Abstract
Acute superior mesenteric venous thrombosis (ASMVT) is an intractable disease with poor prognosis. Argatroban, a direct thrombin inhibitor, may be a novel anticoagulant method in the therapy of ASMVT. The aim of the present study was to assess the efficacy and safety of early argatroban therapy in ASMVT patients. The current retrospective study reviewed a consecutive series of ASMVT patients receiving early argatroban therapy during hospitalization between March 2013 and April 2014, with 18 ASMVT patients included in the study. Of these, 16 patients without hepatic dysfunction underwent anticoagulant therapy with argatroban with a mean dose of 1.57±0.34 µg/kg/min and a mean duration of 12.2±3.7 days, while their activated partial thromboplastin time (aPTT) was elevated to 1.95±0.26 times the baseline value. In addition, 2 hepatic dysfunction patients received therapy with a dose of 0.41 µg/kg/min and 0.46 µg/kg/min, and with aPTT of 1.68 and 1.62 times the baseline value, respectively. Overall, 94% (n=17) of the patients presented clinical improvement, while 88% (n=16) of patients presented partially or completely dissolved thrombus in contrast-enhanced computed tomography images. The incidence of surgery and bowel resection was 6% (excluding 1 case with intestinal necrosis detected on admission). Furthermore, 11% (n=2) of patients experienced a bleeding episode, however no major bleeding or mortality occurred during hospitalization. During the follow-up, the mortality and the recurrence rate were 6% and 11%, respectively. In conclusion, early initiation of argatroban treatment may be an effective and safe therapy in ASMVT, manifesting efficient resolution of the thrombus, rapid improvement of symptoms, low incidence of bowel resection and bleeding complication, and low mortality rate.
Keywords: acute superior mesenteric venous thrombosis, acute mesenteric ischemia, anticoagulation, argatroban
Introduction
Acute superior mesenteric venous thrombosis (ASMVT) is an insidious disease with high mortality, accounting for 1 in 5,000 to 15,000 inpatient admissions, 1 in 1,000 emergency department admissions and 6–9% of all cases of acute mesenteric ischemia (1–3). ASMVT was first reported by Elliot (4), and subsequently Warren and Eberhard indicated that it could be a cause of intestinal infarction distinct from mesenteric arterial occlusion (5).
Although the prognosis has benefited from improvements in diagnostic methods, particularly in contrast-enhanced computed tomography (CT), the prognosis of ASMVT is greatly improved by early diagnosis and early initiation of the treatment. Overall, ASMVT remains an intractable disease with unsatisfactory outcome. The requirement of laparotomy and the mortality rate following therapy with traditional anticoagulant medicines, including unfractionated heparin (UFH), low molecular weight heparin (LMWH) and warfarin, remain high (2,6). The application of these medications is limited due to the occurrence of heparin-associated thrombocytopenia (7).
Thrombectomy is a useful procedure applied in acute large vessel thrombosis (8,9). While transcatheter thrombolysis has been recently considered to be another effective technique (10,11), these procedures are invasive and not substitutes for anticoagulation to diminish high rates of bleeding (12). Prolonged transarterial thrombolysis may even increase the potential risk of thrombosis or embolization in the superior mesenteric artery (SMA), the SMA branches and the common femoral artery near the arteriotomy site (12). Therefore, these invasive treatments are not substitutes for anticoagulation.
More effective and safe treatments are thus pursued. Argatroban, a direct thrombin inhibitor, induces predictable anticoagulant effects (13) and a lower incidence of bleeding complications compared with heparin, as observed in a rat model (14). In addition, its hepatic clearance may be superior in ASMVT therapy over other anticoagulants (15), when adjusting the dose to achieve an activated partial thromboplastin time (aPTT) to a target value. To the best of our knowledge, only three case studies reporting the application of argatroban in ASMVT and/or portal vein thrombosis (PVT) patients have been published to date (16–18).
The present study presents 18 patients with early initiation of argatroban therapy in the management of ASMVT over a 13-month period in a single institution. This is the first reported series of argatroban therapy in ASMVT patients.
Patients and methods
Patients
The present retrospective study was approved by the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University (Chongqing, China). The medical records of the Department of Vascular Surgery (the First Affiliated Hospital of Chongqing Medical University) were searched to identify patients with admitting diagnosis of ASMVT between March 2013 and April 2014. A total of 18 consecutive adult patients with ASMVT who received argatroban as the exclusive anticoagulant were evaluated retrospectively. The selection of alternative anticoagulant agent was at the discretion of the treating physician. The potential risks and benefits of the anticoagulant were explained, and informed consent was obtained from each patient and relatives. Excluded cases included therapy with other anticoagulants or transcatheter thrombolysis, incomplete medical records or imaging data, incomplete follow-up, or allergy to anticoagulant or thrombolytic agents. In total, 26 patients were excluded from the current study.
Data collection
Information was collected regarding the age, gender, past medical history, presenting symptoms and signs, anticoagulant therapy details, operative details, CT scan, outcome of therapy, complications, length of hospital stay and follow-up details of the patients. The follow-up period was between discharge until April 2015.
Treatment, response assessment and follow-up
Once the diagnosis was confirmed by contrast-enhanced CT, all these patients began anticoagulant therapy with argatroban. Continuous intravenous argatroban (Novastan; Mitsubishi Pharma Co., Tokyo, Japan) was initiated at 2 µg/kg/min in patients without hepatic dysfunction, and the initial argatroban dose was 0.5 µg/kg/min in hepatic dysfunction patients. The aPTT was measured prior to and 2 h after argatroban administration, and the dosage was adjusted until the aPTT was 1.5–3.0 times the baseline aPTT value. Next, the aPTT was measured daily, at 2 h after each dosage adjustment. Hepatic dysfunction was defined as a total serum bilirubin level of >25.5 µmol/l (1.5 mg/dl), aspartate aminotransferase level of >100 IU/l, or alanine aminotransferase level of >100 IU/l (19).
In addition, all patients underwent physical examination twice a day, along with evaluation of white blood cell counts and hepatic function test. The non-operative therapy included nasogastric suction, broad-spectrum prophylactic antibiotics and total parenteral nutrition. If intestinal infarction was suspected due to clinical manifestation or radiologic findings, laparotomy was performed.
The mean time for symptoms to be alleviated was 6.3±3.7 days, as presented in Table I. Following the alleviation of symptoms, patients began enteral nutrition support and contrast-enhanced CT was performed for therapeutic effect evaluation. The degree of thrombus lysis was divided into three levels based on CT examination: No lysis, partial lysis and complete lysis. No lysis was defined as thrombus removal <50% or worsening of the patient's condition. Partial lysis indicated 50–90% clot removal. Complete lysis represented >90% clot removal. The degree of lysis in each case was determined by two radiologists independently (10). Once clinical and radiographic improvement was noted, warfarin (Qilu Pharmaceutical Co., Ltd., Jinan, China) therapy was initiated at a dose of 2.5 mg/as a long-term therapy, outside of hospital treatment (20). When the international normalized ratio (INR) reached the target range of 2–3, argatroban was discontinued and warfarin alone was continued. Subsequently, the patient was discharged from hospital. The duration of oral anticoagulation was ~6 months for patients with known reversible factors, however this treatment was lifelong for patients who suffered from the idiopathic or with prothrombotic states (20). Recurrence was defined as the symptoms recurring due to the disease, while other causes were ruled out, and when the CT scan indicated that the thrombus was not significantly altered or was even worsened compared with the observation at hospital discharge.
Table I.
Clinical characteristics, disease causes, CT scan details and disease course of 18 ASMVT patients.
| Parameter | Value |
|---|---|
| Age, years | 50.9±13.9 (30–72) |
| Gender (male/female), n | 12/6 |
| Thrombophilia, n | |
| Protein C or S deficiency | 2 |
| Antithrombin III deficiency | 1 |
| Malignancy | 2 |
| Historical venous thromboembolism | 3 |
| Local factors causing vessel wall injury, n | |
| Intra-abdominal surgery | 6 |
| Pancreatitis | 2 |
| Patients with cirrhosis, n | |
| Hepatitis B virus | 4 |
| Hepatitis C virus | 1 |
| Alcohol | 1 |
| Clinical presentation, n (%) | |
| Abdominal pain | 18 (100) |
| Distention | 9 (50) |
| Nausea | 4 (22) |
| Melena | 3 (17) |
| Ileus | 3 (17) |
| Fever | 2 (11) |
| Lumbodorsal pain | 1 (6) |
| Emesis | 1 (6) |
| Peritonitis | 1 (6) |
| Vessel with thrombosis (CT scan), n | |
| SMV | 2 |
| SMV+PV | 10 |
| SMV+PV+SV | 6 |
| Symptom onset to treatment, days | 5.0±3.5 |
| Surgery, n (%) | 1 (5.9) |
| Length of hospital stay, days | 16.4±7.6 |
| Treatment to symptom remission, days | 6.3±3.7 |
ASMVT, acute superior mesenteric venous thrombosis; SMV, superior mesenteric vein; PV, portal vein; SV, splenic vein.
Complications
Bleeding events were recorded in all patients. Major bleeding was defined as: i) Fatal bleeding; and/or ii) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome; and/or iii) bleeding causing a reduction in hemoglobin level of at least 20 g/l (1.24 mmol/l), or leading to the transfusion of two or more units of whole blood or red cells (21). Minor bleeding was any overt bleeding not fitting the major bleeding definition (22,23). When major bleeding occurred, the administration of argatroban was terminated immediately and was followed by transfusion therapy according to the degree of anemia. When minor bleeding occurred, the administration of argatroban was terminated at the investigator's discretion. The aPTT, INR and hemoglobin level were monitored in both situations.
Statistical analysis
A descriptive retrospective study was conducted. The present study did not include tests of statistical significance due to the limited number of patients. All collected variables were used in descriptive statistical analysis. Numerical data were summarized by means of standard statistics (i.e. mean, standard deviation, minimum, median and maximum). Data were analyzed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Etiology and clinical presentation
A total of 18 ASMVT patients (6 females and 12 males), with a mean age of 50.9±13.9 years (between 30 and 72 years) and a mean weight of 60.7±8.2 kg, were included in the present study. The etiology of ASMVT and the presenting symptoms of the 18 patients are displayed in Table I. In addition, the mean time for symptom remission was 6.3±3.7 days, and the mean length of hospital stay was 16.4±7.6 days.
Dose-response of argatroban
In total, 16 patients without hepatic dysfunction underwent anticoagulant therapy with argatroban for a mean dose of 1.57±0.34 µg/kg/min and mean treatment duration of 12.2±3.7 days (between 7 and 18 days), and the aPTT was elevated 1.95±0.26 times over the baseline value. In addition, 2 hepatic dysfunction patients underwent therapy with an initial dose of 0.5 µg/kg/min, and a mean dose of 0.41 µg/kg/min for 9 days and 0.45 µg/kg/min for 6 days, achieving an aPTT of 1.68 and 1.62 times higher than the baseline value, respectively (Table II). In these 2 patients, the serum bilirubin level was increased to 108.1 µmol/l and 39.3 µmol/l prior to treatment, thus the initial dose was adjusted to 0.5 µg/kg/min.
Table II.
Dose-response of argatroban.
| aPTT (sec) | INR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aPTT (sec) | INR | |||||||||
| Patient | Cirrhosis | Hepatic dysfunction | Prior to argatroban therapy | Following argatroban therapy | Mean value during therapy | Prior to argatroban therapy | Following argatroban therapy | Starting dose (µg/kg/min) | Mean dose (µg/kg/min) | Duration of therapy (days) |
| 1 | − | − | 32.6 | 58.7 | 65.7 | 1.17 | 1.31 | 2 | 1.19 | 18 |
| 2 | − | − | 26.7 | 63.2 | 61.8 | 0.99 | 1.55 | 2 | 2 | 12 |
| 3 | − | − | 24.5 | 68.3 | 60.0 | 1.26 | 1.61 | 2 | 1.52 | 12 |
| 4 | + | + | 40.5 | 84.7 | 68.1 | 1.39 | 2.32 | 0.50 | 0.41 | 9 |
| 5 | − | − | 32.6 | 92.1 | 64.0 | 1.07 | 1.76 | 2 | 1.33 | 14 |
| 6 | − | − | 43.3 | 71.1 | 72.0 | 1.21 | 1.55 | 2 | 1.05 | 16 |
| 7 | − | − | 34.9 | 66.5 | 69.9 | 1.15 | 1.66 | 2 | 2 | 8 |
| 8 | − | − | 28.2 | 66.1 | 67.3 | 1.28 | 1.64 | 2 | 1.61 | 14 |
| 9 | − | − | 41.4 | 67.8 | 73.6 | 1.18 | 1.63 | 2 | 2 | 12 |
| 10 | + | − | 44.2 | 76.4 | 72.2 | 1.48 | 1.82 | 2 | 1.13 | 7 |
| 11 | − | − | 36.4 | 79.1 | 75.8 | 1.28 | 2.49 | 2 | 1.54 | 10 |
| 12 | + | + | 42.1 | 59.4 | 68.4 | 1.31 | 1.5 | 0.50 | 0.45 | 6 |
| 13 | − | − | 39.9 | 62.4 | 77.9 | 0.98 | 1.58 | 2 | 1.53 | 15 |
| 14 | − | − | 45.3 | 68.3 | 74.4 | 1.08 | 1.48 | 2 | 2 | 7 |
| 15 | − | − | 41 | 76.9 | 74.8 | 1.42 | 1.52 | 2 | 1.21 | 7 |
| 16 | + | − | 39 | 76.2 | 78.9 | 1.22 | 2.57 | 2 | 1.58 | 13 |
| 17 | + | − | 45.4 | 78.7 | 74.7 | 1.08 | 1.48 | 2 | 1.41 | 18 |
| 18 | + | − | 39.8 | 77.1 | 70.5 | 1.32 | 1.78 | 2 | 2 | 12 |
| Total | 6+/12- | 2+/16- | 37.7±6.4 | 71.8±9.0 | 70.6±5.4 | 1.22±0.14 | 1.74±0.36 | 1.83±0.49 | 1.44±0.49 | 11.8±3.6 |
aPTT, activated partial thromboplastin time; INR, international normalized ratio.
Details of in-hospital surgery
Intestinal resection surgery was performed in 2/18 patients (11%). One of these patients had been suspected to have intestinal infarction upon admission to our hospital, and underwent laparotomy within 12 h of admission. Extensive intestinal necrosis was found during the surgical procedure, and thus resection of 130 cm of the small bowel and an end-to-end intestinal anastomosis were performed. The other patient had peritonitis on the 4th day after admission, and underwent laparotomy and resection of 90 cm of the jejunum for localized bowel necrosis (Table III). As the intestinal necrosis of the first patient was not ascribed to the failure of argatroban therapy, the incidence of laparotomy during hospitalization was considered to be 6% (1/17 patients).
Table III.
Therapeutic evaluation, complications and follow-up data of argatroban treatment.
| Patient | Surgery in-hospital | Clinical outcome | Thrombolysis degree | Complications | Follow-up duration (months) | Outcome of follow-up |
|---|---|---|---|---|---|---|
| 1 | N/A | Elimination of symptoms | Partial | N/A | 18 | Surgical treatment for jejunum perforation, but no recurrence |
| 2 | N/A | Clinical improvement | Partial | N/A | 18 | No recurrence |
| 3 | N/A | Clinical improvement | Partial | N/A | 15 | No recurrence |
| 4 | N/A | Elimination of symptoms | None | Hematochezia | 18 | Recurrence |
| 5 | N/A | Elimination of symptoms | Partial | N/A | 14 | No recurrence |
| 6 | N/A | Clinical improvement | Partial | N/A | 15 | No recurrence |
| 7 | N/A | Elimination of symptoms | Partial | N/A | 12 | No recurrence |
| 8 | Resection of 130 cm of the small bowel | Clinical improvement | Partial | N/A | 12 | No recurrence |
| 9 | N/A | Clinical improvement | Partial | N/A | 12 | No recurrence |
| 10 | N/A | Clinical improvement | Partial | N/A | 15 | No recurrence |
| 11 | N/A | Clinical improvement | Complete | N/A | 24 | No recurrence |
| 12 | N/A | No improvement | None | Hematochezia | 6 | Recurrence and mortality |
| 13 | Resection of 90 cm of the jejunum | Clinical improvement | Partial | N/A | 20 | No recurrence |
| 14 | N/A | Clinical improvement | Partial | N/A | 20 | No recurrence |
| 15 | N/A | Elimination of symptoms | Partial | N/A | 20 | No recurrence |
| 16 | N/A | Elimination of symptoms | Partial | N/A | 18 | No recurrence |
| 17 | N/A | Clinical improvement | Partial | N/A | 15 | Surgical treatment for intestinal obstruction, but no recurrence |
| 18 | N/A | Clinical improvement | Partial | N/A | 20 | No recurrence |
The serial numbers of the patients are the same as those used in Table II. N/A, not applicable.
Clinical improvement and bleeding episodes
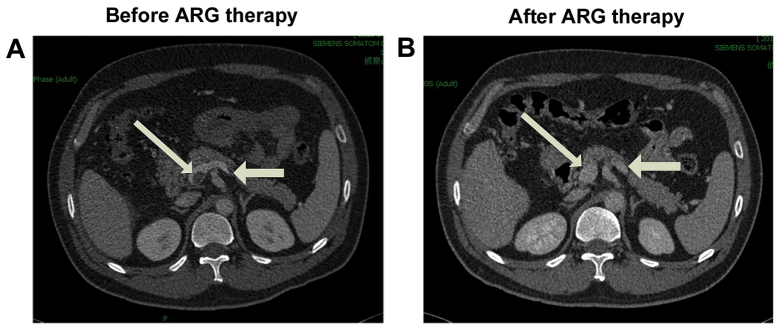
Clinical symptom improvement or elimination was observed in 94% (17/18) of the patients, and no mortality occurred during the hospitalization period. Contrast-enhanced CT images indicated that the thrombus was partially dissolved when compared with the previous CT scan in 83% (15/18) of patients, dissolved completely in 6% (1/18) of patient (Fig. 1), and was not significantly altered in the remaining 11% (2/18) of patients (Table III).
Figure 1.
Contrast-enhanced CT scans of the abdomen in a representative patient with acute superior mesenteric venous thrombosis. (A) Selected axial contrast-enhanced CT image upon admission shows the thrombus located in the superior mesenteric venous trunk, junction of portal (thick arrow) and splenic vein (thin arrow) with bowel edema. (B) CT image at the same level as (A), obtained following argatroban therapy, shows that the thrombus was completely dissolved. CT, computed tomography; ARG, argatroban.
No major bleeding or mortality secondary to bleeding occurred. However, minor bleeding complications occurred in 2 patients (11%) during anticoagulation therapy with argatroban (Table III). Both cases presented hematochezia, thus argatroban treatment was terminated, and aPTT, INR and hemoglobin levels were monitored daily. No transfusion was required in these patients. When the hemorrhage stopped, argatroban therapy was continued, with no further bleeding episodes observed during the remaining of the treatment.
Follow-up results
The median duration of follow-up was 16.2 months (range, 6–24 months). The contrast-enhanced CT examination as the main method was applied in evaluating the recurrence. Overall, recurrence was observed in 11% (2/18) of patients, with 1 patient succumbing to the disease during follow-up; thus, the mortality rate was 6% (1/18 patients). These 2 recurrence cases had received the lower adjusted initial dose of argatroban therapy for hepatic dysfunction, and 1 of these patients received a shorter duration of anticoagulant therapy due to minor bleeding, which may have resulted in an unsatisfactory thrombolysis degree at hospital discharge.
The patient who succumbed 6 months after treatment did not achieve optimal therapy effect during the hospitalization period; however, the abdominal symptoms were relieved gradually after hospital discharge. However, recurrence occurred, which resulted in intestinal infarction and septic shock, leading to mortality.
Another patient required further surgery 1 month following discharge for intestinal obstruction. A segmental inflammatory structured intestine ~10 cm in length with extensive inflammatory adhesion was detected during the surgery. Resection of 30 cm of the small bowel was thus performed. No ASMVT recurrence was indicated by the contrast-enhanced CT scanning, but these inflammatory changes were likely to be a result of SMVT. Furthermore, another patient received jejunum perforation repair after 1 month. It is difficult to determine whether the jejunum perforation was secondary to SMVT or an independent event, since the contrast-enhanced CT imaging showed no recurrence (Table III).
Discussion
ASMVT is an insidious disease that is difficult to diagnose. The most common symptoms include abdominal pain, distention and nausea, which are not specific symptoms and easily mimic other diseases. The duration of symptoms prior to treatment initiation significantly influences the outcome in patients with AMVT, including the mortality rate, possibility of needing a laparotomy and the long-term prognosis (9,24). With the assistance of contrast-enhanced CT in recent years, the time from symptom presentation to diagnosis has decreased from an average of 1 week during 1978–1995 to ~1 day during 1995–2003 (5). However, due to the lack of a standard diagnosis and treatment strategy and the imbalance distribution of medical resources amongst other issues in China, the interval between symptom presentation and treatment is even longer. This interval was found to be 5.0±3.5 days in the present study study.
Even when ASMVT is diagnosed earlier, treatment does not necessarily result in a more satisfactory outcome. Currently, anticoagulation is widely accepted as the first-choice therapy in ASMVT patients (25). Immediate heparinization using UFH or LMWH upon diagnosis of SMVT is considered to be the current standard therapy even in certain patients with bleeding due to surgery or SMVT-induced ischemia. However, the majority of the relevant data published to date demonstrate that UFH or LMWH as traditional anticoagulant medicine present certain limitations in SMVT-PVT therapy, such as heparin-associated thrombocytopenia, high incidence of laparotomy requirement and high mortality rate (2,5). As presented in Table IV, which summarizes the findings of the present and selected previous studies, the incidence of laparotomy is as high as 0–56%, and the 30-day in-hospital mortality ranges between 0 and 25%.
Table IV.
Selected studies reporting the treatment and outcome of acute mesenteric venous thrombosis.
| First author | Median study year | N | Mean age (years) | Mean hospital stay (days) | Surgery (%) | Resection (%) | Anticoagulation therapy (%) | Anticoagulant | Mortality (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Morasch | 1992 | 31 | 49.1 | N/A | 35.5 | 32.3 | 71.0 | UFH | 22.6%a | 30 |
| Alvi | 1996 | 20 | 55.6 | N/A | 40 | 40 | 100 | UFH | 20% | 24 |
| Brunaud | 1997 | 12 | 56.7 | 23.2±8.4 | 8.3 | 8.3 | 100 | UFH | 25.0% | 2 |
| Zhang | 1999 | 28 | 45 | 12.6±4.6 | 32.1 | 17.9 | 100 | UFH+LMWH | 11.0% | 6 |
| Joh | 1999 | 6 | 45 | 31 | 0 | 0 | 100 | UFH or LMWH | 0% | 31 |
| Muñoz | 2000 | 13 | 68 | N/A | 38.5 | N/A | 69.2 | UFH | 38.5%b | 32 |
| Cenedese | 2005 | 9 | 57 | N/A | 55.6 | 55.6 | 100 | N/A | 0% | 33 |
| Present study | 2014 | 18 | 50.9 | 16.4±7.6 | 11.1 | 11.1 | 100 | ARG | 0% | − |
The incidence of surgery, bowel resection and mortality only refer to the in-hospital rates.
Overall mortality was 22.6%, while the mortality of patients initially treated with heparin was 13.6%.
Overall mortality was 38.5%, while the mortality of patients that were initially treated with heparin was 11.1%. N/A, data not available; UFH, unfractionated heparin; LMWH, low molecular weight heparin; ARG, argatroban.
Transcatheter thrombolysis has been recently reported to be a more effective alternative in the management of ASMVT (10,11). The catheter can be inserted via percutaneous transhepatic (26), transfemoral (12), transjugular (27), or transarterial (in the SMA) approaches (12,28). However, numerous hospitals do not have the ability to apply these techniques due to the high technical requirement. In addition, transjugular intrahepatic portosystemic shunt is only recommended when anticoagulation is unsuccessful and the clinical condition worsens (20), due to the technical difficulties in constructing the shunt in the absence of a normal anatomy of the portal and the hepatic vein systems in hepatic and portal vein thrombosis patients (29). The indications include extensive thromboses, severe symptoms, and persistent or worsening symptoms despite anticoagulation (11), and thus the use of this technique is limited and can not be administered to all patients. Furthermore, despite published data only available from specific case reports and small case series, a remarkable high rate of bleeding was noticed, especially via the transhepatic route. For instance, the series reported by Hollingshead et al revealed that 75% of patients (n=15) had partial or complete clot resolution; however, 60% of patients (n=12) developed a major complication, with bleeding the most common complication (12). Therefore, a more effective and safe anticoagulation method is pursued.
In recent years, argatroban, a direct thrombin inhibitor, has been successfully used in numerous thrombotic diseases. Compared with other anticoagulants, argatroban has unique attributes that contribute to its safety and efficacy, including its small molecular weight, peptidomimetic structure, reversible binding to thrombin and nonimmunogenic nature (7). Furthermore, argatroban can be differentiated from other anticoagulants by its hepatic (not renal) clearance (15). In consideration of these characteristics, argatroban may be considered to have certain superiorities in ASMVT therapy over other anticoagulants.
To the best of our knowledge, this is the first reported series to highlight the effectiveness and safety of argatroban therapy in ASMVT patients. The incidence of surgery and bowel resection, and the in-hospital mortality in the current study were found to be 6 (excluding a case presenting intestinal necrosis upon admission, as mentioned earlier) and 0%, respectively. These rates are lower compared with those reported in recent studies involving anticoagulant therapy initiated with UFH or/and LMWH in ASMVT patients within the last few years (Table IV) (2,6,24,30–33). This indicates that argatroban can effectively avoid bowel infarction and mortality in ASMVT patients. Furthermore, the mean time from treatment initiation to symptom remission in the current study was 6.3±3.7 days, which indicated the timely onset of the anticoagulation effect induced by argatroban.
Only 1 patient (5%) had complete thrombolysis in the present study, which is lower than the 15.0% rate reported by Hollingshead et al (12), or the 53.8 and 62.5% rates reported by Yang et al (10,11) conducted via transcatheter thrombolysis and aspiration thrombectomy therapies, respectively. However, if partial thrombolysis cases are considered, then 89% patients in the current study exhibited complete or partial thrombolysis, with 17 (94%) cases having clinical improvement or elimination of symptoms; therefore, this rate is not markedly different from previous studies that similarly presented conclusions which considered partial and complete thrombolysis. However, thrombolysis results in significantly higher risk of bleeding. For instance, 60% of patients in the study by Hollingshead et al (12) experienced bleeding or decreased hematocrit level, while 23.1 and 25% of patients experienced bleeding complications in the studies by Yang et al (10,11).
Bleeding is the primary adverse effect associated with anticoagulation therapy. The incidence of major bleeding in previous studies involving argatroban and UFH used in heparin-induced thrombocytopenia (HIT; 6.9 vs. 6.7%) (23), in HIT with thrombosis syndrome (5.7 vs. 7.0%) (34), in percutaneous coronary intervention (0% vs. 3.0%) (35), and in acute myocardial infarction (19% vs. 20%) (36) do not reveal apparent differences in major bleeding; however, in accordance with various studies, argatroban improves the clinical outcome (23,34,35,36).
The recommendation for the initial dose of argatroban in HIT is 2 µg/kg/min (0.5 µg/kg/min in hepatic dysfunction patients), adjusted to achieve an aPTT value 1.5–3 times of the patient's baseline aPTT (23,34). However, limited data exist regarding dosing patterns, course and safety of argatroban therapy in ASMVT patients. In the current study, the most commonly used initial dose was administered, adjusted to maintain the aPTT at 1.5–3 times the baseline value, and the results presented were satisfactory. Only 2 patients (11%) experienced bleeding complication, and no major bleeding occurred in the current study.
Certain patients included in the current series also suffered from cirrhosis and pancreatitis. Whether cirrhosis patients with portomesenteric venous thrombosis require anticoagulation therapy is controversial due to the high risk of bleeding (37,38). It was also reported that a patient with PVT and acute pancreatitis developed severe hematemesis due to UFH (39). In the present study, there was also a patient with evidently elevated serum bilirubin level, who presented minor bleeding in spite of the initial dose being administered at 0.5 µg/kg/min and adjusted to 0.41 µg/kg/min. The elimination half-life of argatroban is increased when other cofactors are present, such as hepatic dysfunction, renal insufficiency and critical illness (13). Further studies have proven that lower initial dosage is indicated for specific patient populations. An initial dose of 0.2 µg/kg/min in critically-ill patients with multiple organ dysfunction was sufficient and safe for achieving effective anticoagulation (40). However, the extent of association among dose, therapeutic effect and bleeding risk in hepatic dysfunction patients may not be simply concluded owing to the limited sample size in the current study, and thus further studies are required.
All 18 patients were followed up for a median time of 16.2 months, and received at least 6 months of oral-anticoagulant therapy with warfarin, as the majority of studies recommend (3). However, during the follow-up performed in the present study, 2 patients (11%) presented SMVT recurrence, including 1 patient (5%) who succumbed to the disease. This recurrence may be due to various reasons. First, the 2 recurrence patients underwent therapy with an initial dose of 0.5 µg/kg/min lasting for 9 and 6 days; thus, the anticoagulation time was slightly shorter compared with that in other patients in the current study, which may result in insufficient anticoagulation therapy and unsatisfactory thrombus dissolution upon hospital discharge. In addition, both recurrence patients presented ASMVT combined with cirrhosis and hepatic dysfunction. Whether such ASMVT patients have a higher risk of recurrence remains unknown.
The present study has several limitations. Firstly, the study does not provide an answer to the question of whether argatroban is a better option for ASMVT patients due to the absence of a control group, such as a group receiving interventional treatment or anticoagulant therapy with another medicine. A single-center randomized clinical trial on argatroban and LMWH in ASMVT therapy is currently conducted. Furthermore, as a result of the retrospective and cross-sectional nature, and the small number of patients included in the current study, the dosage, course and target value of argatroban therapy remains unknown. Further investigation is required to improve the understanding and management of argatroban therapy in ASMVT patients.
In conclusion, argatroban therapy is effective and safe in patients with ASMVT. It may be another feasible anticoagulant in ASMVT therapy, which is beneficial in that it can rapidly improve symptoms, with low incidence of bowel resection or bleeding complication, and a low mortality rate. However, random-controlled trials on the use of argatroban and other anticoagulants or interventional treatment are needed. In addition, the optimal dose, course and target value of argatroban need to be further researched.
References
- 1.Rhee RY, Gloviczki P. Mesenteric venous thrombosis. Surg Clin North Am. 1997;77:327–338. doi: 10.1016/S0039-6109(05)70552-1. [DOI] [PubMed] [Google Scholar]
- 2.Brunaud L, Antunes L, Collinet-Adler S, Marchal F, Ayav A, Bresler L, Boissel P. Acute mesenteric venous thrombosis: Case for nonoperative management. J Vasc Surg. 2001;34:673–679. doi: 10.1067/mva.2001.117331. [DOI] [PubMed] [Google Scholar]
- 3.Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15:407–418. doi: 10.1177/1358863X10379673. [DOI] [PubMed] [Google Scholar]
- 4.Elliot JW. II. The operative relief of gangrene of intestine due to occlusion of the mesenteric vessels. Ann Surg. 1895;21:9–23. doi: 10.1097/00000658-189521060-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren S, Eberhard TP. Mesenteric venous thrombosis. Surg Gynecol Obstet. 1935;61:102–121. [Google Scholar]
- 6.Zhang J, Duan ZQ, Song QB, Luo YW, Xin SJ, Zhang Q. Acute mesenteric venous thrombosis: A better outcome achieved through improved imaging techniques and a changed policy of clinical management. Eur J Vasc Endovasc Surg. 2004;28:329–334. doi: 10.1016/j.ejvs.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Jeske WP, Fareed J, Hoppensteadt DA, Lewis B, Walenga JM. Pharmacology of argatroban. Expert Rev Hematol. 2010;3:527–539. doi: 10.1586/ehm.10.53. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Sarr M, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–1688. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 9.Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954–968. doi: 10.1016/S0016-5085(00)70183-1. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Liu B, Ding W, He C, Wu X, Li J. Acute superior mesenteric venous thrombosis: Transcatheter thrombolysis and aspiration thrombectomy therapy by combined route of superior mesenteric vein and artery in eight patients. Cardiovasc Intervent Radiol. 2014;38:88–99. doi: 10.1007/s00270-014-0896-z. [DOI] [PubMed] [Google Scholar]
- 11.Yang SF, Liu BC, Ding WW, He CS, Wu XJ, Li JS. Initial transcatheter thrombolysis for acute superior mesenteric venous thrombosis. World J Gastroenterol. 2014;20:5483–5492. doi: 10.3748/wjg.v20.i18.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingshead M, Burke CT, Mauro MA, Weeks SM, Dixon RG, Jaques PF. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:651–661. doi: 10.1097/01.RVI.0000156265.79960.86. [DOI] [PubMed] [Google Scholar]
- 13.Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: Effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20:318–329. doi: 10.1592/phco.20.4.318.34881. [DOI] [PubMed] [Google Scholar]
- 14.Berry CN, Girard D, Lochot S, Lecoffre C. Antithrombotic actions of argatroban in rat models of venous, ‘mixed’ and arterial thrombosis, and its effects on the tail transection bleeding time. Br J Pharmacol. 1994;113:1209–1214. doi: 10.1111/j.1476-5381.1994.tb17126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hursting MJ, Alford KL, Becker JC, Brooks RL, Joffrion JL, Knappenberger GD, Kogan PW, Kogan TP, McKinney AA, Schwarz RP., Jr Novastan (brand of argatroban): A small-molecule, direct thrombin inhibitor. Semin Thromb Hemost. 1997;23:503–516. doi: 10.1055/s-2007-996128. [DOI] [PubMed] [Google Scholar]
- 16.Muta T, Okamura T, Kawamoto M, Ichimiya H, Yamanaka M, Wada Y, Urata M, Kayamori Y, Hamasaki N, Kato K, et al. Successful therapy with argatroban for superior mesenteric vein thrombosis in a patient with congenital antithrombin deficiency. Eur J Haematol. 2005;75:167–170. doi: 10.1111/j.1600-0609.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 17.Dager WE, Gosselin RC, Owings JT. Argatroban therapy for antithrombin deficiency and mesenteric thrombosis: Case report and review of the literature. Pharmacotherapy. 2004;24:659–663. doi: 10.1592/phco.24.6.659.34745. [DOI] [PubMed] [Google Scholar]
- 18.Young SK, Al-Mondhiry HA, Vaida SJ, Ambrose A, Botti JJ. Successful use of argatroban during the third trimester of pregnancy: Case report and review of the literature. Pharmacotherapy. 2008;28:1531–1536. doi: 10.1592/phco.28.12.1531. [DOI] [PubMed] [Google Scholar]
- 19.Levine RL, Hursting MJ, McCollum D. Argatroban therapy in heparin-induced thrombocytopenia with hepatic dysfunction. Chest. 2006;129:1167–1175. doi: 10.1378/chest.129.5.1167. [DOI] [PubMed] [Google Scholar]
- 20.Singal AK, Kamath PS, Tefferi A. Mesenteric Venous Thrombosis. Mayo Clin Proc. 2013;88:285–294. doi: 10.1016/j.mayocp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Kearon. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 22.Doepker B, Mount KL, Ryder LJ, Gerlach AT, Murphy CV, Philips GS. Bleeding risk factors associated with argatroban therapy in the critically ill. J Thromb Thrombolysis. 2012;34:491–498. doi: 10.1007/s11239-012-0758-y. [DOI] [PubMed] [Google Scholar]
- 23.Lewis BE, Wallis DE, Berkowitz SD, Matthai WH, Fareed J, Walenga JM, Bartholomew J, Sham R, Lerner RG, Zeigler ZR, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843. doi: 10.1161/01.CIR.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 24.Alvi AR, Khan S, Niazi SK, Ghulam M, Bibi S. Acute mesenteric venous thrombosis: Improved outcome with early diagnosis and prompt anticoagulation therapy. Int J Surg. 2009;7:210–213. doi: 10.1016/j.ijsu.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis. American college of chest physicians evidence-based clinical practice guidelines. Chest. (9th ed) 2012;141(2 Suppl):e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi N, Kuroki K, Yanaga K. Percutaneous transhepatic mechanical thrombectomy for acute mesenteric venous thrombosis. J Endovasc Ther. 2005;12:508–511. doi: 10.1583/04-1335MR.1. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama S, Murashima N, Isobe Y. Superior mesenteric venous thrombosis treated by direct aspiration thrombectomy. Hepatogastroenterology. 2008;55:367–370. [PubMed] [Google Scholar]
- 28.Poplausky MR, Kaufman JA, Geller SC, Waltman AC. Mesenteric venous thrombosis treated with urokinase via the superior mesenteric artery. Gastroenterology. 1996;110:1633–1635. doi: 10.1053/gast.1996.v110.pm8613072. [DOI] [PubMed] [Google Scholar]
- 29.Riggio O, Ridola L, Lucidi C, Angeloni S. Emerging issues in the use of transjugular intrahepatic portosystemic shunt (TIPS) for management of portal hypertension: Time to update the guidelines? Dig Liver Dis. 2010;42:462–467. doi: 10.1016/j.dld.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Morasch MD, Ebaugh JL, Chiou AC, Matsumura JS, Pearce WH, Yao JS. Mesenteric venous thrombosis: A changing clinical entity. J Vasc Surg. 2001;34:680–684. doi: 10.1067/mva.2001.116965. [DOI] [PubMed] [Google Scholar]
- 31.Joh JH, Kim DI. Mesenteric and portal vein thrombosis: Treated with early initiation of anticoagulation. Eur J Vasc Endovasc Surg. 2005;29:204–208. doi: 10.1016/j.ejvs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz S, Cubo P, González-Castillo J, Nuevo JA, García-Lamberechts EJ, Sanz A. Superior mesenteric venous thrombosis: A retrospective study of thirteen cases. Rev Esp Enferm Dig. 2004;96:385–394. doi: 10.4321/S1130-01082004000600004. [DOI] [PubMed] [Google Scholar]
- 33.Cenedese A, Monneuse O, Gruner L, Tissot E, Mennesson N, Barth X. Initial management of extensive mesenteric venous thrombosis: Retrospective study of nine cases. World J Surg. 2009;33:2203–2208. doi: 10.1007/s00268-009-0168-2. [DOI] [PubMed] [Google Scholar]
- 34.Lewis BE, Wallis DE, Leya F, Hursting MJ, Kelton JG. Argatroban-915 Investigators: Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163:1849–1856. doi: 10.1001/archinte.163.15.1849. [DOI] [PubMed] [Google Scholar]
- 35.Rössig L, Genth-Zotz S, Rau M, Heyndrickx GR, Schneider T, Gulba DC, Desaga M, Buerke M, Harder S, Zeiher AM. ARG-E04 study group: Argatroban for elective percutaneous coronary intervention: The ARG-EO4 multi-center study. Int J Cardiol. 2011;148:214–219. doi: 10.1016/j.ijcard.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 36.Vermeer F, Vahanian A, Fels PW, Besse P, Müller E, Van de Werf F, Fitzgerald D, Darius H, Puel J, Garrigou D, et al. Argatroban and alteplase in patients with acute myocardial infarction: The ARGAMI study. J Thromb Thrombolysis. 2000;10:233–240. doi: 10.1023/A:1026591023462. [DOI] [PubMed] [Google Scholar]
- 37.de Franchis R, V Faculty Baveno. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 38.DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park WS, Kim HI, Jeon BJ, Kim SH, Lee SO. Should anticoagulants be administered for portal vein thrombosis associated with acute pancreatitis? World J Gastroenterol. 2012;18:6168–6171. doi: 10.3748/wjg.v18.i42.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beiderlinden M, Treschan TA, Görlinger K, Peters J. Argatroban anticoagulation in critically ill patients. Ann Pharmacother. 2007;41:749–754. doi: 10.1345/aph.1H569. [DOI] [PubMed] [Google Scholar]