Abstract
One's own face and gaze are never seen directly but only in a mirror. Yet, these stimuli capture attention more powerfully than others' face and gaze, suggesting the self is special for brain and behavior. Synchronous touches felt on one's own and seen on the face of others induce the sensation of including others in one's own face (enfacement). We demonstrate that enfacement may also reduce the overwhelming distracting power of self-gaze. This effect, hereafter called ‘engazement', depends on the perceived physical attractiveness and inner beauty of the pair partner. Thus, we highlight for the first time the close link between enfacement and engazement by showing that changes of the self-face representation induced by facial visuo-tactile stimulation extend to gaze following, a separate process likely underpinned by different neural substrates. Moreover, although gaze following is a largely automatic, engazement is penetrable to the influence of social variables, such as positive interpersonal perception.
The sense of self bears unique importance to humans' survival. Self-related stimuli are processed and recalled faster and more accurately than stimuli related to others, suggesting a special status for the self in cognitive and emotional systems1,2,3,4,5,6,7,8,9.
Self-identity is inherently linked to one's own face. Self-capture and self-advantage effects in processing the self-face have been extensively found: indeed, the self-face is recognized faster10, grabs and retains attention longer11,12 than others - even familiar - faces. At a basic level, self-identity is grounded on the sense of the bodily self, which is based on the integration of congruent spatio-temporal multisensory information13. Indeed, a coherent representation of one's own face (and body) is formed and maintained, because a person's mirror reflection moves at the exact time when he/she moves, and one feels the face being touched at the same time he/she sees the touch occurring.
It is classically held that the visual representation of the self-face is fundamentally stable. However, it has been recently shown that such representation is inherently plastic since experiencing synchronous interpersonal multisensory stimulations (IMS) can alter the ability to discriminate self from other faces14,15, an effect we named ‘enfacement'15. This effect seems, in fact, to derive from IMS-induced updating of the self-face representation to include features of the other's face16. Enfacement is also permeable to the influence of individual, personality and social variables, being stronger in people with low interoceptive sensitivity17, high emphatic traits, and with partners considered physically attractive15. Additionally, experiencing the enfacement can blur the conceptual representations of self and other by inducing a sense of perceived psychological self-other similarity and conformity behavior18 and can cancel baseline differences in remapping onto one's own somatosensory system, tactile stimuli seen on the self and the other's face (as measured at the Visual Remapping of Touch task)19.
What has not been investigated thus far is if enfacement can also reduce the prominence in processing self- as opposed to other-face related stimuli. Here, we focused on self vs. familiar-other gaze shifts considering their pivotal role in human interactions. Directional gaze - even if task-irrelevant - induces reflexive shifts of visuo-spatial attention in observers more than other directional cues20,21 and predominantly when observers watch their own gaze shifts22. Indeed, a very recent gaze-cueing study found that self-gaze can elicit a self-capture effect: observing gaze shifts of the self face with respect to both a familiar and a stranger face, induces a higher number of error rates in detecting targets presented in a position incongruent with the gaze shifts22.
Thus, we combined the enfacement paradigm15 with a gaze-following task20,23,24 to explore if experiencing self-other merging with a close other may reduce the distracting power of one's own gaze22 compared to the friend's gaze, and if social perception variables and individual empathic personality traits may drive the power of this effect, as initially found for the enfacement15.
To this aim, participants sitting in front of each other underwent synchronous (which was the illusory condition) and asynchronous (which was the control condition) visuo-tactile facial stimulations and immediately after were asked to perform a gaze-following task (Figure 1) with 3 different distracting faces: the self-face, the friend's face, and a morphed face (45% self-face and 55% friend's face). The morphed face was included to test whether any effect of IMS on the gaze-following task (GFT) applied also to this ambiguous face where subjects fail to correctly discriminate the amount of self and other facial features after synchronous IMS15.
Figure 1. Experimental Design.
(A) Example of one typical session. A/Synchronous stimulations were administered in separate runs. Each a/synchronous run was composed by three stimulation blocks. (B) Each stimulation block lasted 2 minutes and was followed by 36 trials of the Gaze Following Task (GFT). (C) Timeline of one representative GFT trial. A face with straight gaze stayed on the screen for 500 ms after which the eyes shifted toward left or right, and participants were instructed to ignore this gaze shift. After 75 ms from the gaze shift, an imperative cue (a change in blue/red color of the central black square) signaled to the participants if they had to gaze toward the same (congruent trials) or a different (incongruent trials) direction as the one cued by the eyes.
In the gaze-following task (Figure 1), participants were instructed to look at a central black square positioned in between the eyes of a centrally presented (self/friend/morphed) face gazing straight and to perform a saccade toward a left-sided target when the black square turned into blue, or toward a right-sided target when the central square turned into red. The saccade planning was interfered by the face that shifted the gaze toward a congruent or incongruent direction 75 ms before the central square changed colors.
Results
Enfacement Induction
The perceived phenomenology of the illusion was measured by asking participants to complete an 8-item questionnaire, which is an adapted version15 of the original rubber hand illusion questionnaire25, after either synchronous and asynchronous visuo-tactile facial stimulation. The first 3 questions capture the experience of the illusion by measuring: subjective experience of referred sensation (statements 1 and statement 2) and sense of facial ownership (statement 3).
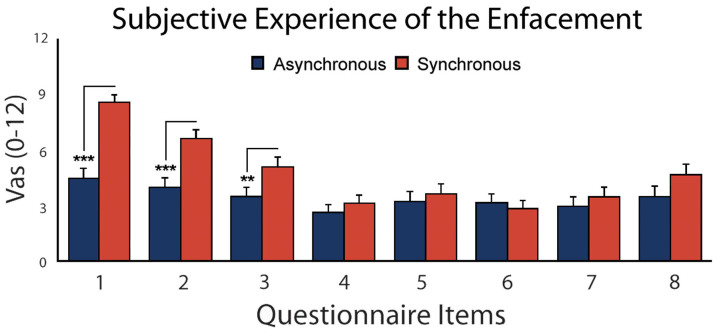
Results confirmed that our visuo-tactile stimulation procedure was effective in inducing the enfacement effect. Repeated measures 2 × 8 ANOVA with Stroking (2 levels: Synch; Asynch) and Item (8 levels) as factors showed a main effect of Stroking (F1, 37 = 19.31, P = .000, ηp2 = 0.34), with higher scores in the synchronous than the asynchronous condition (4.77 ± 0.57 vs. 3.42 ± 0.52; P = .000) and a main effect of Item (F7, 259 = 19.16, P = .000, ηp2 = 0.34). Also the Stroking X Item interaction was significant (F7, 259 = 9.40, P = .000, ηp2 = 0.20). Newman–Keuls post hoc tests show that the significance of the interaction was accounted for by the fact that subjects gave significantly higher scores in the synchronous with regard to the asynchronous condition only in items 1–3 (those describing the illusion: item1: 8.63 ± 0.40 vs. 4.49 ± 0.53; item 2: 6.63 ± 0.50 vs. 3.99 ± 0.52 and item 3: 5.09 ± 0.56 vs. 3.47 ± 0.53; all Ps ≤ .01, see Figure 2 and Table 1 for the list and wording of all items). This indicates that the subjective experience of the illusion explicitly occurred in the synchronous condition and specifically only for the three illusion-related items, not for the control ones.
Figure 2. Subjective experience of the enfacement illusion.
Asterisks indicate significantly higher agreement with the perceptual experience of the illusion in the synchronous (red) with regard to the asynchronous (blue) stimulation conditions for statement 1–3 (** = P ≤ .01; *** = P≤ .001) namely the ones that describe the illusion in its components of referred touch and ownership. Error bars represent standard errors of mean (SEM).
Table 1. List of the 8 statements presented after both the synchronous and asynchronous Interpersonal Multisensory Stimulation (IMS). The questionnaire (adapted by Sforza et al., 2010) included eight statements. The first 3 questions capture the experience of the illusion by measuring: subjective experience of referred sensation (statements 1 and statement 2) and sense of facial ownership (statement 3). Participants were asked to reply to each of the statements using a Visual Analogue Scale (VAS) ranging from 0 (= “completely false”) to 12 (= “completely true”).
| List of the statements | |
|---|---|
| 1. | It seemed as if I was feeling the touch of the paintbrush in the location where I saw the other's face touched. |
| 2. | It seemed as if the touch I felt was caused by the paintbrush touching the other's face. |
| 3. | I felt as if the other's face was my face. |
| 4. | It felt as if my face was drifting toward the other's face. |
| 5. | It seemed as if I might have more than one face. |
| 6. | It seemed as if the touch I was feeling came from somewhere between my own face and the other's face. |
| 7. | It appeared as if the other's face was drifting toward my own face. |
| 8. | The other's face began to resemble to my own face, in terms of shape, skin tone, or some other visual features. |
Gaze following Task
Reaction Times
Results from the 2 × 3 ANOVA (with: Stroking (Synch; Asynch) and Identity (Self; Friend; Morph) as within-subjects factors) performed on the Congruency Effect calculated on the latencies of saccadic responses (RTs in the correct trials only) showed no significant main or interaction effects (all Fs < 1.56, all Ps > .22).
Accuracy
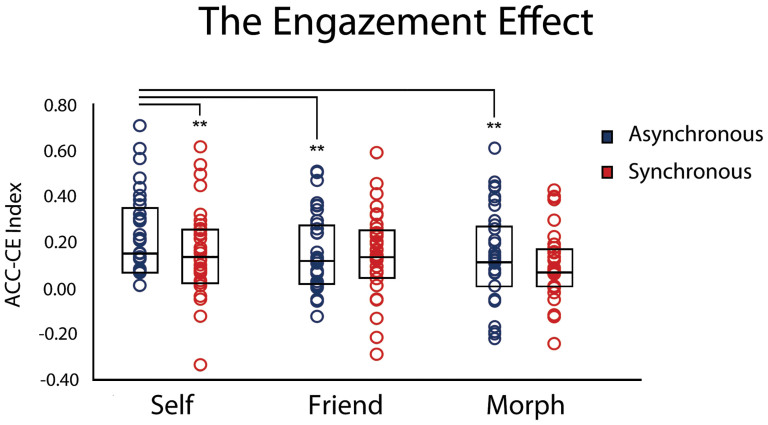
Results on the Congruency Effect calculated on the accuracy of saccadic responses (please see the data analysis section) showed the synchronous stimulation increased overall accuracies, by reducing the overwhelming distracting power of self-gaze (Figure 3). Indeed, Wilcoxon matched pair test indicated higher accuracies after synchronous (median: 0.08; range: −0.04, 0.53) with respect to the asynchronous (median: 0.10; range: −0.08, 0.57) stroking condition (Z = 2.49; P = .01, r = .29), independently of the identity condition. Conversely there were not significant differences between the self, the friend and the morphed faces independently of stroking (χ2(2) = 3.62; P = .16). Crucial for our hypothesis was the significant Friedman-ANOVA (χ2(5) = 18.44; P = .002) in which all our experimental conditions were compared between each other [Stroking (Asynch; Synch) and Identity (Self; Friend; Morph)]. The Friedman-ANOVA revealed a significant difference between our experimental conditions (χ2(5) = 18.44; P = .002). Planned Wilcoxon matched pairs test showed, as predicted, that while the self-gaze was the most distracting identity in the asynchronous control condition [Self Asynch (median: 0.14; range: 0.00, 0.70) vs. Friend Asynch (median: 0.11; range: −0.13, 0.50), Z = 2.94, P .003, r = .34; Self Asynch vs. Morph Asynch (median: 0.11; range: −0.23, 0.60), Z = 2.87, P .004, r = .33], the synchronous interpersonal visuo-tactile stimulation reduced the distracting power of the self gaze [Self Synch (median: 0.13; range: −0.35, 0.61) vs. Self Asynch, Z = 2.69, P = .007, r = .31] and made it similar to that of the friend and of the morphed gazes [Friend Synch (median: 0.13; range: −0.30, 0.58); Morph Synch (median: 0.06; range: −0.25, 0.42); all Zs < 1.95; all Ps > .05], which did not differ between the synchronous and the asynchronous conditions (all Zs < 1.18, all Ps > .24; see Figure 3).
Figure 3. The ‘engazement' effect.
Box Plots of the Accuracy Congruency Effect (ACC-CE) Index for the saccadic responses at the gaze following task after participants received the synchronous (red) and the asynchronous (blue) interpersonal multisensory stimulations. The thicker horizontal lines represent the medians, the boxes the interquartile ranges and the circles the individual ACC-CE values in the different experimental conditions. Wilcoxon matched pair test run after the significant Friedman-ANOVA indicated that the synchronous visuo-tactile stimulation significantly improved the capacity to ignore the (distracting) self-gaze by obliterating the difference between self and other gazes we found in the asynchronous control condition. Asterisks indicate significant differences (** = p < .01).
In analogy with the original ‘Enfacement' effect showing that Synch IMS affects self-face recognition, we named this effect ‘Engazement' to highlight that Synch-IMS reduced the highly distracting power of the self-gaze and made it be as distracting as the other gazes.
Predictors of the Engazement effect
To obtain an index of the Engazement effect, i.e. of the reduction of the distracting power of the self with respect to the other gazes in the synchronous vs. the asynchronous control condition, we calculated the difference in the accuracy of the saccadic responses (ACC-CE values) between the asynchronous (which showed higher distracting power of the self gaze) and the synchronous conditions for the self-identity. Then, to clean this effect from spurious factors at the individual level, we subtracted from this difference the averaged difference between the asynchronous and the synchronous friend and morphed faces (which showed no difference between the stroking conditions). In such a way, the higher the resulting values, the higher the engazement effect.
Then, in order to analyze the factors that predicted the Engazement effect, separated standard multiple regression models were run on the Engazement index, with independent variables: i) the phenomenological experience of the illusion (as measured by the difference between the agreement expressed after synchronous and asynchronous stimulations with statements 1–3 of the adapted Botvinick and Cohen questionnaire25); ii) interpersonal perception (as measured by subjective scores of the 4 questions on interpersonal perception: perceived attractiveness, inner beauty, degree of knowledge and familiarity with the pair partner; iii) and the empathic traits of the participants (as measured by the 4 subscales of the IRI questionnaire, namely: Perspective Taking; Fantasy Scale; Personal Distress; Empathic Concern).
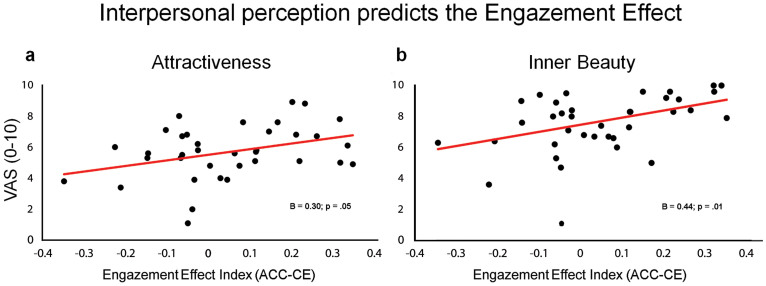
Results show that neither the phenomenological experience of the illusion (R2 = 0.01, F4,34 = 0.15, P = .93) nor the participants' empathy traits (R2 = 0.12, F4,32 = 1.05, P = .40; after removal of one outlier that presented standard residuals above ±2.5 standard deviations) predicted the IMS-dependent reduction of the distracting power of the self-gaze. The standard regression model with interpersonal perception measures as predictors was instead significant (R2 = 0.34, F4,31 = 4.06, P = .01; after removal of two outliers that presented standard residuals above ±2.5 standard deviations) and showed a significant positive regression weight of the attractiveness (β = 0.30, t(31) = 2.05 P = .05; Figure 4A) and of the inner beauty (β = 0.44, t(31) = 2.85, P = .01; Figure 4B) over the Engazement index. Beta scores of the degree of knowledge and familiarity measures were both non-significant (“How deeply they knew each other”: β = 0.07, t(31) = 0.43, P = .67; “How frequently they saw each other”: β = −0.07, t(31) = −0.45, P = .66).
Figure 4. Interpersonal Perception predicts the Engazement effect.
Scatterplots show the relationship between the engazement index calculated on the ACC-CE values and: (A) perceived physical attractiveness and (B) inner beauty of the pair participant. Beta values and associated P values are reported for each predictor.
Discussion
The main result of the present study is that the high capturing power of one's own gaze22 can be reduced by inducing self-other merging through synchronized interpersonal multisensory stimulation between one's own and another person's face. Thus, an entirely novel result of our study is that synchronous IMS brings about fast changes of the way in which self-faces are processed. Moreover, we called the IMS effect on gaze ‘Engazement' and additionally demonstrated that it varies as a function of positive interpersonal perception, as measured by perceived physical attractiveness and inner beauty of the pair partner.
Enfacement reduces the capturing power of the self gaze (Engazement effect)
Subjective reports concerning the phenomenal experience of the enfacement illusion clearly demonstrate that after synchronous interpersonal multisensory stimulation participants affirmed to perceive the face of the other as their own face and started feeling on their face the touch observed on the other's face. After the asynchronous stimulation, instead, they didn't perceive any illusory feeling. Interestingly, the strength of this effect seems to be higher than in previous studies (e.g.15,16,17,18,26,27) probably due to the fact that we used an unpredictable stimulation pattern (please see the Method section), which has been shown to induce stronger rubber hand28 and full body29,30 illusions.
The results of the gaze-following task in the control asynchronous condition confirm previous ones obtained with a gaze-cueing task and without participants undergoing any kind of IMS22. In particular our data indicate that the distracting power of directional gaze is much higher for one's own face than for others' faces (i.e. friend and morphed).
Overall our data suggest that the self-capture effect elicited by one's own gaze is robust since it can be observed in a number of different circumstances, ranging from different response modalities (saccadic and manual responses22) and tasks (e.g. gaze-following and gaze cueing22). Previous studies indicate that observing directionally oriented saccades automatically triggers in an onlooker the tendency to make a saccade directed in the same direction23,24,31 and activates fronto-parietal and temporal cortical regions similar to those recruited during actual execution of eye movements32,33. Here, we found that seeing one's own saccades triggered an oculomotor program that maximally interfered with the ongoing programming and execution of saccades directed in the opposite direction. This result is reminiscent of action observation studies showing that seeing one's own actions might be special compared to perceiving somebody else actions. Indeed, people are better at recognizing34, predicting35 and synchronizing36 with their own with respect to others' movements because seeing one's own actions represents a unique condition in which what is perceived is maximally similar to what is represented. Thus, observing one's own- with respect to others- gaze shifts may activate a ‘mirrored' oculomotor program37 with maximal interfering power.
Importantly, our study demonstrates for the first time that synchronous IMS modulates the prioritizing power of highly distracting self-related stimuli and that this robust self-gaze capture can be highly plastic, as it is strongly reduced by inducing enfacement immediately before the saccadic task is performed. Indeed, in line with our hypotheses we found that synch-IMS modulates the distracting power of the self- but not of the other-gaze. Specifically, after synchronous IMS, the overwhelming distracting power of the self-gaze vanishes to the point to which one's own face becomes as distracting as a friend's (or a morphed) face. Also, the distracting power of the friend and morphed faces was not affected by the synchronous stimulation, which, however, selectively improved the capacity to ignore the attentional capture evoked by self-gaze.
Our results are in line with previous studies on enfacement suggesting that synchronous IMS affects the self-face but not the other-face representation16. Indeed it induces the incorporation of facial features of the other into self-face representation15 and the categorization as self faces of stimuli that contained a higher percentage of other's face14. Interestingly, the matching between felt and observed tactile stimuli over another face induced also the subjective experience of facial self-other similarity, possibly because synchronous IMS changes the mental representation of our physical appearance16,17. Congruently, preliminary electroencephalographic data from our group suggest that the visual processing of the self-face, as indexed by the amplitude of a long latency visual component associated with the stage of self-identification, becomes more similar to processing of the other face as a result of synchronous IMS38. That synchronous IMS affects the self more than the other face representation is completely plausible if we consider that integrative mechanisms detecting congruence of body-related multisensory stimuli are relevant for building, maintaining and updating self - but not others'- face (and body) representations. Indeed, the visual representation of our face is built upon accumulating congruent multisensory experiences, derived by matching one's own sensorimotor experience with the sensorimotor behavior of the face observed in a mirror13,17,39.
Thus, we speculate that the observed IMS-induced changes in the prioritizing power of highly distracting self-related stimuli may be driven by changes in the way the self-face is represented as a result of experiencing synchronous IMS (i.e. the enfacement illusion).
Engazement varies as a function of positive interpersonal perception of the other
Studies indicate that gaze-following, although often triggered automatically, is permeable to the influence of high-order socio-cognitive variables, such as social status40, political affiliation23, age41 and group membership42. The point of novelty of the present study is that attentional capture of the self-gaze becomes similar to the friend (and morphed) face, depending on the interpersonal evaluation of the other's physical and inner beauty. This effect may depend upon the known link between positive interpersonal perception and the enfacement strength15,18,43. Indeed, previous research demonstrated that the misattribution to the self of the other physical feature (i.e. inclusion of the other in the self representation) is higher when the other is positively perceived and absent when the other is negatively perceived43. Also, experiencing enfacement is positively correlated with15 and can even increase18 the perceived physical attractiveness of the other.
It is worth noting that, far from being an entirely low-level variable, perception of physical attractiveness has a close relationship with complex social perceptions and depends largely on intricate, higher-order social influences44. Thus, the present result expands previous research by demonstrating the penetrability of the engazement effect to these social variables. Perceived physical attractiveness determines positive interpersonal perception, namely attractive people are perceived more positively in terms of intelligence, competence and personality traits45,46,47,48. Moreover, perceived physical attractiveness is one of the factors that leads to friendship (or to romantic love)49. Indeed, human beings are initially attracted to those more appealing to the eyes than to emotions. However, inner beauty may become more relevant as intimacy progresses. In this vein, our exploration of the relationship between engazement, physical attractiveness and inner beauty using pairs of individuals known to one another turned out to be crucial. We suggest that the engazement (as well as the enfacement) effect could be related to inner beauty when the relationship between partners in a pair is very strong. Accordingly in fact, our participants reported to deeply know their pair partner and to spend lots of time together (see Table 2). As a matter of fact, unlike our previous study15, here we found that the engazement effect is significantly predicted also by the inner beauty of the other. We speculate that this relationship may become even stronger when the relationship between partners in a pair is even tighter, such as for example in the case of romantic interactions.
Table 2. Mean (±SEM) values of subjective ratings at the Interpersonal Perception and Empathy (IRI) Questionnaires.
| Questionnaire | |
|---|---|
| Interpersonal Perception: | |
| Physical attractiveness (0–10 VAS) | 5.76 (0.28) |
| Inner beauty (0–10 VAS) | 7.72 (0.25) |
| How deeply participants knew the pair partner (0–10 VAS) | 7.21 (0.29) |
| How often participants see the pair partner (1–30 days). | 17.63 (1.09) |
| Empathy traits: | |
| PT | 24.61 (0.47) |
| FS | 23.79 (0.60) |
| EC | 21.29 (0.28) |
| PD | 19.63 (0.41) |
All in all, our study supports the view that experiencing the enfacement illusion as a result of synchronous IMS may alter the processing of very relevant self-related stimuli, such as the self-face and gaze. At a neural level, we can hypothesize that this effect may rely on the activity of brain regions involved in self-recognition, multisensory integration and in reflexive shifts of attention triggered by social signals, such as gaze. Related to this issue, previous research demonstrated that self-recognition relies on a right-dominant (but largely bilaterally distributed) circuits involving occipito-frontal and parietal regions6,7,50,51,52 and that selective disruption of right inferior parietal53 and temporoparietal junction (TPJ)54,55 regions impairs self-other discrimination and self recognition. Interestingly, a recent study showed the strength of the enfacement is related to the activity of right TPJ, intra-parietal sulcus and occipital face area regions56. It is worth noting that the TPJ region is also involved in: i) attributing multisensory stimuli to the self and the other in order to maintain a coherent body perception57; ii) full body58 illusions caused by IMS; and iii) attentional orienting to socially relevant stimuli59. Thus, on the basis of available evidence we can speculate that modulation of activity in the above-mentioned brain circuits involved in self recognition and self identity illusions may ultimately affect the activity of fronto-parietal and temporal brain network involved in execution and observation of eye movements32,33,37. The integrated activity within these circuits may underpin the engazement effect.
Methods
Participants
19 couples of same-sex, right-handed, healthy, normal or corrected to normal vision, naïve, Caucasian friends (26 Females; M: 22.05, SD: 2.73) provided their informed consent to participate in the study. Involving couples of friends minimized the difference in familiarity between the self and other's face. Participants were informed about the study's aim at the end of the experiment. The study was approved by the independent Ethics Committee of the Santa Lucia Foundation (Rome) and in accordance with the Declaration of Helsinki (1964).
Stimuli and Procedures
Stimuli
About one week before the experimental session, photos of each participants' face looking forward, left and right were taken in a room with controlled lighting and modified with Adobe Photoshop® so that only the oval shape and the facial features were shown on a light gray background. Each participant's face was morphed with his/her friends' face using Abrasoft FantaMorph® 4.0 in steps of 1% thus obtaining 100 images. We selected 3 images as stimuli for each participant: (1) 100% self-face, (2) 100% friend's face, and (3) the 45% self-55% friend morphed face where participants reported higher self-attribution ratings after the synchronous IMS with respect to the asynchronous IMS in our previous study15. Thus, we had for each participant 3 identities (Self; Friend; Morph) and 3 gaze directions (Straight; Left; Right).
Experimental Procedure
The experiment consisted of two sessions. Each session (Figure 1A) lasted around one hour and a half and included 2 phases: the enfacement induction and the gaze-following task (which was performed by one participant in the first session, and by the other in the second one).
Enfacement Induction
Participants seated facing the pair other at a distance of about 140 cm. A trained experimenter stood between the two participants and repeatedly touched the subjects' cheeks with two identical paintbrushes (Figure 1B) synchronously or asynchronously. The participant undergoing the gaze-following task after the enfacement induction wore a rigid white-paper funnel around the eyes to avoid seeing the experimenter while focusing on his/her friend's face.
In the synchronous experimental condition, the paintbrush strokes (and taps) were given simultaneously, but their length and direction was changed across strokes to make them unpredictable to enhance the illusion28,29,30. In the control asynchronous condition, the same pattern of stimulation was used, but temporal asynchrony was introduced between strokes on the two faces.
After each type of visuo-tactile stimulation, the adapted version15 of the original rubber hand illusion questionnaire25 was administered to assess the phenomenological experience of the illusion (Table 1). Subjects indicated their response on a seven-step 12-cm long visual-analog scale [(VAS), with 0 = “completely false”; 12 = “completely true”)
Gaze Following Task
Immediately after each stroking block, the participants performed the gaze-following task (GFT). During the GFT, participants sat on a comfortable chair in front of an LCD monitor, positioned at about 60 cm from their eyes. Eye position and movements were measured online monocularly through an infrared video-based system (ASL 504 Remote Tracker, Applied Science Laboratories, USA). The experiment was created with E-Prime 1.1 (Psychology Software Tools, Inc., Pittsburgh, PA) and ran on an IBM compatible computer.
Each trial started with the appearance of a black central fixation square (0.44° × 0.44° in size) presented on a light gray (about 47 cd/m2) background, and two larger lateral black squares (0.67° × 0.67°) presented at 15.1° to the left and right of the center of the display (Figure 1C). The fixation square was presented between the eyes of a face (e.g. either the self, friend, or morph) with a straight gaze. After 575 ms, the central square color changed to either blue or red. This was the imperative signal for the participants to make a saccade toward the direction signaled by the color. The imperative cue remained visible until the end of the trial. 75 ms before the onset of the imperative cue, the distracting face made a left- or right-ward saccadic movement congruent or incongruent with the instruction. The 75 ms interval was chosen because it strongly interferes with participants' automatic oculomotor response20,21,23,24,33. Importantly, the subjects were instructed to ignore the distracting stimulus and to focus on the change in color of the fixation square. To avoid that participants anticipated stimuli, a random inter-trial interval (range: 3000 to 4000 ms) was used. A practice session (discarded from statistical analyses) of 12 trials (i.e., one trial per condition) was given at the beginning of the GFT session.
Experimental Design
During each session, a/synchronous stroking was administered in separate runs. Each run comprised three 2 minutes-stroking blocks. The order of the runs was identical for the two members inside the pairs and counterbalanced across the pairs. Each stroking block was followed by the GFT where the two instruction cues (blue or red), the direction of the gaze (leftward or rightward) and the three faces (self, friend or morph) were equiprobable and presented in a random sequence. Participants performed 36 trials in each block, for a total of 108 trials for each stroking condition. Each level of factors was presented in 18 trials.
Measures of interpersonal perception and personality traits
In the pre-experimental session, subjects were asked to rate the physical attractiveness and the inner beauty15 of their partners along a 10 cm VAS scales (10 = ‘maximally beautiful'; 0 = ‘minimally beautiful'). To control the degree of familiarity between the pairs of friends, we asked each participant (1) how deeply he/she knew the pair partner (on a 10-cm VAS, with 10 = ‘very deeply' and 0 = ‘scarcely') and (2) how many days per month, does he/she see the friend, on average (from 1 to 30). It was emphasized that the partner would never have access to the reports of the rater.
To check that the hypothesized effect was related to empathic traits, participants completed the Interpersonal Reactivity Index60 questionnaire.
Interpersonal Perception and Empathy scores are reported in Table 2.
Data Analysis
Gaze Following Task
Saccade Reaction Times (RTs; defined as the time elapsed between the onset of the imperative cue and the onset of the saccade) and landing coordinates were extracted using a script written in MATLAB. We excluded from the statistical analysis the trials in which the saccade occurred 150 ms before (anticipations) or 550 ms after (delays) the imperative cue onset (<0.1% of the total trials), or where no clear saccade was evident because of a loss of signal or eye blinks (11.6% of the total trials).
Only saccadic RTs for correct trials were considered for the analyses. Trials with saccadic latencies ±3 standard deviations (with respect to the participant's mean) were discarded from the statistical analyses (2.7% of the total across participants).
The Congruency Effect (CE) index
For both Accuracy and RTs of the saccadic movements, we calculated in each subject an index of the interfering power of the observed gazes for each identity and stroking condition. For the Accuracy, we subtracted the mean ACC of the incongruent trials from the congruent ones and we normalized this difference on the mean of the congruent trials. For RTs, the index was computed as above with the only difference that we subtracted the latencies of saccadic responses in the congruent conditions from the ones obtained in the incongruent conditions. Thus, for both CE-ACC and CE-RTs indexes higher positive values indicated higher reflexive attentional capture exerted by the respective distracting gaze.
All statistical analyses were run using STATISTICA 7 software.
The Congruency Effect: Reaction Time
The CEs calculated on the RTs of the saccadic response were normally distributed. Thus, we applied a 2 × 3 repeated measures ANOVA, with Stroking (Synchronous; Asynchronous) and Identity (Self; Friend; Morph) as within-subjects factors. Outlier participants were defined as those presenting mean values above ±2.5 standard deviations from the grand mean of all participants in each single condition, and recoded by using the mean value of the respective condition ±2.5 standard deviations as indicated by61. Level of significance was set to P ≤ 0.05.
The Congruency Effect: Accuracy
The CEs calculated on the Accuracy of the saccadic response were not normally distributed (3 variables out of 6; Kolmogorov-Smirnov test: Ds (38) > .17; all Ps < .005). Thus, we applied the non-parametric Friedman-ANOVA for dependent samples. To test the significance of the differences between pairs of experimental conditions, we used Wilcoxon matched pairs test and when necessary we corrected the significance of the P values for multiple testing according to the FDR method62. Outlier participants were defined according to the Tukey test as being outside the first and the third percentile of the data distribution relative to each condition, and recoded by using the boundary value of the respective condition (i.e., the upper or lower boundary value depending on the participant's own value61).
Predictors of the Engazement
To test which variables predicted the engazement effect, we ran different regression models with the engazement effect-index (please see the Results section) as the dependent variable and with the following independent variables: i) the subjective experience of the illusion; ii) the empathic personality traits; and iii) interpersonal perception measures.
Acknowledgments
This work was funded by the EU Information and Communication Technologies Grant (VERE project, FP7-ICT-2009-5, Prot. Num. 257695) and the Italian Ministry of Health (grant RC11.G and RF-2010-2312912).
Footnotes
The authors declare no competing financial interests.
Author Contributions I.B. and S.M.A. developed the study concept. All authors contributed to the study design. Data collection and analysis were done by B.S.H., G.P., F.C. and I.B. The manuscript was drafted by I.B., B.S.H., G.P., M.T.L. and critically reviewed by SMA. All authors approved the final version of the paper for submission.
References
- Ross M. & Sicoly F. Egocentric biases in availability and attribution. J. Pers. Soc. Psychol. 37, 322–336 (1979). [Google Scholar]
- Wood N. L. & Cowan N. The cocktail party phenomenon revisited: how frequent are attention shifts to one's name in an irrelevant auditory channel? J. Exp. Psychol. Gen. 124, 243–262 (1995). [DOI] [PubMed] [Google Scholar]
- Symons C. S. & Johnson B. T. The Self-Reference Effect in Memory: A Meta- Analysis. Psychol Bull. 121, 371–94 (1997). [DOI] [PubMed] [Google Scholar]
- Keenan J. P., Falk D. & Gallup G. G. J. The face in the mirror: The Search for the origins of consciousness. (Haper Collins Publishers., 2003). [Google Scholar]
- Sugiura M. et al. Cortical mechanisms of visual self-recognition. Neuroimage 24, 143–9 (2005). [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Kaplan J. T., Molnar-Szakacs I., Zaidel E. & Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage 25, 926–35 (2005). [DOI] [PubMed] [Google Scholar]
- Platek S. M. et al. Neural Substrates for Functionally Discriminating Self-Face From Personally Familiar Faces. Hum. Brain Mapp. 27, 91–98 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devue C. et al. Here I am: the cortical correlates of visual self-recognition. Brain Res. 1143, 169–82 (2007). [DOI] [PubMed] [Google Scholar]
- Rosa C., Lassonde M., Pinard C., Keenan J. P. & B. P. Investigations of hemispheric specialization of self-voice recognition. Brain Cogn. 68, 204–14 (2008). [DOI] [PubMed] [Google Scholar]
- Tong F. & Nakayama K. Robust representations for faces: evidence from visual search. J. Exp. Psychol. Hum. Percept. Perform. 25, 1016–35 (1999). [DOI] [PubMed] [Google Scholar]
- Brédart S., Delchambre M. & Laureys S. One's own face is hard to ignore. Q. J. Exp. Psychol. 59, 46–52 (2006). [DOI] [PubMed] [Google Scholar]
- Devue C., Van der Stigchel S., Brédart S. & Theeuwes J. You do not find your own face faster; you just look at it longer. Cognition 111, 114–122 (2009). [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia 48, 703–12 (2010). [DOI] [PubMed] [Google Scholar]
- Tsakiris M. Looking for myself: current multisensory input alters self-face recognition. PLoS One 3, e4040 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza A., Bufalari I., Haggard P. & Aglioti S. M. My face in yours: Visuo-tactile facial stimulation influences sense of identity. Soc. Neurosci. 5, 148–62 (2010). [DOI] [PubMed] [Google Scholar]
- Tajadura-Jiménez A., Grehl S. & Tsakiris M. The other in me: interpersonal multisensory stimulation changes the mental representation of the self. PLoS One 7, e40682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajadura-Jiménez A., Longo M. R., Coleman R. & Tsakiris M. The person in the mirror: using the enfacement illusion to investigate the experiential structure of self-identification. Conscious. Cogn. 21, 1725–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladino M.-P., Mazzurega M., Pavani F. & Schubert T. W. Synchronous multisensory stimulation blurs self-other boundaries. Psychol. Sci. 21, 1202–7 (2010). [DOI] [PubMed] [Google Scholar]
- Cardini F., Tajadura-Jiménez A., Serino A. & Tsakiris M. It feels like it' s me: interpersonal multisensory stimulation enhances visual remapping of touch from other to self. J. Exp. Psychol. Hum. Percept. Perform. 39, 630–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crostella F., Carducci F. & Aglioti S. M. Reflexive social attention is mapped according to effector-specific reference systems. Exp. Brain Res. 197, 143–51 (2009). [DOI] [PubMed] [Google Scholar]
- Ricciardelli P., Bricolo E., Aglioti S. M. & Chelazzi L. My eyes want to look where your eyes are looking: Exploring the tendency to imitate another individual' s gaze. Neuroreport 13, 2259–64 (2002). [DOI] [PubMed] [Google Scholar]
- Hungr C. J. & Hunt A. R. Physical self-similarity enhances the gaze-cueing effect. Q. J. Exp. Psychol. 65, 1250–9 (2012). [DOI] [PubMed] [Google Scholar]
- Liuzza M. T. et al. Follow My Eyes: The Gaze of Politicians Reflexively Captures the Gaze of Ingroup Voters. PLoS One 6, e25117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzza M. T. et al. A look into the ballot box: gaze following conveys information about implicit attitudes toward politicians. Q. J. Exp. Psychol. 66, 209–16 (2013). [DOI] [PubMed] [Google Scholar]
- Botvinick M. & Cohen J. Rubber hands “feel” touch that eyes see. Nature 391, 756 (1998). [DOI] [PubMed] [Google Scholar]
- Mazzurega M., Pavani F., Paladino M. P. & Schubert T. W. Self-other bodily merging in the context of synchronous but arbitrary-related multisensory inputs. Exp. Brain Res. 213, 213–21 (2011). [DOI] [PubMed] [Google Scholar]
- Maister L. & Tsakiris M. My face, my heart: Cultural differences in integrated bodily self-awareness. Cogn. Neurosci. 5, 10–6 (2014). [DOI] [PubMed] [Google Scholar]
- Mohan R. et al. No pain relief with the rubber hand illusion. PLoS One 7, e52400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Perez-Marcos D., Ehrsson H. H. & Sanchez-Vives M. V. Towards a digital body: the virtual arm illusion. Front. Hum. Neurosci. 2, 6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova V. I. & Ehrsson H. H. When right feels left: referral of touch and ownership between the hands. PLoS One 4, e6933 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli P., Carcagno S., Vallar G. & Bricolo E. Is gaze following purely reflexive or goal-directed instead? Revisiting the automaticity of orienting attention by gaze cues. Exp. Brain Res. 224, 93–106 (2013). [DOI] [PubMed] [Google Scholar]
- Grosbras M.-H., Laird A. R. & Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum. Brain Mapp. 25, 140–154 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzato V., Macaluso E., Crostella F. & Aglioti S. M. Mapping reflexive shifts of attention in eye-centered and hand-centered coordinate systems. Hum. Brain Mapp. 33, 165–78 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich G. & Prinz W. Recognition of self-generated actions from kinematic displays of drawing. J. Exp. Psychol. Hum. Percept. Perform. 27, 456–465 (2001). [DOI] [PubMed] [Google Scholar]
- Knoblich G. & Flach R. Predicting the Effects of Actions: Interactions of Perception and Action. Psychol. Sci. 12, 467–472 (2001). [DOI] [PubMed] [Google Scholar]
- Flach R., Knoblich G. & Prinz W. Off-line authorship effects in action perception. Brain Cogn. 53, 503–513 (2003). [DOI] [PubMed] [Google Scholar]
- Shepherd S. V., Klein J. T., Deaner R. O. & Platt M. L. Mirroring of attention by neurons in macaque parietal cortex. Proc. Natl. Acad. Sci. U. S. A. 106, 9489–94 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalari I., Sforza A. L. & Aglioti S. M. My face in yours: Behavioural and Electrophysiological correlates of “enfacement” in 11th Int. Multisensory Res. Forum. 36 (2010). at <http://imrf.mcmaster.ca/IMRF/ocs2/index.php/imrf/2010/paper/view/334>. [Google Scholar]
- Rochat P. Five levels of self-awareness as they unfold early in life. Conscious. Cogn. 12, 717–731 (2003). [DOI] [PubMed] [Google Scholar]
- Dalmaso M., Pavan G., Castelli L. & Galfano G. Social status gates social attention in humans. Biol Lett. 23, 450–452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardo F., Marino B. F., Actis-Grosso R., Rossetti A. & Ricciardelli P. Face age modulates gaze following in young adults. Sci Rep. 4, 4746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan G., Dalmaso M., Galfano G. & Castelli L. Racial group membership is associated to gaze-mediated orienting in Italy. PLoS One 6, e25608 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalari I., Leggenhager B., Porciello G., Serra Holmes B. & Aglioti S. M. Enfacing others but only if they are nice to you. Front. Behav. Neurosci. 8, 102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois J. H., Kalakanis L., Rubenstein A. J., Larson A. & Hallam M, S. M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychol. Bull. 126, 390–423 (2000). [DOI] [PubMed] [Google Scholar]
- Dion K., Berscheid E. & Walster E. What is beautiful is good. J. Pers. Soc. Psychol. 24, 285–290 (1972). [DOI] [PubMed] [Google Scholar]
- Eagly A. H., Ashmore R. D., Makhijani M. G. & Longo L. C. What is beautiful is good but …: A metaanalytic review of research on the physical attractiveness stereotype. Psyhcological Bull. 110, 109–128 (1991). [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annu. Rev. Psychol. 57, 199–226 (2006). [DOI] [PubMed] [Google Scholar]
- Johnson K. L. & Tassinary L. G. Compatibility of basic social perceptions determines perceived attractiveness. Proc. Natl. Acad. Sci. U. S. A. 104, 5246–51 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. S., Perlman D. & Brehm S. S. Intimate relationships. (McGraw-Hill.itle., 2007). [Google Scholar]
- Kircher T. T. et al. Recognizing one's own face. Cognition 78, B1–B15 (2001). [DOI] [PubMed] [Google Scholar]
- Platek S. M., Paul J., Gallup G. G. & Mohamed F. B. Where am I? The neurological correlates of self and other. Cognitive Brain Research 19, 114–122 (2004). [DOI] [PubMed] [Google Scholar]
- Platek S. M., Wathne K., Tierney N. G. & Thomson J. W. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Res. 1232, 173–84 (2008). [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Molnar-Szakacs I., Zaidel E. & Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Soc. Cogn. Affect. Neurosci. 1, 65–71 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch C., Dinse H. R., Tegenthoff M., Juckel G. & Brüne M. An rTMS study into self-face recognition using video-morphing technique. Soc. Cogn. Affect. Neurosci. 6, 442–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch C., Krüger M. C. & Brüne M. Repetitive transcranial magnetic stimulation over the temporoparietal junction influences distinction of self from famous but not unfamiliar others. Behav. Neurosci. 126, 792–6 (2012). [DOI] [PubMed] [Google Scholar]
- Apps M. A. J., Tajadura-Jiménez A., Sereno M., Blanke O. & Tsakiris M. Plasticity in Unimodal and Multimodal Brain Areas Reflects Multisensory Changes in Self-Face Identification. Cereb. Cortex (2013) 10.1093/cercor/bht199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M., Costantini M. & Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia 46, 3014–8 (2008). [DOI] [PubMed] [Google Scholar]
- Ionta S. et al. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 70, 363–74 (2011). [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G. & Shulman G. L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 58, 306–324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126 (1983). [Google Scholar]
- Field A. Discovering Statistics using SPSS. (SAGE Publications Ltd, 2009). [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing″. J. R. Stat. Soc. Ser. B 289–300 (1995). [Google Scholar]