Abstract
Hypervascular hepatocellular carcinoma (HCC) is one of the leading causes of cancer-associated mortality. Angiogenesis is an important contributor to HCC progression and metastasis; therefore, inhibiting angiogenesis may be an effective method of treating HCC. Tumstatin is a novel type of efficient endogenous vascular endothelial cell growth inhibiting factor. The anti-angiogenic activity of tumstatin is localized to the 54–132 amino acid region (Tum-5). In a previous study performed by our group, the gene fragment encoding Tum-5 was cloned and inserted into a pLXSN retroviral vector. In the present study, the anti-angiogenic effects of Tum-5 and the antitumor effects exerted by the pLXSN-Tum-5 vector in vivo were investigated. The results demonstrated that pLXSN-Tum-5 significantly inhibited the growth of human umbilical vein endothelial cells compared with pLXSN, but had no obvious effect on HepG2 cell growth. Moreover, the antitumor and anti-angiogenic activity of Tum-5 was examined in vivo using a xenograft of H22 HCC cells. The results indicated that pLXSN-Tum-5 significantly inhibited tumor growth following 5 injections over 10 days. The size and weight of tumors in the pLXSN-Tum-5 group were lower than those in the saline and pLXSN groups. Furthermore, immunohistochemical analysis with CD31 antibodies indicated that the average microvessel density in the pLXSN-Tum-5 group were significantly lower than that in the saline and pLXSN groups. These results suggested that Tum-5 exerts its antitumor activity by suppressing vascular endothelial cells. The gene fragment of Tum-5 may be developed as an effective inhibitor of angiogenesis and used to treat patients with HCC.
Keywords: tumor angiogenesis, tumstatin, hepatocellular carcinoma, retroviral vector
Introduction
Hepatocellular carcinoma (HCC), one of the most common types of cancer in the world, is a hypervascular carcinoma. Angiogenesis serves an important role in HCC progression, malignancy, metastasis and high rates of recurrence (1–3). Solid tumors may not grow bigger than 2–3 mm3 if tumor angiogenesis is blocked (4). Therefore, the development of genetic engineering technologies to target angiogenesis may be a novel and effective method of treating HCC.
Angiogenesis enables tumor growth and metastasis to occur. Moreover, it is the process by which tumor cells are provided with a supply of blood and nutrients (5–7). Due to its extensive role in inducing cancer cell growth, tumor angiogenesis has become a novel and promising target for anticancer therapy. Inhibiting tumor angiogenesis may be an efficient way of preventing tumor occurrence and progression.
Tumstatin is a novel factor that inhibits vascular endothelial cell growth. It belongs to the NC1 domain of α3 chain of type-IV collagen and exerts anti-angiogenic activity (8,9). Tumstatin binds to endothelial cell surface integrins and exerts its effects through multiple mechanisms, including inhibition of endothelial cell protein synthesis, which results in endothelial cell apoptosis, inhibition of tumor blood vessel formation, and inhibition of tumor cell growth, invasion and metastasis (9). The anti-angiogenic activity of tumstatin is localized to its 54–132 amino acid region (Tum-5), which exhibits similar biological activity to the parent protein (10–12).
The aim of the present study was to evaluate whether gene therapy with Tum-5 is an effective strategy to treat patients with HCC. The Tum-5 gene fragment was cloned and inserted into a pLXSN retroviral vector following the protocol of a previous study by our group (13). To identify the role Tum-5 serves in the process of HCC growth, the anti-angiogenic and antitumor effects of pLXSN-Tum-5 virus transfection were assessed in vitro and in vivo.
Materials and methods
Reagents
The rabbit anti-mouse CD31 monoclonal antibody was purchased from eBioscience, Inc. (cat. no. 13-0311-81; San Diego, CA, USA). The Ready-to-Use Immunohistochemistry Hypersensitivity UltraSensitive™ S-P kit was purchased from Maixin Biotech. Co., Ltd. (Fuzhou, China). MTT and Polybrene® were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Cells and culture
The retroviral packaging mouse fibroblast cell line PA317, NIH3T3 fibroblasts, human umbilical vein endothelial cells (HUVECs), the HepG2 human hepatocarcinoma cell line and the H22 mouse hepatocarcinoma cell line were all obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 37°C with 5% CO2 in complete Dulbecco's Modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
Production of viral particles and determination of viral titer
The human pLXSN-Tum-5 plasmid was constructed as previously described (13). A total of 5×105 PA317 packaging cells were seeded in 6-well plates. After 24 h, pLXSN-Tum-5 and pLXSN were transfected into the PA317 cells using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h. Infected cells were split and grown with 400 µg/ml G418-containing medium. After 2 weeks, G418-resistant colonies were picked, the virus-containing supernatant was collected and passed through a 0.45 µm filter, subsequently frozen and stored at −80°C. The titer of viral stocks was determined by infection of the NIH3T3 cells using a previously described technique (14). The titers of pLXSN-Tum-5 and pLXSN virus were 8.2×106 colony-forming units/ml and 7.6×106 colony-forming units/ml, respectively.
MTT assay for cell proliferation in vitro
Cell growth was evaluated using an MTT assay. HUVECs or HepG2 cells were seeded in 96-well plates at 8×103 cells/well (0.2 ml/well) and cultured at 37°C in 5% CO2 overnight to allow for cell attachment. Cells were subsequently infected with pLXSN-Tum-5 virus at 0, 1, 5, 10, 25 and 50 multiplicity of infection (MOI) in the presence of 5 µg/ml Polybrene. Following 72 h of incubation, the supernatant was removed and serum-free culture medium containing 5 mg/ml MTT was added to each well. Following 4 h of incubation with MTT, the supernatant was discarded and 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck Millipore) was added for 10 min. The optical density (OD) of each well was measured at 570 nm using a Bio-Rad 2550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All experiments were performed in triplicate.
Antitumor effects in vivo
The antitumor effects of retroviral vectors containing Tum-5 gene were examined in vivo using a H22 mouse HCC xenograft implanted in Kunming (KM) female mice (55–70 days old; weight, 15–20 g) that were obtained from Jilin University (Changchun, China). The mice were implanted subcutaneously with 1×106 H22 cells in 0.1 ml serum-free medium to produce a subcutaneous tumor xenograft. The mice were housed in sterile prebedded plastic cages and maintained at 20°C with a 12 h light/12 h dark cycle and had free access to mouse food and water. When the tumor size reached 30–70 mm3, 15 xenograft-bearing mice were randomly divided into three groups: Saline (n=5), pLXSN (empty virus; n=5) and pLXSN-Tum-5 (n=5). Injections of saline, pLXSN and pLXSN-Tum-5 were administered on days 0, 2, 4, 6 and 8 into tumor tissues at a MOI of 5 per mouse. The tumor size and body weight of each mouse were recorded every other day. The antitumor effects were determined by measuring the tumor dimensions via vernier caliper to the nearest 0.1 mm, and calculating the volume using the following equation: V=ab2/2, where a and b represent the length and width of tumor, respectively. After 10 days, mice under pentobarbital anesthesia (80 mg/kg body weight; Sigma-Aldrich) were sacrificed by cervical dislocation, and tumor tissues were carefully excised from the body and weighed. All animal experiments were performed in compliance with the NIH guidelines for the care and use of laboratory animals. The animal experiments in this study were approved by the Animal Ethics Committee of Beihua University (Jilin City, China).
Immunohistochemical staining for CD31
Tumor tissues from the H22 tumor-bearing mice (saline, pLXSN and pLXSN-Tum-5 groups) were fixed in 10% formalin at room temperature for 24 h, embedded in paraffin and cut into 4-µm consecutive sections. Following deparaffinization and antigen retrieval, immunohistochemical staining was performed using the Ready-to-Use Immunohistochemistry Hypersensitivity UltraSensitive™ S-P kit according to the manufacturer's instructions. Sections were treated with 3% hydrogen peroxide for 10 min at room temperature to block the activity of endogenous peroxidase. The sections were washed with phosphate-buffered saline (PBS) for 5 min and blocked with normal goat serum (provided with the kit) for 10 min at room temperature. The sections were subsequently incubated with a 1:100 dilution of the monoclonal antibody for CD31 at 4°C overnight. The sections were then washed with PBS and treated with biotinylated secondary antibody (provided with the kit) for 10 min, followed by further incubation with streptavidin-horseradish peroxidase complex. Following additional washing, diaminobenzidine was used as a chromogen and counterstaining was performed using hematoxylin. Sections were dehydrated, cleared and mounted with resin.
Microvessel density (MVD)
From the CD31-stained sections, the MVD was determined at the hot spot through light microscopy examination (BX43F; Olympus Corporation, Tokyo, Japan). For each section, positively stained microvessels were counted from 5 high-power fields (HPF; magnification, ×400). The average count was regarded as the MVD per HPF.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences version 17.0 (SPSS, Inc., Chicago, IL, USA). All experiments were performed at least three times and all values were expressed as the mean ± standard deviation. Student's t-test was used to compare values between two groups and one-way analysis of variance (ANOVA) was used for more than two groups. For all analyses, P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of Tum-5 transfection on HUVEC growth in vitro
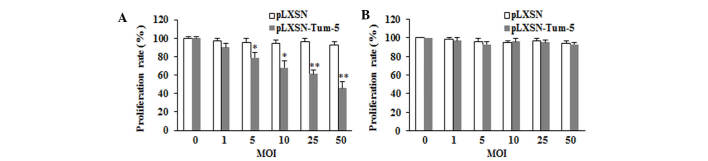
To examine the role of Tum-5 in tumor angiogenesis, HUVECs were used to mimic the tumor environment. HUVECs were transfected with pLXSN-Tum-5 or pLXSN virus at different MOIs of 0, 1, 5, 10, 25 and 50. The proliferation rate was measured using the MTT assay at 72 h. The results demonstrated that cells in the pLXSN-Tum-5 group exhibited a significantly lower proliferation rate compared with the pLXSN group (P<0.05). Tum-5 significantly inhibited the proliferation of HUVECs in a titer-dependent manner (Fig. 1A).
Figure 1.
Effect of Tum-5 on cell growth in vitro. The proliferation rate of (A) human umbilical vein endothelial cells and (B) HepG2 cells was assessed following pLXSN-Tum-5 and pLXSN virus transfection at MOIs of 0, 1, 5, 10, 25 and 50 for 72 h. *P<0.05 and **P<0.01, compared with the pLXSN group. Tum, tumstatin; MOI, multiplicity of infection.
Effect of Tum-5 transfection on HepG2 cell growth in vitro
Based on the anti-angiogenesis activity of Tum-5 in HUVECs, it was investigated whether Tum-5 exerts antitumor activity in tumor cells in vitro. HepG2 cells were transfected with Tum-5 virus at different MOIs of 0, 1, 5, 10, 25 and 50 for 72 h. The results demonstrated that the growth of the pLXSN-Tum-5 group was not significantly different from that of the pLXSN group (P>0.05; Fig. 1B). This result indicated that the introduction of Tum-5 into HepG2 cells did not affect HepG2 cell proliferation.
Antitumor effect of Tum-5 in vivo
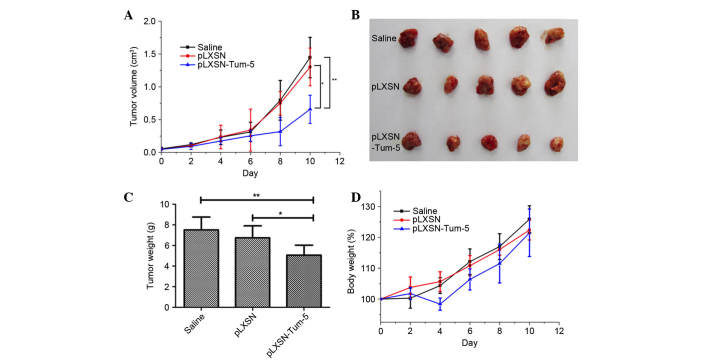
To test if the retrovirus containing the Tum-5 gene was able to inhibit tumor growth in vivo, a H22 mouse HCC xenograft model was employed. When the tumor size reached 30–70 mm3, the 15 mice were randomly divided into three equal groups. Tumors were injected with pLXSN or pLXSN-Tum-5 retroviral vectors (MOI of 5 per mouse) on days 0, 2, 4, 6 and 8. The results indicated that, compared with saline (P<0.01) and empty retroviral vectors (P<0.05), pLXSN-Tum-5 significantly inhibited tumor growth on day 10, after the administration of 5 injections (Fig. 2A). Considering that pLXSN had no effect on tumor growth, it was concluded that tumor growth was inhibited by the Tum-5 gene carried by the retroviral vector. Tumor tissues were excised on the 10th day and weighed. The size and weight of tumors from mice in the pLXSN-Tum-5 group were significantly lower than those of mice in the saline (P<0.01) and pLXSN groups (P<0.05) (Fig. 2B and C). Changes in body weight were used to evaluate the toxicity of the retroviral vectors. The results demonstrated no significant weight loss in mice in the pLXSN-Tum5 group compared with mice from the other groups following 10 days of treatment, suggesting that retroviral vectors containing the Tum-5 gene exhibit low toxicity (Fig. 2D). The notable weight loss of the pLXSN-Tum-5 group at day 4 may be attributed to the inhibited tumor growth caused by Tum-5.
Figure 2.
Antitumor efficacy of Tum-5 in vivo. (A) Tumor volume of H22 tumor-bearing mice treated with saline, pLXSN and pLXSN-Tum-5 measured over 10 days. Mice received injections on days 0, 2, 4, 6 and 8. (B) Images of excised tumors on day 10. (C) Weight of excised tumors. (D) Body weight of H22 tumor-bearing mice treated with saline, pLXSN and pLXSN-Tum-5 over 10 days. *P<0.05 for the pLXSN-Tum-5 group vs. the pLXSN group and **P<0.01 for the pLXSN-Tum-5 group vs. the saline group. Tum, tumstatin.
Expression of CD31 in tumor tissue
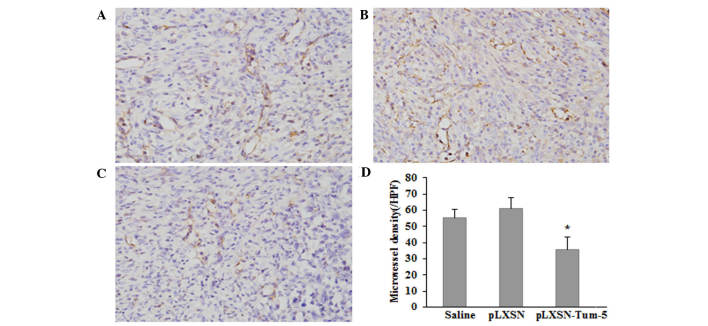
As no direct antitumor effect of Tum-5 was detected in vitro, it was speculated that the antitumor effect of Tum-5 observed in vivo was ascribed to the failure of angiogenesis in H22 tumor-bearing mice. Therefore, the endothelial cell marker CD31, was used to analyze tumor angiogenesis. Tumor tissues from H22 tumor-bearing mice were immunohistochemically stained with an antibody against CD31. The average MVD of CD31-stained sections in the pLXSN-Tum-5 group was significantly lower than that in the saline and pLXSN groups (P<0.05; Fig. 3). These results suggested that Tum-5 may decrease HCC tumor growth by inhibiting angiogenesis in vivo.
Figure 3.
Immunostaining for CD31 in tumor tissues from H22 xenograft mice in (A) the saline group, (B) the pLXSN group and (C) the pLXSN-Tum-5 group (magnification, ×400). (D) Graph of average microvessel density in CD31-stained sections. *P<0.05 compared with saline and pLXSN groups. HPF, high-power field; Tum, tumstatin.
Discussion
Tumor angiogenesis is a key process for the majority of solid tumors, since the growth and metastasis of malignant cells require the formation of new blood vessels (5–7,15,16). Tumstatin specifically inhibits endothelial cell proliferation and induces apoptosis by interacting with αVβ3 integrin (12,17). Deletion mutagenesis analysis has revealed that the anti-angiogenic activity of tumstatin is localized to the 54–132 amino acid region (Tum-5) (11). It is easier to transfer smaller fragments into tissue or cells, and in the present study, Tum-5 cDNA was therefore transfected into the retrovirus plasmid pLXSN to construct pLXSN-Tum-5 (13), a carrier which can effectively transfect host cells to stably express Tum-5 (18). To determine whether Tum-5 exhibits anti-angiogenic activity, HUVECs were transfected with pLXSN-Tum-5 virus in vitro. The results of the MTT assay indicated that the transfection of pLXSN-Tum-5 virus significantly decreased HUVEC proliferation in a titer-dependent manner and that the Tum-5 fragment, like full-length tumstatin, exhibits proliferation-inhibitory activity.
It has been demonstrated that tumstatin inhibits tumor growth in a number of different types of cancer, including human renal carcinoma, prostate carcinoma, lung carcinoma and glioma (11,12,19–22). As a gene fragment encoding the region of tumstatin with anti-angiogenic activity, Tum-5 has been identified to exhibit antitumor activity by exerting anti-angiogenic activity, but does not act directly on tumor cells (10–12). However, it has been reported that the Tum-5 gene may significantly inhibit gastric cancer cell proliferation and promote cell apoptosis in vitro (23). Therefore, the present study evaluated the antitumor effects of Tum-5 on HepG2 cells in vitro. The results showed that the proliferation of HepG2 cells transfected with Tum-5 did not significantly differ from that of cells transfected with retroviral vector, suggesting that Tum-5 had no direct antitumor activity on HepG2 cells. It was considered that Tum-5 may have different effects on gastric cancer and HCC cells. However, Tum-5 did not exert an antitumor effect on H22 cells in vitro in our prelimary experiment. In blood vessels formed during tumor angiogenesis, vascular endothelial cells may serve important roles in several steps of tumor cell activation, proliferation, migration, invasion and tubule formation (24,25). Considering the rich blood vessels observed in HCC, specific angiogenesis-targeting interventions provide a possible treatment for HCC by inhibiting the abovementioned processes (26–28). Therefore, the inhibition of angiogenesis represents a potential therapeutic target for HCC that may reduce the mortality of patients with HCC. The present study investigated the inhibitory effects of Tum-5 on HCC growth in vivo by injecting pLXSN-Tum-5 virus into KM mice bearing H22 tumors, and a significant antitumor effect was observed. The size and weight of tumors from mice in the pLXSN-Tum-5 group were significantly lower than those from the pLXSN and wild-type H22 HCC groups, suggesting that Tum-5 is effective at inhibiting HCC tumor growth.
The rate of angiogenesis is typically estimated by measuring the MVD of fixed tissue immunostained for endothelial markers, including CD31, CD34 and factor VIII. CD31 is a pan-endothelial marker for small and large vessels (29). In order to assess the MVD, CD31 and associated antibodies are used to mark tumor vascular endothelial cells and the capillary number per unit area is counted (30). In the present study, immunohistochemical staining for CD31 revealed that the average MVD in the pLXSN-Tum-5 group was significantly lower compared with that in the pLXSN and wild-type H22 HCC groups. The results indicated that Tum-5 may have a significant anti-angiogenic effect on the neovascular endothelial cells of HCC.
In conclusion, the present study determined that Tum-5 specifically inhibited HUVEC proliferation, but did not have any direct effect on HCC cell growth in vitro. The in vivo study demonstrated that Tum-5 exerted stronger antitumor activity through suppression of vascular endothelial cells compared with direct action on HCC cells. Taken together, the results of the present study suggested that the gene fragment of Tum-5 may be an effective angiogenesis inhibitor and may be developed as novel therapeutic strategy to treat patients with HCC.
Acknowledgements
The present study was supported by the Science and Technology Department of Jilin province (grant nos. 20110728 and 20150101128JC) and by the Health Department of Jilin Province (grant no. 20122120).
References
- 1.Yuan MM, Xu YY, Chen L, Li XY, Qin J, Shen Y. TLR3 expression correlates with apoptosis, proliferation and angiogenesis in hepatocellular carcinoma and predicts prognosis. BMC Cancer. 2015;15:245. doi: 10.1186/s12885-015-1262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L, Zeng J, Hong Z, Peng J. Livistona chinensis seeds inhibit hepatocellular carcinoma angiogenesis in vivo via suppression of the Notch pathway. Oncol Rep. 2014;31:1723–1728. doi: 10.3892/or.2014.3051. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Li GY, Zhu JY, Huang DB, Zhou HC, Zhong W, Ji CS. Overexpression of AGGF1 is correlated with angiogenesis and poor prognosis of hepatocellular carcinoma. Med Oncol. 2015;32:131. doi: 10.1007/s12032-015-0574-2. [DOI] [PubMed] [Google Scholar]
- 4.Lewis CE, Leek R, Harris A, McGee JO. Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. J Leukoc Biol. 1995;57:747–751. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 7.Bielenberg DR, Zetter BR. The contribution of angiogenesis to the process of metastasis. Cancer J. 2015;21:267–273. doi: 10.1097/PPO.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/S1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudhakar A, Boosani CS. Inhibition of tumor angiogenesis by tumstatin: Insights into signaling mechanisms and implications in cancer regression. Pharm Res. 2008;25:2731–2739. doi: 10.1007/s11095-008-9634-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J Biol Chem. 2001;276:15240–15248. doi: 10.1074/jbc.M007764200. [DOI] [PubMed] [Google Scholar]
- 11.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 12.Maeshima Y, Colorado PC, Kalluri R. Two RGD-indepentdent alpha vbeta3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- 13.Gai XD, Luo H, Li C, Feng K. The construction of recombined retroviral plasmid with human Tum-5 gene and packing cell line. Zhouguo Lao Nian Xue Zazhi. 2009;29:1194–1196. (In Chinese) [Google Scholar]
- 14.Bodine DM, McDonagh KT, Brandt SJ, Ney PA, Agricola B, Byrne E, Nienhuis AW. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci USA. 1990;87:3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisacchi D, Benelli R, Vanzetto C, Ferrari N, Tosetti F, Albini A. Anti-angiogenesis and angioprevention: Mechanisms, problems and perspectives. Cancer Detect Prev. 2003;27:229–238. doi: 10.1016/S0361-090X(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Gou X, Ke X, Cui H, Chen Z. Human tumor cells induce angiogenesis through positive feedback between CD147 and insulin-like growth factor-I. PLoS One. 2012;7:e40965. doi: 10.1371/journal.pone.0040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedchenko V, Zent R, Hudson BG. Alpha(v)beta3 and alpha(v)beta5 integrins bind both the proximal RGD site and non-RGD motifs within noncollagenous (NC1) domain of the alpha3 chain of type IV collagen: Implication for the mechanism of endothelia cell adhesion. J Biol Chem. 2004;279:2772–2780. doi: 10.1074/jbc.M311901200. [DOI] [PubMed] [Google Scholar]
- 18.McTaggart S, Al-Rubeai M. Retroviral vectors for human gene delivery. Biotechnol Adv. 2002;20:1–31. doi: 10.1016/S0734-9750(01)00087-8. [DOI] [PubMed] [Google Scholar]
- 19.Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M, Dickerson WM, Kalluri R. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J Biol Chem. 2001;276:31959–31968. doi: 10.1074/jbc.M103024200. [DOI] [PubMed] [Google Scholar]
- 20.Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A, Kalluri R. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci USA. 2005;102:2934–2939. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye HX, Yao Y, Jiang XJ, Yuan XR. Tumstatin transfected into human glioma cell line U251 represses tumor growth by inhibiting angiogenesis. Chin Med J (Engl) 2013;126:1720–1725. [PubMed] [Google Scholar]
- 22.You Y, Xue X, Li M, Qin X, Zhang C, Wang W, Giang C, Wu S, Liu Y, Zhu W, et al. Inhibition effect of pcDNA-tum-5 on the growth of S180 tumor. Cytotechnology. 2008;56:97–104. doi: 10.1007/s10616-007-9117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J. A study on the influence of proliferation and apoptosis for Tum-5 gene to human gastric carcinoma cell. Fuzhou, China: 2010. (In Chinese) [Google Scholar]
- 24.Jahroudi N, Greenberger JS. The role of endothelial cells in tumor invasion and metastasis. J Neurooncol. 1995;23:99–108. doi: 10.1007/BF01053415. [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Dong YY, Wang WM, Xie XY, Wang ZM, Chen RX, Chen J, Gao DM, Cui JF, Ren ZG. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. 2013;32:51. doi: 10.1186/1756-9966-32-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang ZL, Zhang JF, Yuan YF, He YM, Liu QY, Mao XW, Ai YB, Liu ZS. Suppression of angiogenesis and tumor growth in vitroin vivo using an anti-angiopoietin-2 single-chain antibody. Exp Ther Med. 2014;7:543–552. doi: 10.3892/etm.2014.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grothey A, Galanis E. Targeting angiogenesis: Progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6:507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- 28.Sugimachi K, Tanaka S, Taguchi K, Aishima S, Shimada M, Tsuneyoshi M. Angiopoietin switching regulates angiogenesis and progression of human hepatocellular carcinoma. J Clin Pathol. 2003;56:854–860. doi: 10.1136/jcp.56.11.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cârţână T, Săftoiu A, Gruionu LG, Gheonea DI, Pirici D, Georgescu CV, Ciocâlteu A, Gruionu G. Confocal laser endomicroscopy for the morphometric evaluation of microvessels in human colorectal cancer using targeted anti-CD31 antibodies. PLoS One. 2012;7:e52815. doi: 10.1371/journal.pone.0052815. [DOI] [PMC free article] [PubMed] [Google Scholar]