi.12342. [DOI] [PubMed] [Google Scholar]
- Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy P., Sankaran S., Marshel J.H., Kim C.K., Ferenczi E., Lee S.Y., Berndt A., Ramakrishnan C., Jaffe A., Lo M., Liston C., Deisseroth K. Projections from neocortex mediate top-down control of memory retrieval. Nature. 2015;526(7575):653–659. doi: 10.1038/nature15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga D., Matos M.R., Glas A., Smit A.B., Spijker S., Van den Oever M.C. Optogenetic dissection of medial prefrontal cortex circuitry. Front. Syst. Neurosci. 2014;8:230. doi: 10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S., Neale B.M., Corvin A., Walters J.T., Farh K.H., Holmans P.A. Working group of the psychiatric genomics consortium. Biological insights from 108 schizophrenia-associated genetic loci. Schizophr. Nat. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson O.J., Krimsky M., Lieberman L., Allen P., Vytal K., Grillon C. Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala “aversive amplification” circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry. 2014;1(4):294–302. doi: 10.1016/S2215-0366(14)70305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg C., Horner A.E., Bussey T.J., Saksida L.M. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer's disease. Neurobiol. Aging. 2013;34(3):731–744. doi: 10.1016/j.neurobiolaging.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A. Targeting the “species gene ensemble”. Hippocampus. 2002;12(1):105–108. doi: 10.1002/hipo.10000. [DOI] [PubMed] [Google Scholar]
- Rutter M., Dunn J., Plomin R., Simonoff E., Pickles A., Maughan B., Ormel J., Meyer J., Eaves L. Integrating nature and nurture: implications of person-environment correlations and interactions for developmental psychopathology. Dev. Psychopathol. 1997;9(2):335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Saddoris M.P., Cacciapaglia F., Wightman R.M., Carelli R.M. Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J. Neurosci. 2015;5(33):11572–11582. doi: 10.1523/JNEUROSCI.2344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsom J.N., Wong A.H. Schizophrenia and depression co-morbidity: what we have learned from animal models. Front. Psychiatry. 2015;6:13. doi: 10.3389/fpsyt.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino K.J. Behavioral genetics and child temperament. J. Dev. Behav. Pediatr. 2005;26(3):214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr E., Millan M.J., Bahn S., Bertolino A., Turck C.W., Kapur S., Möller H.J., Dean B. Biomarkers for Psychiatry: the journey from fantasy to fact, a report of the 2013 CINP Think Tank. Int. J. Neuropsychopharmacol. 2015;8(10) doi: 10.1093/ijnp/pyv042. pii: pyv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K. Grune and Stratton; New York: 1959. Clinical Psychopathology. [Google Scholar]
- Schultz W., Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schumann G., Binder E.B., Holte A., de Kloet E.R., Oedegaard K.J., Robbins T.W. Stratified medicine for mental disorders. Eur. Neuropsychopharmacol. 2014;24(1):5–50. doi: 10.1016/j.euroneuro.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Segal D.S., Geyer M.A. Animal models of psychopathology. In: Judd L.L., Groves P.M., editors. Psychobiological Foundations of Clinical Psychiatry. J.B. Lippincott Co; Philadelphia: 1985. pp. 1–14. (Chapter 46) [Google Scholar]
- Seidenbecher T., Remmes J., Daldrup T., Lesting J., Pape H.C. Distinct state anxiety after predictable and unpredictable fear training in mice. Behav. Brain Res. 2016;1(304):20–23. doi: 10.1016/j.bbr.2016.02.009. 2016 May. [DOI] [PubMed] [Google Scholar]
- Shemesh Y., Sztainberg Y., Forkosh O., Shlapobersky T., Chen A., Schneidman E. High-order social interactions in groups of mice. Elife. 2013;2:e00759. doi: 10.7554/eLife.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Höflich A., Küblböck M., Kraus C., Baldinger P., Moser E., Lanzenberger R., Windischberger C. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for FMRI. Cereb. Cortex. 2015;25(4):895–903. doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery D.A., Neumann I.D. Oxytocin and major depressive disorder: experimental and clinical evidence for links to aetiology and possible treatment. Pharm. (Basel) 2010;3(3):702–724. doi: 10.3390/ph3030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.S., Berridge K.C., Aldridge J.W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. U.S. A. 2011;108(27):E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller J.W., Ripke S., Lee P.H., Neale B., Nurnberger J.I., Santangelo S. Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on 5 major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Miyake A., Hankin B.L. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front. Psychol. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Miller G.A., Heller W., Banich M.T. Flexible brain network reconfiguration supporting inhibitory control. Proc. Natl. Acad. Sci. U. S. A. 2015;112(32):10020–10025. doi: 10.1073/pnas.1500048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A.M., Kalueff A.V. Developing better and more valid animal models of brain disorders. Behav. Brain. Res. 2015;276:28–31. doi: 10.1016/j.bbr.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Tovote P., Fadok J.P., Lüthi A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tupes E.C., Christal R.E. Personnel Laboratory, Air Force Systems Command; Lackland Air Force Base, TX: 1961. Recurrent Personality Factors Based on Trait Ratings. Technical Report ASD-tr-61-97. [Google Scholar]
- van Goethem N.P., Rutten K., van der Staay F.J., Jans L.A., Akkerman S., Steinbusch H.W., Blokland A., van't Klooster J., Prickaerts J. Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav. Brain Res. 2012;232(2):323–334. doi: 10.1016/j.bbr.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Vermunt M.W., Tan S.C., Castelijns B., Geeven G., Reinink P., de Bruijn E., Kondova I., Persengiev S., Netherlands Brain Bank, Bontrop R., Cuppen E., de Laat W., Creyghton M.P. Epigenomic annotation of gene regulatory alterations during evolution of the primate brain. Nat. Neurosci. 2016;9(3):494–503. doi: 10.1038/nn.4229. [DOI] [PubMed] [Google Scholar]
- Vervliet B., Raes F. Criteria of validity in experimental psychopathology: application to models of anxiety and depression. Psychol. Med. 2013;43(11):2241–2244. doi: 10.1017/S0033291712002267. [DOI] [PubMed] [Google Scholar]
- Voelkl B., Würbel H. Reproducibility crisis: are we ignoring reaction norms? Trends Pharmacol. Sci. 2016;37(7):509–510. doi: 10.1016/j.tips.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Westbrook A., Braver T.S. Dopamine does double duty in motivating cognitive effort. Neuron. 2016;89(4):695–710. doi: 10.1016/j.neuron.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83(1):1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Willner P. Animal models of depression. In: den Boer J.A., Ad Sitsen J.M., editors. Handbook of Depression and Anxiety. A Biological Approach. Marcel Dekker; New York-Basel-Hong Kong: 1994. pp. 291–316. [Google Scholar]
- Willner P., Belzung C. Treatment-resistant depression: are animal models of depression fit for purpose? Psychopharmacol. Berl. 2015;232(19):3473–3495. doi: 10.1007/s00213-015-4034-7. [DOI] [PubMed] [Google Scholar]
- Yen Y.C., Anderzhanova E., Bunck M., Schuller J., Landgraf R., Wotjak C.T. Co-segregation of hyperactivity, active coping styles, and cognitive dysfunction in mice selectively bred for low levels of anxiety. Front. Behav. Neurosci. 2013;7:103. doi: 10.3389/fnbeh.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen Y.C., Gassen N.C., Zellner A., Rein T., Landgraf R., Wotjak C.T., Anderzhanova E. Glycogen synthase kinase-3β inhibition in the medial prefrontal cortex mediates paradoxical amphetamine action in a mouse model of ADHD. Front. Behav. Neurosci. 2015;9:67. doi: 10.3389/fnbeh.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B., Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci. 2013 doi: 10.3389/fnhum.2013.00609. 7,609. [DOI] [PMC free article] [PubMed] [Google Scholar]