Abstract
The present study aimed to analyse the effects of six weeks of strength training (ST), with and without blood flow restriction (BFR), on torque, muscle activation, and local muscular endurance (LME) of the knee extensors. Thirty-seven healthy young individuals were divided into four groups: high intensity (HI), low intensity with BFR (LI+BFR), high intensity and low intensity + BFR (COMB), and low intensity (LI). Torque, muscle activation and LME were evaluated before the test and at the 2nd, 4th and 6th weeks after exercise. All groups had increased torque, muscle activation and LME (p<0.05) after the intervention, but the effect size and magnitude were greater in the HI, LI+BFR and COMB groups. In conclusion, the groups with BFR (LI+BFR and COMB) produced magnitudes of muscle activation, torque and LME similar to those of the HI group.
Keywords: Therapeutic occlusion, Electromyography, Ischemia, Quadriceps muscle, Exercise tolerance
INTRODUCTION
In recent years, low-intensity (LI) training with blood flow restriction (BFR) has attracted much attention, both as a possible alternative to high-intensity (HI) exercise (≥70% of one-repetition maximum [1RM]) in the context of rehabilitation and as a training method to increase strength and muscle hypertrophy in healthy subjects [27, 38]. Additionally, this method has been used to increase torque [14, 19, 34, 36], muscle activation [15, 17, 38–42], local muscular endurance (LME) [6, 7, 11, 13, 20, 36], and maximum strength [16, 35, 37]. Fujita et al. [9] reported that the reason for these increases is that LI strength training (ST) combined with BFR increases endurance, phosphorylation and muscle protein synthesis and promotes increased strength while providing the same gains as conventional ST with high loads.
In this regard, several studies have reported the acute [15, 17, 23–26, 38] and chronic [3, 4, 6, 7, 11, 13, 16, 20, 30, 35, 36, 42] effects of ST with BFR. Thus, some studies have evaluated the chronic effects of ST with BFR on isometric torque [14, 19, 34, 36] and LME [6, 7, 13, 20, 36] after application of LI ST with BFR. It was observed that only one study has evaluated the chronic effect of a unilateral knee extension isotonic training programme on isometric torque [14], and one study has investigated the effects of combined LI and HI ST with and without BFR in alternating sessions on muscle strength and size [43], but the exercise utilized was for upper limbs (bench press). Therefore, there is a lack of studies aiming to investigate the effects of combined LI and HI ST with and without BFR on torque, LME and muscle activation in alternating sessions of the lower limbs. Additionally, understanding the chronic effect of BFR in the lower limbs in alternate sessions on the torque, LME and muscle activation could help the scientists and health professionals that work with this training method, and it would be an excellent strategy of intervention for the knee extensor muscles for different population groups. For example, the combination of HI and low load with the BFR sessions may be a good intervention option for highly trained people who cannot easily increase the strength and muscle hypertrophy, and this combination could generate different stimuli (tension and metabolic, respectively) since both are performed with HI.
Therefore, the aim of this study was to analyse the effects of six weeks of ST, with and without BFR, on torque, muscle activation, and LME of the knee extensors in healthy young individuals. We hypothesized that LI ST with BFR would promote increases in torque, muscle activation and LME similar to HI training without BFR.
MATERIALS AND METHODS
Subjects
The study included individuals a) who were untrained in ST for at least six months; b) who were apparently healthy, aged between 18 and 30 years, and of both genders; c) with no history of osteo-articular problems in the lower limbs; d) who responded negatively to the Physical Activity Readiness Questionnaire (PAR-Q) [33]; e) who had no history of cardiovascular disease; and f) who had an ankle-brachial index (ABI) in the range 0.90–1.40 [28]. Individuals who did not regularly attend two consecutive training sessions per week were excluded.
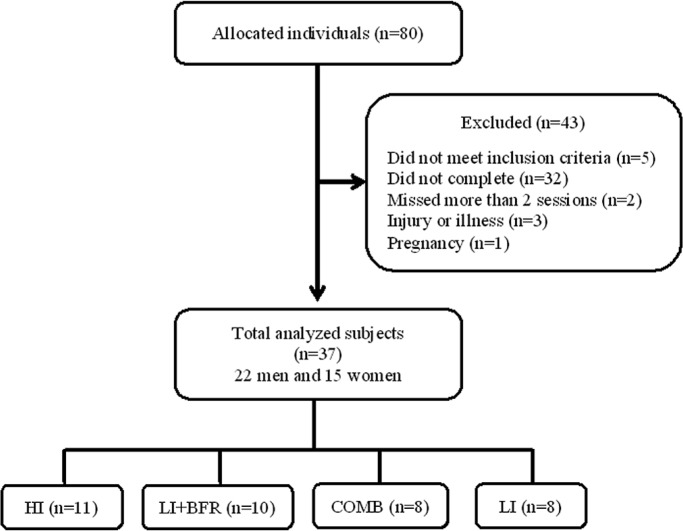
Of the total 80 allocated individuals, 43 were excluded and 37 were randomized to the four groups that completed the study (Figure 1): a) High intensity at 80% of 1RM (HI, n=11); b) Low intensity combined with BFR at 30% of 1RM (LI+BFR; n=10); c) HI combined with LI+BFR (COMB; n=8); and d) Low intensity at 30% of 1RM (LI; n=8), as shown in Table 1.
FIG. 1.
Sample flowchart.
Note: HI=high-intensity group; LI+BFR=low-intensity with blood flow restriction group; COMB=combined group; LI=low-intensity group.
TABLE 1.
Anthropometric sample characteristics.
| Variables | HI (men=5; women=6) | LI+BFR (men=7; women=3) | COMB (men=5; women=3) | LI (men=5; women=3) | ICC | P |
|---|---|---|---|---|---|---|
| Age (years) | 20.73 ± 3.79 | 23.70 ± 4.90 | 24.75 ± 4.86 | 22.38 ± 2.61 | 0.949 | <0.001 |
| BM (kg) | 64.30 ± 12.76 | 76.09 ± 21.88 | 66.82 ± 11.55 | 62.58 ± 9.31 | 0.801 | 0.002 |
| Height (m) | 1.69 ± 0.12 | 1.72 ± 0.09 | 1.69 ± 0.09 | 1.65 ± 0.08 | 0.978 | <0.001 |
| BMI (kg ∙ m-2) | 22.4 ± 3.79 | 25.18 ± 4.98 | 23.17 ± 2.14 | 22.93 ± 3.88 | 0.967 | <0.001 |
Note: HI=high-intensity group; LI+BFR=low-intensity with blood flow restriction group; COMB=combined group; LI=low-intensity group; BM=body mass; BMI=body mass index.
The sample design was constructed with the aid of G*Power 3.1 software [8] and according to the procedures suggested by Beck [5]. Based on post-hoc analysis, for a sample of 37 individuals, with an effect size of 0.50, an α = 0.05 and a correlation coefficient of 0.5, a statistical power of 87.4% was obtained.
Subjects were informed about risks and benefits of the study and gave informed consent. The study was approved by the Ethics Committee on Research Involving Human Beings under protocol number 0389/2011, and all the procedures were performed in agreement with the Declaration of Helsinki.
Study design
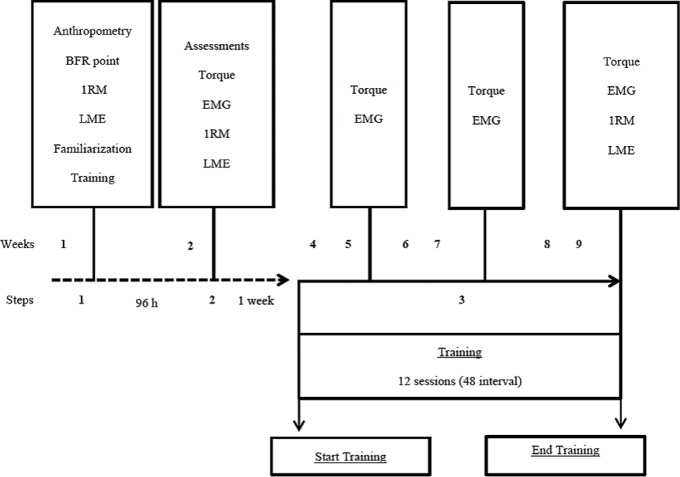
Anthropometry, determination of BFR point, 1RM, LME, and familiarization of training were assessed at the first visit to the laboratory. On the second visit, evaluations of torque, EMG, 1RM retest, and LME (pre-test) were conducted. The interval between steps 1 and 2 was 96 hours and between steps 2 and 3 one week. In step three, there was a 48-hour interval between training sessions. After pre-test analysis, the experimental groups were trained for six weeks. Every 2 weeks of training torque and electromyographic (EMG) assessments were conducted. Post-testing was completed 48 h following the last training session (Figure 2).
FIG. 2.
Procedures in timeline.
Note: BFR=blood flow restriction 1RM=one repetition maximum; LME=local muscular endurance; EMG=electromyography.
Determination of blood flow restriction pressure (BFR)
External compression for BFR during the intervention was performed according to Laurentino et al. [16]. To do so, we used a specially adapted sphygmomanometer (18 cm wide x 80 cm long). This device was placed on the most proximal portion of the thigh and inflated until interruption of the auscultatory pulse of the tibial artery, established with the aid of a portable vascular Doppler (MedPeg DV -2001, Ribeirão Preto, SP, Brazil). The cuff pressure used during exercise for the LI+BFR and COMB groups was 80% of the total BFR pressure in the resting state, which was kept constant throughout the exercise. The average pressures on the lower limbs used throughout the experiments were 141.56 ± 12.19 and 129.83 ± 11.87 mm Hg for the LI+BFR and COMB groups, respectively.
Torque and surface electromyogram (EMG)
For data recording, subjects were seated and had their knee flexed at an angle of 60° and the ankle fixed to the resistance arm bracket of the power system with trunk and pelvis maintained at approximately 90º, set by bands in the trunk, pelvis and thigh to avoid compensatory movements. Subjects were then asked to perform three maximal voluntary isometric contractions (MVICs) each lasting five seconds at intervals of 60 seconds [31].
Knee extension isometric torque (vastus medialis [VM], rectus femoris [RF] and vastus lateralis [VL]) was recorded using an EMG-Force integrated system, composed of a Bonett chair with strain gages fixed on the chair’s resistance arms [21]. The amplified signal was digitized using a 16-channel A/D converter board with 12-bit resolution at a sampling frequency of 1000 Hz.
The software used for capturing and processing strength signals and EMG was the BioMed (digital polygraph) application. Surface EMG signals (root mean square [RMS] and median frequency [Fmed]) were captured simultaneously with strength using a system with the following settings: biological amplifier (CMRR > 95 dB), high input impedance (10 MΩ), low noise (<5 μV RMS), bandwidth 10-490 Hz and 1500x gain.
Active bipolar electrodes (Skintact, Austria), with a distance of 20 mm between poles, were placed in the centre of the VM, RF and VL muscles [12] to record electrical activity of the quadriceps, which was calculated by averaging the values obtained from the sum of the three evaluated muscles and normalized according to the peak MVIC divided by the pre-test value. To ensure that the electrodes were placed at the same points in all assessments, volunteers were marked with a long-lasting dye (henna), and when it had nearly disappeared a new layer was applied, which ensured that the electrodes were placed in the same place. Furthermore, during the entire test week, marking was performed.
The entire EMG signal capture process was performed in accordance with Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) recommendations [12].
Maximum dynamic strength test (1RM)
The 1RM was determined three weeks before the start of the ST. Procedures followed American College of Sport Medicine recommendations [2], in which subjects were instructed not to perform any physical activity or strenuous effort for at least 24 hours prior to testing.
For the warm-up, each subject performed a set of 5 to 10 repetitions with a load of 40% of perceived maximum weight; after one minute of rest, the individual performed the second set of three to five repetitions with 60% to 80% of perceived maximum weight.
The 1RM test procedures were then initiated, in which each volunteer performed up to five attempts of a unilateral knee extension (dominant limb) on the extensor chair (Body fitness, Brazil) with intervals of three to five minutes between attempts. The 1RM test was performed at pre-test and at the 2nd, 4th and 6th weeks, while the training load was adjusted at each evaluation on each occasion based on the previous evaluation.
Local muscular endurance (LME) and training volume
LME was calculated as the maximum number of repetitions of the unilateral knee extension achieved in a single set with a fixed load of 40% of 1RM. The articular range of motion (ROM) was from 90° to 0°, at a speed controlled by a metronome (Tagima, Brazil), with total movement execution time of four seconds (two seconds for concentric action and two seconds for eccentric action) until the point that concentric failure occurred. When the individual could not maintain the repetition cycle within the sets to the established cadence and amplitude, the concentric point of failure was determined, and the highest number of successfully performed repetitions was computed.
Strength training (ST) programme
ST was performed over two sessions per week, with 48-hour intervals between them, for six weeks (totalling 12 sessions). The exercise used was a unilateral knee extension (dominant limb), which was trained using a conventional knee extension machine (Body fitness, Brazil). Individuals were trained using only the dominant leg in order to prevent a cross-transfer effect [19]. All groups performed four sets until concentric failure.
The HI group performed traditional multiple sets training, with a load of 80% of 1RM without BFR and with a two-minute interval. The LI+BFR group executed an exercise protocol with BFR using the same BFR determining cuff, at an intensity of 30% of 1RM and intervals of 30 seconds between sets. The COMB group trained with a mixed approach, combining an HI ST session (four sets at 80% of 1RM with an interval of two minutes) with an LI+BFR session (four sets at 30% of 1RM with a 30-second interval) executed alternately. The LI group performed traditional multiple sets training, at 30% of 1RM and a rest interval of 30 seconds.
Statistical analyses
All analyses were performed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL). Data normality was verified using the Shapiro-Wilk test and the homogeneity of the variances using the Levene test. All the variables showed normal distribution (p>0.05). The reliability of the sample characteristics (age, body mass, height, and BMI) was verified by the interclass correlation coefficient (ICC) (Table 1). A two-way ANOVA of repeated measures [groups (HI vs. LI+BFR vs. COMB vs. LI) x time (pre-test vs. 2nd week vs. 4th week vs. 6th week)] was used to evaluate the effects of training on all dependent variables, followed by the Tukey post hoc test to locate the main differences. As the RMS variable did not fulfil the assumption of homogeneity, we used the delta variation to avoid erroneous comparisons (2nd/pre-test; 4th/pre-test; 6th/pre-test; 4th/2nd; 6th/2nd; 6th/4th), so one-way ANOVA was used to compare the groups. Effect sizes (ES) were calculated as [(Post-training Mean – Pre-training)/Pre-Training SD], and classified according to the magnitude [trivial < 0.50, small = 0.50 to 1.25, moderate = 1.25-1.90, and large > 2.0] [29], and percentage changes (Δ%) were used to express possible differences between the first (pre-test) and fourth (6th week – post-test) evaluations. A significance level of 5% was adopted for all comparisons.
RESULTS
Total training volume (12 sessions) was determined by load x repetitions. The average volume for each group was as follows: HI=584.05 kg; LI+BFR=295.63 kg; LI=310.0 kg; and COMB=432.8 kg. There were significant increases between HI vs. LI+BFR (p<0.001), HI vs. LI (p<0.001), and HI vs. COMB (p=0.045).
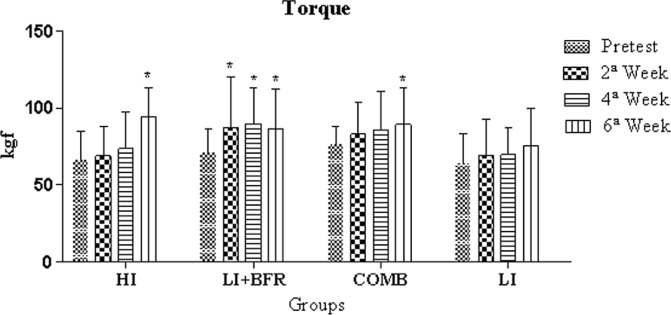
There were no statistically significant differences for the post-test in the isometric torque for the intergroup analysis (p>0.05). In the intra-group analysis, significant increases were observed in the HI group between the pre-test and the 6th week (p<0.001, Δ=43%), the 2nd and the 6th weeks (p<0.001, Δ=36.4%) and the 4th and 6th weeks (p<0.001, Δ=28%). There were also significant increases between evaluations for the pre-test and the 2nd week, the pre-test and the 4th week, and the pre-test and the 6th week (p=0.003, Δ=24.2%; p<0.001, Δ=27.5%; and p=0.004, Δ=22.7%, respectively) for the LI+BFR group. There was also a significant increase between the pre-test and the 6th week (p=0.027, Δ=17.4%) in the COMB group, as shown in Figure 3.
FIG. 3.
Comparative analysis of isometric torque between experimental group evaluations.
* Significant differences to the pre-test
Note: HI=high-intensity group; LI+BFR=low-intensity with blood flow restriction group; COMB=combined group; LI=low-intensity group.
In the intergroup analysis of RMS by one-way ANOVA, no differences were observed between groups when the difference between the percentags deltas was compared (2nd/pre-test; 4th/pre-test; 6th/pre-test; 4th/2nd; 6th/2nd; 6th/4th; p>0.05). In the EMG signal RMS intra-group analysis, significant increases were observed in the COMB group between the pre-test and the 2nd week (p=0.032; Δ=51.3%); the pre-test and the 4th week (p<0.05; HI: Δ=52.9%, LI+BFR: Δ=98.6%, COMB: Δ=77.2%, LI: Δ=4,.8%) and the pre-test and the 6th week (p<0.05; HI: Δ=55.3%, LI+BFR: Δ=80.9%, COMB: Δ=80.9% LI: Δ=36.7%). Significant increases were also detected in the HI group between the 2nd and 4th weeks (p=0.003; Δ=51.1%) and the 2nd and 6th weeks (p=0.002; Δ=53.5%), as shown in Table 2 and Table 3.
TABLE 2.
Comparative analysis of root mean square (RMS) between experimental group evaluations.
| Groups | |||||
|---|---|---|---|---|---|
| Evaluations | HI | LI + BFR | COMB | LI | P |
| Pre-test | 2.51 ± 1.23 | 1.52 ± 0.47 | 2.20 ± 0.84 | 3.90 ± 2.04 | 0.004 |
| 2nd week | 2.54 ± 1.56 | 2.42 ± 1.21 | 3.33 ± 2.06* | 2.75 ± 1.00 | 0.147 |
| 4th week | 3.84 ± 2.07* | 3.02 ± 1.21* | 3.90 ± 2.16* | 3.98 ± 2.10* | 0.246 |
| 6th week | 3.90 ± 2.04* | 2.75 ± 1.00* | 3.98 ± 2.10* | 4.58 ± 1.16* | 0.134 |
| Delta percentage (post / pre) | HI | LI+BFR | COMB | LI | P |
| 2nd / Pre-test | 1.07 ± 0.56 | 1.68 ± 0.88 | 1.46 ± 0.51 | 1.27 ± 0.53 | 0.203 |
| 4th / Pre-test | 1.54 ± 0.30 | 2.08 ± 1.04 | 1.72 ± 0.57 | 1.50 ± 0.60 | 0.241 |
| 6th / Pre-test | 1.63 ± 0.48 | 1.85 ± 0.57 | 1.78 ± 0.67 | 1.48 ± 0.62 | 0.562 |
| 4th / 2nd | 1.71 ± 0.65 | 1.40 ± 0.67 | 1.22 ± 0.43 | 1.28 ± 0.51 | 0.283 |
| 6th / 2nd | 1.77 ± 0.70 | 1.37 ± 0.84 | 1.31 ± 0.65 | 1.26 ± 0.41 | 0.335 |
| 6th / 4th | 1.04 ± 0.17 | 0.95 ± 0.20 | 1.12 ± 0.61 | 1.03 ± 0.33 | 0.771 |
Note: HI=high-intensity group; LI+BFR=low-intensity with blood flow restriction group; COMB=combined group; LI=low-intensity group.
Significant differences to the pre-test.
TABLE 3.
Effect size values (magnitude) between the pre-test and the 6th week of muscle activation (RMS and Fmed), LME and muscle strength (1RM) between experimental groups.
| Variables | HI (n=11) | LI+BFR (n=10) | COMB (n=08) | LI (n=08) |
|---|---|---|---|---|
| RMS (RMS%MVC) ES |
1.13 | 2.61 | 2.11 | 1.05 |
| Magnitude | Small | Large | Large | Small |
| Fmed (Hz) ES |
5.09 | 4.91 | 6.02 | 2.38 |
| Magnitude | Large | Large | Large | Large |
| LME (Repetitions) ES |
27.5 | 26.1 | 19.3 | 22.7 |
| Magnitude | Large | Large | Large | Large |
| Strength (Kg) ES |
1.49 | 1.02 | 1.08 | 0.59 |
| Magnitude | Moderate | Small | Small | Small |
Note: HI=high-intensity group; LI+BFR=low-intensity with blood flow restriction group; COMB=combined group; LI=low-intensity group; RMS=root mean square; ES=effect size; Fmed=median frequency; LME= local muscular endurance.
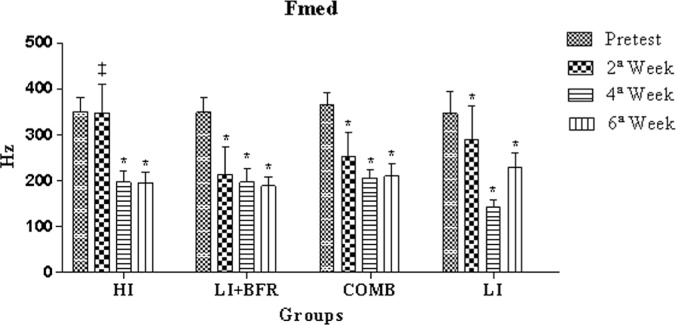
Statistically significant differences were observed in the Fmed intergroup analysis between HI and LI+BFR at the 2nd week (p<0.001). Significant reductions were observed in the HI group in the intra-group analysis between the pre-test and the 4th week, the pre-test and the 6th week, the 2nd and 4th weeks, and the 2nd and 6th weeks (p<0.001, Δ=43.7%; p<0.001, Δ=44.0%; p<0.001, Δ=43.2%; and p<0.001, Δ=43.4%, respectively). There were also significant reductions between evaluations for the pre-test and the 2nd week, the pre-test and the 4th week, and the pre-test and the 6th week (p<0.001, Δ=38.6%; p<0.001, Δ=43.4%; and p<0.001, Δ=45.8%, respectively) in the LI+BFR group. There were also significant reductions between evaluations for the pre-test and the 2nd week, the pre-test and the 4th week, and the pre-test and the 6th week in the COMB group (p<0.001, Δ=30.8%; p<0.001, Δ=43.4%; and p<0.001, Δ=42.6%, respectively). In the LI group, there were significant reductions between evaluations for the pre-test and the 2nd week, the pre-test and the 4th week, the pre-test and the 6th week, the 2nd and 6th weeks, and the 4th and 6th weeks (p=0.036, Δ=16.3%; p<0.001, Δ=58.5%; p<0.001, Δ=33.7%; p<0.001, Δ=50.4%; p=0.021, Δ=20.8%; and p<0.001, Δ=59.7%, respectively), as shown in Figure 4.
FIG. 4.
Comparative analysis of Fmed between experimental group evaluations.
Note: * Significant differences to the pre-test; ‡ Significant difference between HI and LI+BFR in the 2nd week.
HI=high-intensity group; LI+BFR=low-intensity with blood flow restriction group; COMB=combined group; LI=low-intensity group.
Table 3 shows percentage changes (Δ%), effect sizes (ES) and magnitudes for evaluations in all groups, pre-test and post-test (6 weeks).
DISCUSSION
This study analysed the effects of six weeks of ST, with and without BFR, on torque, muscle activation, and LME of the knee extensors in healthy young individuals. To our knowledge, this was the first study to verify the influence of LI and HI ST with BFR in alternating sessions on torque, muscle activation, and LME in lower limbs. The main findings of this study were: a) significant increases in isometric torque in the HI, LI+BFR, and COMB groups after intervention and b) significant increases in RMS, Fmed, and LME for all groups.
Some studies have assessed the chronic effect of LI ST with BFR on muscle torque [14, 36, 42]. Yasuda et al. [42] investigated the effects of six weeks of concentric and eccentric LI ST with BFR on the muscle torque of ten young men performing elbow flexion exercises three days a week for six weeks. Each individual performed four sets (30x15x15x15) of unilateral elbow flexions (30% of 1RM), with one arm trained prioritizing the concentric phase and the other prioritizing the eccentric phase. The authors observed significant increases in torque (8.6%) for the concentric phase after training but not for the eccentric phase (3.8%). Comparing these results with those of the present study, it can be observed that for exercise performed unilaterally, irrespective of the body section (upper or lower), ST performed with BFR appears to improve torque.
Furthermore, some studies that determined that ST with BFR had an acute effect on muscle activation [39, 40] also observed that BFR increased muscle activation compared to training without BFR, with greater amplitude of the EMG signal in the concentric phase [42]. This increased muscle activation can lead to acute changes that are reflected in increases in muscle size and muscle cell swelling, which may be important factors in promoting muscle hypertrophy [42]. One study evaluated the chronic effect of ST with BFR on muscle activation [14]. The authors found that low-load ST with BFR did not affect motor unit activation of muscle and tendon properties, but there was an increase to high intensity, which diverges from our findings. It is speculated that it may have occurred because in the study by Kubo et al. [14] subjects trained with one leg using a low load with BFR and the other leg at high intensity without BFR; however, in our study, different groups were used, thus avoiding the cross-training effect [19].
One study investigated the effects of combined LI and HI ST with and without BFR in alternating sessions on muscle strength and size, but the exercise used was the bench press [43]. The findings of the study by Yasuda et al. [43] corroborate those in the present study (inferior limbs). The authors obtained similar results for the combined and high intensity groups for maximum strength and MVIC. It can be observed with these results that the combination of low load strength exercise with BFR and high loads can be an excellent alternative for improving performance of maximum muscle strength and MVIC in upper and lower limbs. This may have occurred due to the variation of the stimulus (tensional and metabolic), which appears to be an excellent option for strength conditioning professionals. It is worth mentioning that we did not find in our study significant increases for the LI group with BFR, which trained to fatigue, which differs from the findings of Mitchell et al. [22] and Fahs et al. [7]. This may have occurred because the training proposed by Mitchell et al. [22] and Fahs et al. [7] used different training for each leg, which may have promoted a cross training effect and thus promoted an increase in torque or muscle strength in the LI group without BFR similar to the group with BFR [19].
Intra-group analysis revealed significant increases in LME in the groups using BFR. In this regard, the results of the few studies that have evaluated LME after training with BFR [6, 7, 11, 13, 20, 36] corroborate the findings of this study. Kacin and Strazar [13] studied LME after subjecting 10 men to four ST sessions per week for four weeks, with and without BFR. Four unilateral knee extension sets were performed in each protocol (at 15% MVIC) until muscle fatigue. One limb was trained with BFR (≥230 mm Hg), and the opposite limb was trained without BFR. The LME of each limb was recorded before and after training, regardless of the training protocol. The authors observed significant increases in both limbs, but with significantly higher increases for the limb that trained with BFR (63% and 27%) vs. without BFR (36% and 11%).
Takarada et al. [36] also evaluated LME after subjecting 17 male athletes to two weekly sessions of LI ST over eight weeks with and without BFR. Each group performed four sets of bilateral knee extensions (at 50% 1RM) until muscle fatigue. The LI+BFR group improved LME. Analysing the results of studies by Kacin and Strazar [13] and Takarada et al. [36] and comparing them with the present study, it can be observed that regardless of form (unilateral or bilateral), load percentage (30-50% of 1RM or 15% of MVIC), and training volume and duration (4, 6 and 8 weeks), ST performed with BFR seems to improve LME in apparently healthy male and female athletes.
Furthermore, some studies have found an acute effect of ST with BFR on LME [38], and it has been observed that BFR can significantly reduce LME compared to ST without BFR. This reduction in LME can be explained by increased muscle fatigue and metabolite accumulation promoted by BFR, as these factors have an inverse relationship with increased LME [32]. Thus, this relationship, when detected in the acute phase, can justify the chronic benefits of ST with BFR on increasing LME.
It is thought that LI ST with BFR reduces myostatin expression in a manner similar to HI training [16], increasing metabolite accumulation and the expression of factors of the mammalian target of rapamycin signalling pathway (mTOR), muscle protein synthesis (S6K1), lactate, growth hormone, insulin-like growth factor (IGF-1), satellite cells and heat shock proteins [9, 18, 38]. In acute and chronic forms, it can increase torque and LME, in addition to reducing muscle fatigue after 12 weeks of intervention.
The current study has some limitations. Firstly, the number of sessions was low, as a greater number of sessions (16-36) is effective in increasing strength and muscle hypertrophy [1, 16]; however, twelve sessions are useful for increasing strength and muscle hypertrophy [10]. Secondly, the pressure was based on arterial occlusion that was measured in the supine position, but the exercises were performed in the standing position. This method was chosen based on previous literature [16]. Although this may have been a limitation, our stimulus appeared adequate given the beneficial response we observed in strength. Finally, there was no measurement of muscle size.
CONCLUSIONS
In conclusion, LI ST with BFR over six weeks increased LME and isometric torque and reduced muscle fatigue, with results approaching the levels of those found in HI training. Therefore, it is important to conduct new experiments to study the physiological and biochemical aspects of LME and fatigue, especially involving different individuals, exercises and intensities, for a better understanding of the process underlying muscular adaptation to ST with BFR.
Conflicts of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100:1460–6. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 2.ACSM. American College of Sports Medicine (ACSM’s). Guidelines for exercise testing and prescription. 9 ed. Baltimore: Lippincott Williams and Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 3.Araujo JP, Neto GR, Loenneke JP, Bemben MG, Laurentino G, Batista G, Silva JCG, Freitas E, Cirilo-Sousa MS. The effects of water aerobics in combination with blood flow restriction on strength and functional capacity in post-menopausal women. AGE. 2015 doi: 10.1007/s11357-015-9851-4. 10.1007/s11357-015-9851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araújo JP, Neto GR, Silva JCG, Silva HG, Neto EAP, Marconio J, Torres VB, Poderoso R, Cirilo-Sousa MS. Does water aerobics with blood flow restriction change the body composition? J Exerc Physiol Online. 2015 [Google Scholar]
- 5.Beck TW. The importance of a priori sample size estimation in strength and conditioning research. J Strength Cond Res. 2013;27:2323–37. doi: 10.1519/JSC.0b013e318278eea0. [DOI] [PubMed] [Google Scholar]
- 6.Cook SB, Brown KA, DeRuisseau K, Kanaley JA, Ploutz-Snyder LL. Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J Appl Physiol. 2010;109:341–9. doi: 10.1152/japplphysiol.01288.2009. [DOI] [PubMed] [Google Scholar]
- 7.Fahs CA, Loenneke JP, Thiebaud RS, Rossow LM, Kim D, Abe T, Beck TW, Feeback DL, Bemben DA, Bemben MG. Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct Imaging. 2015;35:167–76. doi: 10.1111/cpf.12141. [DOI] [PubMed] [Google Scholar]
- 8.Faul F, Erdfelder E, Lang A, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 9.Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–10. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Brechue WF, Kurita K, Sato Y, Abe T. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J Kaatsu Train Res. 2008;4:1–8. [Google Scholar]
- 11.Gil ALS, Neto GR, Sousa MSC, Dias I, Vianna J, Nunes RAM, Novaes JS. Effect of strength training with blood flow restriction on muscle power and submaximal strength in eumenorrheic women. Clin Physiol Funct Imaging. 2015:35. doi: 10.1111/cpf.12291. [DOI] [PubMed] [Google Scholar]
- 12.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 13.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. 2011;21:231–41. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 14.Kubo K, Komuro T, Ishiguro N, Tsunoda N, Sato Y, Ishii N, Kanehisa H, Fukunaga T. Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. J Appl Biomech. 2006;22:112–9. doi: 10.1123/jab.22.2.112. [DOI] [PubMed] [Google Scholar]
- 15.Labarbera KE, Murphy BG, Laroche DP, Cook SB. Sex differences in blood flow restricted isotonic knee extensions to fatigue. J Sports Med Phys Fitness. 2013;53:444–52. [PubMed] [Google Scholar]
- 16.Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves M, Jr, Aihara AY, RCF A, Tricoli V. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012;44:406–12. doi: 10.1249/MSS.0b013e318233b4bc. [DOI] [PubMed] [Google Scholar]
- 17.Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, Bemben DA, Bemben MG. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve. 2015;51:713–21. doi: 10.1002/mus.24448. [DOI] [PubMed] [Google Scholar]
- 18.Loenneke JP, Wilson GJ, Wilson JM. A mechanistic approach to blood flow occlusion. Int J Sports Med. 2010;31:1–4. doi: 10.1055/s-0029-1239499. [DOI] [PubMed] [Google Scholar]
- 19.Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc. 2008;40:258–63. doi: 10.1249/mss.0b013e31815c6d7e. [DOI] [PubMed] [Google Scholar]
- 20.Manimmanakorn A, Hamlin MJ, Ross JJ, Taylor R, Manimmanakorn N. Effects of low-load resistance training combined with blood flow restriction or hypoxia on muscle function and performance in netball athletes. J Sci Med Sport. 2013;16:337–42. doi: 10.1016/j.jsams.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Mendonça GLF, Leite JTF, Alencar JF. Force transduction in a Bonnet chair adapted for evaluation and muscle strengtheningly exercising. Rev Bras Eng Bioméd. 2000;16:63–7. [Google Scholar]
- 22.Mitchell CJ, Churchward-Venne TA, West DWD, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol. 2012;113:71–7. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriggi JR, Di Mauro HS, Dias SC, Matos JM, Urtado MB, Neto NFCS, Nascimento DC, Tibana RA, Assumpção CO, Prestes J. Similar hypotensive responses to resistance exercise with and without blood flow restriction. Biol Sport. 2015;32:289–94. doi: 10.5604/20831862.1163691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neto GR, Santos HH, Sousa JBC, Júnior ATA, Araújo JP, Aniceto RR, Sousa MSC. Effects of high-intensity blood flow restriction exercise on muscle fatigue. J Hum Kinet. 2014;41:163–72. doi: 10.2478/hukin-2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neto GR, Sousa MSC, Costa PB, Salles BF, Novaes GS, Novaes JS. Hypotensive effects of resistance exercises with blood flow restriction. J Strength Cond Res. 2015;29:1064–70. doi: 10.1519/JSC.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 26.Neto GR, Sousa MSC, Silva GVC, Gil ALS, Salles BF, Novaes JS. Acute resistance exercise with blood flow restriction effects on heart rate, double product, oxygen saturation and perceived exertion. Clin Physiol Funct Imaging. 2016;36:53–9. doi: 10.1111/cpf.12193. [DOI] [PubMed] [Google Scholar]
- 27.Pope ZK, Willardson JM, Schoenfeld BJ. Exercise and blood flow restriction. J Strength Cond Res. 2013;27:2914–26. doi: 10.1519/JSC.0b013e3182874721. [DOI] [PubMed] [Google Scholar]
- 28.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality the strong heart study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 29.Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18:918–20. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- 30.Santos AR, Neves MT, Gualano B, Laurentino GC, Lancha AH, Ugrinowitsch C, Lima FR, Aoki MS. Blood flow restricted resistance training attenuates myostatin gene expression in a patient with inclusion body myositis. Biol Sport. 2014;31:121–4. doi: 10.5604/20831862.1097479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos HH, Ávila MA, Hanashiro DN, Camargo PR, Salvini TF. The effects of knee extensor eccentric training on functional tests in healthy subjects. Braz J Phys Ther. 2010;14:276–83. doi: 10.1590/s1413-35552010005000014. [DOI] [PubMed] [Google Scholar]
- 32.Schott J, McCully K, Rutherford OM. The role of metabolites in strength training. Eur J Appl Physiol Occup Physiol. 1995;71:337–41. doi: 10.1007/BF00240414. [DOI] [PubMed] [Google Scholar]
- 33.Shephard RJ. PAR-Q, Canadian home fitness test and exercise screening alternatives. Sports Med. 1988;5:185–95. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 34.Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur J Appl Physiol Occup Physiol. 1998;77:189–91. doi: 10.1007/s004210050319. [DOI] [PubMed] [Google Scholar]
- 35.Silva JCG, Neto GR, Freitas E, Neto E, Batista G, Torres M, Sousa MSC. Chronic effect of strength training with blood flow restriction on muscular strength among women with osteoporosis. J Exerc Physiol Online. 2015;18:33–41. [Google Scholar]
- 36.Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86:308–14. doi: 10.1007/s00421-001-0561-5. [DOI] [PubMed] [Google Scholar]
- 37.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88:2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 38.Wernbom M, Järrebring R, Andreasson MA, Augustsson J. Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J Strength Cond Res. 2009;23:2389–95. doi: 10.1519/JSC.0b013e3181bc1c2a. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda T, Brechue WF, Fujita T, Sato Y, Abe T. Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J Sports Sci Med. 2008;7:467. [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda T, Fukumura K, Fukuda T, Iida H, Imuta H, Sato Y, Yamasoba T, Nakajima T. Effects of low-intensity, elastic band resistance exercise combined with blood flow restriction on muscle activation. Scand J Med Sci Sports. 2014;24:55–61. doi: 10.1111/j.1600-0838.2012.01489.x. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda T, Loenneke JP, Ogasawara R, Abe T. Influence of continuous or intermittent blood flow restriction on muscle activation during low-intensity multiple sets of resistance exercise. Acta Physiol Hung. 2013;100:419–26. doi: 10.1556/APhysiol.100.2013.4.6. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda T, Loenneke JP, Thiebaud RS, Abe T. Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. Plos One. 2012;7:52843. doi: 10.1371/journal.pone.0052843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasuda T, Ogasawara R, Sakamaki M, Ozaki H, Sato Y, Abe T. Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol. 2011;111:2525–33. doi: 10.1007/s00421-011-1873-8. [DOI] [PubMed] [Google Scholar]