Abstract
Background
Noise acts as a stressor and is reported to have impact on individual health depending on nature, type, intensity and perception. Modern medicine has no effective drugs or cure to prevent its consequences. Being an environmental stressor noise cannot be avoided; instead minimizing its exposure or consuming anti-stressor and adaptogens from plants can be considered.
Objectives
The present study was carried out to evaluate the anti-stressor, adaptogen and immunostimulatory activity of Scoparia dulcis against noise-induced stress in Wistar rat models.
Material and methods
Noise stress in rats was created by broadband white noise generator, 100 dB A/4 h daily/15 days and S. dulcis (200 mg/kg b.w.) was administered orally. 8 groups of rats were used consisting of 6 animals each; 4 groups for unimmunized and 4 groups for immunized. For immunization, sheep red blood cells (5 × 109 cells/ml) were injected intraperitoneally.
Results
Sub-acute noise exposed rats showed a significant increase in corticosterone and IL-4 levels in both immunized and unimmunized rats whereas lymphocytes, antibody titration, soluble immune complex, IL-4 showed a marked increase with a significant decrease in IL-2, TNF-α, IFN-γ cytokines only in unimmunized rats. Immunized noise exposed rats presented increased leukocyte migration index and decreased foot pad thickness, IL-2, TNF-α, IFN-γ with no changes in the lymphocytes.
Conclusion
S. dulcis (SD) has normalized and prevented the noise induced changes in cell-mediated and humoral immunity and it could be the presence of anti-stressor and immuno stimulant activity of the plant.
Keywords: Noise stress, Cell-mediated immunity, Humoral immunity, Cytokines, Scoparia dulcis
Highlights
-
•
Noise being an environmental stressor, it cannot be avoided. Hence this study reveals the immunotoxic effects of noise.
-
•
We examine the changes in the adaptive immunity after immunization with sheep RBCs along with noise exposure.
-
•
TH1 and TH2 cytokine profiles were examined in the spleen as well as serum to determine the alteration in immune pathways.
-
•
To examine the effect of Scoparia dulcis as an anti stressor (adaptogen) and immunomodulatory property.
1. Introduction
In modern world noise is one of common stressors. Noise impact depends on its nature, type, intensity and the perception of an individual. Modern medicines have no effective drugs to prevent or cure the stress and its consequences. Despite the noise harmful effect on all body systems, differential effect of immune responsiveness in animals as well as in humans is still under investigation. It may be because of the involvement of hormones secreted via hypothalamic-pituitary-adrenal (HPA) axis and sympathetic adrenal medullary axis related with the limbic system in response to stress [1]. Third stress axis, which involves neuropeptides, neurotrophins, and the sensory neuropeptide substance P activates the neurokinin 1 receptor and is generally described to act locally as a pro-inflammatory way in close contact between substance P containing peptidergic nerve fibers with mast cells and subsequent neurogenic inflammation [2]. The key mechanisms involved in transformed immune competence and neuronal plasticity after stress experience could be due to the result of HPA axis and sympathetic adrenal-medullary axis; it generates Th2 bias on one hand and local pro-inflammatory NNA activation on the other hand [3], [4]. Noise being an environmental stressor and immunotoxin [5] cannot be avoided, instead attenuatation or minimizing the exposure to environmental noise can be considered. For effective immune protection the recruitment of leukocytes in the target area from circulation or lymphoid organs is needed. The leukocyte trafficking and infiltration in response to the antigen were intermediated by the cytokines of TH1 subsets (IL-2, IFN-γ and TNF-α) and TH2 subsets of IL-4 [6]. In acute stress, enhancement of cell-mediated immunity [7] was observed whereas, chronic stress depleteted the immune system [8]. Acute stress has been reported to increase both cortisol and levels of plasma cytokines [9]. Even perceived stress is associated with elevated maternal serum pro-inflammatory cytokines, lower anti-inflammatory cytokines, and exaggerated production of the pro-inflammatory cytokines by lymphocytes stimulated in vitro [10]. There is evidence which shows that cytokine HPA axis circuit plays a relevant role in controlling excessive inflammatory reactions and the non-specific expansion of immune cells with no or low affinity for the antigen that triggers an immune response [11]. Further, stressful conditions affect the body by the excessive free radical production via increase in glucocorticoids [12]. Free radicals act continuously like a chain reaction until the unpaired electron is paired or blocked. Studies show that there is an inverse relationship between the dietary intake of antioxidants from foods and incidence of human diseases. The oxidative stress also reported to stimulate the adaptive immunity that results in pathogenesis of the disease [13]. Hence it is very important to search for plants as a source of antioxidants because they have few side effects and are more effective because of their synergistic effects. Scoparia dulcis (SD) is a herb widely distributed in tropical and subtropical regions. S. dulcis is a species of flowering plant belonging to the Family Plantaginaceae. It is an erect annual herb, producing white flowers that grows to a height of 0.5 m with various indigenous tribes in the rain-forest zone are well-documented [14]. Aqueous extracts of S. dulcis possess important hepatoprotective and antioxidant activity [15]; traditional practitioners have claimed that the in vitro antioxidant activity supports the therapeutic effects [16]. Phytochemical screening of S. dulcis revealed that it is rich in flavonoids and terpenes that are believed to be responsible for its pharmacological action [17]. It has been used for blood cleansing in childbirth and acts as a general tonic [18]. S. dulcis plants have been traditionally used as remedies for stomach trouble [19]. The indigenous tribes in Nicaragua use a hot water infusion or decoction of the leaves or the whole plant for stomach pain, menstrual disorder, as an aid in child birth, as a blood purifier, for insect bites, fever, heart problems, liver and stomach disorders, malaria, sexually transmitted diseases and as a general tonic [20]. Based on these earlier usages of S. dulcis, a medicinal plant with anti-oxidative, anti-inflammatory and neuro-protective properties [21] that have been studied in vitro as well as in in vivo models, it has been analyzed for its immune modulatory property specific towards the cytokine profile and modulation of adaptive immunity after subjected to noise stress. In this present study noise exposure on cytokine mediated immune functions are focused.
2. Materials and methods
2.1. Plant materials and preparation
S. dulcis was collected from Tampcol, Chennai, India after it was confirmed by the botanist and a sample was deposited in herbarium (Reg no: NIS/MB/63/2012) of National Institute of Siddha, Chennai. 100 g of air dried leaves portions of SD were soaked in 1 L of distilled water overnight and filtered subsequently into a beaker using filter paper and funnel. The filtrate was concentrated at 40 °C to constant weight using a Rotavapor apparatus. The residue was collected and stored at −4 °C. The concentrate was then reconstituted into a stock solution of 200 mg/ml in distilled water. The required volume of this solution (calculated on the basis of animal weight) was administered orally daily using a gavage.
2.2. Experimental animals and groupings
The study was initiated with proper approval by the Institutional Animal Ethics Committee (IAEC No 01/19/2013). Healthy adult male Wistar rats weighing about 180–200 g were used for this study. Animals were housed in groups of three per cage and maintained at a constant temperature controlled room 22 ± 5 °C, of relative humidity 55± 5% with a 12-h light: 12-h dark cycle and allowed free access to food and water. The experimental animals were randomly divided into 8 groups (6 rats in each group). Group 1 served as the control, Group 2 animals were subjected to noise-stress for 4 h daily for 15 days (Sub-acute exposure), Group 3 control treated with SD for 48 days and experiments were carried out on 49th day and Group 4 consisted of noise stress + SD-treated animals. The SD dosage used in this study was 200 mg/kg/b.w. and was based on an earlier report [22]. These animals were pre-treated with SD for 33 days and then exposed to noise stress for 15 days. Group 5 Immunized control, Group 6 Immunized Noise alone (animals were subjected to noise-stress for 4 h daily for 15 days), Group 7 Immunized control treated with SD for 48 days and experiments were carried out on 49th day and Group 8 Immunized noise stress + SD-treated animals. A combined pre- and post treatment regimen of each extract may also prove to be more effective [23] and in the current study the animals were pre-treated with SD for 33 days and then exposed to noise stress for 15 days.
2.3. Noise stress procedure
Noise was produced by a loud speaker (15 W), installed at a distance of 30 cm above the cage, and driven by a white noise generator emitting all the frequencies in the range 0–20 kHz. A precision sound level meter was used to monitor the intensity of sound to 100 dB uniformly in the cage. During the experiment, the noise level peaked at 100 dB immediately after the generator was switched on and continued daily for 4 h for 15 days. The controls were also kept in these cages without noise stress exposure [24].
2.4. Immunization
The sheep red blood cells (SRBC) were collected in a sterile Alsever's solution. It was centrifuged and washed thrice using phosphate buffered saline to get clear red blood cell suspension by removing the supernatant. Then the red cell suspension was prepared in a series of dilutions with methylene blue and the cells were counted using Neubauer chamber under the microscope. The dilution count of 5 × 109 cells per ml was identified and one ml was administered intraperitoneally (i.p) to rats. The day of immunization was considered as day 0 and the blood samples were collected from jugular vein on the 5th day to assess the primary immune response [25]. After blood collection the rat was sacrificed under deep anesthesia and the immune organs were harvested for the assays.
2.5. Assay of corticosterone [26]
This method is based on the oxidation of corticosteroids with ferric iron (III) in acidic medium and subsequent complex with ferrous iron (II) and potassium hexacyanoferrate. 0.5 μl of sample was mixed with appropriate volumes of working solutions of corticosterone. 2 ml of sulphuric acid and 2 ml ferric chloride were added to 0.5 ml of potassium hexacyanoferrate (III) solution. The mixture was heated in a water-bath maintained at 70 ± 2 °C for 30 min with occasional shaking. The absorbance was measured at 780 nm against the reagent blank.
2.6. Soluble immune complex
A simple turbidometric assay [27] based on the precipitation of immune complex by low concentration of polyethylene glycol (PEG) which, is an uncharged, water soluble, linear polymer was carried out. Control as well as test serum were diluted to 1:3 with borate buffer; 0.22 ml of this diluted serum was added to tube A and tube B. 2 ml of borate buffer was added to tube A and this served as control. To tube B, 2 ml of PEG borate buffer solution was added and this served as test; it was mixed well and incubated at room temperature for 1 h. The absorbance of the test and the control tube was measured at 450 nm (E450) in a spectrophotometer and the result was expressed as PEG index derived by the formula, i.e. PEG index = (E450 with PEG − E450 with PBS) × 1000.
2.7. Cell-mediated immunity
Leukocyte migration index and footpad thickness in rats were carried out according to the method described by Tewari et al. [28].
2.8. Leukocyte migration index
Spleen and thymus were weighed individually and single cell suspension was prepared by teasing and pressing through the mesh in a Petri dish containing medium. Erythrocytes were removed from the spleen cell suspension using 0.83% ammonium chloride and this forms the effectors of lymphocytes. Thymus and spleen cell suspensions were washed thrice with medium, mixed in 1:3 ratio respectively and were divided into two parts. The first part remains unaltered whereas in the second part 0.1 ml of antigen SRBC (1.5 × 106 SRBC/ml) was added. The suspensions were mixed well and drawn into a capillary which was then heat sealed at one end and centrifuged (1000 g for 2 min) at room temperature. Then at the capillary fluid interface it was cut and mounted in the sealed chamber separately in different chambers marked as ‘with Ag’ and ‘without Ag’ with the help of silicon grease. Immediately the chamber was filled with medium taking great care to exclude air bubbles. The plates were incubated in an incubator at 37 °C overnight (15–18 h). After incubation the migration area from the capillary was projected to a screen with the help of camera Lucida and the area of migration was plotted in a paper. Using planimeter the area of migration was calculated. The average of 4–6 capillaries was taken as the mean area of migration. The migration index was calculated as follows:
| The migration index = Mean area of migration in presence of antigen/Mean area of migration in absence of antigen |
2.9. Footpad thickness test
FPT was studied only in immunized animals as this needs sensitization. Immunization was done with SRBC and the day of immunization was considered as day ‘0’. Four days later the left hind foot pad was challenged by an injection of 0.1 ml of 20% SRBC subcutaneously suspended in normal saline. The right hind paw received saline alone and served as control. The increase in foot pad thickness was measured 24 h after SRBC challenge by Vernier calliper and the values were reported as index.
| DTH = The thickness of SBRC injected foot pad/Thickness of the saline injected food pad × 100 |
2.10. Humoral immunity
2.10.1. Antibody titre [29]
It is a direct hemagglutination reaction that is commonly performed by mixing various dilutions of antiserum with a suspension of particulate antigens. The specific agglutination of red cells constitutes a highly sensitive method for detection of antibody titer by observing till the low concentration in the serum that shows agglutination.
2.10.2. Quantification of cytokine
Enzyme Linked Immunosorbent Assay (ELISA) (Peprotech, Rocky Hill, New Jersey USA) kits were used to quantify Th1 by assessing TNF-α (M900-M73),IL-2 (M900-M205), and IFN-γ (M900-M98) whereas Th2 (ELS-IL4-001), (IL-4 Ray biotech USA) cytokines within serum as well as in tissue. Measurements were performed according to the manufacture's procedural instructions.
2.10.3. Statistical analysis
Each of the experiment was carried out in triplicate and the results are expressed as mean ± standard deviation (S.D.). All data were analyzed with the SPSS for windows statistical package (version 20.0, SPSS Institute Inc., Cary, North Carolina). The statistical significance between the four different groups was analyzed by using one way ANOVA test followed by Tukey's multiple comparison tests and the significance level was fixed at p < 0.05.
3. Results
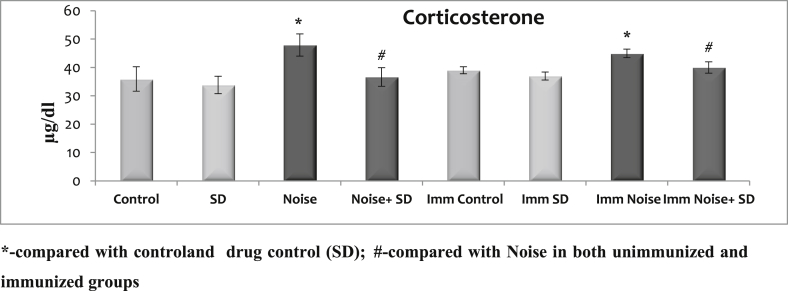
3.1. Effect of sub-acute noise on corticosterone levels
The data are summarized in (Fig. 1) with mean ± S.D. There were no significant changes observed in the corticosterone level between control and control treated with SD of unimmunized or immunized rats. However, noise stress increased the corticosterone levels irrespective of whether unimmunized or immunized. But the treatment with SD to noise stressed group whether immunized or un immunized could maintain the corticosteroid level at normal level during noise stress exposure.
Fig. 1.
Effect of sub-acute noise on corticosterone levels.
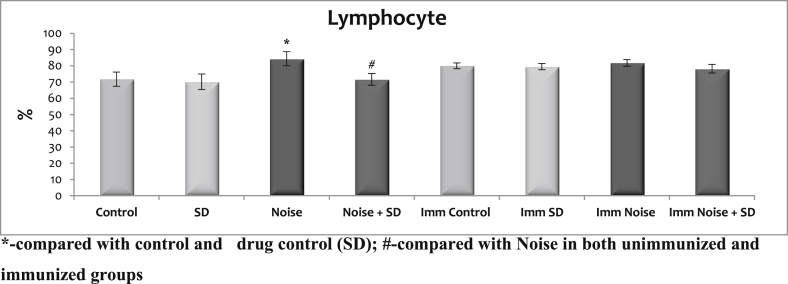
3.2. Effect of sub-acute noise on lymphocytes
The data are summarized in (Fig. 2) with mean ± S.D. SD when given to normal animals, did not alter the lymphocyte numbers from controls. The noise stress only increased the lymphocyte levels in unimmunized rats but not in immunized animals. However, treatment with SD during noise exposure in unimmunized could maintain the lymphocytes at normal level during noise stress exposure.
Fig. 2.
Effect of sub-acute noise on lymphocytes.
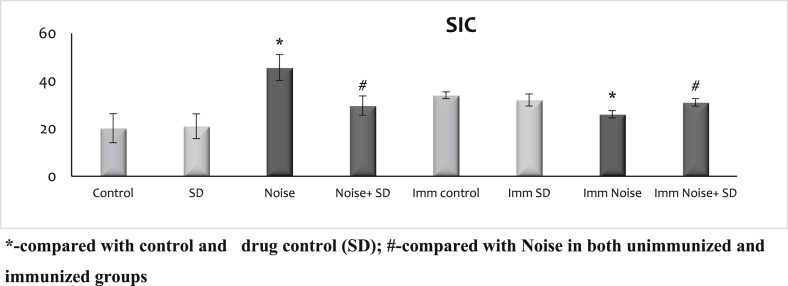
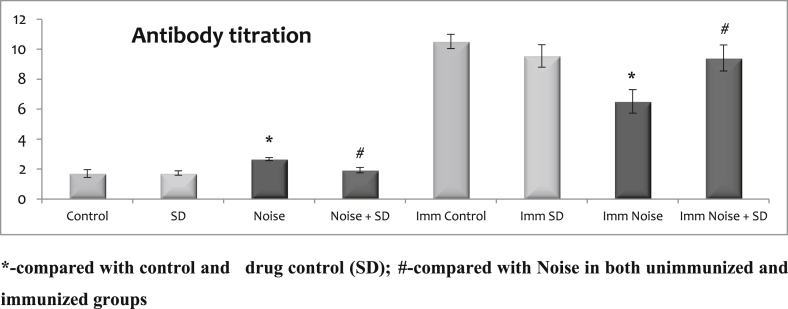
3.3. Effect of sub-acute noise on humoral immunity
Antibody titration and soluble immune complex is given in Fig. 3, Fig. 4. There was no difference observed between the control and control treated with SD irrespective of whether immunized or unimmunized. The noise stressed animals showed a marked increase in the soluble immune complex and antibody titration of humoral immunity in unimmunized whereas it was decreased in the immunized. However, treatment with SD during noise exposure whether immunized or unimmunized could maintain the soluble immune complex and antibody titration at normal levels during noise stress exposure.
Fig. 3.
Effect of sub-acute noise on soluble immune complex.
Fig. 4.
Effect of sub-acute noise on antibody titration.
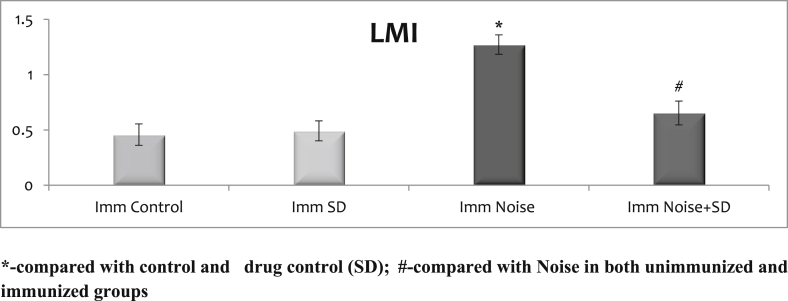
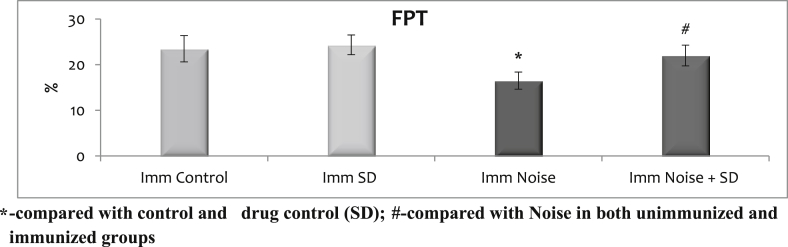
3.4. Effect of sub-acute noise on cell-mediated immunity: leukocyte migration index (LMI) and footpad thickness (FPT)
It was carried out only on immunized animals and the data are summarized in Fig. 5, Fig. 6 with mean ± S.D. SD when given to normal animals did not alter the level LMI and FPT levels from controls. There were no significant differences observed between the control and control treated with SD. Only in the immunized noise stressed animals there was an increase in LMI levels and decreased FPT when compared with control, control treated with SD and SD treated noise stressed animals.
Fig. 5.
Effect of sub-acute noise on leukocyte migration index (LMI).
Fig. 6.
Effect of sub-acute noise on footpad thickness (FPT).
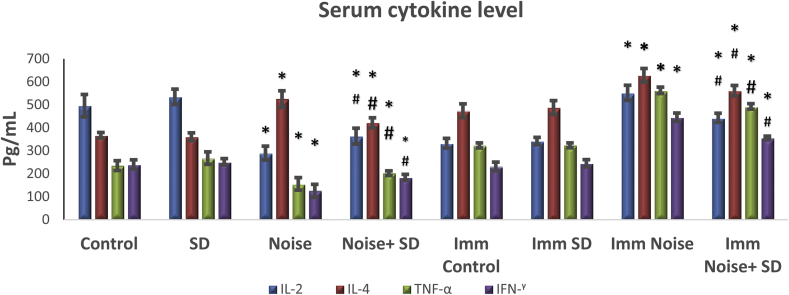
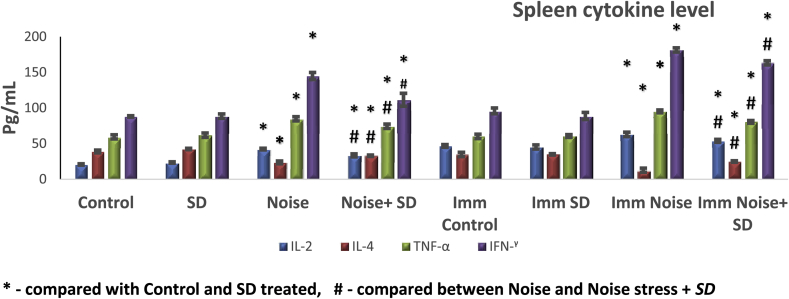
3.5. Effect of sub-acute noise on cytokines levels
Fig. 7, Fig. 8 show the descriptive statistics of the cytokine concentrations in serum as well as in spleen.
Fig. 7.
Protective effect of S. dulcis (SD) serum cytokines in albino rats exposed to noise-stress in unimmunized and immunized (Imm) groups. Values are expressed as mean ± S.D. from six animals.
Fig. 8.
Protective effect of S. dulcis (SD) spleen cytokines in albino rats exposed to noise-stress in unimmunized and immunized (Imm) groups. Values are expressed as mean ± S.D. from six animals.
In serum of unimmunized noise exposed animals, IL-4 is increased and IL-2, TNF-α and IFN-γ is decreased compared to control and control treated with S. dulcis and vice versa in case of unimmunized spleen. In case of immunized groups, the level of cytokines IL-2, TNF-α and IFN-γ in noise exposed animals were increased irrespective of spleen and serum, however, there was a significant change only with IL-4 which showed a marked increase in serum and a significant decrease in the spleen when compared to control and control treated with S. dulcis. However when the noise exposed animals treated with S. dulcis brought back the cytokine levels nearly to control level irrespective of immunized or unimmunized animals, still it deviated from the controls and control treated with S. dulcis in serum and spleen.
4. Discussion
The present study clearly indicates that noise exposure acts as a stressor and was not adopted even after repeated exposures. The elevated corticosteroid level in noise exposed animals further reflects this and could be the possible reason for immune system and inflammatory responses. Noise exposure induces the glucocorticoids increase [30]. Increased corticosteroid level reported as an immunosuppressor in both humans and animals [2]. However, treatment with SD could normalize the corticosteroid level. Hence, it may be inferred that the constituents in SD may be involved in regulating the HPA axis and corticosteroid induced alteration in the immunity. The decrease in the circulating lymphocytes after noise exposure only in the unimmunized animals suggests that, it may be due to redistribution of leukocytes. According to Nagaraja et al. [31], abnormal distribution of leukocytes may be due to local chemotaxis that causes the cell retention in several organs. The leukocyte messengers such as cytokines, lymphokines, monokines are able to convey information to neuro and endocrine structures about the present state of activity of the immune system is stressed and also been associated classically with activation and regulation of the immune system and inflammatory responses [32]. At the early stage of stress, the activated HPA axis and sympathetic nervous system (SNS) axis can upregulate the levels of glucocorticoids (GCs) and catecholamines (CAs), respectively, and in turn inhibit the secretion of pro-inflammatory cytokines directly or indirectly while promoting the secretion of anti-inflammatory cytokines. It is essential to note that noise stress decreases the serum IL-2, IFN-γ and TNF-α level. IFN-γ reported to shift towards Th2 cell activity which tends to be a strong immunosuppressive effect [33], despite increase in IL-4 was observed. Studies showed that, IL-4 inhibits the production of TNF-α, IL-1 and IL-6 by macrophages [12]. Hence, the increase in IL-4 may be one of the reasons for the decrease in TNF-α observed in this present study. It is obvious that this study shows the conflicting results between the immunized and unimmunized rats on Th1/Th2 subtypes. However, it may be possible due to the action of noise exposure on immune-neuroendocrine interactions for immunoregulation and host defenses, which play an active role of the immune system in mediating metabolic and homeostatic adjustments or derangements during the course of certain infections and inflammation [34]. Th1 cells secrete IL-2, IFN-γ and participate in cell-mediated immunity [10], whereas, Th2 cells secrete IL-4 and play a role in humoral immunity. The cytokine balance of Th1/Th2, (i.e, pro-inflammatory and anti-inflammatory cytokines) plays a major role in neuroimmunological responses [35] and this has been used to assess various stressor levels. The noise exposure acts as stressor and in turn alters the activated HPA axis and SNS axis which can upregulate the levels of glucocorticoids (GCs) and catecholamines (CAs), respectively [36]. However the lasting impacts of noise exposure on HPA axis, glucocorticoid resistance, and activated NF-кB on cytokines cause the pro-inflammatory cytokines to increase further, which to a certain level induce inflammatory response. In addition, IL-1β;(beta), IL-6, TNF-α, and other cytokines in turn activate NF-кB, in which the mechanism includes the pro-inflammatory cytokines leading to phosphorylation and loss of I-κB [37]. All the steps induce continued increased pro-inflammatory cytokines, and finally inflammation, which may induce various diseases [38].
The increase in SIC and antibody titer only in the unimmunized animals after noise stress exposure indicate a non-specific reaction, because there was a decrease in antibody titer observed after immunization in these noise stressed animals. As the antigen used is the T dependent antigen, it is essential to consider the cell-mediated as well as the cytokine profile in the noise stressed animals.
The present study finding also in concurrence with the Tewari et al. [28], report the inverse correlation existing between the LMI and FPT. The present observation in noise stressed animal is further correlated with Srikumar et al. [24], where noise stress induced a decrease in FPT with an enhancement in LMI. The decrease in foot pad thickness after stress could be due to the effect of stress on the inflammatory, mediatory and adhesion molecules on the endothelial cell and extra vascular space. Leukocyte migration acts as an index for cell-mediated immunity, the normal migration depends on the balance of these two opposing factors (migration inhibitory factor) and (migration stimulating factor). Migration stimulation factor (MStF) is from the suppressor cells and migration inhibition factor (MIF) is from the helper cells [8].
The neural modulation of immunity might play an essential role during the noise stress. Hence it can be concluded that stress induced alterations are multifactorial. Overall changes in the parameters of immune system in noise stress exposed animals were prevented except the cytokine profile. When the noise exposed animals were treated with S. dulcis the cytokine profile was tried to maintain near normal. It may be due to the action of S. dulcis, rich in phytochemicals like flavonoids, terpenoids etc. Further the presence of catecholamines in SD which has many another positive factors in regulating the immune response and acute phase proteins against the action of glucocorticoids. It is essential to consider that, when SD fed to normal animals none of the parameters studied were deviated from control which further reflects the action of SD can be considered as an adapogen. Hence this study recommends the use of SD as a prophylactic to the unavoidable environmental stressor such as noise.
5. Conclusion
In conclusion aqueous extracts of S. dulcis shows immune modulatory and stimulatory effect on cell-mediated and humoral immunity by exhibiting the antioxidant, anti-stressor, immuno stimulant activity by normalizing and preventing the changes in cell-mediated and humoral immunity induced by noise stress increased corticosterone and free radicals in the Wistar rats.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgement
The author likes to acknowledge the financial support provided by the UGC DST-INSPIRE (IF 130064), India and to the University of Madras, Chennai Tamil Nadu, India.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Ader R., Felten D.L., Cohen N. 3rd ed. Academic Press; San Diego, CA: 2001. Psychoneuroimmunology. [Google Scholar]
- 2.Ambrose J., Wolstenholme G.E.W., Knight, editors. Hormones and the immune response. J. and A. Churchill; London: 1970. pp. 100–116. [Google Scholar]
- 3.Arndt J., Smith N., Tausk F. Stress and atopic dermatitis. Curr Allergy Asthma Rep. 2008;8:312–317. doi: 10.1007/s11882-008-0050-6. [DOI] [PubMed] [Google Scholar]
- 4.Baker M.E. Adrenal and sex steroid receptor evolution: environmental implications. J Mol Endocrinol. 2001;26:119–125. doi: 10.1677/jme.0.0260119. [DOI] [PubMed] [Google Scholar]
- 5.Prasher D. Is there evidence that environmental noise is immunotoxic? Noise Health. 2009;1(11):151. doi: 10.4103/1463-1741.53361. [DOI] [PubMed] [Google Scholar]
- 6.Chida Y., Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- 7.Coussons-Read Mary E., Okun M.L., Nettles C.D. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Dhabhar F.S., Mcewen B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 9.Dhabhar F.S. A hassle a day may keep the pathogens away: the fight-or-flight stress response and the augmentation of immune function. Integr Comp Biol. 2009;49(3):215–236. doi: 10.1093/icb/icp045. [DOI] [PubMed] [Google Scholar]
- 10.Douglas F., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 11.Fox R.A., Rajaraman K. A link between helper and suppressor factors and the lymphokines migration inhibition factor and migration stimulation factor. Cell Immunol. 1981;59:448–454. doi: 10.1016/0008-8749(81)90424-x. [DOI] [PubMed] [Google Scholar]
- 12.Hart P.H., Vitti G.F., Burgess D.R., Whitty G.A., Piccoli D.S., Hamilton J.A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci. 1989;86:3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ising H., Kruppa B. Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health. 2002;6:5. [PubMed] [Google Scholar]
- 14.Branch L.C., Silva M.D. Folk medicine of Alter do chao, Para, Brazil. Acta Amaz. 1983;13:737–797. [Google Scholar]
- 15.Langeswaran K., Jagadeesan A., Vijayaprakash J. Hepatoprotective and antioxidant activity of Scoparia dulcis Linn, against N-Nitrosodiethylamine (DEN) induced hepatotoxicity in experimental rats. Int J Drug Dev Res. 2012;4:295–303. [Google Scholar]
- 16.Ratnasooriya W.D., Jayakody J.R.A.C., Premakumara G.A.S., Ediriweera E.R.H.S.S. Antioxidant activity of water extract of Scoparia dulcis. Fitoterapia. 2005;6:220–222. doi: 10.1016/j.fitote.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T., Masaru K., Yoshihisa M., Tooru T., Naokata M. Antiviral agents of plant origin. III. Scopadulin, a novel tetracyclic diterpene from S. dulcis. Chem Pharm Bull. 1990;38:945–947. doi: 10.1248/cpb.38.945. [DOI] [PubMed] [Google Scholar]
- 18.Edeoga H.O., Okwu D.E., Mbaebie B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–688. [Google Scholar]
- 19.Satyanarayana S., Sushruta K., Sarma G.S., Srinivas N., Raju G.S. Antioxidant activity of the aqueous extracts of spicy food additives—evaluation and comparison with ascorbic acid in in vitro systems. J Herb Pharmacother. 2004;1(4):1–10. [PubMed] [Google Scholar]
- 20.Taylor L. Square One Pub; 2005. The healing power of rainforest herbs: a guide to understanding and using herbal medicinals. [Google Scholar]
- 21.Murti K., Panchal M., Taya P., Singh R. Pharmacological properties of Scoparia dulcis: a review. Pharmacologia. 2012;3:344–347. [Google Scholar]
- 22.Latha M., Pari L. Effect of an aqueous extract of Scoparia dulcis on blood glucose, plasma insulin and some polyol pathway enzymes in experimental rat diabetes. Braz J Med Biol Res. 2004;37:577–586. doi: 10.1590/s0100-879x2004000400015. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y., Eve D.J., Yu S., Shojo H., Bae E.C., Park D.H. Acute treatment with herbal extracts provides neuroprotective benefits in in vitro and in vivo Stroke models, characterized by reduced ischemic cell death and maintenance of motor and neurological functions. Cell Med. 2010;1:137. doi: 10.3727/215517910X552818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikumar R., Parthasarathy N.J., Manikandan S., Muthuvel A., Rajamani R., Sheeladevi R. Immunomodulatory effect of Triphala during experimentally induced noise stress in albino rats. J Health Sci. 2007;53:142–145. [Google Scholar]
- 25.Srinivasan S., Loganathan S., Wankhar W., Rathinasamy S., Rajan R. Stress effect on humoral and cell mediated immune response: indispensable part of corticosterone and cytokine in neutrophil function. Trials Vaccinol. 2016;5:61–70. [Google Scholar]
- 26.Singh D.K., Verma R. Spectrophotometric determination of corticosteroids and its application in pharmaceutical formulation. Iran J Pharmacol Ther. 2008;7:61–65. [Google Scholar]
- 27.Seth P., Srinivas R.V. Circulating immune-complexes in cervical-cancer-simple method for detection and characterization. Indian J Med Res. 1981;73:926–929. [Google Scholar]
- 28.Tewari S., Seshadri M., Poduval T.B. Migration inhibition of normal rat thymocytes as an in-vitro method of detecting cell mediated immunity in rat and mouse. J Immunol Methods. 1982;51:231–239. doi: 10.1016/0022-1759(82)90262-9. [DOI] [PubMed] [Google Scholar]
- 29.Puri A., Saxena R., Saxena R.P., Saxena K.C., Srivastava T., Andon J.S. Immuno stimulant activity of Nyctanthes arbor-tristis L. J Ethnopharmacol. 1994;42:31–37. doi: 10.1016/0378-8741(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 30.Zheng K.C., Makoto A. Modulations of immune functions and oxidative status induced by noise stress. J Occup health. 2007;49:32–38. doi: 10.1539/joh.49.32. [DOI] [PubMed] [Google Scholar]
- 31.Nagaraja H.S., Anupama B.K., Jeganathan P.S. Stress responses in albino rats. Thai J Physiol Sci. 2006;19:8–15. [Google Scholar]
- 32.Hopkins S.J., Rothwell N.J. Cytokines and the nervous system I: expression and recognition. Trends Neurosci. 1995;18(2):83–88. [PubMed] [Google Scholar]
- 33.Verhoef C.M., van Roon J.A., Vianen M.E., Lafeber F.P., Bijlsma J.W. The immune suppressive effect of dexamethasone in rheumatoid arthritis is accompanied by upregulation of interleukin 10 and by differential changes in interferon γ and interleukin 4 production. Ann Rheumatic Dis. 1999;58:49–54. doi: 10.1136/ard.58.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Besedovsky H.O., Del Rey A. Immune-neuroendocrine circuits: integrative role of cytokines. Front Neuroendocrinol. 1992;13(1):61–94. [PubMed] [Google Scholar]
- 35.Plata-Salaman C.R., Turrin N.P. Cytokine interactions and cytokine balance in the brain: relevance to neurology and psychiatry. Mol Psychiatry. 1999;1:4. [PubMed] [Google Scholar]
- 36.Tian R., Hou G., Li D., Yuan T.F. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Sci World J. 2014:3. doi: 10.1155/2014/780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beg A.A., Ruben S.M., Scheinman R.I., Haskill S., Rosen C.A., Baldwin A.S. I κ B interacts with the nuclear localization sequences of the subunits of NF-κ B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct 1;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 38.Rahman I., Gilmour P.S., Jimenez L.A., MacNee W. Oxygen/nitrogen radicals: cell injury and disease. Springer; US: 2002. Oxidative stress and TNF-a induce histone acetylation and NF-кB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation; pp. 239–248. [PubMed] [Google Scholar]