Abstract
This study aimed to apply the dead space fraction [ratio of dead space to tidal volume (VD/VT)] to titrate the optimal positive end-expiratory pressure (PEEP) in a swine model of acute respiratory distress syndrome (ARDS). Twelve swine models of ARDS were constructed. A lung recruitment maneuver was then conducted and the PEEP was set at 20 cm H2O. The PEEP was reduced by 2 cm H2O every 10 min until 0 cm H2O was reached, and VD/VT was measured after each decrement step. VD/VT was measured using single-breath analysis of CO2, and calculated from arterial CO2 partial pressure (PaCO2) and mixed expired CO2 (PeCO2) using the following formula: VD/VT = (PaCO2 - PeCO2)/PaCO2. The optimal PEEP was identified by the lowest VD/VT method. Respiration and hemodynamic parameters were recorded during the periods of pre-injury and injury, and at 4 and 2 cm H2O below and above the optimal PEEP (Po). The optimal PEEP in this study was found to be 13.25±1.36 cm H2O. During the Po period, VD/VT decreased to a lower value (0.44±0.08) compared with that during the injury period (0.68±0.10) (P<0.05), while the intrapulmonary shunt fraction reached its lowest value. In addition, a significant change of dynamic tidal respiratory compliance and oxygenation index was induced by PEEP titration. These results indicate that minimal VD/VT can be used for PEEP titration in ARDS.
Keywords: acute respiratory distress syndrome, dead space fraction, positive end-expiratory pressure, recruitment maneuver
Introduction
Acute respiratory distress syndrome (ARDS) is a severe and life-threatening medical condition that is common in critically ill patients and has a high mortality rate (1). It is a main reason for acute respiratory failure, and is characterized by widespread inflammation in the lungs (1,2). ARDS can induce pathophysiological mechanisms of alveolar collapse, hyoxemia, vascular dysfunction and elevated dead space fraction [the ratio of dead space volume to tidal volume (VD/VT)] (3,4).
Currently, the lung-protection strategy for ventilation involves the use of high positive end-expiratory pressure (PEEP) levels combined with low tidal volumes to prevent end expiratory alveolar collapse, increase functional residual capacity, reduce VD/VT and attenuate hypoxemia (5,6). However, the application of higher levels of PEEP may not be necessarily beneficial, since it increases the inflation of lung regions. Additionally, it will also increase the risk of hemodynamic abnormalities as well as the lung injury induced by ventilation (7,8). Numerous studies have attempted to define the optimal PEEP level on the basis of a variety of methods during a recruitment maneuver (RM) with decreasing PEEP (9–11).
A number of studies have applied the VD/VT method to assess the effects of lung recruitment and PEEP titration in patients with severe ARDS (9–11). VD/VT is a specific value based on the relatively high diffusibility of CO2 across tissue membranes (12), and the exchange of CO2 depends strictly on alveolar ventilation volume (13). However, some studies did not find a similar effect on VD/VT during PEEP titration (14,15). Therefore, the application of the lowest VD/VT method to titrate the optimal PEEP in patients with ARDS remains to be investigated.
In the present study, an oleic acid lung-injury model in swine was used to evaluate the effect of varying the PEEP level on dead space fraction. The aim was to realize the changes in VD/VT induced by different PEEP levels in the ARDS swine model and to explore the feasibility of using the VD/VT ratio to guide the optimal PEEP titration.
Materials and methods
Animals and anesthesia
The study was a prospective, sham-controlled and in vivo animal study, and was approved by the animal ethics committee of Beijing Shijitan Hospital, affiliated to Capital Medical University (Beijing, China).
Twelve healthy male swine (age, 11–13 months) with an average weight of 39.13±3.27 kg were provided by the animal center of Pinggu Hospital of Capital Medical University [licence: SYXK (B) 2010–0016]. The animals were housed at 21–27°C with a humidity of 45–55%, with free access to food and water. Swine were fasted for 24 h and then were orotracheally intubated in the supine position during deep intramuscular anesthesia with ketamine (35 mg/kg; Jiangsu Hengrui Medicine Co. Ltd., Lianyungang, China), 3% pentobarbital sodium (30 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and diazepam (1.5 mg/kg; Sigma-Aldrich). A double cavity central venous catheter was inserted into the right internal jugular vein using the Seldinger technique (16) and connected to the monitoring system. Following line placement, the anesthetic was switched to total intravenous anesthesia with continuous infusion of pentobarbital sodium (2 mg/kg/h), ketamine (3 mg/kg/h) and pipecurium bromide (0.03 mg/kg/h; Gedeon Richter Plc., Budapest, Hungary). In all swine, a 4-French gauge arterial thermodilution catheter was inserted via the left femoral artery. The arterial catheter was connected to a computer for pulse contour analysis (Pulsion Medical Systems, Munich, Germany) for the clinical monitoring of hemodynamic measurements.
Monitoring
The respiration parameters of alveolar partial pressure of O2 (PAO2)/fraction of inspiration O2 (FiO2) ratio (P/F), arterial CO2 partial pressure (PaCO2) and arterial O2 saturation (SaO2) were directly measured through arterial blood gas analysis using the (GEM Premier 3000; Instrumentation Laboratory, Inc., Lexington MA, USA) (17). Lung maximum dynamic tidal respiratory compliance (Cdyn) was monitored using a SERVI-i ventilator (Siemens Maquet Critical Care AB, Slona, Sweden).
The VD/VT ratio was measured via the single-breath analysis of CO2 (18) (NICO Cardiopulmonary Management System; Novametrix; Philips Respironics, Murrysville, PA, USA). With this method, the partial pressure of mixed-expired CO2 was calculated followed by the Enghoff modification of the Bohr equation as follows: VD/VT=(PaCO2-PeCO2)/PaCO2, where PeCO2 represents mixed expired CO2 (19). An arterial blood gas sample was obtained when the PeCO2 variability on the NICO monitor was ≤1 mmHg within 5 min. The NICO sensor fitted between the Y-piece and the endotracheal tube.
The intrapulmonary shunt fraction (Qs/Qt) was calculated according to the standard formulae:
Qs/Qt = (CcO2 - CaO2)/(CcO2 - CvO2)
CaO2 = Hb × SaO2 × 1.34 + PAO2 × 0.0031
CvO2 = Hb × SvO2 × 1.34 + PvO2 × 0.0031
CcO2 = Hb × ScO2 × 1.34 + PcO2 × 0.0031
ScO2 ≈ 100%
PcO2 ≈ PAO2
PAO2 = PiO2 - PaCO2/R
PiO2 = (PB - PH2O) × FiO2
where Qs represents shunted pulmonary blood flow; Qt represents total pulmonary blood flow; CcO2 represents pulmonary capillary O2 content; PvO2 represents mixed venous O2 partial pressure; CaO2 represents arterial O2 content; CvO2 represents mixed venous O2 content; Hb represents hemoglobin; PiO2 represents partial pressure of inspired O2; R represents respiratory quotient (0.8); PB represents barometric pressure (~100 kPa on sea level); PH2O represents saturation vapor pressure (6.3 kPa at 37°C).
The hemodynamic parameters of cardiac output index (CI), global end-diastolic volume index (GEDI), extravascular lung water index (ELWI), intra-thoracic blood volume index (ITBI) and systemic vascular resistance index (SVRI) were directly measured by the thermodilution method (20) using the PICCO system (Pulsion Medical Systems). The central venous pressure (CVP) was monitored with the central venous catheter in the right internal jugular vein.
Protocol
Following intubation, lungs were ventilated in a volume-controlled ventilation mode, with the following initial parameters: VT of 8 ml/kg, FiO2 of 1.0, PEEP of 5 cm H2O, respiratory rate of 40 breaths/min, and inspiratory to expiratory time ratio (I:E) of 1:2. These settings were maintained for 30 min to achieve stabilization.
ARDS induction
After recording pre-injury hemodynamic, gas exchange, respiratory mechanics measurements and oxygen metabolism, 0.2 ml/kg oleic acid (Sigma-Aldrich) in 40 ml saline was slowly (within 15 min) injected in the right atrium via the central venous catheter. After a 90-min injury stabilization period, the experimental protocol was initiated. A successful model of ARDS was defined by P/F <200 mmHg for 90 min following oleic acid infusion (21). Each swine was infused continuously with intravenous saline at a rate of 100 ml/h.
Lung recruitment maneuver
The swine were stabilized for 15 min on the following ventilator settings and followed by data gathering: Pressure control ventilation (PCV) peak pressure, 35 cm H2O; PEEP, 20 cm H2O; inspiratory time, 0.6 sec; rate, 40/min and FiO2 1.0. The PEEP was then set to 20 cm H2O and pressure control set to a peak airway pressure of 40 cm H2O. These settings were maintained for 2 min, followed by a 15-min stabilization period with a peak pressure 35 cm H2O. Data were gathered if PAO2 + PaCO2 was >400 mmHg. If PAO2 + PaCO2 was <400 mmHg, the PEEP setting remained unchanged and pressure control was increased to obtain a peak airway pressure of 45 cm H2O. This pattern was sustained for 2 min, followed by a 15-min stabilization period with peak pressure 35 cm H2O. If PAO2 + PaCO2 was >400 mmHg, the lung recruitment was considered complete (22,23).
PEEP titration
When the sum of PAO2 and PaCO2 was >400 mmHg, all swine underwent a decremental PEEP titration in volume control mode. PEEP was decreased in 2 cm H2O steps (from 20 to 0 cm H2O) and was maintained at each level for 10 min. Cdyn was measured at each step using a VT of 8 ml/kg and a frequency of 40/min. Additionally, the physiological data including Cdyn, VD/VT and P/F were gathered following each step. The optimal open-lung PEEP was identified by the lowest VD/VT method, which was achieved as determined by a reduction in VD/VT and then a rise with each PEEP step.
The study consisted of the following seven experimental periods: i) Pre-injury period, which involved introduction of catheters and mechanical ventilation using the initial parameters; ii) injury period, when ARDS was induced by the intravenous administration of oleic acid; iii) PEEP period 1 (Po-4), 4 cm H2O below the optimal PEEP; iv) PEEP period 2 (Po-2), 2 cm H2O below the optimal PEEP; v) PEEP period 3 (Po), optimal PEEP; vi) PEEP period 4 (Po+2), 2 cm H2O above the optimal PEEP; vii) PEEP period 5 (Po+4). 4 cm H2O above the optimal PEEP.
Statistical analysis
All data were analyzed using IBM-SPSS version 19.0 statistics software (IBM SPSS, Armonk, NY, USA) and were expressed as mean ± standard deviation. Analysis of non-parametric repeated-measures ANOVA test was used for comparison of all variables collected during the seven assessment periods. P<0.05 was considered to indicate a statistically significant difference.
Results
Optimal PEEP
The optimal PEEP identified by the lowest VD/VT method was 13.25±1.36 cm H2O.
VD/VT changes induced by different PEEP levels
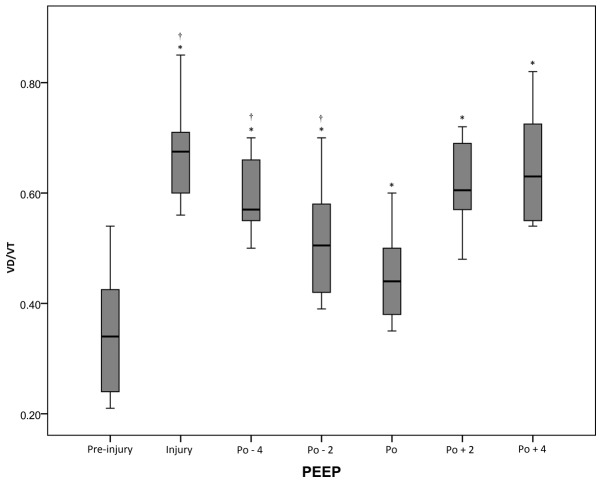
There was a significant (P<0.05) increase in VD/VT from the pre-injury period (0.35±0.11) to the injury period (0.68±0.10). Following the RM, VD/VT decreased to the lowest value of 0.44±0.08 (vs. injury, P<0.05) at the optimal PEEP. When PEEP decreased to Po-4 cm H2O, VD/VT significantly increased to 0.60±0.07 (P<0.05). However, at the Po+4 cm H2O, VD/VT was higher (0.64±0.10; Fig. 1 and Table I).
Figure 1.
VD/VT of the acute respiratory distress syndrome swine model under different conditions. *P<0.05 vs. pre-injury; †P<0.05 vs. injury. VD/VT, ratio of dead space volume to tidal volume (dead space fraction); PEEP, positive end-expiratory pressure; Po, optimal PEEP; -n, n cm Hg below Po; +n; n cm Hg above Po.
Table I.
Respiration parameters of the acute respiratory distress syndrome swine model under different conditions (mean ± standard deviation).
| Parameter | Pre-injury | Injury | Po-4 | Po-2 | Po | Po+2 | Po+4 |
|---|---|---|---|---|---|---|---|
| VD/VT | 0.35±0.11 | 0.68±0.10a | 0.60±0.07a,b | 0.52±0.11a,b | 0.44±0.08a,b | 0.61±0.08a | 0.64±0.10a |
| PaCO2 (mmHg) | 34.80±6.73 | 43.81±8.02a | 47.73±10.33a | 49.05±12.47a | 49.19±11.82a | 47.71±12.28a | 47.62±12.89a |
| SaO2 (%) | 99.86±0.05 | 88.40±11.25a | 96.79±1.52b | 98.58±0.48b | 98.75±2.55b | 97.78±0.96b | 96.34±2.50b |
| Qs/Qt (%) | 1.88±1.14 | 21.06±15.62a | 7.50±4.14a,b | 5.01±1.53b | 2.77±2.53b | 6.68±2.86b | 9.55±5.85a,b |
| P/F (mmHg) | 562±162 | 75±21a | 166±109a | 291±62a,b | 342±144a,b | 365±133a,b | 294±170a,b |
| Cdyn (ml/cm H2O) | 38.17±6.97 | 15.17±5.37a | 17.83±3.81a | 18.00±4.97a | 20.67±5.58a,b | 19.33±4.44a,b | 17.50±3.34a |
P<0.05 vs. pre-injury
P<0.05 vs. injury. VD/VT, dead space volume/tidal volume (dead space fraction); PaCO2, alveolar partial pressure of carbon dioxide; SaO2, arterial oxygen saturation; Qs/Qt, intrapulmonary shunt fraction; P/F, alveolar partial pressure of oxygen/fraction of inspiration oxygen (oxygenation index); Cdyn, dynamic tidal respiratory compliance; Po, optimal positive end-expiratory pressure; -n, n cm Hg below Po; +n; n cm Hg above Po.
Changes of P/F during PEEP decrement
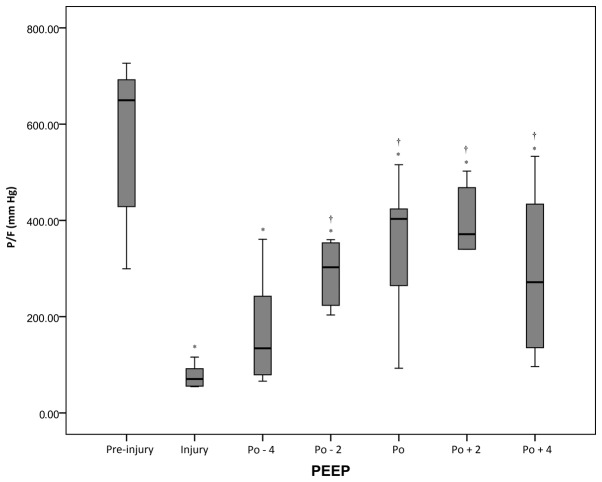
There was a statistically significant (P<0.05) reduction in P/F from the pre-injury period (562±162 mmHg) to the injury period (75±21 mmHg). Following the RM, P/F values significantly increased from 166±109 to 365±133 mmHg when the PEEP increased from the Po-4 cm H2O to Po+2 cm H2O. However, P/F decreased again at Po+4 cm H2O (Fig. 2 and Table I).
Figure 2.
P/F ratio of the acute respiratory distress syndrome swine model under different conditions. *P<0.05 vs. pre-injury; †P<0.05 vs. injury. P/F, alveolar partial pressure of oxygen/fraction of inspiration oxygen (oxygenation index); PEEP, positive end-expiratory pressure; Po, optimal PEEP; -n, n cm Hg below Po; +n; n cm Hg above Po.
Changes of Cdyn and Qs/Qt during PEEP decrement
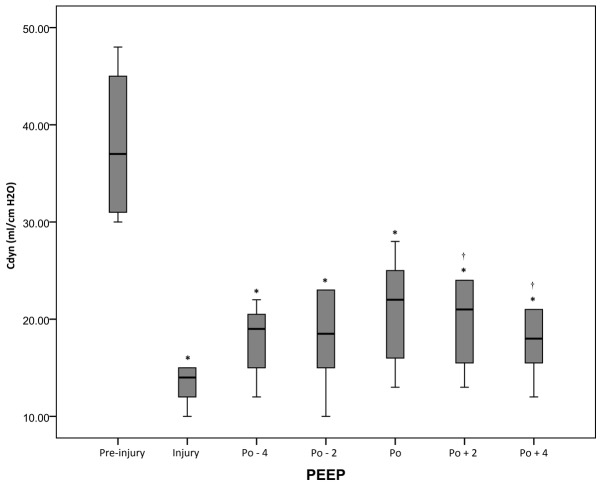
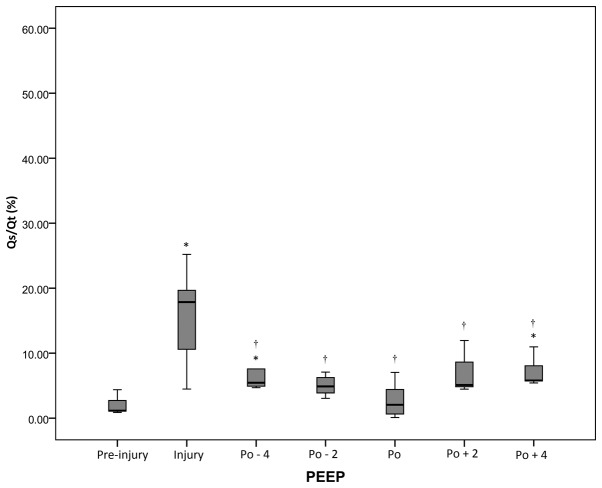
At all periods after injury, the Cdyn values were decreased compared with the pre-injury value (P<0.05), but the highest post-RM values were observed at the pressure level of optimal PEEP. However, Qs/Qt values were significantly (P<0.05) lower at the pressure level of optimal PEEP compared with the levels at the injury period (Figs. 3 and 4 and Table I).
Figure 3.
Cdyn of the acute respiratory distress syndrome swine model under different conditions. *P<0.05 vs. pre-injury; †P<0.05 vs. injury. Cdyn, dynamic tidal respiratory compliance; PEEP, positive end-expiratory pressure; Po, optimal PEEP; -n, n cm Hg below Po; +n; n cm Hg above Po.
Figure 4.
Qs/Qt of the acute respiratory distress syndrome swine model under different conditions. *P<0.05 vs. pre-injury; †P<0.05 vs. injury. Qs/Qt, intrapulmonary shunt ratio; PEEP, positive end-expiratory pressure; Po, optimal PEEP; -n, n cm Hg below Po; +n; n cm Hg above Po.
Hemodynamic changes induced by different PEEP levels
The CI, ITBI, GEDI and SVRI did not change significantly during the pre-injury, injury and variable PEEP periods, although a downtrend was observed in CI with the increase of PEEP. For CVP, a significant (P<0.05) increment was observed during the variable PEEP period relative to the pre-injury and injury period. In addition, CVP increased markedly as PEEP increased. In comparison with the pre-injury period, EVLWI values were significantly higher during the injury and variable PEEP periods (P<0.05; Table II).
Table II.
Hemodynamics parameters of the acute respiratory distress syndrome swine model under different conditions (mean ± standard deviation).
| Parameter | Pre-injury | Injury | Po-4 | Po-2 | Po | Po+2 | Po+4 |
|---|---|---|---|---|---|---|---|
| CVP (mmHg) | 6.58±2.08 | 8.67±2.84a | 10.67±1.72a,b | 10.83±2.67a,b | 10.75±2.83a,b | 11.33±2.06a,b | 11.83±1.90a,b |
| CI (l/min/m2) | 4.84±2.08 | 3.36±1.62 | 3.80±1.86 | 3.75±2.00 | 3.75±2.09 | 2.96±1.46 | 2.67±1.22 |
| ITBI (ml/m2) | 694±219 | 664±201 | 561±240 | 690±229 | 611±202 | 597±201 | 590±130 |
| GEDI (ml/m2) | 555±175 | 532±161 | 449±192 | 552±183 | 488±162 | 478±160 | 472±104 |
| EVLWI (ml/kg) | 10.69±4.01 | 17.61±5.71a | 15.33±3.00a | 17.14±7.13a | 16.85±6.05a | 16.69±4.97a | 16.94±4.81a |
| SVRI (dyn.sec.cm−5.m2) | 1,430±590 | 2,113±1,012 | 2,469±948 | 2,541±1,366 | 2,179±1,439 | 2,152±1,532 | 1,970±1,237 |
P<0.05 vs. pre-injury
P<0.05 vs. injury. CVP, central venous pressure; CI, cardiac output index; ITBI, intra-thoracic blood volume index; GEDI, global end-diastolic volume index; ELWI, extravascular lung water index; SVRI, systemic vascular resistance index; Po, optimal positive end-expiratory pressure; -n, n cm Hg below Po; +n; n cm Hg above Po.
Discussion
Currently, many methods exist in the literature for identifying the PEEP to set in patients with ARDS following a lung RM. The detection parameters include Cdyn, PAO2, maximum PAO2 + PaCO2, as well as the inflation lower inflection point (Pflex) and deflation upper Pflex on the pressure-volume curve (22). However, controversy over the approach for setting PEEP has existed since 1967 when Ashbaugh et al (24) first used PEEP to manage ARDS. A previous study has reported that an increased VD/VT ratio is one of the markers of early ARDS, and furthermore, an elevated VD/VT ratio is associated with an increased risk of mortality (10). In the present study, a decremental PEEP procedure was performed following an RM in swine with ARDS. It was observed that PEEP caused significant changes of VD/VT, Qs/Qt, Cdyn and P/F. The results indicated that in cases of recruitment maneuver and a PEEP titration procedure, VD/VT might become a clinically useful tool for assessing collapsed alveolar opening and titrating the optimal PEEP in ARDS.
A markedly elevated VD/VT may be detected in early ARDS, which is suggested to be due to the obstruction of pulmonary blood flow in the extra-alveolar pulmonary circulation (25), and increasing areas with a low ventilation (26). Importantly, injury of pulmonary capillaries by inflammation and thrombus can also result in increased VD/VT (27,28). As shown in Fig. 1, VD/VT was significantly higher during the injury period than during the pre-injury period, which was in accordance with results from previous studies (29,30). In addition, the results showed that different PEEP levels following RM caused significant changes in VD/VT, which was in agreement with the findings of Maisch et al (31), who showed that different PEEP levels after RM caused significant changes in VD/VT and P/F, as well as compliance in patients with ARDS. With the increase of PEEP, VD/VT showed a trend of decline. However, higher PEEP may lead to an increase of the VD/VT ratio, which might be caused by the regional over-distention of well-ventilated alveoli (26) or by a reduction in cardiac output (32). As can be seen from the formula used to calculate VD/VT, VD/VT is inversely related to CO2 elimination. The elimination of CO2 by the lung is influenced by effective alveolar surface area, alveolar ventilation and cardiac output (33,34). Following the RM and optimal PEEP, the CO2 elimination capacity of the lung is increased, because alveolar ventilation is markedly increased. The present study also showed that in the PEEP levels ranging from Po-4 to Po, VD/VT was gradually reduced. However, when the PEEP levels ranged from Po+2 to Po+4 cm H2O, VD/VT increased again. A previous study demonstrated that VD was significantly increased in piglets with higher PEEP (20 cm H2O), which was induced by hyperinflation of the lung region (35).
In ARDS, the alveolar collapse causes a deficiency of alveolar ventilation while the blood flow does not significantly decrease and VD/VT increases, which leads to a decline of the ventilation and blood flow and increase of Qs/Qt. In the present study, the Qs/Qt ratio achieved its maximum value with the increase of VD/VT in ARDS conditions. Under the application of PEEP, the Qs/Qt ratio showed a trend of decline to approach the base value with the reduction of VD/VT. Furthermore, Qs/Qt reached its minimum under the optimal PEEP state.
Higher PEEP increases VD/VT via a reduction in cardiac output (32,36). In the present study, following the application of PEEP, CVP increased as the PEEP increased, indicating that PEEP significantly affected the loading conditions of the right atrium to reduce the volume of returned blood. The increase of CVP might be due to the augmentation of intrapleural pressure and vena cava reflux resistance that were induced by the high PEEP levels. Moreover, the reduction of returned blood volume gives rise to a reduction of left atrium cardiac output, which is in accordance with the present study's findings that the CI gradually declined with the increase of PEEP. Notably, CI had no evident significant difference among PEEP states, because the PEEP range in this study was <20 cm H2O.
In conclusion, measurement of VD/VT is valuable in assessing the effects of lung recruitment. The minimal VD/VT can be used as one of many options for the assessment of PEEP titration in ARDS. In the context of RM and a PEEP titration procedure, a reduction in VD/VT and Qs/Qt, and an increase in Cdyn and P/F indicate a maximum amount of effectively expanded alveoli. The VD/VT may be prospectively used in future clinical trials, particularly when the goal is to evaluate the benefit of an open-lung protective ventilation strategy in patients with ARDS.
However, there are certain limitations to the present study. In the context of RM and PEEP, alveolar ventilation volume could not be assessed by direct computed tomography methods. In addition, PEEP was not evaluated at >20 cm H2O after RM in the ARDS model, so it is impossible to comment on the effect of higher PEEP on VD/VT and Qs/Qt under those circumstances. Finally, the sample size of 12 swine is relatively small, and arguably underpowered to detect an important effect.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81372043).
References
- 1.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 2.Petty TL. Acute respiratory distress syndrome (ARDS) Dis Mon. 1990;36:1–58. [PubMed] [Google Scholar]
- 3.Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, et al. The ALIEN study: Incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 4.Bull TM, Clark B, McFann K, Moss M. National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network: Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182:1123–1128. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18:319–321. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 6.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 7.Levy MM. PEEP in ARDS-how much is enough? N Engl J Med. 2004;351:389–391. doi: 10.1056/NEJMe048103. [DOI] [PubMed] [Google Scholar]
- 8.Sakuramoto H, Shimojo N, Jesmin S, Unoki T, Kamiyama J, Oki M, Miya K, Kawano S, Mizutani T. Repeated open endotracheal suctioning causes gradual desaturation but does not exacerbate lung injury compared to closed endotracheal suctioning in a rabbit model of ARDS. BMC Anesthesiol. 2013;13:47. doi: 10.1186/1471-2253-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charron C, Repesse X, Bouferrache K, Bodson L, Castro S, Page B, Jardin F, Vieillard-Baron A. PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: A physiological study. Crit Care. 2011;15:R175. doi: 10.1186/cc10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fengmei G, Jin C, Songqiao L, Congshan Y, Yi Y. Dead space fraction changes during PEEP titration following lung recruitment in patients with ARDS. Respir Care. 2012;57:1578–1585. doi: 10.4187/respcare.01497. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 12.Tusman G, Suarez-Sipmann F, Böhm SH, Pech T, Reissmann H, Meschino G, Scandurra A, Hedenstierna G. Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med. 2006;32:1863–1871. doi: 10.1007/s00134-006-0371-7. [DOI] [PubMed] [Google Scholar]
- 13.Uttman L, Bitzén U, De Robertis E, Enoksson J, Johansson L, Jonson B. Protective ventilation in experimental acute respiratory distress syndrome after ventilator-induced lung injury: A randomized controlled trial. Br J Anaesth. 2012;109:584–594. doi: 10.1093/bja/aes230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beydon L, Uttman L, Rawal R, Jonson B. Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med. 2002;28:1239–1245. doi: 10.1007/s00134-002-1419-y. [DOI] [PubMed] [Google Scholar]
- 15.Blanch L, Lucangelo U, Lopez-Aguilar J, Fernandez R, Romero P. Volumetric capnography in patients with acute lung injury: Effects of positive end-expiratory pressure. Eur Respir J. 1999;13:1048–1054. doi: 10.1034/j.1399-3003.1999.13e19.x. [DOI] [PubMed] [Google Scholar]
- 16.Higgs Z, Macafee D, Braithwaite B, Maxwell-Armstrong C. The Seldinger technique: 50 years on. Lancet. 2005;366:1407–1409. doi: 10.1016/S0140-6736(05)66878-X. [DOI] [PubMed] [Google Scholar]
- 17.Marshall W. Arterial blood gas analysis. Ann Clin Biochem. 2010;47:283–283. doi: 10.1258/acb.2010.201005. [DOI] [Google Scholar]
- 18.Fletcher R, Jonson B, Cumming G, Brew J. The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth. 1981;53:77–88. doi: 10.1093/bja/53.1.77. [DOI] [PubMed] [Google Scholar]
- 19.Fowler WS. Lung function studies; The respiratory dead space. Am J Physiol. 1948;154:405–416. doi: 10.1152/ajplegacy.1948.154.3.405. [DOI] [PubMed] [Google Scholar]
- 20.Hofer CK, Furrer L, Matter-Ensner S, Maloigne M, Klaghofer R, Genoni M, Zollinger A. Volumetric preload measurement by thermodilution: A comparison with transoesophageal echocardiography. Br J Anaesth. 2005;94:748–755. doi: 10.1093/bja/aei123. [DOI] [PubMed] [Google Scholar]
- 21.Quintel M, Pelosi P, Caironi P, Meinhardt JP, Luecke T, Herrmann P, Taccone P, Rylander C, Valenza F, Carlesso E, Gattinoni L. An increase of abdominal pressure increases pulmonary edema in oleic acid-induced lung injury. Am J Respir Crit Care Med. 2004;169:534–541. doi: 10.1164/rccm.200209-1060OC. [DOI] [PubMed] [Google Scholar]
- 22.Caramez MP, Kacmarek RM, Helmy M, Miyoshi E, Malhotra A, Amato MB, Harris RS. A comparison of methods to identify open-lung PEEP. Intensive Care Med. 2009;35:740–747. doi: 10.1007/s00134-009-1412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, Souza CE, Victorino JA, Kacmarek RM, Barbas CS, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:268–278. doi: 10.1164/rccm.200506-976OC. [DOI] [PubMed] [Google Scholar]
- 24.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 25.Greene R, Zapol WM, Snider MT, Reid L, Snow R, O'Connell RS, Novelline RA. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124:593–601. doi: 10.1164/arrd.1981.124.5.593. [DOI] [PubMed] [Google Scholar]
- 26.Dantzker DR, Brook CJ, Dehart P, Lynch JP, Weg JG. Ventilation-perfusion distributions in the adult respiratory distress syndrome. Am Rev Respir Dis. 1979;120:1039–1052. doi: 10.1164/arrd.1979.120.5.1039. [DOI] [PubMed] [Google Scholar]
- 27.Idell S, Mazar AP, Bitterman P, Mohla S, Harabin AL. Fibrin turnover in lung inflammation and neoplasia. Am J Respir Crit Care Med. 2001;163:578–584. doi: 10.1164/ajrccm.163.2.2005135. [DOI] [PubMed] [Google Scholar]
- 28.Tomashefski JF, Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 29.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 30.Lucangelo U, Bernabè F, Vatua S, Degrassi G, Villagrà A, Fernandez R, Romero PV, Saura P, Borelli M, Blanch L. Prognostic value of different dead space indices in mechanically ventilated patients with acute lung injury and ARDS. Chest. 2008;133:62–71. doi: 10.1378/chest.07-0935. [DOI] [PubMed] [Google Scholar]
- 31.Maisch S, Reissmann H, Fuellekrug B, Weismann D, Rutkowski T, Tusman G, Bohm SH. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anaesth Analg. 2008;106:175–181. doi: 10.1213/01.ane.0000287684.74505.49. [DOI] [PubMed] [Google Scholar]
- 32.Coffey RL, Albert RK, Robertson HT. Mechanisms of physiological dead space response to PEEP after acute oleic acid lung injury. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1550–1557. doi: 10.1152/jappl.1983.55.5.1550. [DOI] [PubMed] [Google Scholar]
- 33.Breen PH, Mazumdar B. How does positive end-expiratory pressure decrease CO2 elimination from the lung? Respir Physiol. 1996;103:233–242. doi: 10.1016/0034-5687(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 34.Anderson CT, Breen PH. Carbon dioxide kinetics and capnography during critical care. Crit Care. 2000;4:207–215. doi: 10.1186/cc696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang R, Huang Y, Chen Q, Hui X, Li Y, Yu Q, Zhao H, Yang Y, Qiu H. The effect of alveolar dead space on the measurement of end-expiratory lung volume by modified nitrogen wash-out/wash-in in lavage-induced lung injury. Respir Care. 2012;57:2074–2081. doi: 10.4187/respcare.01800. [DOI] [PubMed] [Google Scholar]
- 36.Suwa K, Hedley-Whyte J, Bendixen HH. Circulation and physiologic dead space changes on controlling the ventilation of dogs. J Appl Physiol. 1966;21:1855–1859. doi: 10.1152/jappl.1966.21.6.1855. [DOI] [PubMed] [Google Scholar]