Abstract
As a modern phenomenon, there is currently limited understanding of the possible toxic effects and broader implications of electronic nicotine delivery systems (ENDS). Large volumes of aerosolized particles are inhaled during “vaping” and there are now an increasing number of case reports demonstrating toxic effects of ENDS, as well as human studies demonstrating impaired lung function in users. This article presents a case of respiratory bronchiolitis interstitial lung disease (RB‐ILD) precipitated by vaping in a 33‐year‐old male with 10 pack years of traditional cigarette and prior treatment for mixed germ cell tumour. The patient had started vaping 10–15 times per day while continuing to smoke 10 traditional cigarettes per day. After 3 months of exposure to e‐cigarette vapour, chest computed tomography demonstrated multiple new poorly defined pulmonary nodules with fluffy parenchyma opacification centred along the terminal bronchovascular units. Video‐assisted thoracoscopy with lung biopsy of the right upper and right middle lobes was undertaken. The microscopic findings were overall consistent with RB‐ILD. This case demonstrates toxicity with use of ENDS on open lung biopsy with resolution of radiographic findings on cessation. We believe that this is the first case where open lung biopsy has demonstrated this and our findings are consistent with RB‐ILD.
Keywords: Electronic nicotine delivery systems, RB‐ILD, interstitial lung disease, lung injury, thoracic surgery
Introduction
As a modern phenomenon, there is currently limited understanding of the possible toxic effects and broader implications of electronic nicotine delivery systems (ENDS). There is evidence that their prevalence has risen rapidly since introduction in 2007 among a broad range of users 1. This is further complicated by the existing and increasing heterogeneity of these products 2. ENDS have been marketed and perceived as a healthier replacement for conventional smoking, or quit smoking aids; however, there is growing concern that usage patterns are not clearly reflective of this 1. Large volumes of aerosolized particles are inhaled during “vaping” and there are now an increasing number of case reports demonstrating toxic effects of ENDS 3, 4 as well as human studies demonstrating impaired lung function in users 5.
Case Report
This article presents a case of respiratory bronchiolitis‐interstitial lung disease (RB‐ILD) precipitated by vaping in a 33‐year‐old male with 10 pack years of traditional cigarette smoking.
The patient had completed chemotherapy for tumour marker (serum alphafetoprotein (AFP) and beta human chorionic gonadotropin (BHCG)) negative mixed germ cell tumour with embryonal carcinoma (70%) and seminoma (30%) components 9 months prior. At diagnosis positron emission tomography fludeoxyglucose (PET‐FDG) staging demonstrated multiple FDG‐avid pulmonary metastases. After three courses of BEP (bleomycin, etoposide, cisplatin) chemotherapy, there was full resolution of previously FDG‐avid lung metastases with no evidence of residual disease 6 months after completion of chemotherapy. Pulmonary function tests were performed throughout chemotherapy and during follow up. Flow and volume values were within normal limits on all pulmonary function tests (PFTs). At the conclusion of chemotherapy, they were: forced expiratory volume in 1 second (FEV1) 3.72 L (94% pred), forced vital capacity (FVC) 4.84 L (103% pred), total lung capacity (TLC) 6.58 L (100% pred), and diffusion capacity of carbon monoxide (DLCo) 19.9 mL/min/mmHg (61% pred).
Following this, in an attempt to reduce smoking traditional cigarettes, the patient had started vaping. A “Royale Refillable E‐Vapour” device with “Tsunami White Spirits Vaporizer E‐Liquid E‐Juice” was used by the patient 10–15 times per day while continuing to smoke 10 traditional cigarettes per day.
Nine months post‐treatment, as part of routine follow up, computed tomography (CT) was performed. This was following 3 months of exposure to e‐cigarette vapour. Chest CT demonstrated multiple new poorly defined pulmonary nodules with fluffy parenchyma opacification centred along the terminal bronchovascular units (Fig. 1). Lesions were considered concerning for but not typical of tumour recurrence. Tumour markers remained negative as did CT of abdomen and pelvis. The patient was minimally symptomatic during this time period with some shortness of breath on retrospective questioning. PFTs were performed during this time, with following findings: FEV1 3.83 L (98% pred), FVC 4.92 L (106% pred), TLC 6.62 L (101% pred), and DLCo 22.39 mL/min/mmHg (69% pred).
Figure 1.

Pre‐biopsy computed tomography (CT) imaging demonstrating poorly defined pulmonary nodules with fluffy parenchyma opacification centred along the terminal bronchovascular units.
Investigation and management of the case involved a multidisciplinary team including surgeons, physicians, and radiologists with an interest in ILD. Bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsy were non‐diagnostic and the decision to perform open lung biopsy was made at an expert radiology and multidisciplinary meeting (MDM). Video‐assisted thoracoscopy with lung biopsy of the right upper and right middle lobes was then undertaken.
Macroscopically, the right upper lobe biopsy demonstrated multiple areas of black pigmentation and a few bullae ranging in size from 7 to 12 mm maximally at the pleural surface. Microscopic examination of both specimens showed intra‐bronchiolar and adjacent intra‐alveolar accumulation of abundant pigmented macrophages (Fig. 2). There was mild interstitial fibrosis, with interstitial accumulation of macrophages, mild lymphocytic infiltration, and occasional plasma cells. No polarisable particles or well‐formed granuloma were seen. Moderate sub‐pleural and paraseptal emphysema were also present. No evidence of malignancy was seen. Specimen slides obtained at open lung biopsy were also reviewed at subsequent MDM. The microscopic findings were considered by the group to be consistent with RB‐ILD, with fibrosis being consistent with the earliest stages of desquamative interstitial pneumonitis. The patient was advised in the first instance to cease use of the e‐cigarette and traditional cigarettes.
Figure 2.
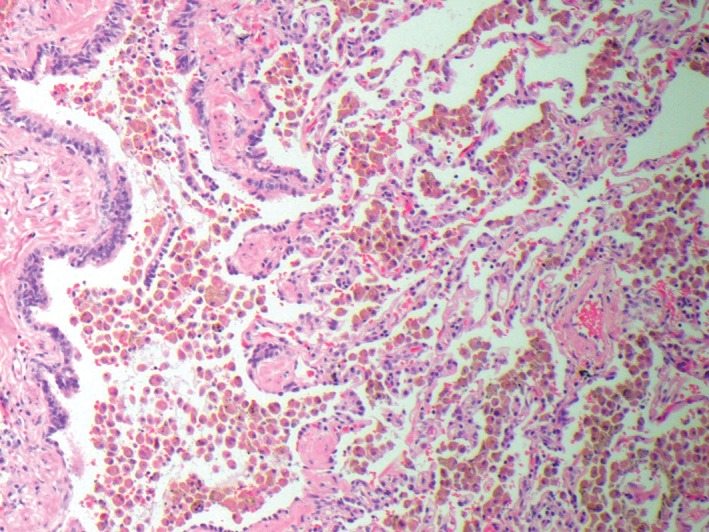
Open lung biopsy showing intrabronchial and alveolar macrophages × 10 microscopy.
One month later the patient reported having smoked only 10 traditional cigarettes and not having used e‐cigarettes in that time. There was significant radiographic improvement with resolution of all previous changes apart from a small residual nodular radio density seen abutting the oblique fissure on the right side. Subsequent PFTs 1 and 3 months after remained unchanged.
Close examination of the case time course lent weight to the diagnosis of vaping‐associated RB‐ILD. The differential was initially broad given the non‐specific nature of the imaging changes present and included cancer recurrence, bleomycin lung injury, and tobacco smoking lung injury among others. The changes were not considered to be consistent with bleomycin lung injury as they occurred later than would be expected for this pathology. PFTs remained unchanged and biopsy did not support this diagnosis. Traditional smoking has been well recognized as a cause for RB‐ILD. However, this usually occurs with significantly higher exposure than was seen in this case. Further to this, smoking of traditional cigarettes was significantly reduced in the months prior to the changes and changes resolved with ongoing low level tobacco smoke exposure. While these exposures increased the complexity of the case, there was a high level of confidence from the multidisciplinary team in the diagnosis of RB‐ILD and that vaping was the most likely precipitant.
Discussion
While there were confounding exposures associated with the case, RB‐ILD related to the use of ENDS was considered the most likely diagnosis by the MDT involved. The impacts of traditional cigarette smoke and exposure to prior bleomycin treatment are impossible to define accurately, however, traditional cigarette smoke exposure patterns had remained unchanged for many years preceding these changes. Importantly the RB‐ILD worsened after commencement and improved with cessation of vaping. We believe this may represent the first case where open lung biopsy has demonstrated this and our findings are consistent with RB‐ILD.
With increasing use of ENDs and uncertainty about their impacts and the possibility of direct toxic effects, we believe that this is an important area for future research as well as a potential diagnosis for respiratory physicians to be aware of.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Flower, M. , Nandakumar, L. , Singh, M. , Wyld, D. , Windsor, M. and Fielding, D. (2017) Respiratory bronchiolitis‐associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirology Case Reports, 5 (3), e00230. doi: 10.1002/rcr2.230.
Associate Editor: Tamera Corte
References
- 1. Drummond MB, and Upson D. 2014. Electronic cigarettes. Potential harms and benefits. Ann. Am. Thorac. Soc. 11(2):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu S‐H, Sun JY, Bonnevie E, et al. 2014. Four hundred and sixty brands of e‐cigarettes and counting: implications for product regulation. Tob. Control 23:iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkins G, and Drescher F. 2015. Acute inhalational lung injury related to the use of electronic nicotine delivery system (ENDS). Chest 148(4):83A. doi:10.1378/chest.2281610. [Google Scholar]
- 4. Hureaux J, Drouet M, and Urban T. 2014. Subacute bronchial toxicity induced by an electronic cigarette: take home message. Thorax 69:588. [DOI] [PubMed] [Google Scholar]
- 5. Vardavas CI, Anagnostopouloss N, Kougias M, et al. 2012. Short‐term pulmonary effects of using an electronic cigarette impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141(6):1400–1406. [DOI] [PubMed] [Google Scholar]


