Fig. 4.
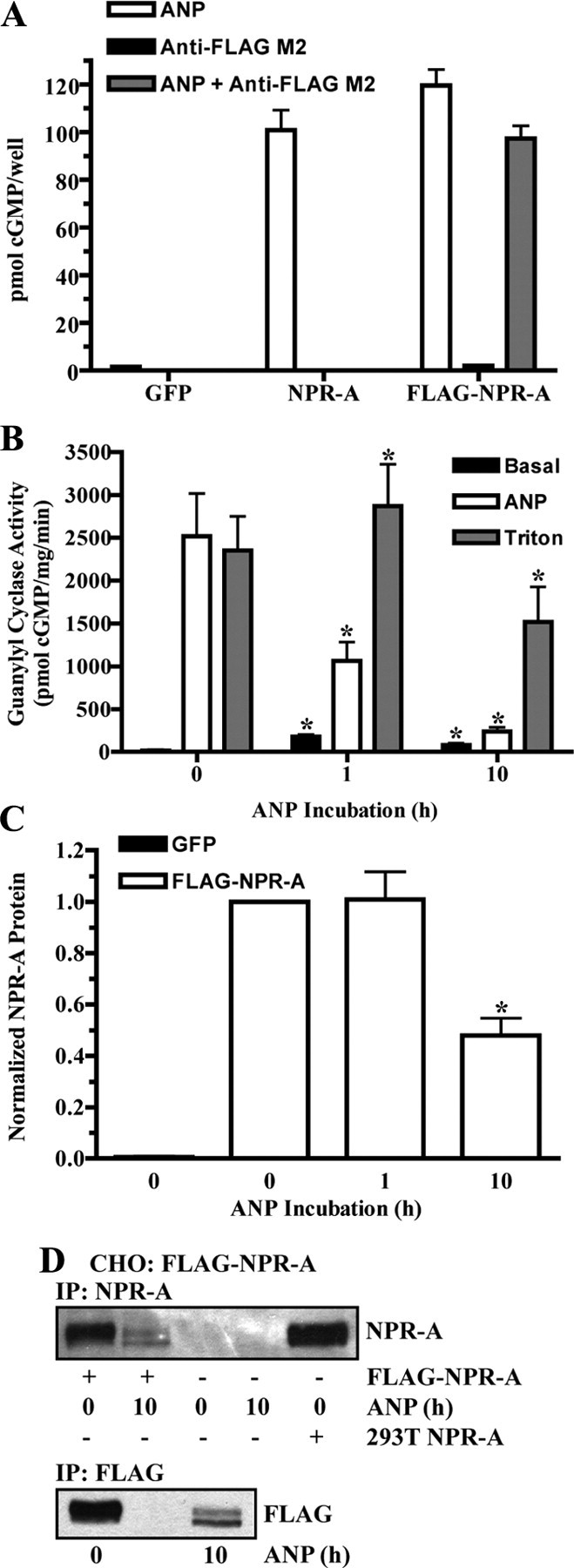
ANP stimulates FLAG-NPR-A down-regulation. A, 293 cells transiently transfected with GFP, NPR-A (wild type), or FLAG-NPR-A were stimulated with 1 μm ANP for 3 min, and cGMP levels were measured. FLAG-NPR-A transfected cells were also stimulated with anti-FLAG M2 antibody (1:2000) or both ANP and anti-FLAG M2 antibody. Data points are represented as mean ± sem, where n = 8. B–D, CHO cells were transiently transfected with or without FLAG-NPR-A and incubated with 10 μg/ml cycloheximide in the absence or presence of 200 nm ANP. B, Membranes were prepared and assayed for guanylyl cyclase activity after basal, ANP, or Triton X-100 stimulation. Data points are represented as mean ± sem, where n = 6. C, FLAG-NPR-A was immunoprecipitated with antibody to the FLAG epitope and detected by immunoblot using antibody to NPR-A. Protein levels were quantitated, normalized, and presented in graphical form as a mean ± sem, where n = 4. D, FLAG-NPR-A was immunoprecipitated and detected by immunoblot using antibody to both NPR-A (top) and the FLAG epitope (bottom). NPR-A isolated from 293T NPR-A cells was used as a positive control. Statistical significance was determined by a paired t test (vs. 0 h ANP), where *, P < 0.01 (B) and P < 0.005 (C).
