Abstract
To the best of our knowledge, the effect of pre-emptively blocking pain transmission on acute postoperative cognitive dysfunction (POCD) has not yet been assessed. Therefore, the present study aimed to investigate the effect of pre-emptive analgesia via a continuous femoral nerve block (CFNB) on postoperative pain and early cognitive function following total knee arthroplasty (TKA) surgery in elderly patients. CFNB was performed prior to TKA surgery in the pre-emptive analgesia group (n=30) and following TKA surgery in the control group (n=30). POCD was defined as a two-point reduction in the postoperative score compared with the preoperative score in the mini-mental state examination. The visual analog scale (VAS) was used to evaluate the intensity of pain at rest and during exercise. The intraoperative dose of remifentanil in the pre-emptive analgesia group was significantly lower than in the control group (P<0.01). In the preemptive analgesia group, VAS scores at three days post-surgery were lower than those in the control group (P<0.01). The incidence of POCD on the third postoperative day was slightly lower in the pre-emptive analgesia group compared with the control group. In conclusion, the results demonstrate that pre-emptive analgesia by CFNB may promote the recovery of early cognitive function following TKA in elderly patients.
Keywords: femoral nerve block, pre-emptive analgesia, total knee arthroplasty, postoperative cognitive dysfunction
Introduction
Postoperative cognitive dysfunction (POCD) describes the memory and intellectual impairment that occurs in certain individuals during the perioperative period (1,2). A major risk factor for POCD is age; therefore, it is particularly prevalent in the elderly (2). Pain is also considered to be a risk factor for POCD as the areas of the brain involved in pain perception and cognitive control overlap (2,3). Total knee arthroplasty (TKA) has been reported to be one of the most painful orthopedic surgeries (4).
Postoperative pain is intensified by sensitization of the pain receptors in the spinal cord and brain (5). This sensitization may be due to a perioperative inflammatory response, in which inflammatory mediators released at the operative site sensitize peripheral pain receptors (6). The ensuing increased nociceptive input to the spinal cord sensitizes the pain receptors there and this sensitization, in turn, sensitizes the pain receptors in the brain.
If pain impulses from the operative site were interrupted throughout as well as following surgery, both intra- and postoperative peripheral pain stimuli could be blocked from transmitting to the central nervous system, thus reducing sensitization of pain receptors in the brain. If the occurrence of POCD is associated with the development of this sensitization, as has been suggested previously (6), initiating a blockade of pain transmission prior to, rather than following, surgery may decrease the incidence of POCD.
Femoral nerve block (FNB) reduces pain transmission from the TKA operative area (7) and when FNB is administered during TKA, following spinal anesthesia postoperative pain is reduced (4). In tendon graft repair, the use of postoperative femoral nerve infusion of ropivacaine reduces early postoperative pain (2 h following surgery) more effectively when administered pre-emptively rather than when administered at the end of surgery (8). It has been demonstrated that femoral nerve infusion of ropivacaine improves pain control for one day after TKA treatment, compared with epidural ropivacaine infusion, which continues for 6 h (9).
Studies of POCD have been conducted at a later time post-surgery; however, to the best of our knowledge, not during the initial days following surgery (6,10–12). Furthermore, to the best of our knowledge, no studies have yet investigated the effect of pre-emptively blocking pain transmission on acute POCD in the first days following surgery. In the present study the effect of a femoral nerve infusion of ropivacaine on TKA initiated prior to surgery and at the end of surgery was compared, with regard to the incidence of POCD and pain in the initial seven days following surgery.
Patients and methods
Patients
The present study is a prospective, randomized, double blind, controlled study. A total of 60 patients (13 males and 47 females) aged >65 years who underwent primary TKA under intravenous anesthesia at the General Hospital of Ningxia Medical University (Ningxia, China) between April and September 2014 were enrolled in the current study. All patients were examined in accordance with the American Society of Anesthesiologists (ASA) physical status classification and belonged to an ASA grade II or I. Exclusion criteria were as follows: A mini mental state examination (MMSE) score lower than the minimum score for each education level (illiterate, ≤17 points; primary school, ≤20 points; secondary school, ≤22 points; college, ≤23 points), nervous system disease, serious visual disorders and inability to communicate normally, a history of mental disease or of having received relevant medication, coagulation disorders, opioid abuse, inability to carry out effective oral communication with doctors, inability to complete the MMSE, inability to undergo surgery due to various reasons, including pulmonary infection, high blood pressure and disturbance of blood coagulation, voluntarily withdrawal from the study and refusal to take the MMSE. The current study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University. Informed consent was obtained from each participant.
Patient grouping
Patients were randomly divided into two groups: A pre-emptive analgesia group and a control group (n=30 in each). Continuous FNB (CFNB) was performed in the pre-emptive and control groups, but initiated prior to surgery in the pre-emptive analgesia group and following surgery in the control group. The same anesthesiologist was responsible for the management of anesthesia for all patients and the same group of orthopedic surgeons performed TKA on all patients. The individuals collecting data and the patients were blinded to the grouping.
Administration of anesthesia
All patients underwent surgery under intravenous anesthesia alone. Prior to anesthetic, 1 mg penehyclidine hydrochloride (Chengdu List Pharmaceutical Co., Ltd., Chengdu, China) was injected intramuscularly. The patients were then taken to surgery where the basilic vein was percutaneously cannulated with 20 gauge intravascular catheters, and non-invasive blood pressure, electrocardiogram, peripheral capillary oxygen saturation, partial pressure of exhaled carbon dioxide (PETCO2) and bispectral index (BIS) were monitored routinely. Midazolam (0.05–0.1 mg/kg, Nhwa Pharmaceutical Co., Ltd., Jiangsu, China), sufentanil (0.3–0.4 µg/kg, Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China), etomidate (0.1–0.2 mg/kg, Nhwa Pharmaceutical Co., Ltd.) and cis-atracurium (0.15–0.2 mg/kg, Shanghai Pharmaceuticals Holding Co., Ltd., Shanghai, China) were injected intravenously for induction of anesthesia. A laryngeal mask airway was applied 3 min later. Oxygen flow was set to 1.5 l/min, tidal volume 6–8 ml/kg, respiratory rate 12–14 times/min and PETCO2 35–45 mmHg. Anesthesia was maintained by continuous intravenous infusion of propofol (4–8 mg/kg/h, Corden Pharma, Caponago, Italy) and remifentanil (0.1–0.2 µg/kg/min, Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China). Muscle relaxation was maintained by intermittent (40–60 min) injection of cis-atracurium (0.05 mg/kg, Shanghai Pharmaceuticals Holding Co., Ltd.). The intraoperative BIS value was maintained at 40–60. A mean arterial pressure 20% lower than the basal value or a systolic blood pressure <90 mmHg (1 mmHg=0.133 kPa) was considered to represent hypotension, and 3–6 mg of ephedrine (Northeast Pharmaceutical Group, Shenyang, China) was injected intravenously. A heart rate of <60 beats/min was considered to indicate bradycardia and 0.25–0.5 mg atropine (Tianjin Pharmaceutical Group Co., Ltd., Tianjin, China) was injected intravenously. No additional cis-atracurium was injected 30 min prior to the end of surgery. Infusion of propofol and remifentanil was stopped at the end of surgery and neostigmine (0.02 mg/kg, Runhong Pharmaceutical Co., Ltd., Xinzheng, China) and atropine (0.01 mg/kg, Tianjin Pharmaceutical Group Co., Ltd.) were injected intravenously to antagonize the residual effect of the muscle relaxants. The laryngeal mask was removed and the patient was transferred to post-anesthesia care unit until the recovery of consciousness, determined by the patient being able to open their eyes upon instruction, tidal volume of unassisted respiration ≥6 ml/kg and a respiratory rate ≥10 times/min. When completely awake, patients returned to the orthopedic ward and were discharged after 7–10 days of recovery.
FNB and perioperative pain control
For the FNB procedure, patients were placed in the supine position with the affected leg slightly abducted. The femoral artery was palpated under the inguinal ligament and the entry point was marked: 1 cm lateral to the femoral artery parallel to the upper part of the pubic symphysis. A Braun nerve stimulator (B. Braun Medical, Ltd., Melsungen, Germany) with a pulse duration of 0.1 msec and a stimulation frequency of 2 Hz was used to assist with positioning of the entry point. The initial current was 1 mA and the threshold current at the time of local anesthetic injection was 0.3 mA. A needle was inserted vertically into the previously marked entry point and if a current <0.3 mA could still induce quadricep contraction and patella fluctuation, 15 ml of a 1:1 mixture of 2% lidocaine (Shiyao Yinhu Pharmaceutical Co., Ltd., Yunchen, China) and 0.75% ropivacaine (AstraZeneca AB, Sodertalje, Sweden) was injected. The needle was inserted slowly, 2 mm into the entry point at a cephalic angle of 30° and the core was withdrawn. A 20G cannula for arterial puncture was placed into the core and 5 ml 1:1 lidocaine/ropivacaine mixture was injected again. A disposable patient controlled analgesia pump was connected (0.75% ropivacaine 20 ml + sufentanil 40 µg) and used for 50 h with self-controlled femoral nerve analgesia following surgery.
Intraoperative multi-point intraarticular injection of ropivacaine (150 mg/100 ml) was also conducted. The injection sites were the posterior and medial sides of the knee joint, medial and lateral sides of the articular capsule, origin and insertion sites of the collateral ligaments, extensor apparatus and infrapatellar fat pad and subcutaneous tissue. Other perioperative pain control managements included cyclooxygenase 2 (COX-2) inhibitors (parecoxib sodium or celecoxib; Pharmacia and Upjohn LLC, Kalamazoo, MI, USA) administered following surgery twice a day for all patients.
Patient clinicopathological characteristics
Age, gender, education level, body mass index (BMI), comorbidities, anesthesia duration, operative time, anesthetic dosage, tourniquet time and perioperative analgesic use were recorded (Table I).
Table I.
Baseline characteristics of study population.
| Parameter | Pre-emptive (n=30) | Control (n=30) |
|---|---|---|
| Age, years | 68.6±5.6 | 67.8±4.3 |
| Male (%) | 7 (23.3) | 6 (20.0) |
| BMI, kg/m2 | 25.3±3.4 | 26.1±3.6 |
| Anesthesia time, min | 128.2±24.4 | 123.2±22.5 |
| Operative time, min | 90.2±17.4 | 92.3±15.6 |
| Tourniquet time, min | 60.9±17.9 | 60.6±12 |
| ASA grade (%) | ||
| I | 5 (16.7) | 6 (20.0) |
| II | 25 (83.3) | 24 (80.0) |
| Education (%) | ||
| Illiteracy | 12 (40) | 10 (33.3) |
| Elementary school | 8 (26.7) | 7 (23.3) |
| High school or above | 10 (33.3) | 13 (43.3) |
| Comorbidity (%) | ||
| Cardiovascular disease | 3 (10.0) | 3 (10.0) |
| Hypertension | 12 (40.0) | 12 (40.0) |
| Diabetes | 4 (13.3) | 4 (13.3) |
Continuous data are expressed as mean ± standard deviation and tested by independent t-test. Categorical variables are presented as number (%) and tested by χ2 test. BMI, body mass index; ASA, American Society of Anesthesiologists.
Pain ratings
The Visual Analog Score (VAS) at rest and during exercise was used to estimate pain strength. In this system, the patient rates pain on a score of 0–10, 0 representing no pain and 10 representing the worst pain imaginable. VAS scores were recorded at rest and during activity for patients in both groups prior to surgery and 1, 3, 5 and 7 days following surgery. For these ratings, pain during rest refers to the severity of pain after 30 min rest following joint movement; pain during exercise refers to pain when the knee joint performs maximum flexion. Functional exercise of the knee joint was started day 1 postoperation. The patients were asked to stand for 3–5 min and the exercise training included quadricep and tibialis anterior muscle isometric contraction. From day 2 postoperation, exercise training included knee extension and flexion exercises to straight leg raising, 4–5 times (at least 15 min per leg raising) a day.
Cognitive function and dysfunction
The MMSE was used to evaluate cognitive function prior to surgery to provide a baseline value and was measured 1, 3, 5 and 7 days following surgery (13). A decrease in the MMSE scale was used to determine the presence of POCD. Previous studies have used a postoperative MMSE score >1 standard deviation below the preoperative score to define POCD (14). In the present study, 1 standard deviation of preoperative MMSE score is 2 points, so a postoperative MMSE score ≥2 points lower than the preoperative score was defined as POCD.
Statistical analysis
Data regarding patient age, BMI, anesthesia time, surgery time and tourniquet-bearing time were presented as mean ± standard deviation and an independent t-test was used for statistical examination. Due to the skewed distribution of the data, data for drug administration are presented as median and interquartile ranges, and the Wilcoxon rank-sum test was used for statistical examinations. Categorical variables were expressed by count (%) and examined statistically using the χ2 test. Group differences for time trends among VAS at rest and during activity, and the MMSE were examined by generalized estimation equation. Interactions within each corresponding time and group were included in the models. If a significant interaction item was revealed followed by a Bonferroni post-hoc test. All statistical assessments were evaluated using SPSS software, version 22 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Case selection
A total of 83 patients were initially enrolled. Patients were excluded due to an inability to communicate normally (one case), MMSE score lower than the threshold value of the individual's education level (seven cases) and the presence of neurological disorder (one case). In addition, nine patients were excluded because they could not cooperate on the follow-up survey and one patient voluntarily withdrew from the study. Finally, four patients were dropped following frequency matching with three comorbidities (cardiovascular disease, hypertension and diabetes). Therefore, a total of 60 patients were included in the final analysis of the results and 30 patients were included in each group (Fig. 1).
Figure 1.
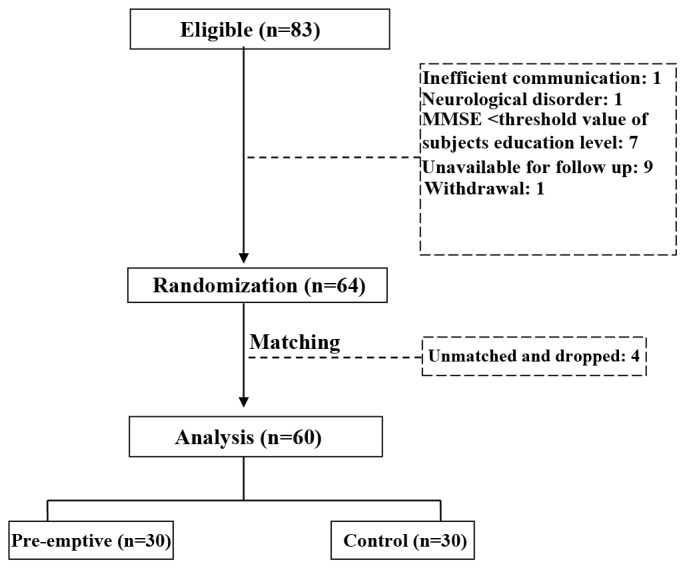
Diagram of patient grouping. MMSE, mini mental state examination.
Patient demographics
Patients' baseline characteristics are summarized in Table I. There were no significant differences between the two groups in any baseline characteristics. Among all patients, 21.7% were males, 81.7% had ASA at II and 61.7% had an education level of elementary school or below. In the study population, the prevalence of cardiovascular disease was 10%, hypertension was 40% and diabetes was 13.3%. The mean age, BMI, anesthesia time, surgery time and tourniquet-bearing time were 68.2±5.0 years, 25.7±3.5 kg/m2, 125.7±23.4, 91.3±16.4 and 60.7±15.1 min, respectively.
Medicine administration between study populations
Midazolam, sufentanil, cis-atracurium, etomidate, propofol, remifentanil, celecoxib and parecoxib sodium were used in the present study (Table II). During the perianesthesia period, the dose of remifentanil used in the pre-emptive analgesia group was significantly lower, compared with the control group (P=0.019). No significant differences between groups were observed in the doses of any other medication.
Table II.
Medication administered to individuals in the present study.
| Parameter | Pre-emptive (n=30) | Control (n=30) | P-value |
|---|---|---|---|
| Midazolam, mg | 4 (3–4) | 4 (3–4) | 0.893 |
| Sufentanil, mg | 25 (20–30) | 25 (20–30) | 0.464 |
| Cisatracurium besylate, mg | 20 (15–20) | 20 (15–20) | 0.804 |
| Etomidate, mg | 18 (10–20) | 20 (10–20) | 0.107 |
| Propofol, mg | 400 (350–500) | 480 (400–500) | 0.124 |
| Celecoxib, g | 6 (4.8–6) | 6 (5.4–6) | 0.499 |
| Parecoxib sodium, mg | 40 (40–40) | 40 (40–80) | 0.299 |
| Remifentanil, mg | 1.8 (1.5–2.0) | 2.0 (1.8–2.0) | 0.019a |
P<0.05, indicating a statistically significant difference between the groups. Data are presented as median (interquartile range) and tested with the Wilcoxon rank-sum test.
Differences in the incidence of POCD
In each group, the incidence of POCD was similar on day 1 (Table III) and then decreased with time. This decrease was faster in the group administered a pre-emptive FNB: By day 3 the incidence in this group was significantly lower than in the control group (6.7 vs. 26.7%, P=0.038; Table III). By day 5, there were no cases of POCD in the pre-emptive analgesia group and no cases of POCD in the control group by day 7.
Table III.
Occurrence of POCD in patients following surgery.
| P-value | Time and group | Pre-emptive (n=30) | Control (n=30) |
|---|---|---|---|
| Day 1 | 0.999 | ||
| No POCD | 19 (63.3) | 19 (63.3) | |
| POCD | 11 (36.7) | 11 (36.7) | |
| Day 3 | 0.038a | ||
| No POCD | 28 (93.3) | 22 (73.3) | |
| POCD | 2 (6.7) | 8 (26.7) | |
| Day 5 | 0.313 | ||
| No POCD | 30 (100.0) | 29 (96.7) | |
| POCD | 0 (0.0) | 1 (3.3) | |
| Day 7 | NA | ||
| No POCD | 30 (100.0) | 30 (100.0) | |
| POCD | 0 (0.0) | 0 (0.0) |
P<0.05, indicating a statistically significant difference between the groups. All data are presented as count (%) and tested using the χ2 test. POCD, postoperative cognitive dysfunction; NA, not available due to no identified cases of POCD in control group.
Differences in the VAS score
Pain (based on the VAS score) at rest and during exercise for the pre-emptive and control groups is presented in Fig. 2A and B. Pain at rest (standstill) increased significantly following surgery in both groups (P<0.05) and remained at stable levels in subsequent days (Fig. 2A). However, differences in pain intensity between the groups at each time point were not significant (Fig. 2A). Average pain intensity during days 1–7 in the two groups was scored as ~3, a level considered moderate. By contrast, pain during activity decreased from a relatively high preoperative level (6–7) to a level near 4 on day 1, then increased significantly in the control group from day 3 onward, but did not exhibit a significant increase in the pre-emptive FNB group until day 5 (Fig. 2B).
Figure 2.
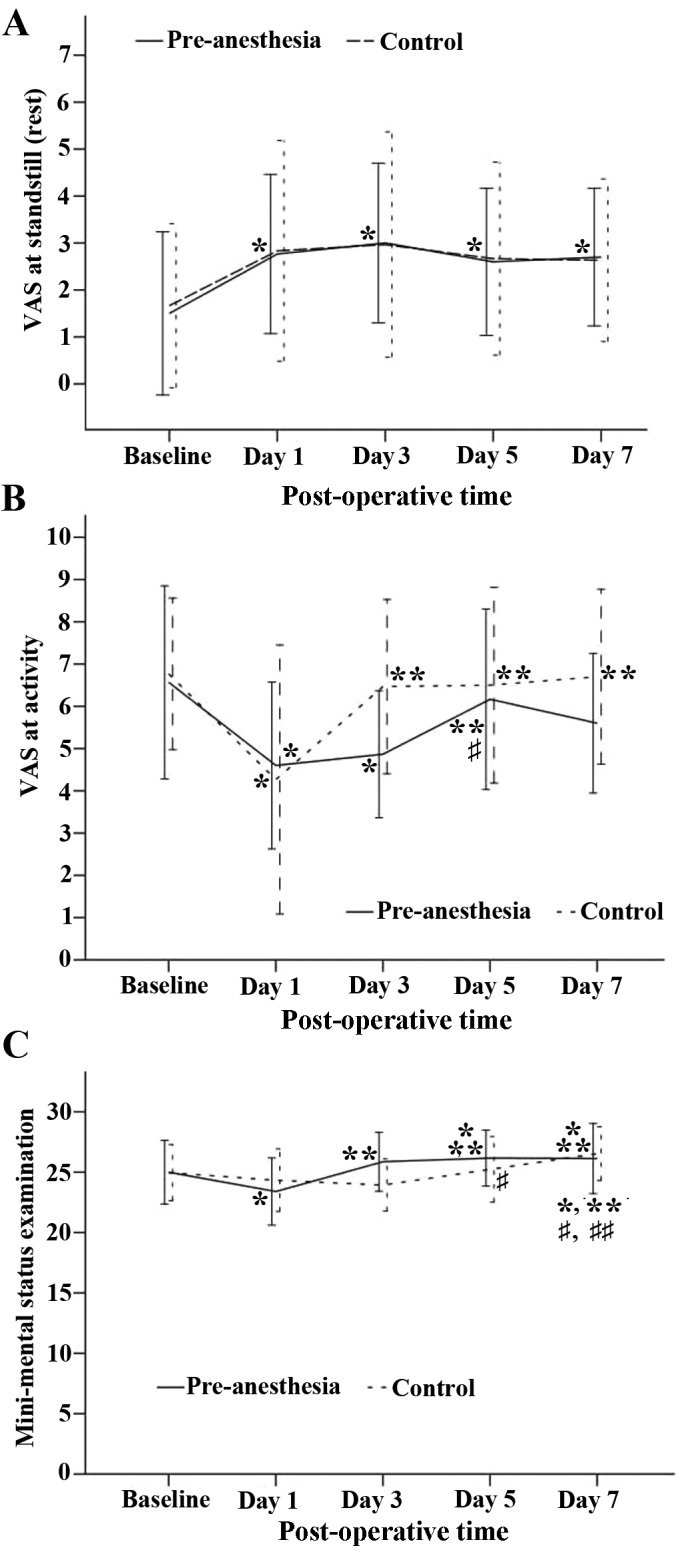
Changes in VAS score between days 1–5. (A) Changes in VAS at rest and (B) during activity. (C) The mini mental state examination of all patients over time. Data are expressed as mean ± standard deviation and tested by generalized estimation equation. The post hoc test of time effect was examined using the Bonferoni correction. The data from the pre-anesthesia group are presented as solid lines and the results from the control group are presented as dotted lines. P<0.05 vs. *baseline, **day 1, #day 3 and ##day 5. VAS, visual analog scale.
Differences in MMSE
Changes in the MMSE over time for the two groups are presented in Fig. 2C. The time trend for the two groups was similar (P=0.568), but the MMSE scores differed significantly by group (P<0.05). Both groups demonstrated a decrease from baseline on day 1, but the pre-emptive FNB group exhibited recovery from this decrease by day 3, while it took the control group until day 7 to achieve recovery from the day 1 value (Fig. 2C).
Discussion
The present study demonstrated that in TKA, the pre-emptive infusion of remifentanil to the femoral nerve decreased VAS scores during activity more than femoral nerve infusion initiated at the end of surgery. These results suggest that a pre-emptive femoral blockade of pain impulse transmission during surgery prevented CNS hypersensitization.
Throughout the present study, patients in the pre-emptive analgesia group had a lower VAS score (postoperative pain sensitivity) during activity than patients in the control group with the exception of day 1. The incidence of POCD at day 1 following TKA surgery was similar in both groups. However, at day 3 following surgery, the incidence of POCD was decreased in the pre-emptive analgesia group compared with the post-surgery analgesia group. This difference was not significant; however, it suggests that the pain control of pre-emptive analgesia may promote an early recovery of postoperative cognitive function. This mild improvement in early postoperative cognitive function recovery may therefore reduce duration of hospitalization and total medical expenses of the individual. The present study used a short time period following surgery to measure POCD; this allowed the observation of a short-term phenomenon that studies at a later time period may not detect. An advantage of using this early time period is that there is a low rate of patients lost to follow-up (15).
In the present study, other factors that may affect POCD were controlled; these include demographic risk factors including age, hypertension, diabetes and coronary heart disease (16). The same anesthesiologist managed the anesthesia for all patients in the present study and the same group of orthopedic surgeons performed TKA on all patients. The depth of anesthesia, doses of preanesthetic anesthetics (with the exception of remifentanil), development of hypotension and bradycardia (changes in the circulatory function) were similar in the two groups. Anesthesia time, operative time and tourniquet time were also similar. Thus, the severity of intraoperative tissue injury was generally consistent, avoiding any differences in the degree of inflammation caused by tissue injury between the two groups. The current study assessed potentially confounding factors, suggesting that the decrease in VAS and POCD incidence observed in the pre-emptive analgesia group may be solely due to the pre-emptive timing of the analgesia.
However, the dose of remifentanil used in the pre-emptive analgesia group was significantly lower (P<0.05) than the dose used in the control group. It has previously been demonstrated that remifentanil may induce hyperalgesia in a dose-dependent manner (17). Due to the dose of intraoperative remifentanil in the pre-emptive group being lower compared with the control group, the incidence and severity of remifentanil-induced hyperalgesia may have been lower. This may be an additional reason, along with the timing of analgesia, for the relatively low VAS score during exercise in the pre-emptive group.
The incidence of early POCD in the pre-emptive analgesia group was lower than previously reported by Rodriguez et al (10), but the incidence of early POCD in the control group was higher in the present study. This comparison indirectly confirms that pre-emptive analgesia by CFNB may reduce the incidence of postoperative early POCD following TKA.
All patients in the present study underwent multimodal analgesia including preoperative and postoperative regular application of COX-2 inhibitors, intraoperative multipoint local injection of 0.15% ropivacaine around the knee joint and the application of 0.15% ropivacaine and low-dose opioids for patient-controlled nerve analgesia (PCNA). Therefore, there was no significant difference in the VAS score at rest between the two groups, which indicates that multimodal analgesia achieved satisfactory postoperative analgesia for patients in the two groups. The VAS score during exercise was relatively low in the pre-emptive analgesia group, suggesting that pre-emptive analgesia may reduce pain during exercise. This may be one reason for the slightly lower incidence of POCD in the pre-emptive analgesia group compared with the control group. Restlessness following general anesthesia is an independent risk factor in the development of POCD (18) and pre-emptive analgesia may prevent the occurrence of agitation during the period of recovery (19). This further indicates that pre-emptive analgesia may promote an early recovery of postoperative cognitive function.
One limitation of the present study is that patients had to be tested a number of times within a short period and individuals became familiar with the content of the cognitive test and thus may have learned the appropriate responses. However, this type of learning cannot be avoided in studies associated with POCD. The results collected suggest that the incidence of POCD at day 3 after TKA is a temporary condition in the acute postoperative period, regardless of whether analgesia was administered pre- or postoperation. Furthermore, when patients were at standstill, pain was not a substantial risk factor of POCD. As the incidence of POCD did not steadily increase by day 7 after TKA in the post-analgesia group, the doctor was unable to predict long-term cognitive dysfunction using the results of the current study. It has been suggested that the early cognitive function may predict long-term cognitive dysfunction (20). Therefore, the impact of relevant interventions on long-term POCD should be further studied.
In conclusion, pre-emptive analgesia by CFNB may promote recovery from early POCD in elderly patients who have undergone TKA. This effect may be associated with reduced pain experienced during postoperative exercise by individuals pre-emptively treated with analgesia.
References
- 1.Dodds C, Allison J. Postoperative cognitive deficit in the elderly surgical patient. Br J Anaesth. 1998;81:449–462. doi: 10.1093/bja/81.3.449. [DOI] [PubMed] [Google Scholar]
- 2.Cohendy R, Brougere A, Cuvillon P. Anesthesia in the older patient. Curr Opin Clin Nutr Metab Care. 2005;8:17–21. doi: 10.1097/00075197-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative effect, pain and cognitive control in the cingulated cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen HW, Liu SS, Ware PD, Nairn CS, Owens BD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesth Analg. 1998;87:93–97. doi: 10.1213/00000539-199807000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Meftah M, Wong AC, Nawabi DH, Yun RJ, Ranawat AS, Ranawat CS. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics. 2012;35:e660–e664. doi: 10.3928/01477447-20120426-19. [DOI] [PubMed] [Google Scholar]
- 6.Xie G, Zhang W, Chang Y, Chu Q. Relationship between perioperative inflammatory response and postoperative cognitive dysfunction in the elderly. Med Hypotheses. 2009;73:402–403. doi: 10.1016/j.mehy.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 7.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, Murthy Y. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: A meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–1162. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 8.Roseag OP, Krepski B, Cicutti N, Dennehy KC, Liu AC, Johnson DH. Effect of preemptive multimodal analgesia for arthroscopic knee repair. Reg Anesth Pain Med. 2001;26:125–130. doi: 10.1097/00115550-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Qian X, Qu YX, Jiang T, Xu JD, Zheng C. Patient-controlled femoral nerve analgesia and epidural analgesia for postoperative pain after total knee arthroplasty. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15:3053–3056. (In Chinese) [Google Scholar]
- 10.Rodriguez RA, Tellier A, Grabowski J, Fazekas A, Turek M, Miller D, Wherrett C, Villeneuve PJ, Glachino A. Cognitive dysfunction after total knee arthroplasty: Effects of intraoperative cerebral embolization and postoperative complications. J Arthroplasty. 2005;20:763–771. doi: 10.1016/j.arth.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Shoair OA, Ii MP Grasso, Lahaye LA, Daniel R, Biddle CJ, Slattum PW. Incidence and risk fastors for postoperative cognitive dysfuntion in older adults undergoing major noncardiac surgery: A prospective study. J Anaesthesiol Clin Pharmacol. 2015;31:30–36. doi: 10.4103/0970-9185.150530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai TL, Sands LP, Leung JM. An update on postoperative cognitive dysfunction. Adv Anesth. 2010;28:269–284. doi: 10.1016/j.aan.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, Vellas B, Touchon J. MCI Working Group of the European Consortium on Alzheimer's Disease (EADC): Mild cognitive impairment (MCI) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw PJ, Bates D, Cartlidge NE, French JM, Heaviside D, Julian DG, Shaw DA. Neurologic and neuropsychological morbidity following major surgery: Comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700–707. doi: 10.1161/01.STR.18.4.700. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Ding S, Zhang L. Risk factors for developing postoperative cognitive dysfunction in elderly patients after general anesthesia. Zhonghua Mazuixue Zazhi. 2013;33:31–33. (In Chinese) [Google Scholar]
- 16.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Cabañero D, Campillo A, Célérier E, Romero A, Puig MM. Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: Effect of a second surgery. Anesthesiology. 2009;111:1334–1345. doi: 10.1097/ALN.0b013e3181bfab61. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Wei CW, Yu YG, Ni XL, Xiong LZ. Correlation between the agitation during the anesthesia recovery period and the postoperative cognitive dysfunction after general anesthesia. Zhonghua Mazuixue Zazhi. 2013;33:34–36. (In Chinese) [Google Scholar]
- 19.Wang Q, Yu YW, Yao SL. Preemptive analgesia effects of flurbiprofen axetil or tramadol in patients undergoing sevoflurane general anesthesia. Guoji Mazuixue Yu Fusu Zazhi. 2013;34:1079–1082. (In Chinese) [Google Scholar]
- 20.Van Dijk D, Moons KG, Keizer AM, Jansen EW, Hijman R, Diephius JC, Borst C, de Jaegere PP, Grobbee DE, Kalkman CJ. Octopus Study Group: Association between early and three month cognitive outcome after off-pump and on-pump coronary bypass surgery. Heart. 2004;90:431–434. doi: 10.1136/hrt.2003.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]


