Abstract
Pythium insidiosum immunomodulatory vaccine (PiV) has been tested in clinical and experimental pythiosis. Previous data showed that P. insidiosum immunogens have the ability to switch the Th2 immune response, normally in place during pythiosis, to a curative Th1 response. Pythiosis cannot be reproduced in experimental rodents with the exception of rabbits, and thus thorough evaluation of PiV´s immunomodulatory properties has been limited by the lack of a compatible inbred mouse model. In this study, we took advantage of the murine BALB/c Leishmania infection model, where infected mice produce a Th2 response, to evaluate the PiV Th2 to Th1 immunomodulatory potential. Twenty-one days following challenge with L. major, large cutaneous granulomas developed in control mice, consistent with the expected Th2 response. In contrast, Leishmania-induced cutaneous lesions in PiV-immunized mice were minimal or absent. Flow cytometry analysis of spleen cells from mice immunized with PiV and subsequently challenged with L. major displayed more CD4+ and CD8+ cells than the control group. Moreover, spleen cells from mice that were immunized with PiV then challenged with L. major secreted high levels of IFN-γ, with a moderate IL-2, IL-4, and IL-10 mixed cytokine profile upon in vitro re-stimulation with PiV. Anti-P. insidiosum IgG1 in immunized animals was present at low titers suggesting a minor immunological role for this Ig isotype in this model. Our preliminary data showed that BALB/c mice challenged with L. major represent an attractive model in which to study PiV´s immunomodulatory properties.
Keywords: Immunology, Physiology, Infectious diseases
1. Introduction
Pythium insidiosum, the etiologic agent of pythiosis, is a fungal-like organism in the kingdom Straminipila developing hypha-like elements in the infected host, with a strong tropism for arteries in otherwise healthy humans and lower animals [1]. In infected tissue, P. insidiosum triggers a typical Th2 inflammatory response with numerous eosinophils, mast cells, basophils, and giant cells, and induces the up-regulation of key Th2 cytokines such as IL-4, IL-5, and IL-10, and high levels of IgE [2]. Straminipila microbes lack ergosterol in their outer membranes (the target of most antifungal drugs), thus complicating the management of pythiosis in infected individuals. First Miller [3] and then Mendoza and Alfaro [4] independently reported that the mixture of proteins extracted from cultures of P. insidiosum (P. insidiosum-immunomodulatory vaccine; PiV) injected in horses with proven cutaneous pythiosis cured 55% of the immunized horses. These authors linked the curative properties of P. insidiosum’s extracts to a change from an eosinophilic granuloma to a lymphocytic mononuclear cell response, which they proposed was key in the killing of P. insidiosum’s hyphae in infected tissues [3, 4]. Since then, PiV immunogens have been successful in humans [5, 6, 7, 8], cattle [2], dogs [9, 10, 11] and horses [12, 13, 14] with pythiosis. It was later found in successfully treated mammals that in addition to the switching from an eosinophilic to a mononuclear cell response, a shift from a Th2 to a Th1 cytokine profile occurred [2]. Moreover, when this hypothesis was tested in the experimental rabbit model, a similar profile was found with the expression of lymphocytic NTPDase [15] and ecto-adenosine deaminase [16], also pointing to a Th2 down-regulation.
PiV immunomodulatory properties in humans and animals with pythiosis have been known for years [1, 2, 10, 12]. Nonetheless, its specificity recently came into question when vaccinated hosts displaying Th2 subsets in processes other than those triggered by P. insidiosum, also produced Th1 responses after challenges with multiple PiV injections (Richard Hansen, Bob Glass personal communication). This unpublished data suggested that cases of equine atopic dermatitis, canine allergic otitis, some cases of equine sarcoid, a case of a dog with mast cell tumors and a dairy cow with Aspergillus fumigatus mastitis, all originally displaying a strong Th2 response, converted to a Th1 response after several PiV injections, resulting in the resolution of their clinical symptoms. The results suggest that PiV proteins have the ability to modulate a Th2 to a Th1 response, in a manner that can affect immune responses against antigens from other infections unrelated to P. insidiosum.
The main challenge evaluating PiV immunotherapeutic properties has been the lack of an appropriate inbred mouse model in which to study PiV’s immunomodulatory features [2, 13]. To test PiV´s Th2 to Th1 immunomodulatory capabilities, a Leishmania murine model [17] displaying a typical Th2 response in infected tissues was investigated. Our study showed that the Th2 response produced by BALB/c mice during L. major infection was replaced by a Th1 response in mice previously immunized with PiV. This Th2 to Th1 switch resulted in the reduction of cutaneous granulomas, and up-regulation of IFN-γ, a key Th1 cytokine. Data shows that Leishmania infected BALB/c mice are an attractive model for studying the immunomodulatory mechanisms triggered by PiV proteins.
2. Materials and methods
2.1. Leishmania major and murine strains
Adult female BALB/c mice (4–6 weeks) were purchased from Centro de Bioterismo of “Instituto de Ciências Biológicas − Federal University of Minas Gerais (UFMG-Belo Horizonte, MG, Brazil)”, and maintained following standard laboratory animal regulations. The strain of Leishmania major (WHO MHOM/IL/80/Friedlin) was provided by Dr. Leda Quércia Vieira at the Biochemistry and Immunology Department (ICB/UFMG, Belo Horizonte, Brazil). Promastigotes of L. major were cultivated in Grace’s Insect Medium (Gibco®, Grand Island, NY, USA) supplemented with 20% heat-inactivated fetal bovine serum (Cultilab, Campinas, SP, Brazil). The promastigotes were harvested from culture medium after 6 days of incubation at 23 °C and then rinsed twice with PBS. The infective dose was standardized to 1 × 107 promastigotes/100 μL/mouse, and each mouse was infected following the protocol published by Mayrink et al. [18].
2.2. Pythium insidiosum immunomodulatory vaccine (PiV) preparation
PiV was prepared as per Mendoza et al., 1992. P. insidiosum (ATCC 58643) was cultured at 37 °C in 2% Sabouraud dextrose broth for 5 days under shaking conditions (150 rpm). The cell mass was separated by filtration and then ground in the presence of liquid nitrogen. The resulting powder was mixed with the above broth medium after the broth had been filtered. A total protein mix was precipitated with 2.0 L of acetone and the precipitated proteins were centrifuged at 5,000 x g for 15 min. The pellet containing proteins was re-suspended in 100 ml of a sterile 154 mM NaCl solution and centrifuged at 5,000 x g for 15 min to remove remaining insoluble proteins from the liquid phase. The supernatant containing soluble P. insidiosum proteins, was then dialyzed (Cut-off 10,000 MW; Spectrum, Rancho Dominguez, CA. USA) against sterile phosphate buffered saline (PBS). PiV total protein quantity was determined by using the 2D Quanti Kit® (GE-Healthcare, USA), following the manufacturer’s instructions. Aliquots of the soluble proteins were sterilized by Gamma radiation (5000 Gray for 2 h) and stored at −80 °C.
2.3. Polyacrylamyde Gel Electrophoresis (SDS-PAGE)
To verify the presence of soluble P. insidiosum proteins (PiV) previously described by Mendoza et al., [10, 14], fifty micrograms of the above extracted proteins were run in 10% polyacrylamide gel electrophoresis under reducing conditions, according to Laemmli [19] and then stained using silver nitrate following methods published by Wray et al. [20].
2.4. Leishmania infection protocol, mouse immunization with PiV and control groups
BALB/c female mice (n = 10) were subcutaneously immunized using 100 μl of the PiV (20 mg/ml) each week (7-day intervals) for three weeks. On day 28, each mouse was injected in the right foot pad with 1 × 107 L. major promastigotes. Challenged animals were evaluated weekly for the following 12 weeks. Controls included L. major challenged (1 × 107/mouse promastigotes) (n = 10) and non-immunized, non-challenged mice (n = 10). Data was collected using a calibrate ruler (Digimess- Brazil) to measure the development of edema, indurations, and site thickness of the infected paws. The size of the granulomas was calculated using the difference in millimeters between the infected and the uninfected paws.
2.5. CD3, CD4 and CD8 Flow Cytometry analysis and cytokine expression in spleen cell cultures
Intracellular cytokine detection was carried out according to Oliveira et al. [21]. Briefly, after 6 h of culture, Brefeldin A (1 mg/mL) was added, after which the cells were harvested and rinsed with PBS. Cells were stained with anti-CD3-Alexa Fluor 488A and PerCP-Cy5-5A labeled anti-CD4 or APC-A labeled anti-CD8 monoclonal antibodies [22] (Becton-Dickson, USA). Cells were then fixed with 2% paraformaldehyde and washed again. Aliquots of 1 × 106 permeabilized cells (50 μL) were incubated with either PeCy7-labeled anti-mouse interferon-γ (IFN-γ) or phycoerythrin-labeled anti-IL-10 monoclonal antibodies (Becton-Dickinson, San Jose, CA, USA). Finally, the cells were rinsed and immediately analyzed by flow cytometry (LSR Fortessa™, Becton-Dickinson Biosciences, USA). Cell phenotype analysis was performed using Flow-Jo software, version 9.5.1 Tree Star.
Seven days after completion of the immunization schedule, spleens of immunized and challenged mice as well as those from the control animals were collected for an in vitro cytokine assay as per Oliveira et al., 2000 [21]. Briefly, cell suspension from a pool of 10 spleens per group from BALB/C mice were obtained and cultured at 5 × 106/mL in the presence of PiV (30 μg/well) or without PiV (control). The non-specific stimulation effect of Concanavalin A (ConA) (10 μg/well) for 72 h was used as a positive control. Supernatant was collected and evaluated for cytokine expression. In vitro expression of the cytokines was assayed by ELISA according to the manufacturer’s instructions (R& D Systems, MN, USA).
2.6. Anti-P. insidiosum IgG ELISA
Blood samples for the detection of anti-P. insidiosum IgG were also collected from the orbital venous plexus with a Pasteur pipette in the immunized (n = 10) and the control (n = 10) groups. Serum was separated and stored at −20 °C. Presence of anti-P. insidiosum IgG1 was determined in serum samples collected seven days after completion of the three PiV immunizations using an “in house” standardized ELISA protocol. Briefly, microtiter plates (Corning − NY − USA) were coated with 2 μg/well of PiV followed by overnight incubation in 0.05 M carbonate buffer (pH9.6) at 4 °C, and plates were rinsed. Sera from mice in each group was pooled, and then serial dilutions were prepared (from 1:2 to 1:640) in PBS/0.05% Tween 20/0.1% bovine serum albumin (BSA). 100 μl of diluted serum was added to individual wells, incubated for 1 h at room temperature, and then washed. One hundred microliters of peroxidase-conjugated goat anti-mouse IgG1 (1:5,000) (Sigma Aldrich, USA) was added for 1 h at room temperature and then washed. Peroxidase activity was assayed by adding 150 μL of o-phenylenediamine dihydrochloride (OPD) solution (34 mg OPD and 20 μL of hydrogen peroxidase to 100 mL citrate/phosphate buffer pH 5.0). Color development was stopped by the addition 50 μL of 2 N H2SO4. Optical density was measured at 492 nm with an ELISA reader (Spectramax-Plus 384, Molecular Devices, USA).
2.7. Statistical analysis
Student’s t-Test and a multiple-comparisons Student-Newman-Keuls (SNK) test were used to analyze data from the measurements of foot-pad granulomas, using STATA software. For cytokine evaluation in cell culture supernatant, One Way ANOVA was used followed by Student’s t-Test, using Prism software. “p” values lower than 0.05 were considered significant.
2.8. Ethics
The protocols involving animals, including euthanasia, were approved by the Ethics Committee on Animal Experimentation at the Federal University of Minas Gerais (CETEA/UFMG) under Protocol Number 313/2014.
3. Results
3.1. Polyacrylamide gel electrophoresis (SDS-PAGE)
To visualize the presence of PiV soluble proteins, SDS-PAGE analysis and silver staining was performed. Fig. 1 shows that the soluble protein extract contains a mixture of high, medium and low molecular weight proteins, each displaying qualitative and quantitative differences, with a greater number of proteins in the range of 45 to 97 kDa.
Fig. 1.

SDS-PAGE electrophoresis of the PIV soluble proteins. The PiV soluble proteins are shown on 10% SDS-PAGE followed by silver nitrate staining. The numbers indicate the molecular weight in kilo-Daltons (kDa).
3.2. Leishmania major infection
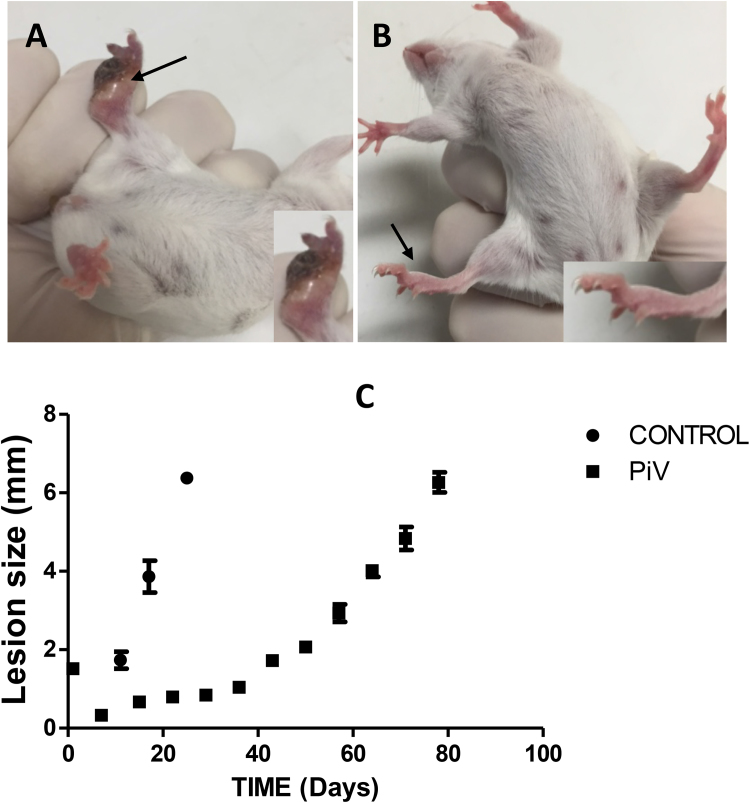
BALB/c control mice challenged with 1 × 107 infective L. major promastigotes showed the development of large granulomas consisting of necrotic tissue (dark spots) (Fig. 2 A). Numerous L. major promastigotes were present in the affected tissue. These control animals were sacrificed on Day 20 due to their granuloma sizes. Granulomas were eight-fold smaller or absent (Fig. 2 B) in PiV immunized mice. The chart in Fig. 2 panel C shows the difference in granuloma size in both PiV and control-challenged mice at different times. Interestingly, a dramatic decrease in granuloma size was observed in mice previously immunized with PiV, challenged with L. major and then re-immunized at least three times after Day 20 (data not shown).
Fig. 2.
Mice in the control group challenged with L. major and/or PiV immunogens. Panel A depicts a mouse from the control group inoculated with L. major displaying granulomatous lesions (arrows) 20 days after challenge. Panel B shows PiV immunized mice previously challenged with L. major. Note the presence of necrotic tissue (dark areas) and the edema of the infected pads (Panel A) whereas panel B shows the absence of necrotic tissue and mild edema in PiV-immunized mice. The figures in the lower section of Panels A and B show a close-up of the infected areas. Granuloma size measurements of BALB/c mice in the control group (● = same as Fig. 1, panel A) and PiV-immunized mice and then challenged with L. major promastigotes (■ = same as Fig. 1 panel B) is shown in Panel C. Note the difference in granuloma size between the control group and PiV-immunized mice and then challenged with L. major on days 20 and 30. After day 30 PiV immunized mice slowly increased the size of their granulomas.
3.3. Flow cytometry and cytokine analysis from spleen cell culture
Table 1 displays the percentage of CD4+ and CD8+ cells expressing IL-10 or IFN-γ. Flow cytometry analysis shows that both helper T cells and cytotoxic lymphocytes were activated in response to PiV (Table 1). IL-10 was expressed by T helper lymphocytes (Th) but the majority of IFN-γ was expressed in CD8+ cytotoxic lymphocytes (Figs. 3, 4).
Table 1.
Flow Cytometer analysis showing the percentage of mice splenic cells CD4+, CD8+ expressing IFN-γ or IL-10 in control group (non-immunized, non-infected) and immunized (PiV only).
| Mice group |
CDC4+ Phenotype |
CDC8+ Phenotype |
||
|---|---|---|---|---|
| IFN-γ | IL-10 | IFN-γ | IL-10 | |
| Control | 0.05 | 0.2 | 0.046 | 0.0 |
| PiV | 1.44 | 0.65 | 6.67 | 0.0 |
Fig. 3.
Flow Cytometry analysis of data from PIV-immunized mice previously challenged with Leishmania major. The figure shows data analyzed by FlowJo software from the splenocyte pool of 10 previously PiV-immunized mice challenged with L. major. Panel A shows the lymphocyte population depicting the lymphocytic region of the mouse spleen cells (square window). Panel B depicts the acquisition window in the region of CD3+ cells. The distribution of two acquisition windows characterizing the CD4+ and CD8+ lymphocytic populations is shown in Panel C. Panel D shows the acquisition window for CD8+ cells expressing intracellular IFN-γ (Panel D).
Fig. 4.
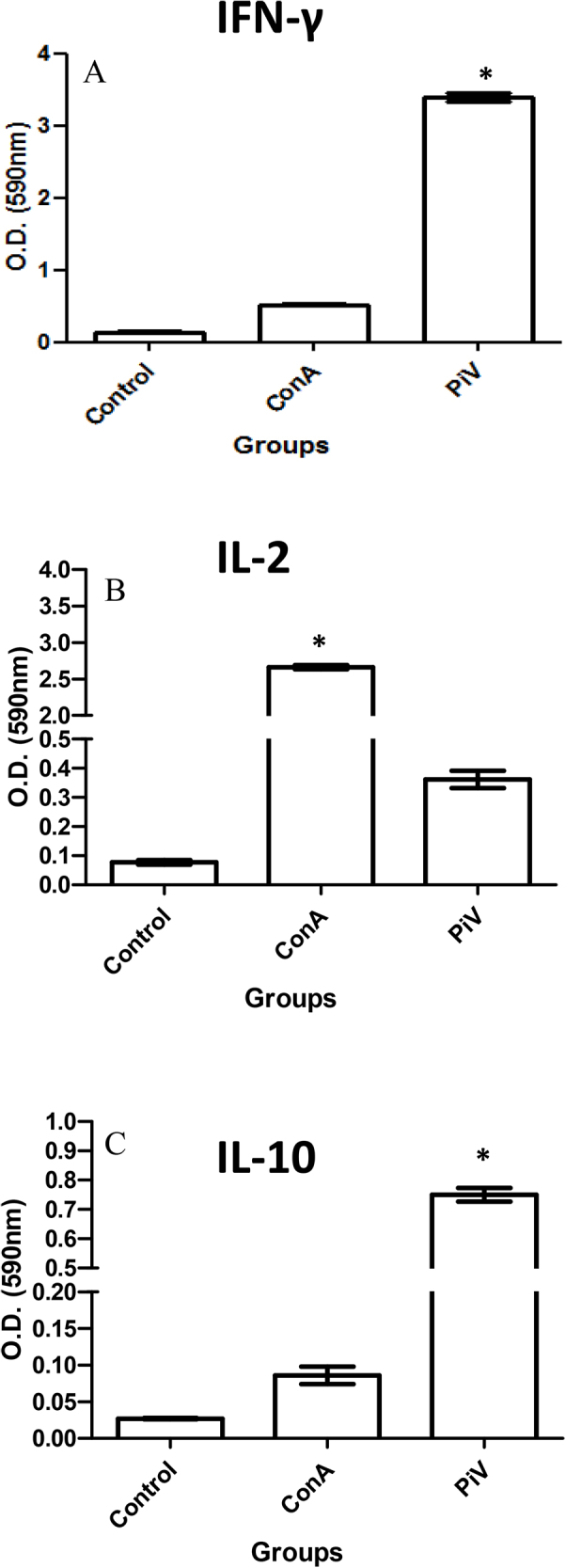
ELISA analysis of cytokines from the control group and the PiV- and Leishmania major-challenged mice. This figure shows that the control group, challenged only with L. major, exhibited significant differences in cytokine production compared with mice challenged with both PiV and L. major. The expression of IFN-γ (ELISA) in the supernatant of mouse splenic cells after 72 h of stimulation with PiV (OD 3.3 = 660 pg/ml) or Concanavalin A (ConA) (OD 0.4 = 111 pg/ml) is shown in Panel A. The graph in panel A displays contrasting differences with the control group challenged with L. major only (undetectable levels, below sensitivity of the test). Panel B depicts expression of IL-2 (ELISA) in the supernatant of mouse splenic cells stimulated with PiV (OD 0.39 = 150 pg/ml) or with ConA (OD 2.7 = 1000 pg/ml) after 72 h of stimulation. This panel also shows significant differences with the control group challenged only with L. major (OD 0.09 = 15 pg/ml). Panel C shows the expression of IL-10 in ELISA of splenocytes in PiV-immunized, L. major challenged BALB/c mice after 72 h of stimulation with PiV (OD 0.79 = 450 pg/ml) or ConA (OD 0.10 = 50 pg/ml). IL-10 detection in non-immunized mice was low (OD 0.03 = 15 pg/ml).
Measured by ELISA, there was a strong expression of IFN-γ by splenocytes from mice immunized with PiV compared to that seen in splenocytes from control mice (challenged with L. major only) treated with the non-specific mitogen ConA (p <0.0001) (Fig. 4A). The mean concentration of IFN-γ in PiV stimulated cells (from PiV immunized mice) was 660 pg/ml (OD = 3.3), whereas the mean concentration of cytokine produced by control cells (from unimmunized mice) stimulated with ConA was 111 pg/ml (OD = 0.4). The concentration of IFN-γ in supernatants of cells from non-immunized control mice (challenged with L. major only) was below the sensitivity threshold of the R & D Systems kit (San Jose, CA, USA) used in this study. IL-2 (Fig. 4B) was expressed at significant levels by the splenic cells from immunized mice after in vitro stimulation with PiV (p < 0.005) (150 pg/ml; OD = 0.39) (Fig. 4B). In contrast, ConA triggered higher levels of IL-2 (1000 pg/ml; OD = 2.7), but these mouse spleen cells did not trigger significant levels of IFN-γ as per Fig. 4A. The average concentration of IL-2 expressed by cell supernatant in non-immunized animals (controls challenged with L. major only) was 15 pg/ml (OD = 0.09) (Fig. 4B). The expression of IL-10 in spleen cells from PiV mice immunized and then challenged with L. major is shown in Fig. 4C. IL-10 levels were significantly higher in PiV immunized mice (450 pg/ml; OD = 0.79) than non-immunized mice (15 pg/ml; OD = 0.03) (p < 0.005). The average concentration of IL-10 expressed by ConA nonspecific stimulation was 50 pg/ml (OD = 0.10).
3.4. Anti-P. insidiosum IgG ELISA
High optical density values were recorded at 1:10 to 1:80 dilutions in the pooled sera of immunized mice tested several times. The anti-P. insidiosum IgG1 levels in the sera of immunized and non-immunized mice are shown in Fig. 5. A contrasting difference was found between the levels of anti-P. insidiosum IgG1 in mice immunized with PiV and then challenged with L. major than those in non-immunized control group (p < 0.05).
Fig. 5.
ELISA detection of anti-P. insidiosum IgG1 in challenged mice. The figure depicts the anti-P. insidiosum IgG1 levels in sera collected seven days after the end of the immunization protocol in mice PiV immunized and then challenged with the L. major (●) and mice in the control group challenged only with L. major (■). As expected, anti-P. insidiosum IgG1 was found only in PiV immunized mice.
4. Discussion
Previous data support claims that PiV proteins have the ability to modulate the Th2 response in cases of pythiosis to a Th1 response after successful immunotherapy [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 23]. Using the mouse Leishmania model, we demonstrated that the Th2 response in place during experimental L. major mice experimental infection of BALB/c mice, switched to a polarized Th1 response. The ex vivo detection of CD4+ and CD8+ splenic cells expressing IL2 and IFN-γ from immunized mice strongly supports this hypothesis. Of interest was the flow cytometry finding that PiV immunized mice activated both T helper cells and cytotoxic lymphocytes in response to in vitro stimulation with PiV antigens. Furthermore, the majority of the labeled IFN-γ was expressed by CD8+ cytotoxic lymphocytes, but not by the CD4+ Th lymphocytic subset as shown in Fig. 3D. This new finding suggests that in mammalian hosts treated by PiV immunotherapy, cytotoxic lymphocytes may play an important role in the downregulation of the Th2 response and the killing of P. insidiosum hyphae. IL-2 was another cytokine expressed in vitro in the supernatant of splenic cell cultures from mice immunized with PiV (p < 0.005) (Fig. 4B). Although ConA triggered higher levels of IL-2 (1000 pg/ml) than those induced by PiV antigens (150 pg/ml), ConA did not trigger significant levels of IFN-γ as shown in Fig. 4A, indicating that the PiV immunogens indeed play a key role in stimulating the expression of IFN-γ, which facilitates Th1 polarization. High levels of IFN-γ, low levels of IL-2 and the expression of IL-10 during in vitro stimulation of splenic cells in PiV-challenged mice suggests a switch from Th2 (BALB/c response to Leishmania) to Th1 (PiV-induced response to Leishmania).
Mendoza et al., 1992 [14] found that the inclusion of several immunodominant proteins of P. insidiosum, including the 28, 30/32 kDa molecular weight, increased the efficacy of management of acute and chronic cases of horse pythiosis. The PiV protein profile in SDS-PAGE used in this study included these antigenic proteins. Our data also showed that the mixture of these P. insidiosum proteins seems to trigger a Th1 cytokine profile in successfully PiV-immunized mice challenged with L. major. The Leishmania mouse model confirmed previous reports [2, 4, 8, 9, 10, 11, 12, 14, 15, 16, 23, 24, 25] indicating that the mixture of P. insidiosum proteins was sufficient to stimulate an immunomodulatory response in the immunized mice challenged with L. major.
Although there are no previous data on the humoral response in murine models immunized with the PiV, it is well known that humans and animals with pythiosis develop high titers of anti-P. insidosum IgG [1, 2, 4, 25]. Snapper et al., 1988 [26] reported that the detection of high IgG1 titers correlated to the presence of a solid Th2 response. Interestingly, in our study the presence of low Th2 profile consistently correlated with low IgG1 titers in PiV-immunized Leishmania mice (maximum titer of 320). This finding suggests that a decrease in IL-10 levels could be related to the polarization to a Th1 subset, and thus the low IgG1 titers detected in this study furthers supports the notion of switching to a Th1 response.
The finding in this study of a Th1 polarization in PiV immunized mice is in agreement with the expression of key cytokines reported in clinical cases of pythiosis treated with PiV [1, 2, 8]. These data are also supported by recent studies in experimentally infected rabbits with P. insidiosum [12, 15, 16, 24]. The latter studies showed that during rabbit experimental pythiosis an increase in E-NTPDase (an enzyme that is present in lymphocytes and is involved in the immune response) and the decrease in ATP or ADP in sera could be involved in the binding of P2Y receptors expressed on the surface of lymphocytes, which in turn could activate a Th2 response [15]. The ecto-adenosine deaminase (E-ADA) present in low concentration in a typical Th2 inflammation increased after immunotherapy, substantiating the hypothesis that during immunotherapy a switching from Th2 to Th1 subset occurs in successfully treated hosts [16]. Our study strongly supports the data indicating that P. insidiosum proteins have the ability to modulate the host T helper response independently of the antigenic stimulus triggering such a response. Our study suggests that the immunological mechanisms by which the PiV modulates the immune response in mammalian hosts with allergies and those with infectious (other than pythiosis) and non-infectious diseases could be investigated using this mouse model.
Immunotherapy with microbial antigens to treat infectious and non-infectious diseases is not a new idea. In fact, Busch in 1868 [27], Fehleisen in 1882 [28] and Coley in 1893 [29] used this approach in the management of sarcoma patients. Robert Koch in 1890 [30] in Germany also reported some success using this approach in patients with tuberculosis. The first two authors [27, 28] noticed tumor regression after erysipelas infections due to Streptococcus pyogenes, whereas Coley [29] prepared extracts of S. pyogenes and vaccinated sarcoma patients. The main problem in the early accounts was the strong patient adverse reaction, including high fever and chills in S. pyogenes-challenged patients [31]. Conversely, Robert Koch [32] reported that several of the treated patients strongly reacted to Mycobacterium antigens resulting in the death of some of the treated patients. Soon after these adverse events, the idea of using microbial antigens for immunotherapy was virtually abandoned [33].
A recent review on the use of immunotherapy to treat infectious and non-infectious diseases highlights the fascinating capabilities that some microbial proteins possess to modulate the immune response [34, 35, 36]. However, few immunotherapeutic proteins of microbial origin are presently being considered for immunotherapy; one of them is PiV, while the rest represent antigenic epitopes harvested from cancer patients [33, 37]. So far, PiV antigenic preparation has shown minimal or no side effects in experimental animals (1,2,10,13, this study), and in cured humans and animals with pythiosis [3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 23]. Based on these results, we anticipate further interest in PiV immunomodulatory capabilities applied to other infectious and non-infectious processes that otherwise involve a non-curative Th2 response.
Declarations
Author contribution statement
Tatiana Maria Inêz-Ferreira: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Leonel Mendoza: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Raquel Vilela: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hélida Monteiro de Andrade, João Paulo Haddad, Isabela Moreira Gondim, Tânia Mara Pinto Dabés Guimarães: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Fernanda Freire Campos Nunes: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Vicente de Paulo Coelho Peixoto de Toledo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported in part by the department of Microbiology and Molecular Genetics, Michigan State University and by Solid Tech Animal Health, Inc., Newcastle, Oklahoma, USA.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Drs. Richard Hansen and Kathy Hoag for the critic and suggestions to the manuscript. The authors thank also the case information provided by Drs. R. Hansen and Robert Glass.
References
- 1.Gaastra W., Lipman L.J., De Cock A.W., Exel T.K., Pegge R.B., Scheurwater J., Vilela R., Mendoza L. Pythium insidiosum: an overview. Vet. Microbiol. 2010;146:1–16. doi: 10.1016/j.vetmic.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza L., Newton J.C. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Med. Mycol. 2005;43:477–486. doi: 10.1080/13693780500279882. [DOI] [PubMed] [Google Scholar]
- 3.Miller R.I. Treatment of equine phycomycosis by immunotherapy and surgery. Aust. Vet. J. 1981;57:377–382. doi: 10.1111/j.1751-0813.1981.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza L., Alfaro A.A. Equine pythiosis in Costa Rica: Report of 39 cases. Mycopathologia. 1986;94:123–129. doi: 10.1007/BF00437377. [DOI] [PubMed] [Google Scholar]
- 5.Sermsathanasawadi N., Praditsuktavorn B., Hongku K., Wongwanit C., Chinsakchai K., Ruangsetakit C., Hahtapornsawan S., Mutirangura P. Outcomes and factors influencing prognosis in patients with vascular pythiosis. J. Vasc. Surg. 2016;64(2):411–417. doi: 10.1016/j.jvs.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Permpalung N., Worasilchai N., Plongla R., Upala S., Sanguankeo A., Paitoonpong L., Mendoza L., Chindamporn A. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: a retrospective study of 18 patients. J. Antimicrob. Chemother. 2015;70:1885–1892. doi: 10.1093/jac/dkv008. [DOI] [PubMed] [Google Scholar]
- 7.Wanachiwanawin W., Mendoza L., Visuthisakchai S., Mutsikapan P., Sathapatayavongs B., Chaiprasert A., Suwanagool P., Manuskiatti W., Ruangsetakit C., Ajello L. Efficacy of immunotherapy using antigens of Pythium insidiosum in the treatment of vascular pythiosis in humans. Vaccine. 2004;22:3613–3621. doi: 10.1016/j.vaccine.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Thitithanyanont A., Mendoza L., Chuansumrit A., Pracharktam R., Laothamatas J., Sathapatayavongs B., Lolekha S., Ajello L. Use of an immunotherapeutic vaccine to treat a life-threatening human arteritic infection caused by Pythium insidiosum. Clin. Infect. Dis. 1998;27:1394–1400. doi: 10.1086/515043. http://www.jstor.org/stable/4481737 [DOI] [PubMed] [Google Scholar]
- 9.Hensel P., Greene C.E., Medleau L., Latimer K.S., Mendoza L. Immunotherapy for treatment of multicentric cutaneous pythiosis in a dog. J. Am. Vet. Med. Assoc. 2003;223:215–218. doi: 10.2460/javma.2003.223.215. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza L., Mandy W., Glass R. An improved Pythium insidiosum-vaccine formulation with enhanced immunotherapeutic properties in horses and dogs with pythiosis. Vaccine. 2003;21:2797–2804. doi: 10.1016/s0264-410x(03)00225-1. [DOI] [PubMed] [Google Scholar]
- 11.Schmiedt C.W., Stratton-Phelps M., Torres B.T., Bell D., Uhl E.W., Zimmerman S., Epstein J., Cornell K.K. Treatment of intestinal pythiosis in a dog with a combination of marginal excision, chemotherapy, and immunotherapy. J. Am. Vet. Med. Assoc. 2012;241:358–363. doi: 10.2460/javma.241.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Santurio J.M., Leal A.T., Leal A.B., Festugatto R., Lubeck I., Sallis E.S., Copetti M.V., Alves S.H., Ferreiro L. Three types of immunotherapics against pythiosis insidiosi developed and evaluated. Vaccine. 2003;21:2535–2540. doi: 10.1016/s0264-410x(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 13.Dixon D.M., Casadevall A., Klein B., Mendoza L., Travassos L., Deepe G.S., Jr. Development of vaccines and their use in the prevention of fungal infections. Med. Mycol. 1998;36(Suppl. 1):57–67. https://www.ncbi.nlm.nih.gov/pubmed/9988493 [PubMed] [Google Scholar]
- 14.Mendoza L., Villalobos J., Calleja C.E., Solis A. Evaluation of two vaccines for the treatment of pythiosis insidiosi in horses. Mycopathologia. 1992;119:89–95. doi: 10.1007/BF00443939. https://www.ncbi.nlm.nih.gov/pubmed/1435952 [DOI] [PubMed] [Google Scholar]
- 15.Bach B.C., Leal D.B., Ruchel J.B., Souza Vdo C., Maboni G., Dal Pozzo M., Schlemmer K.B., Alves S.H., Santurio J.M. Immunotherapy for pythiosis: Effect on NTPDase activity in lymphocytes of an experimental model. Biomed. Pharmacother. 2010;64:718–722. doi: 10.1016/j.biopha.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Bach B.C., Leal D.B., Jaques J.A., Souza Vdo C., Ruchel J.B., Schlemmer K.B., Zanette R.A., Hecktheuer P.A., de Lima Pereira P., Casali E.A., Alves S.H., Santurio J.M. E-ADA activity in lymphocytes of an experimental model of pythiosis treated with immunotherapy. Cell Biochem. Funct. 2013;31:476–481. doi: 10.1002/cbf.2921. [DOI] [PubMed] [Google Scholar]
- 17.Sacks D., Noben-Thrauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 18.Mayrink W., Machado-Coelho G.L., Guimarães T.M.P.D., Andrade H.M., Peres E.C., da Costa C.A., Toledo V.P.C.P. Immuno-biochemical evaluations of phenol and thimerosal as antigen preservatives in Montenegro skin test. Acta Trop. 2006;98:87–93. doi: 10.1016/j.actatropica.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli V.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. https://www.ncbi.nlm.nih.gov/pubmed/5432063 [DOI] [PubMed] [Google Scholar]
- 20.Wray W., Boulikas T., Wray V.P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. https://www.ncbi.nlm.nih.gov/pubmed/6175245 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira M.A.P., Santiago H.C., Lisboa C.R., Ceravollo I.P., Trinchieri G., Gazzinelli R.T., Vieira L.Q. Leishmania sp: Comparative study with Toxoplasma gondii and Trypanosoma cruzi in their ability to initialize IL-12 and IFN-γ synthesis. Exp. Parasitol. 2000;95:96–105. doi: 10.1006/expr.2000.4523. [DOI] [PubMed] [Google Scholar]
- 22.Campi-Azevedo A.C., de Almeida Estevam P., Coelho-Dos-Reis J.G., Peruhype-Magalhães V., Villela-Rezende G., Quaresma P.F., Maia Mde L., Farias R.H., Camacho L.A., Freire Mda S., Galler R., Yamamura A.M., Almeida L.F., Lima S.M., Nogueira R.M., Silva Sá G.R., Hokama D.A., de Carvalho R., Freire R.A., Filho E.P., Leal Mda L., Homma A., Teixeira-Carvalho A., Martins R.M., Martins-Filho O.A. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect. Dis. 2014;14:391. doi: 10.1186/1471-2334-14-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira D.I., Botton S.A., Azevedo M.I., Motta M.A., Lobo R.R., Soares M.P., Fonseca A.O., Jesus F.P., Alves S.H., Santurio J.M. Canine gastrointestinal pythiosis treatment by combined antifungal and immunotherapy and review of published studies. Mycopathologia. 2013;176:309–315. doi: 10.1007/s11046-013-9683-7. [DOI] [PubMed] [Google Scholar]
- 24.Zanette R.A., Alves S.H., Polottto M.B., Weiblen C., Figuera R.A., Wolkmer P., Flores M.M., Santurio J.M. Iron chelation therapy as a treatment for Pythium insidiosum in an animal model. J. Antimicrob. Chemother. 2013;68:1144–1147. doi: 10.1093/jac/dks534. [DOI] [PubMed] [Google Scholar]
- 25.Chindamporn A., Vilela R., Hoag K.A., Mendoza L. Antibodies in the sera of host species with pythiosis recognize a variety of unique immunogens in geographically divergent Pythium insidiosum strains. Clin. Vaccine Immunol. 2009;16:330–336. doi: 10.1128/CVI.00429-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snapper C.M., Finkelman F.D., Paul W.E. Differential regulation of IgG1 and IgE by synthesis of Interleukin 4. J. Exp. Med. 1988;167:183–196. doi: 10.1084/jem.167.1.183. https://www.ncbi.nlm.nih.gov/pubmed/3257252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busch W. Aus der Sitzung der medicinischen. Section vom 13 November 1867. Berl. Klin. Wochenschr. 1868;5:137–139. [Google Scholar]
- 28.Fehleisen F. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Übertragbarkeit auf den Menschen. Dtsch. Med. Wochenschr. 1882;8:553–554. [Google Scholar]
- 29.Coley W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am. J. Med. Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 30.Koch R. An address on bacteriological research delivered before the International Medical Congress, held in Berlin, August 1980. Br. Med. J. 1890;2:380–383. doi: 10.1136/bmj.2.1546.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiemann B., Starnes C.O. Coley's toxins, tumor necrosisfactor and cancer research: a historical perspective. Pharmacol. Ther. 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-x. https://www.ncbi.nlm.nih.gov/pubmed/7724661 [DOI] [PubMed] [Google Scholar]
- 32.Koch R. A further communication on a remedy for tuberculosis. Br. Med. J. 1890;2:1193–1195. [Google Scholar]
- 33.Stanford J.L. Immunotherapy for leprosy and tuberculosis. In: Ernst Jucker, editor. Vol. 33. Birkhauser Verlag Basel; Boston, Berlin: 1989. pp. 415–447.http://link.springer.com/chapter/10.1007%2F978-3-0348-9146-2_13#page-1 (Progress in Drug Research). [DOI] [PubMed] [Google Scholar]
- 34.Nishat S., Andreana P.R. Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines. 2016;4:19. doi: 10.3390/vaccines4020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldarouish M., Wang C. Trends and advancements in tumor immunology and lung cancer immunotherapy. J. Exp. Clin. Cancer Res. 2016;35:157. doi: 10.1186/s13046-016-0439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamarin D., Jazaeri A.A. Leveraging immnunotherapy for the treatment of gynecologic cancers in the era of precision medicine. Gynecol. Oncol. 2016;141:86–94. doi: 10.1016/j.ygyno.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch D., Murphy A. The emerging role of immunotherapy in colorectal cancer. Ann. Transl. Med. 2016;4:305. doi: 10.21037/atm.2016.08.29. [DOI] [PMC free article] [PubMed] [Google Scholar]