Abstract
The aim of the present study was to examine the effect of an intensified training phase followed by a tapering phase on the salivary immunoglobulin A concentration and on the upper respiratory tract infection (URTI) symptoms in young male basketball players. The session rating of perceived exertion method was used to quantify the internal training load, and the Wisconsin Upper Respiratory Symptom Survey-21 questionnaire was used to assess URTI symptoms. The Yo-Yo IR1 test and saliva collection were carried out at the beginning of the study (T1), after the intensified phase (T2), and after tapering (T3). A higher internal training load was observed for the intensified phase compared with the tapering phase (t=19.10; p<0.001), and a significant decrease in salivary immunoglobulin A concentration was detected (F=7.48; p=0.004) at T3 compared to T1 (p=0.02) and T2 (p=0.05). However, there was no significant difference between phases for severity of URTI (χ2= 2.83; p=0.242). The Yo-Yo IR1 test performance increased from T2 and T3 compared to T1 (F=58.24; p<0.001). There was no significant effect of aerobic fitness level on salivary immunoglobulin A response (F=1.095; p=0.344). In summary, the present findings suggest that an intensified training load followed by a tapering period negatively affects the mucosal immune function with no significant change in severity of URTI in young basketball players.
Keywords: Periodization, Mucosal immunity, Athletes
INTRODUCTION
Periodization is the appropriate manipulation of training stress to optimize gains in athletic performance [1, 2]. Additionally, training periodization has also been referred to as a common strategy to organize and manipulate training loads (TL) in order to ensure a proper balance between training stress and recovery. Such balance may optimize the adaptive responses and athletic performance whilst avoiding negative consequences, which could lead to non-functional overreaching or overtraining syndrome [2–7]. One periodization strategy that has been employed to maximize the effects of the training stimulus involves the use of periods of intensified physical training at certain stages followed by recovery periods (tapering) [3, 7]. An insufficient period of recovery between successive training sessions likely may cause the suppression of the immune system [8] and may lead to an increase of the risk of illness, notably, for greater incidence of upper respiratory tract infections (URTI). It has also been demonstrated in adult athletes that strenuous training might have an increased risk of infection because of transient immune suppression [9–11].
Despite the relevance of these associations between training load and immunity, there is limited information regarding young athletes. It is noteworthy that nowadays, young athletes are also involved in intensive training programmes [11] and frequently participate in official competitive matches separated by short time intervals [12]. Among the few studies that have investigated the effect of a competition with matches separated by short time intervals (congested schedule) in youth team sport athletes, Mortatti et al. [12] demonstrated a significant relationship between the salivary immunoglobulin A (SIgA) decrease and the incidence of URTI symptoms in young soccer players, monitored through an official competition, which included 7 matches played over 20 days. However, as yet little is known regarding how youth team sport athletes cope with intensified periods of training load, and particularly how this periodization strategy might affect their mucosal immunity.
A transient fall in SIgA has been shown to be a good predictor of increased risk of URTI [13]. The role of SIgA is to prevent viral replication and to inhibit the attachment of bacteria and viruses at the mucosal epithelium in the mouth, throat, and upper respiratory tract [14]. Therefore, a decrease in the production and/or concentration of this antibody due to intensified training load periods would represent a risk factor for subsequent URTI episodes in athletes [9]. Moreover, Kunz et al. [15] investigated the effect of fitness level on the acute salivary antimicrobial proteins response, including SIgA, of highly experienced cyclists at various exercising workloads. The results of their study revealed that highly fit cyclists exhibited a greater post-exercise increase in the SIgA concentration. The reported percentage difference between highly fit and less fit cyclists was +181%. Interestingly, this result suggests that the fitness level would affect the SIgA response, at least for an acute exercise bout.
However, up to now, the SIgA response during a deliberately intensified training phase followed by a tapering period in young basketball players is still unknown. Moreover, there is scarce knowledge about the influence of the player’s aerobic fitness level on SIgA response and URTI symptoms in this population. Therefore, the aim of the present study was to examine the effect of an intensified training phase followed by a tapering phase on the SIgA concentration and on the occurrence and severity of URTI symptoms in elite young male basketball players. It was hypothesized that an intensified training phase would elicit a greater decrease in SIgA concentration, which would increase the severity of URTI symptoms compared to the tapering phase. Additionally, it was expected that the SIgA response (SIgA concentration) would be affected by aerobic fitness level.
MATERIALS AND METHODS
Participants
Twenty-three male basketball players (mean ± SD: age, 15.8 ± 0.8 years; body mass, 82.7 ± 13 kg) volunteered to participate in this investigation. The basketball players were from an under-17 age group and regularly competed in a major State Basketball Championship in São Paulo, Brazil. The inclusion criteria for the present investigation were: participants had to be familiar with training exercises used in the investigation and to have been performing strength training regularly for at least 6 months before the commencement of the study. To include the participants’ data in the final analysis, additional criteria were adopted: completion of at least 75% of the training programme, completion of session rating of perceived exertion (session-RPE) and a weekly questionnaire for URTI symptoms (WURSS-21), and they had to perform the field test and provide saliva samples at the three testing occasions (T1, T2, and T3). Data of players who had injuries during the investigation period were excluded. Data from 23 basketball players were retained for analysis according to these additional criteria. All 23 players whose data were considered completed all the planned training sessions (100%). Data from 2 players were excluded due to players’ commitments. This research conforms to the ethical principles, which was approved by the local University Research Ethics Committee. All experimental procedures, risks and benefits were explained in detail to the athletes and their respective parents or their legal guardians, and written informed consent was obtained from each participant and their respective parents or guardians.
Internal training load (ITL)
The session-RPE method was used to determine the ITL through the product of session duration (in minutes) and session-RPE score for both strength training and skill-conditioning sessions in accordance with the method adopted previously in basketball players [16–18]. The averages of accumulated weekly ITL for each phase were retained for analysis.
Upper respiratory tract infection (URTI) symptoms
Symptoms and functional impairments associated with the common cold were assessed using the Wisconsin Upper Respiratory Symptom Survey-21 (WURSS-21) questionnaire. The WURSS-21 questionnaire was filled out on a weekly basis at the end of every assessed week. The validity of this questionnaire has been demonstrated earlier by Barrett et al. [19] and was adopted with young team sport athletes by Freitas et al. [4]. The questionnaire includes 1 global question, 10 symptom-based questions, 9 functional impairment/quality-of-life questions, and 1 global change question. The severity of each reported symptom was rated on a 7-point Likert scale: 1 (very mild), 3 (mild), 5 (moderate), and 7 (severe). Symptoms not experienced were recorded as 0. A global score for general symptoms was calculated from the sum of the 10 symptom-based and the 9 functional impairment/quality-of-life questions as proposed by Barrett et al. [19].
Performance test (aerobic fitness test)
Yo-Yo Intermittent Recovery Test (Yo-Yo IR1)
To assess players’ aerobic fitness, the level 1 version of the Yo-Yo Intermittent Recovery (Yo-Yo IR1) test was applied according to previously described methods [20]. All basketball players were familiar with the testing procedures. The players performed repeated 20-m shuttle runs, back and forth between the starting line and finish line marked by cones, at progressively increasing speeds dictated by an audio bleep emitted from a CD player. Between each shuttle, the players had a 10-s period of walking around a cone placed 5 m from the starting line. Failure to achieve the shuttle run on two successive occasions resulted in termination of the test. Total distance covered represented the test result. All the Yo-Yo IR1 tests were performed in the same basketball court where the basketball players usually trained. Reliability of the Yo-Yo IR1 test, calculated as the coefficient of variation, was shown to be 4.9% for the total distance [20].
Aerobic fitness level determination
Subjects were divided by a median split into high (HG) and low (LG) aerobic fitness groups based on their Yo-Yo IR1 performance at T1 (commencement of the study).
Saliva collection and assays
Saliva collection required the subjects to passively drool directly into sterile 15-ml centrifuge tubes over a 5-minute period. The samples were collected with the participants in a seated position, with eyes open, head tilted slightly forward with a minimal orofacial movement. Immediately after collection, the saliva samples were frozen and stored at -80°C until analysis for SIgA concentration. The samples were tested for SIgA concentrations in duplicate using enzyme-linked immunosorbent assays (s-IgA EIA kit, ELISA, Salimetrics, USA) in accordance with previous methods [21–23]. The average intra-assay coefficient of variation for SIgA was 6.0%.
Procedures
The 8-week investigation period comprised 1 familiarization week, and 4 weeks of intensified training phase followed by a 3-week tapering phase, as described previously [6]. Briefly, training was intensified with the inclusion of 4 more strength training sessions (STS) on top of players’ usual 2 STS per week routine. These sessions included more strength exercises and sets within each session, when compared to the “normal” training period (habitual players’ programme) that were performed before the commencement of the experimental period. The STS in week 1, before the commencement of the intensified training phase (week 2 to 5), was conducted as a “familiarization period”. It is important to highlight that all the assessed basketball players were, however, already familiar with the selected strength training exercises. Tactics and technical skills were also part of the training programme. Non-STS were classified as skills-conditioning training (SKS). During the tapering period (TP), the external training load returned to 2 STS a week although the intensity of these sessions was maintained at an elevated level. All participants were submitted to the session-RPE method to quantify the internal training load (ITL) in each session. The WURSS-21 questionnaire was completed on a weekly basis in order to assess URTI symptoms. All the athletes were familiar with all these procedures. Performance tests and saliva collection were carried out at the beginning of the study (T1), after the intensified phase (T2), and after the tapering phase (T3). For the saliva collection and performance assessment, athletes were required to have eaten their last meal (lunch) at least 2 hours before and not exercise for 48 hours. The samples were always collected at the team’s training facility at 3:00 PM.
Statistical analysis
SPSS 19.0 was used for all statistical analyses. The Gaussianity of data and homogeneity of variance were analysed using the Shapiro- Wilk test and Levene’s test, respectively. A one-way ANOVA with repeated measures was used to compare SIgA concentration at T1, T2 and T3. Another ANOVA with repeated measures was used to compare Yo-Yo IR1 values at the three time points. In case of significant F-values the least significant difference (LSD) post hoc test was used for multiple comparison purposes. The Friedman test for non-parametric data was used to compare time points (T1, T2, and T3) for severity of URTI. If necessary, the Wilcoxon test as post-hoc analysis with Bonferroni adjustment was used for multiple comparisons. The average ITL accumulated for each training phase (intensified vs tapering phases) was compared through a paired t-test. A two-way ANOVA was used to compare the difference between groups (HG vs. LG) and test occasion (T1, T2, and T3) for SIgA concentration. In the case of violation of the assumption of sphericity, the significance was established by using the Greenhouse-Geisser correction. For all statistical analyses, the significance level was set at p ≤ 0.05.
RESULTS
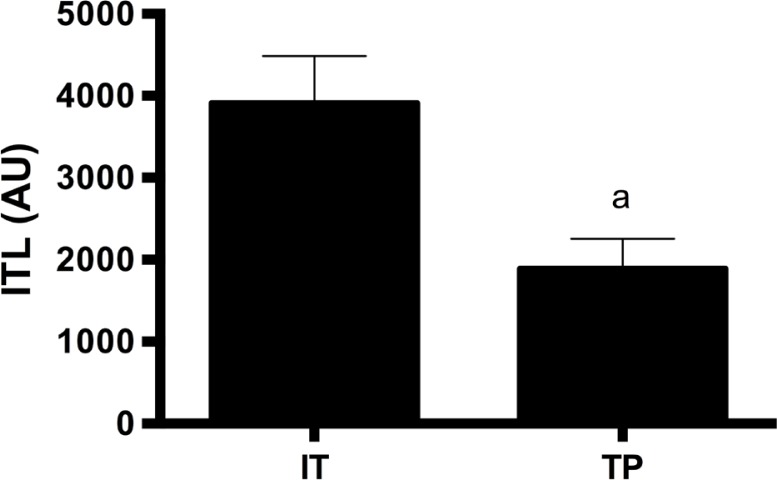
Figure 1 presents ITL during the intensified and tapering phases. As expected, a higher ITL was observed for the intensified phase compared with the tapering phase (t=19.10; p < 0.001).
FIG. 1.
Internal training load (ITL) during the intensified training period (IT) and the tapering period (TP). AU = arbitrary units; a – significant difference compared to intensified phase (IT) (p < 0.05).
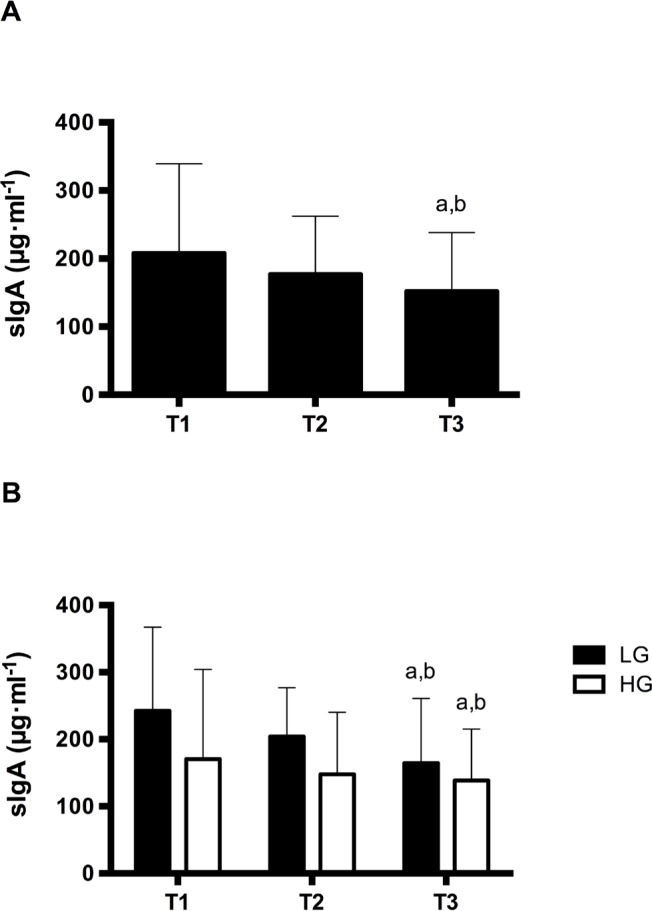
The SIgA response is presented in Figure 2. A significant decrease in SIgA concentration (Figure 2A) (F=7.48; p = 0.004) was observed from T1 to T3 (p = 0.002) and T2 (p = 0.05).
FIG. 2.
SIgA concentration (A whole sample and B aerobic fitness level: low [LG] and high [HG]) at the beginning of the study (T1), after the intensified training period and after the tapering period). a – significant difference from T1; b – significant difference from T2.
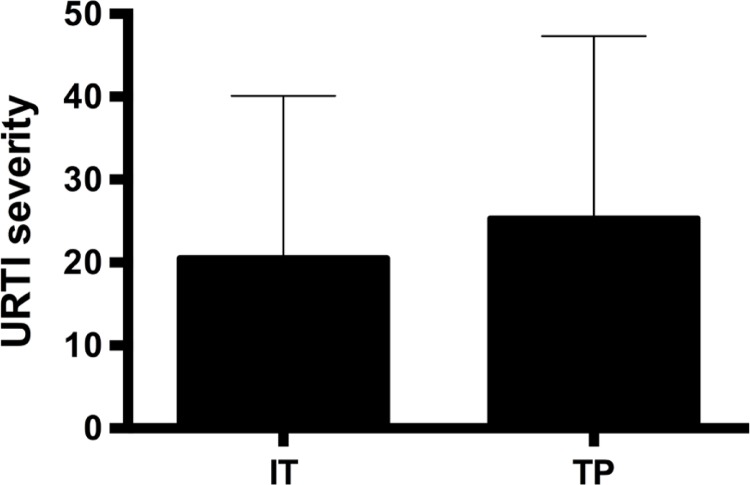
Figure 3 presents the URTI severity. Despite the SIgA decrease observed, there was no significant difference between training phases for severity of URTI (χ2= 2.83; p=0.242). Table 1 presents the results of the Yo-Yo IR1 test. A significant difference between time points was observed for the Yo-Yo IR1 test (F=58.24; p < 0.001). A greater distance covered in the Yo-Yo IR1 test was identified at T2 (p < 0.001) and T3 (p < 0.001) compared to T1. However, no significant difference was noted for Yo-Yo IR1 test performance between T2 and T3 (p = 0.061). In addition, no significant group interaction (aerobic fitness level; HG vs LG) x occasion (T1, T2, and T3) was detected for SIgA (F=1.095; p = 0.344) (Figure 2B).
FIG. 3.
URTI severity during intensified training (IT) and tapering period (TP).
TABLE 1.
Yo-Yo IR1 test distance at the three time points.
| T1 | T2 | T3 | |
|---|---|---|---|
| Yo-Yo IR1 (m) | 897.39 ± 70.40 | 1196.52 ± 101.30a | 1266.08 ± 90.86a |
Note: The values are mean ± SD; IR1: Yo-Yo Intermittent Recovery Test level 1; a – significant difference to baseline; T1 = beginning of the study; T2 = after intensified phase; T3 = after tapering phase.
DISCUSSION
The primary aim of this study was to examine the effects of an intensified training phase followed by a tapering phase on the SIgA concentration and severity of URTI symptoms in elite young male basketball players. In addition, the effect of aerobic fitness level on SIgA response was also investigated. As expected, the intensification phase led to a greater ITL when compared to the tapering phase. Therefore, it is possible to affirm that the planned external training load was successfully implemented in order to generate a deliberate intensified training phase followed by a tapering phase. The 4-week intensified training load followed by a 3-week tapering led to a significant decrease in SIgA concentration from T1 to T3 and T2 to T3. Despite the SIgA concentration decrease, the severity of URTI did not change across the investigation period. Moreover, contrary to the study’s hypothesis, the aerobic fitness level did not influence the SIgA response.
Some previous findings have consistently suggested that there might be an inverse relationship between SIgA and URTI [9, 13]. Moreover, it has also been demonstrated that training load may affect URTI incidence in both men and women involved in endurance-based physical activity [24]. These associations among training load, SIgA and URTI symptoms have also been reported in some observational studies with basketball players [25, 26]. For instance, Moreira et al. [21] conducted a study with a similar sample used in the present study to investigate the effect of a 4-week training period, which included both intensified training weeks and tapering weeks. These authors reported that the young basketball players presented a greater number of URTI episodes and a lower stress tolerance during the higher training load week (2nd week of the investigation period). There was also reported a significant decrease in SIgA when comparing values found at the start with those observed after the end of the investigation.
In another study with basketball players, 8 members of the National Taichung University (NTCU) basketball team in Taiwan participated in a 4-week intensive training programme in preparation for a national tournament [25]. A significantly lower level of SIgA concentration was reported for the intensive training period when compared to a recovery period which was undertaken after the competition phase. The results from the current experimental study not only corroborate the findings from Moreira et al. [21] and He et al. [25] but also add new evidence for the effect of a deliberate intensified training phase followed by a tapering period on SIgA response of young basketball players. Taking together the findings from the current study and those reported previously [21, 25], it is possible to conclude that intensified periods of training may compromise the mucosal immunity of both young and adult basketball players, thereby exposing athletes to a greater risk of URTI.
Interestingly, contrary to the hypothesis of the current study, the observed impairment of mucosal immune function (decrease in SIgA concentration) across the investigation period did not induce a significant change in severity of URTI. However, it is noteworthy that whilst some studies demonstrated an association between SIgA and URTI in team sport athletes [9, 12, 27], others did not find such a relationship [26, 28]. For example, Moreira et al. [26] reported a decrease in both SIgA absolute concentration and SIgA secretion rate in basketball players and staff members of a national basketball team, during a 17-day training period prior to an international competition. However, these authors did not observe an association between SIgA and URTI occurrence. The authors pointed out that, possibly, a more frequent saliva sampling protocol would be required to establish a relationship between salivary SIgA and URTI, which is in accordance with the findings of Gleeson et al. [29]. Therefore, one could argue that more frequent saliva sampling over the 7-week experimental period of the present study might demonstrate this relationship. Nevertheless, it is noteworthy that the severity of URTI was tracked across the 7 weeks of the study, and not only at the three saliva sample collecting points. Additionally, no significant change in severity of URTI across the entire study was noted, regardless of the periodization of training loads (intensification phase vs tapering phase).
In another investigation with team sport athletes, Milanez et al. [28] conducted an observational study to investigate the associations between training load, stress tolerance, URTI and SIgA response in a group of female futsal players in preparation for a competition. These authors monitored 5 training weeks. Stress symptoms were assessed using the DALDA questionnaire, and symptoms of URTI were assessed using the WURSS-21. The questionnaires and saliva sample for determining SIgA concentration were measured on a weekly basis. The results of their study also revealed no significant relationship between SIgA and symptoms of URTI. Despite the significant decrease in SIgA, which was aligned with a greater training load, the expected relationship between SIgA decrease and URTI increase was not observed in their study. Therefore, it is reasonable to suppose that whilst SIgA may be used as a useful and valid indicator of mucosal immune function related to periodization of training loads, there might not be a direct relationship between these variables and URTI symptoms, mainly because of the effect of factors other than salivary antibodies which may contribute to infection development [30]. Indeed, the contribution of other salivary antimicrobial proteins, such as lactoferrin, lysozyme, and alpha-amylase, to maintaining mucosal immune integrity [31] may influence the likelihood of identifying the association between reduced mucosal immunity and URTI symptoms.
An additional aim of the current study was to investigate whether aerobic fitness level may influence SIgA response. In order to address this issue, players were divided into high (HG) and low (LG) aerobic fitness based on their Yo-Yo IR1 performance. Previous studies in both young and adult team sport athletes have demonstrated the adequate reproducibility of the Yo-Yo IR1 test [20, 32]. This test has been used in other studies of young team athletes in order to assess the prolonged, high-intensity intermittent running capacity and aerobic performance [33–35]. It has been proposed that basketball players should have a good aerobic capacity [31, 36]. Indeed, the aerobic capacity has been shown to be strongly correlated with high-intensity running during matches [31, 37].
Taking these features into account, it was expected that the mucosal immunity function of players in the HG would be less affected by the intensified training phase compared to the LG. Despite the unknown influence of the fitness level in SIgA response in a similar homogeneous sample, the hypothesis was based on previous results with athletes from studies which had assessed different physiological responses. For instance, a previous study [38] reported that Australian football players with better 6 min run performance presented smaller disturbances in blood creatine kinase prior to the competition. Indeed, another study [39] showed that fitter team sport athletes experience smaller metabolic disturbances and less acute fatigue following high-intensity activity; moreover, greater aerobic fitness has been shown to affect repeated-sprint performance, reducing the associated decrement in this test.
As a matter of fact, it is important to highlight that the effects of athletic training on SIgA response have been investigated through cross-sectional (athletes vs. non-athletes) [40] and longitudinal studies with different populations and varying experimental durations [12, 23, 27, 41]. Whilst it has been shown that periods of congested schedule competition may reduce SIgA concentration [12, 23] in young and professional soccer players, respectively, and that a heavy training load may also impact SIgA response in team athletes [27], none of these studies took into account the fitness level of the participants, except when comparing sedentary or non-athletes individuals with athletes [40, 42]. Therefore, the present findings are novel and suggest that in a relatively homogeneous sample, like that usually found for team sport athletes, the differences between the high aerobic fitness level athletes and their less fit counterparts are not enough to discriminate or predict the magnitude of SIgA decrease. Nevertheless, it is noteworthy that this result might be attributed, at least in part, to the homogeneity of the analysed sample.
CONCLUSIONS
In summary, the present findings suggest that an intensified training load period followed by a 3-week tapering period negatively affects the mucosal immune function of young basketball players. Nevertheless, regardless of the observed decrease in SIgA concentration, there was no significant change in severity of URTI, and yet the performance in the aerobic fitness test increased. These findings suggest that there may be an association between increased training load and reduced SIgA response and that the delayed effect of a training load intensification period may be relatively long, despite subsequent reduction in training load. Finally, based on the current results, it seems that the initial fitness level does not affect the SIgA response during the intensification period followed by a tapering period in young basketball players.
Acknowledgements
The authors wish to acknowledge the committed participation of all the basketball players, the support staff and coaches involved in this study, particularly those of Bernardo Miloski, Telma Tavernari, David Pelosini, Murilo Drago, and Luiz Carlos Souza Junior.
Conflict of interests
the authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Coutts A, Reaburn P, Murphy A, Watsford M, Spurrs R. Changes in physiological characteristics of semi-professional rugby league players in relation to training load: a case study. J Sci Med Sport. 2003;6(4):37. [Google Scholar]
- 2.Moreira A, Coutts AJ, Moreira A, Bilsborough JC, Sullivan CJ, Cianciosi M, Aoki MS, Coutts AJ. Training Periodization of Professional Australian Football Players During an Entire Australian Football League Season. Int J Sports Physiol Perform. 2015;10:566–71. doi: 10.1123/ijspp.2014-0326. [DOI] [PubMed] [Google Scholar]
- 3.Coutts AJ, Slattery KM, Wallace LK. Practical tests for monitoring performance, fatigue and recovery in triathletes. J Sci Med Sport. 2007;10(6):372–81. doi: 10.1016/j.jsams.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Freitas CG, Aoki MS, Franciscon C, Arruda AFS, Carling C, Moreira A. Psychophysiological responses to overloading and tapering phases in elite young soccer players. Pediatr Exerc Sci. 2014;26(2):195–202. doi: 10.1123/pes.2013-0094. [DOI] [PubMed] [Google Scholar]
- 5.Issurin VB. New horizons for the methodology and physiology of training periodization. Sport Med. 2010;40(3):189–206. doi: 10.2165/11319770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Miloski B, Aoki MS, de Freitas CG, de Arruda AFS, de Moraes HS, Drago G, Borges TO, Moreira A. Does testosterone modulate mood states and physical performance in young basketball players? J Strength Cond Res. 2015;29(9):2474–81. doi: 10.1519/JSC.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 7.Mujika I. Intense training: the key to optimal performance before and during the taper. Scand J Med Sci Sports. 2010;20:24–31. doi: 10.1111/j.1600-0838.2010.01189.x. [DOI] [PubMed] [Google Scholar]
- 8.Bishop N, Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. Front Biosci. 2009;14:4444–56. doi: 10.2741/3540. [DOI] [PubMed] [Google Scholar]
- 9.Fahlman MM, Engels H-J. Mucosal IgA and URTI in American College Football Players: A Year Longitudinal Study. Med Sci Sport Exerc. 2005;37(3):374–80. doi: 10.1249/01.mss.0000155432.67020.88. [DOI] [PubMed] [Google Scholar]
- 10.Pyne DB, Mcdonald WA, Gleeson M, Flanagan A, Clancy RL, Fricker PA. Mucosal immunity, respiratory illness, and competitive performance in elite swimmers. Med Sci Sports Exerc. 2000;33(3):348–54. doi: 10.1097/00005768-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson M, Bishop NC. URI in Athletes : Are Mucosal Immunity and Cytokine Responses Key Risk Factors? Exerc Sport Sci Rev. 2013;41(10):2–7. doi: 10.1097/JES.0b013e3182956ead. [DOI] [PubMed] [Google Scholar]
- 12.Mortatti A, Moreira A, Aoki MS, Crewther B, Castagna C, Arruda A, Filho J. Effect of Competition on Salivary Cortisol, Immunoglobulin A, and Upper Respiratory Tract Infections in Elite Young Soccer Players. J Strength Cond Reserach. 2012;26(5):1396–401. doi: 10.1519/JSC.0b013e31822e7b63. [DOI] [PubMed] [Google Scholar]
- 13.Neville V, Gleeson M, Folland JP. Salivary IgA as a Risk Factor for Upper Respiratory Infections in Elite Professional Athletes. Med Sci Sport Exerc. 2008;40(7):1228–36. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- 14.Mackinnon LT. Immunoglobulin, antibody and exercise. Exerc Immunol Rev. 1996;2:1–35. [Google Scholar]
- 15.Kunz H, Bishop NC, Spielmann G, Pistillo M, Reed J, Ograjsek T, Park Y, Mehta SK, Pierson DL, Simpson RJ. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur J Appl Physiol. 2015;115(5):1015–27. doi: 10.1007/s00421-014-3082-8. [DOI] [PubMed] [Google Scholar]
- 16.Moreira A, Crewther B, Freitas C, Arruda A, Costa E, Aoki MS. Session RPE and salivary immune-endocrine responses to simulated and official basketball matches in elite young male athletes. J Sports Med Phys Fitness. 2012;52:682–7. [PubMed] [Google Scholar]
- 17.Moreira A, Franchini E, de Freitas CG, Schultz de Arruda AF, de Moura NR, Costa EC, Aoki MS. Salivary cortisol and immunoglobulin A responses to simulated and official Jiu-Jitsu matches. J Strength Cond Res. 2012;26(8):2185–91. doi: 10.1519/JSC.0b013e31823b8702. [DOI] [PubMed] [Google Scholar]
- 18.Nunes JA, Moreira A, Crewther BT, Nosaka K, Viveiros L, Aoki MS. Monitoring training load, recovery-stress state, immune-endocrine responses and physical performance in elite female basketball players during a periodized training program. J Strength Cond Res. 2014;28(10):2973–80. doi: 10.1519/JSC.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 19.Barrett B, Brown RL, Mundt MP, Thomas GR, Barlow SK, Highstrom AD, Bahrainian M. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21) Health Qual Life Outcomes. 2009;7:76. doi: 10.1186/1477-7525-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krustrup P, Mohr M, Amstrup T, Rysgaard T, Johansen J, Steensberg A, Pedersen Pk, Bangsbo J. The Yo-Yo Intermittent Recovery Test: Physiological Response, Reliability, and Validity. Med Sci Sport Exerc. 2003;35(4):697–705. doi: 10.1249/01.MSS.0000058441.94520.32. [DOI] [PubMed] [Google Scholar]
- 21.Moreira A, Arsati F, de Oliveira Lima-Arsati YB, Simões AC, de Araújo VC. Monitoring stress tolerance and occurrences of upper respiratory illness in basketball players by means of psychometric tools and salivary biomarkers. Stress Heal. 2011;27(3):e166–72. [Google Scholar]
- 22.Moreira A, Mortatti AL, Arruda AFS, Freitas CG, de Arruda M, Aoki MS. Salivary IgA response and upper respiratory tract infection symptoms during a 21-week competitive season in young soccer players. J Strength Cond Res. 2014;28(2):467–73. doi: 10.1519/JSC.0b013e31829b5512. [DOI] [PubMed] [Google Scholar]
- 23.Morgans R, Orme P, Anderson L, Drust B, Morton JP. Research in Sports Medicine : An An Intensive Winter Fixture Schedule Induces a Transient Fall in Salivary IgA in English Premier League Soccer Players. Res Sport Med. 2014;22(4):346–54. doi: 10.1080/15438627.2014.944641. [DOI] [PubMed] [Google Scholar]
- 24.Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P. Sex differences in immune variables and respiratory infection incidence in an athletic population. Exerc Immunol Rev. 2011;17:122–35. [PubMed] [Google Scholar]
- 25.He C-S, Tsai M-L, Ko M-H, Chang C-K, Fang S-H. Relationships among salivary immunoglobulin A, lactoferrin and cortisol in basketball players during a basketball season. Eur J Appl Physiol. 2010;110(5):989–95. doi: 10.1007/s00421-010-1574-8. [DOI] [PubMed] [Google Scholar]
- 26.Moreira A, Arsati F, Cury PR, Franciscon C, Simões AC, de Oliveira PR, de Araújo VC. The impact of a 17-day training period for an international championship on mucosal immune parameters in top-level basketball players and staff members. Eur J Oral Sci. 2008;116(5):431–7. doi: 10.1111/j.1600-0722.2008.00558.x. [DOI] [PubMed] [Google Scholar]
- 27.Cunniffe B, Griffiths H, Proctor W, Davies B, Baker JS, Jones KP. Mucosal immunity and illness incidence in elite rugby union players across a season. Med Sci Sports Exerc. 2011;43(3):388–97. doi: 10.1249/MSS.0b013e3181ef9d6b. [DOI] [PubMed] [Google Scholar]
- 28.Milanez VF, Ramos SP, Okuno NM, Boullosa DA, Nakamura FY. Evidence of a Non-Linear Dose-Response Relationship between Training Load and Stress Markers in Elite Female Futsal Players. J Sport Sci Med. 2014;13(1):22–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Gleeson M, Pyne D, Austin J, Lynn FJ, Clancy R, McDonald W, Fricker P. Epstein-Barr virus reactivation and upper-respiratory illness in elite swimmers. Med Sci Sports Exerc. 2002;34(3):411–7. doi: 10.1097/00005768-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Diamond G, Beckloff N, Ryan L. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87(10):915–27. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben Abdelkrim N, Castagna C, Jabri I, Battikh T, El Fazaa S, El Ati J. Activity Profile and Physiological Requirements of Junior Elite Basketball Players in Relation to Aerobic-Anaerobic Fitness. J Strength Cond Res. 2010;24(9):2330–42. doi: 10.1519/JSC.0b013e3181e381c1. [DOI] [PubMed] [Google Scholar]
- 32.Deprez D, Coutts AJ, Lenoir M, Fransen J, Pion J, Philippaerts R, Vaeyens R. Reliability and validity of the Yo-Yo intermittent recovery test level 1 in young soccer players. J Sports Sci. 2014;32:903–10. doi: 10.1080/02640414.2013.876088. [DOI] [PubMed] [Google Scholar]
- 33.Figueiredo AJ, Coelho e Silva MJ, Malina RM. Predictors of functional capacity and skill in youth soccer players. Scand J Med Sci Sports. 2011;21(3):446–54. doi: 10.1111/j.1600-0838.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreira A, Mortatti A, Aoki M, Arruda A, Freitas C, Carling C. Role of free testosterone in interpreting physical performance in elite young brazilian soccer players. Pediatr Exerc Sci. 2013;25(2):186–97. doi: 10.1123/pes.25.2.186. [DOI] [PubMed] [Google Scholar]
- 35.Sirotic A, Coutts AJ. Physiological and performance test correlates of prolonged, high-intensity, intermittent running performance in moderately trained women team sport athletes. J Strength Cond Reserach. 2007;21(1):138–44. doi: 10.1519/00124278-200702000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery PG, Pyne DB, Minahan CL. The physical and physiological demands of basketball training and competition. Int J Sports Physiol Perform. 2010;5(1):75–86. doi: 10.1123/ijspp.5.1.75. [DOI] [PubMed] [Google Scholar]
- 37.Castagna C, Manzi V, D’Ottavio S, Annino G, Padua E, Bishop D. Relation between maximal aerobic power and the ability to repeat sprints in young basketball players. J Strength Cond Res. 2007;21(4):1172–6. doi: 10.1519/R-20376.1. [DOI] [PubMed] [Google Scholar]
- 38.Hunkin S, Fahrner B, Gastin P. Creatine kinase and its relationship with match performance in elite Australian Rules football. J Sci Med Sport. 2014;17(3):332–6. doi: 10.1016/j.jsams.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Stone N, Kilding A. Aerobic Conditioning for team sport athletes. Sport Med. 2009;39(8):615–42. doi: 10.2165/00007256-200939080-00002. [DOI] [PubMed] [Google Scholar]
- 40.Tomasi T, Trudeau F, Czerwinski D, Erredge S. Immune parameters in athletes before and after strenuous exercise. J Clin Immunol. 1982;3(2):173–8. doi: 10.1007/BF00915219. [DOI] [PubMed] [Google Scholar]
- 41.Tiollier E, Gomez-Merino D, Burnat P, Jouanin J-C, Bourrilhon C, Filaire E, Guezennec CY, Chennaoui M. Intense training: mucosal immunity and incidence of respiratory infections. Eur J Appl Physiol. 2005;93(4):421–8. doi: 10.1007/s00421-004-1231-1. [DOI] [PubMed] [Google Scholar]
- 42.Gleeson M, Pyne DB. Exercise effects on mucosal immunity. Immunol Cell Biol. 2000;78(5):536–44. doi: 10.1111/j.1440-1711.2000.t01-8-.x. [DOI] [PubMed] [Google Scholar]