Abstract
Caveolae, an almost ubiquitous, structural component of the plasma membrane, play a critical role in many functions essential for proper cell function, including membrane trafficking, signal transduction, extracellular matrix remodeling, and tissue regeneration. Three main types of caveolin proteins have been identified from caveolae since the discovery of caveolin-1 in the early 1990s. All three (Cav-1, Cav-2, and Cav-3) play crucial roles in mammalian physiology, and can effect pathogenesis in a wide range of human diseases. While many biological activities of caveolins have been uncovered since its discovery, their role and regulation in embryonic develop remain largely poorly understood, although there is increasing evidence that caveolins may be linked to lung and brain birth defects. Further investigations are clearly needed to decipher how caveolae/caveolins mediate cellular functions and activities of normal embryogenesis and how their perturbations contribute to developmental disorders.
Keywords: plasma membrane, caveolin-1, caveolae, cholesterol, membrane signaling, embryonic development, mechanosensing, human diseases, birth defects
Introduction
The plasma membrane is a dynamic multidomain membrane system participating in numerous cellular processes including, but not limited to, endocytosis, transcytosis, signal transduction and receptor recycling in eukaryotic cells (Jasmin et al., 2012). In many cell types, the plasma membrane is heavily decorated with specialized plasma membrane subdomains called caveolae, which are small, flask-shaped invaginations 50–100 nm in diameter (Palade, 1953; Yamada, 1955). Despite the initial discovery of caveolae more than six decades ago, their secrets have only gradually been revealed. Caveolae are present virtually ubiquitously in many cell types, most prominently in adipocytes, vascular endothelial cells (ECs), fibroblasts, and epithelial cells, and their classically described functions include endocytosis, cell signaling, and lipid and cholesterol regulation (Cohen et al., 2004a; Jasmin et al., 2012). Recent studies have elucidated additional crucial biological roles for caveolae in plasma membrane organization, signal transmission to the cell interior, remodeling extracellular matrix (ECM), and in tissue regeneration response to the injury. Moreover, caveolae mutations and dysfunction have been linked to a wide range of human diseases, including cancer, pulmonary disease, lipodystrophy, and muscular dystrophy (Cohen et al., 2004a; Jasmin et al., 2012). Here, we describe the role of caveolae and caveolae proteins in mammalian physiology and their localization and expression patterns during embryonic development. Finally, we review the current, although limited, literature that describes caveolae-related human diseases and birth defects.
Caveolae and Caveolins
DISCOVERY AND MORPHOLOGY
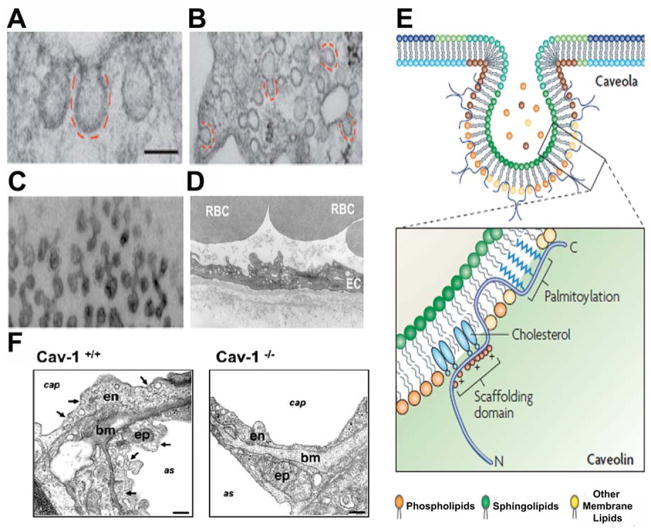
Caveolae, originally named plasmalemmal vesicles, appear as uncoated vesicles, distinct from the larger electron-dense clathrin-coated pits (CCPs). They were first morphologically identified as early as 1955 by Yamada in gall bladder epithelium (Yamada, 1955) and again by Palade in 1961 in blood capillaries (Palade, 1953). Until the 1990s, researchers thought caveolae was an electron microscopy (EM) fixation artifact because EM was the only tool available to study this organelle at the time (Severs, 1988). However, almost 40 years after their initial discovery, discovery of the most important structural protein of caveolae, named caveolins by Rothberg et al., greatly advanced the study of caveolae (Rothberg et al., 1992). Since then, biochemical studies have revealed that caveolae are detergent resistant, highly hydrophobic membrane micro-domains of lipid rafts composed of mainly sphingolipids and cholesterol (Smart et al., 1999). Caveolae have since been described in nearly all cell types as non-clathrin coated membrane invaginations. Generally, isolated caveolae appear as a variety of shapes, including flat, vesicular or U-shaped, or tubular structures possessing a distinctive granular appearance under transmission electron microscopy or low-angle platinum shadowing (Fig. 1A–D Anderson, 1998). Caveolae can be singular or found in grape-like clusters and tubules with sizes significantly >100 nm by fusion of individual caveola (Anderson, 1998).
FIGURE 1.
Caveolae and caveolin-1. Electron micrographs showing the ultrastructure of caveolae in (A) fibroblast, (B) adipocytes, (C) skeletal muscle, and (D) ECs. RBC: red blood cell. Caveolae appear as ~70 nm invaginations of plasma membrane. Scale bar, 100 nm. (E) Caveolin domains and membrane topology. Caveolae are enriched in glycosphingolipids and cholesterol. Cav-1 has its hairpin domain embedded within the membrane and both the amino terminus and the carboxyl terminus are located on the cytoplasmic side of the membrane. (F) Electron micrographs of lung tissue from wild type (Cav-1+/+) and Cav-1 deficient (Cav-1−/−) mice. Cav-1 gene KO results in the absence of caveolae in lung tissue. Arrows indicate caveolae. cap: blood capillary; as: alveolar space; bm: basal membrane; en: endothelium; ep: epithelium. Scale bars, 200 nm. Figures modified from: (A–C) Parton and del Pozo (2013); (D) Navarro et al. (2004); (E) Parton and Simmons (2007); and (F) Le Lay and Kurzchalia, (2005).
CAVEOLINS AND TISSUE DISTRIBUTION
The discovery of caveolins, the principal membrane protein components of caveolae, subsequently allowed the function of caveolae to be dissected further. Caveolin proteins are integral membrane proteins with an unusual hairpin domain embedded within the membrane where both the amino terminus and the carboxyl terminus are exposed to the cytoplasmic side of the membrane (Fig. 1E; Monier et al., 1995). To date, three members of the caveolin gene family (Caveolin-1, −2, −3) with unique tissue distributions have been identified (Table 1; Scherer et al., 1995, 1996).
TABLE 1.
Tissue Distribution of Caveolin Genes Expression
| Genes | Tissue Expression |
|---|---|
| Cav-1 | ECs, smooth muscle cells, macrophages, cardiac fibroblasts, adipocytes |
| Cav-2 | Same as Cav-1 |
| Cav-3 | Vascular smooth muscle cells, cardiac myocytes, skeletal muscle |
Caveolin-1
Originally reported as “caveolin,” the 22-kDa protein was first discovered in the early 1990s. Ultrastructural analysis by immuno-EM using freeze-fracture replicas showed caveolin as a multistriated filamentous structure, coating the cytoplasmic surface of caveolae (Rothberg et al., 1992; Fujimoto et al., 2000). Later, it was re-named as caveolin-1 (Cav-1) following the discovery of two other caveolin genes, caveolin-2 (Cav-2) and caveolin-3 (Cav-3) (Scherer et al., 1996; Tang et al., 1996). Recent studies with generation of Cav-1 knockout (KO) mice, which completely lack caveolae in the respective tissues (Fig. 1F), clearly demonstrated that Cav-1 expression is essential and required for caveoliogenesis, the formation of morphologically identifiable caveolae (Li et al., 1996; Razani et al., 2002a). Cav-1, together with Cav-2, is expressed in a wide range of tissues, including ECs, smooth muscle cells, macrophages, cardiac fibroblasts, and adipocytes. There are two isoforms of Cav-1: Cav-1α and Cav-1β, the latter lacking a part of the N-terminus (Razani et al., 2002c). It has been reported that the two isoforms of Cav-1 have different caveoliogenesis potentials and that the molecular composition of shallow and deep caveolae may vary depending on the expression levels of the two isoforms (Fujimoto et al., 2000).
Caveolin-2
Human Cav-2 is about 38% identical and about 58% similar to human Cav-1(Cohen et al., 2004a). Cav-2 was identified through microsequencing of adipocyte-derived caveolin-enriched membranes (Scherer et al., 1996). Cav-2 and Cav-1 are coexpressed, forming a hetero-oligomeric complex in many cell types with the highest levels of expression in adipocytes, fibroblasts, and smooth-muscle cells. However, no Cav-2 protein expression is found in skeletal muscle fibers and cardiac myocytes (Scherer et al., 1994, 1997). Despite the colocalization of Cav-1 and Cav-2, it appears that Cav-2 expression is regulated independently of either Cav-1 or Cav-3 expressions. For example, Cav-2 protein levels remain unchanged while Cav-1 expression is down-regulated in response to oncogenic transformation of the mouse embryonic fibroblastic NIH 3T3 cell lines (Scherer et al., 1996). Moreover, no change in Cav-2 protein level was observed in myoblast during myotube formation while significantly increased Cav-3 protein levels were observed (Song et al., 1996). These results indicate that the expression of Cav-1, Cav-2, and Cav-3 can be regulated independently in response to a variety of distinct cellular signals (Scherer et al., 1997). Interestingly, although Cav-2 has a similar expression profile to Cav-1, Cav-2 has been considered to be dispensable for caveolae formation. Generation of Cav-2 KO mice demonstrated that in the absence of Cav-2 protein, caveolae still formed and Cav-1 maintained proper membrane localization (Razani et al., 2002b; Le Lay and Kurzchalia, 2005), suggesting that functional redundancy exists between Cav-1 and Cav-2 proteins. Moreover, previous studies reported that Cav-2 played no observable role in the pathogenesis of any abnormalities observed in Cav-1 KO mice (Scherer et al., 1996; Le Lay and Kurzchalia, 2005). However, more recent studies have reported that Cav-2 KO mice do display marked lung pathology, similar to that described in Cav-1 KO mice (Razani et al., 2001a,b), suggesting that Cav-2 may have a role in lung function independent of Cav-1.
Caveolin-3
Human Cav-3 is about 65% identical and about 85% similar to Cav-1 (Cohen et al., 2004a). Cav-3 is predominantly expressed in striated muscle, in vascular smooth muscle, and skeletal and cardiac muscle (Tang et al., 1996). In muscle cells, Cav-3 is localized to the sarcolemma and coincides with the distribution of dystrophin, the muscle-specific plasma membrane protein. Cav-3 directly interacts with dystrophin and forms the dystrophin complex which includes dystrophin, α-sarcoglycan, and β-dystroglycan, suggesting an essential role for Cav-3 in muscle biology (Song et al., 1996). Genetic ablation of Cav-3 causes loss of caveolae in muscle tissues, indicating that Cav-3 is also essential for caveoliogenesis (Le Lay and Kurzchalia, 2005).
FUNCTIONAL ROLES OF CAVEOLAE AND CAVEOLINS
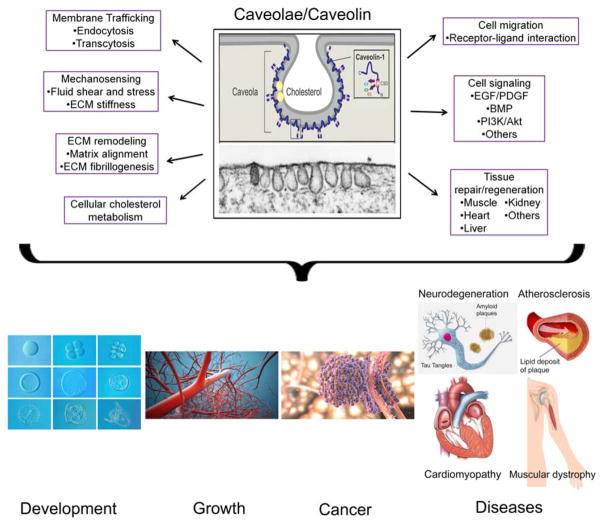
Caveolae have been implicated in numerous functions, including membrane trafficking via endocytosis and transcytosis, mechanosensing, ECM remodeling, cell migration, cell signaling, lipid metabolism, and tissue regeneration. Here, we summarize currently available literature about the function of caveolae in mammalian physiology.
Membrane trafficking
While clathrin-mediated endocytosis constitutes the classical pathway for the internalization of many extracellular ligands and plasma membrane components (Mayor and Pagano, 2007), an alternative caveolae-mediated mechanism also exists. Initially, caveolae had been described as a relatively static structure and their proposed function was limited to the process of pinocytosis, or cellular drinking (Jasmin et al., 2012). However, with the development of new tools to investigate their function, many studies in the last 25 years have identified the endocytotic role of caveolae. These studies have demonstrated that caveolae contain several molecules involved in vesicle docking and fusion shared by CCPs as well as the small GTPase dynamin, which is known to be involved in the internalization of CCPs (Schnitzer et al., 1995; Henley et al., 1998; Oh et al., 1998). These observations strongly suggest a possible role of caveolae in endocytosis. Indeed, several ligands, including membrane components (glycosphingolipids and glycosylphosphatidylinositol-anchored proteins), extracellular ligands (folic acid, albumin, autocrine motility factor, alkaline phosphatase, lactosyl ceramide), bacterial toxins (cholera toxin and tetanus toxin), and several nonenveloped viruses (Simian virus 40, Polyoma virus, respiratory syncytial virus, and HIV virus) are known to be internalized by caveolae (Henley et al., 1998; Lajoie and Nabi, 2010; Jasmin et al., 2012).
Kinases and phosphatases regulate caveolae budding and pinching off from the plasma membrane. Phosphorylation of caveolins, recruitment and activation of dynamin at the caveolar neck, and a subsequent reorganization of the actin cytoskeleton are all essential for caveolae fission from the plasma membrane (Mayor and Pagano, 2007; Jasmin et al., 2012). Upon endocytosis, caveolae can be targeted to early endosomes and then be recycled back to the surface, or targeted to multivesicular bodies or late endosome for degradation (Schnitzer et al., 1995; Jasmin et al., 2012). Although starting to emerge, the mechanism of internalization and subsequent intracellular pathways taken by the internalized substances is still not entirely clear.
As a vesicular transporter, caveolae are also reported to function in transcytosis, the uptake and the transport of macromolecule from the blood-space to the tissue-space via membrane bound vesicles (Simionescu et al., 1975). In the early 1950s, caveolae were first implicated in the transcytosis of molecules across capillary ECs. Of particular note, the uptake of albumin, an abundant serum protein that functions as a carrier for fatty acids, steroids, and thyroid hormones, was reported as a major protein component of caveolae purified from lung tissue (Vasile et al., 1983; Ghitescu et al., 1986). In the early 2000s, two groups reported that ECs derived from caveolae-deficient mice showed defects in the uptake and transport of albumin in vivo (Schubert et al., 2001; McIntosh et al., 2002), strongly suggesting the role of caveolae in transcytosis. In addition to albumin, caveolae also transcytose low density lipoproteins (LDLs) and insulin (Vasile et al., 1983; Ghitescu et al., 1986).
Mechanosensing
In addition to participating in cell transport and membrane trafficking, caveolae also behave as flow sensor converting mechanical stimuli into chemical signals and adapting to laminar flow. Mechanosensing refers to mechanisms that cells use to detect and respond to outside forces. In 1975, Dulhunty et al. first proposed that muscle caveolae would break-open in response to stretching of frog skeletal muscle fibers (Dulhunty and Franzini-Armstrong, 1975), and since then, several studies have reported a role for caveolae in protecting cells from plasma membrane damage (Czarny and Schnitzer, 2004; Yu et al., 2006; Kozera et al., 2009; Sinha et al., 2011). In summary, these studies demonstrate that caveolae play an important role in sensing force or external stimuli such as flow, shear stress, and stretch, and that they are required for further signaling events in response to mechanical cues. In several cells types, including ECs, skeletal muscle cells, adipocytes, cardiomyocytes, and fibroblasts, the morphology of caveolae changes—flattening and/or disassembly—in response to stretching of the plasma membrane (Yu et al., 2006; Kozera et al., 2009; Sinha et al., 2011). Recent studies have revealed that upon mechanical force, such as shear stress for ECs or stretch for vascular smooth muscle cells, caveolae flatten and disassemble in an ATP-and actin-independent manner (Sinha et al., 2011). These changes in morphology and a subsequent increase in the effective cell surface area could buffer and tolerate membrane tension during mechanical stress, providing a way to physically prevent rupture of the plasma membrane or cell lysis (Lee et al., 2002). Flattening and dissociation of caveolae could release factor(s) into the ECM that may modulate cellular response to mechanical stimuli at the molecular level. Additional studies are clearly needed to elucidate the nature and activity of these mechanically released factors from the caveolae.
ECM remodeling
The ECM is a highly dynamic structure present in all tissues and continuously undergoes controlled remodeling processes where ECM components are deposited, degraded or modified (Hynes, 2009; Bonnans et al., 2014). ECM remodeling is critically required in the regulation of cell growth and differentiation during the maintenance of stem cell niches, angiogenesis, bone remodeling, tissue regeneration, and wound repair (Bonnans et al., 2014). Dysregulation of ECM components, structure, stiffness, and abundance contribute to various cytopathological conditions, such as aberrant cell proliferation and apoptosis, fibrosis, and invasive cancer (Berardi et al., 2004; Fukumoto and Yamada, 2005; Hynes, 2009; Bonnans et al., 2014). In cancer progression, tumor microenvironment is also very important for tumor invasion and metastasis, since tumor ECM, consisting mainly of collagen fibers, forms migration tracks and niches for cancer cells in addition to providing survival and proliferative signals (Bonnans et al., 2014). Moreover, solid tumors are generally stiffer than normal tissue, and the mechanism behind the increased matrix stiffness is via induction of Rho-ROCK mediated actomyosin contraction (Goetz et al., 2011; Bonnans et al., 2014). Recently, Cav-1 has been shown to activate Rho signaling pathway by regulating its endogenous inhibitor p190Rho-GAP. The activation of Rho with phosphorylation of Cav-1 at Tyr14 has proven to induce matrix alignment and force-dependent actomyosin contraction, which further promotes ECM fibrillogenesis and stiffens the microenvironment (Goetz et al., 2008, 2011). The precise role of Cav-1 in tumor progression is unclear since current studies show both increased and decreased gene/protein expression profiles in various types of cancers. It appears that Cav-1 acts as a tumor suppressor at early stages of cancer progression, but is upregulated in several metastatic cancer cell lines, including breast and prostate cancers (Shatz and Liscovitch, 2008). In fact, remodeling of ECM by Cav-1 has been reported to assist cell elongation, integrin-mediated adhesiveness and focal adhesion stabilization required for directional migration of cancer cells (Goetz et al., 2011). Other than tumor microenvironment, Cav-1 also regulates fibronectin matrix turnover, which, along with collagen type I, is required to preserve the structural and functional integrity of tissues (Sottile and Chandler, 2005). Sottile and Chandler reported that down-regulation of Cav-1 by RNAi inhibited the loss of fibronectin matrix fibrils, fibronectin internalization and fibronectin degradation, and that these processes could be restored by re-expressing Cav-1 (Sottile and Chandler, 2005). It is still unknown whether these ECM remodeling processes depend solely on Cav-1 or on the caveolae as well. Further studies are required to understand the exact role of Cav-1 or caveolae in regulation of matrix-dependent cell behaviors.
Cell migration
Cell migration is an important cellular function, contributing to many physiological and pathological processes. Up to date, many reports have provided evidence suggesting multiple roles of caveolae and caveolins in cell migration, which are particularly well-studied in ECs in the context of angiogenesis (Wei et al., 1999; Navarro et al., 2004; Grande-Garcia et al., 2007). Although some discrepancies regarding the regulatory effect of Cav-1 exist, the majority of studies agree that Cav-1 and caveolae play a crucial role in cell movement through control of cell membrane composition as well as membrane surface expansion, polarization of signaling molecules, compartmentalization of intra-cellular signaling pathways, and cytoskeletal remodeling.
Cell migration, whether influenced by chemotaxis or not, is a highly regulated and polarized process in terms of speed and directionality (Kimmel and Firtel, 2004; Manahan et al., 2004). In migrating ECs, Cav-1 and caveolae are also polarized, further supporting its role in cell locomotion (Navarro et al., 2004). Moreover, Cav-1 can influence cell migration by facilitating intracellular compartmentalization of signaling pathways, as Cav-1 protein oligomers form a scaffold for assembly of signaling molecules (Smart et al., 1996; Shaul and Anderson, 1998). Included among the many proteins required for cell motility that are detected in association with caveolae are endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor receptor 2, epidermal growth factor (EGF) receptor, platelet-derived growth factor (PDGF) receptor, Ca2+-ATPase, and members of the Rho family of small GTPases (Shaul and Anderson, 1998; Navarro et al., 2004; Parton et al., 2006).
Several studies have reported that caveolin-deficient mice displayed phenotypes that could be associated with cell migratory defects. For example, Cav-1 KO mice demonstrated impaired angiogenic response to exogenous stimuli, reduced blood vessel formation after Matrigel plug implantation, and delayed skin wound healing (Woodman et al., 2003; Grande-Garcia et al., 2007). EC migration is an essential component of endothelial regeneration and angiogenesis (Woodman et al., 2003), and therefore, results from the aforementioned studies suggest that the angiogenic defects shown in vivo are most likely the consequence of impaired endothelial migration caused by loss of Cav-1 expression.
Cav-1 has been shown to interact with β-1 integrin, one of the important cell membrane components implicated in cell movement, in a complex that regulates adhesion and signaling through Src family kinases and focal adhesion kinase (Preissner et al., 2000). Formation of Cav-1 and integrin complexes contributes to cell adhesive processes, migration, and invasion (Preissner et al., 2000). Previously, Wei et al. demonstrated that Cav-1 depletion with antisense full-length Cav-1 mRNA resulted in loss of focal adhesion, indicating that Cav-1 could regulate integrin function (Wei et al., 1999). In addition, three-dimensional EM has revealed complex interactions between caveolae and the cytoskeleton through actin filaments, microtubules, and intermediate filaments. Cav-1 anchors caveolae to the actin cytoskeleton and caveolae further modulate interactions between the cell and ECM under the regulation of Rho signaling pathways (Parton and del Pozo, 2013). Caveolae are also involved in transporting cholesterol and participating in membrane recycling. Cav-1, a structural protein of caveolae, can directly bind to cholesterol and functions in transport of newly synthesized cholesterol from endoplasmic reticulum (ER) to plasma membrane (Smart et al., 1996; Uittenbogaard et al., 1998). Therefore, Cav-1 is capable of participating in the dynamics of plasma membrane composition through cytoplasmic transport and membrane surface delivery of cholesterol. Cholesterol plays a key role in the plasma membrane in regulating cell adhesion and migration. For instance, depletion of cholesterol from the plasma membrane of cells caused changes in cell morphology, decreases in cell adhesion in monolayer and motility (Ramprasad et al., 2007). In addition, cholesterol depletion has been shown to increase cell stiffness due to the interaction between the actin cytoskeleton and the plasma membrane (Norman et al., 2010). These results demonstrate that modification of cellular cholesterol content controlled by Cav-1 and/or caveolae may regulate cell signaling and migration.
Cell signaling
Caveolae serve as a platform for many different signaling pathways and the role of caveolin proteins in cell signaling regulation has been discussed as both a promoter and inhibitor. Cav-1 has been shown to regulate various signaling proteins and signaling pathways associated with cell proliferation, survival, and differentiation.
Cav-1 recruits and regulates many different signaling molecules, including tyrosine kinases, GTP-binding proteins, Ca2+, lipids and lipid-anchored proteins, etc. (Anderson, 1998; Jasmin et al., 2012). In the cases of EGFR, PDGF, and the small GTPase H-Ras molecules, Cav-1 interacts with these molecules through its scaffolding domain and further regulates other signaling molecules located in caveolae (Patel et al., 2008; Jasmin et al., 2012). For example, Cav-1 can directly bind EGFR to negatively regulate EGF signaling and EGF-mediated cell migration (Couet et al., 1997; Jasmin et al., 2012). In ECs, eNOS is localized to caveolae by acylation. In caveolae, interactions between eNOS and Cav-1 through the Cav-1 scaffolding domain have proven to be sufficient to inhibit eNOS activation and function in vivo (Garcia-Cardena et al., 1997).
Cav-1 also regulates cell fate during developmental and pathological processes by participating in cell proliferation, survival, death, and differentiation. Cav-1 has been reported as an anti-proliferative factor, where it induces cell cycle arrest by upregulating p53 and p21 while down-regulating Cyclin D1 expression in human breast cancer cells (Lee et al., 1998; Galbiati et al., 2001c). Cav-1 has been observed as both proapoptotic and prosurvival in different model systems. Although very limited, a few reports have illustrated Cav-1 as a pro-apoptotic factor, since Cav-1 can inhibit cell survival signals by negatively regulating β-catenin/TCL-LEF transcription activity in several different tumor cell lines (Torres et al., 2006, 2007). However, Cav-1 has been mainly described as a prosurvival molecule. Cav-1 is shown to promote cell survival by inducing MAP kinase p38 activity and Src-mediated PI3K and ERK1/2 pathway in heart and intestinal epithelial cells, respectively (Loza-Coll et al., 2005; Das et al., 2007).
Lastly, Cav-1 may be involved in cell signaling pathways regulating cell differentiation processes. Previously, Cav-1 was thought to be implicated in neuronal, adipocytes, vessel, and bone differentiation. For example, several studies suggested that overexpression of Cav-1 inhibited nerve growth factor receptors Trk A and p75 and significantly decreased neuritic processing and neuronal differentiation, suggesting that Cav-1might be a negative regulator (Bilderback et al., 1999; Huang et al., 1999). However, in adipocytes, induction of caveolin expression is essential and necessary during adipogenesis, suggesting that Cav-1 might be a positive regulator for fat/lipid formation (Scherer et al., 1994). Recently, we have demonstrated that Cav-1 expression increases during, and knockdown of Cav-1 expression enhances, osteogenic differentiation potential of human bone marrow derived mesenchymal stem cells (MSCs) (Baker et al., 2012, 2015). Based on our observation, we have proposed that during osteogenic differentiation in MSCs, Cav-1 is upregulated as a negative feedback mechanism to suppress continued osteogenesis. Cav-1 further inhibits PI3K/Akt signaling pathways, whose activation is required for the induction of osteogenesis in human MSCs (Baker et al., 2015). Therefore, regulation of PI3K/Akt signaling by Cav-1 may play a key role in stem cell-mediated bone regeneration.
Tissue regeneration
Recently, several studies have reported that Cav-1 may play an important role in tissue repair and wound healing. Interestingly, these studies indicate that Cav-1 may play either a positive or negative role in tissue repair, depending on the tissue type. Here, we discuss available literature about positive or negative roles of caveolins in skeletal muscle, heart, liver, and kidney as well as in blood vessel reconstruction.
Muscle
Cav-1 is expressed in murine muscle satellite cells and myogenic precursor cells (MPCs), but not in mature muscle fibers (Volonte et al., 2005). During post-injury muscle regeneration, down-regulation of Cav-1 in satellite cells and MPCs are pre-requisite for promoting their activation, proliferation, migration, and differentiation both in vivo and in vitro (Volonte et al., 2005). Following terminal muscle differentiation, Cav-3, which was not expressed in MPCs initially, is now upregulated and remains expressed in mature multinucleated myotubes (Volonte et al., 2005). The importance of down-regulation of Cav-1 during muscle repair is supported again when muscle regeneration is delayed in Cav-1 overexpressing mice and when MPCs derived from those mice fail to migrate to the wounded area, to proliferate, and to differentiate to repair wounds in vitro (Volonte et al., 2005). These results highlight the role of Cav-1 as a mediator of satellite cell activation and postnatal muscle regeneration.
Heart
Other reports have stated that Cav-1 has a direct role in cardiac remodeling after injury. Miyasato et al. examined the role of Cav-1 in cardiac repair and demonstrated that after injury, Cav-1 KO mice displayed enhanced TGF-β1 signaling, which resulted in more widespread collagen deposition in the heart compared to that of their WT counterparts (Miyasato et al., 2011). The authors reported that Cav-1 expression was reduced in mouse hearts 3 days following cryoinjury, and that the return of Cav-1 expression to normal levels was important for later stages of cardiac repair (Miyasato et al., 2011). Cav-1 is known to be a negative regulator of TGF-β1 signaling (Razani et al., 2001b) and therefore, restoration of Cav-1 expression is necessary for the inhibition of the TGF-β1 signaling to reduce collagen deposition during cardiac remodeling. In fact, when injured Cav-1 KO mice were injected with Cav-1 scaffolding domain peptide, collagen deposition was significantly reduced (Miyasato et al., 2011). Taken together, these findings strongly suggest that Cav-1 may be positively involved in cardiac tissue remodeling and repair.
Liver
Cav-1 may also be essential for tissue repair and regeneration in the liver. Fernandez et al. studied the regeneration process triggered by partial hepatectomy in the liver of Cav-1 deficient mice. The authors reported that Cav-1 KO mice showed increased mortality and decreased liver regeneration capacity (Fernandez et al., 2006). Hepatocytes derived from Cav-1 KO mice showed atypical cell activation, proliferation, and differentiation potentials. Additionally, these cells failed to accumulate lipid droplets, which indicated that lipid metabolism is dysregulated in regenerated liver, and moreover, cells did not advance through the cell division cycle (Fernandez et al., 2006). These data suggest that Cav-1 plays a crucial role in coordinating liver lipid metabolism and hepatocyte proliferation in response to cellular injury.
Kidney
In addition to heart and liver regeneration, Cav-1 has been reported to affect kidney regeneration. Fujigaki et al. reported that Cav-1α expression and caveolae formation appeared on the cell plasma membrane exclusively in the early proliferating phase of regenerating proximal tubules after gentamicine-induced acute kidney failure in rats (Fujigaki et al., 2007). This transient expressed Cav-1α isoform appeared to interact with receptors for renotropic growth factors, including PDGF receptor-β and EGF receptor, suggesting that Cav-1 may positively contribute to the regenerating process of kidney through modulating growth factor receptors (Fujigaki et al., 2007).
Blood vessels
Finally, Cav-1 is required for the formation of new blood vessels. Several studies have shown that Cav-1 KO or downregulation impairs angiogenesis both in vitro and in vivo (Griffoni et al., 2000; Woodman et al., 2003; Milovanova et al., 2008). For example, Cav-1 KO mice, which completely lack endothelial caveolae, displayed alterations in tight-junction morphology and suffered from microvascular hyperpermeability (Schubert et al., 2002). Furthermore, these mice showed incomplete capillary formation, as the nascent capillaries formed within implanted tumors were clearly morphologically underdeveloped or were very poorly organized (Woodman et al., 2003). More recently, Milovanova et al. reported that Cav-1 is needed for the induction of mouse pulmonary microvascular EC proliferation in response to an abrupt disruption in laminar flow, suggesting that Cav-1 may function as a shear sensor in flow-adapted ECs (Milovanova et al., 2008). Taken together, these studies provide strong evidence that Cav-1 is essential for new blood vessel formation.
In summary, Cav-1 plays an important role in tissue repair and regeneration. In skeletal muscle, down-regulation of Cav-1 expression is a key to MPC migration to the injury site, proliferation, and differentiation. However, in heart, liver, kidney, and blood vessels Cav-1 positively mediates the regeneration processes and its expression is crucial.
Lipid and cholesterol homeostasis
Caveolae and lipid rafts are specialized membrane domains enriched in cholesterol and sphingolipid, and have been identified in most cell types (Smart et al., 1999; Parton and Richards, 2003). Multiple lines of evidence have demonstrated that these specialized membrane domains function in the regulation of lipid and cholesterol homeostasis. First of all, proteins associated with caveolae and lipid rafts confer the stability of lipid rafts. One of the most common modifications found in proteins associated with lipid rafts is acylation, and Cav-1 has been shown to be acylated at three different sites and then inserted into caveolae (Uittenbogaard et al., 1998). This allows Cav-1 to interact with other caveolae-associated proteins involved in cholesterol metabolism and trafficking, such as actin, annexin II, and the scavenger receptor class B type I (SR-BI) (Babitt et al., 1997; Graf et al., 1999; Smart et al., 1999). SR-BI, a high density lipo-protein (HDLs) receptor, is localized to caveolae and has been shown to mediate selective cholesterol ester uptake and cellular cholesterol efflux to HDLs (Babitt et al., 1997; Graf et al., 1999). Moreover, interactions between Cav-1 and these caveolae-associated proteins generate signaling platforms and confer lipid raft stability by allowing the formation of larger aggregates of lipid rafts (Mundy et al., 2002; Parton and Richards, 2003). Newly synthesized cholesterol from the ER is transported through the cytosol to the plasma membrane at caveolae (Uittenbogaard et al., 1998), and transporting cholesterol between caveolae and intracellular sites involves two types of chaperone complexes. Chaperone complex I is for cholesterol trafficking to plasma membrane surface caveolae from the ER, while chaperone complex II is required for cholesterol trafficking from surface caveolae to ER (Everson and Smart, 2001). Acylatation of Cav-1 is also important in this chaperone complex-mediated cholesterol trafficking because different modifications of Cav-1 can regulate the activity of these complexes and their routing to specific intracellular domains within the ER or the Golgi (Uittenbogaard and Smart, 2000).
Both in vitro and in vivo experiments have shown more direct effects of caveolae/Cav-1 in lipid and cholesterol metabolism. For example, two groups demonstrated that transgenic expression of Cav-1 in cells that do not normally express endogenous caveolins significantly increased uptake of fatty acids into cells and enhanced free cholesterol efflux (Fu et al., 2004; Meshulam et al., 2006). These studies provided strong evidence that transport of fatty acids and free cholesterol across the plasma membrane is modulated by Cav-1. In vivo experiments further elucidate caveolae in lipid and cholesterol regulation. It has been reported that Cav-1 KO mice show decreases in fat mass, in the level of free cholesterol in lipid droplets, in cholesterol synthesis and in lipolytic activity (Razani et al., 2002a; Le Lay and Kurzchalia, 2005). Cav-1 deficiency in mice resulted in accumulation of free cholesterol in mitochondrial membrane and caused mitochondrial dysfuction and apoptotic susceptibility via reactive oxygen species-induced cell death (Bosch et al., 2011; Asterholm et al., 2012). Taken together, these studies suggest that caveolae may coordinate lipid and cholesterol uptake, storage and transport, and that the mutation or loss of Cav-1 in patients could be associated with lipid/cholesterol metabolic disorders, which will be discussed in detail in a later section of this review.
Developmental Expression of Murine Caveolin Genes
There is relatively sparse information about expression of caveolin genes during mouse development and embryogenesis. In this section, we will discuss developmental expression patterns of the caveolin gene family and caveolin protein expressions based on currently available literature.
Developmentally, all three caveolins, Cav-1, −2, and −3, are expressed in the mouse embryo in a tissue-specific manner after gastrulation begins on embryonic day (E) 6.5 (Fig. 2; Table 2). Northern blot analysis of mRNA isolated from whole mouse embryos demonstrated that mRNA for both Cav-1 and Cav-2 were expressed on E7, down-regulated by E11, up-regulated by E15, and remained elevated on E17 (Engelman et al., 1998b). In terms of tissue-specific expression, in situ hybridization revealed prominent expressions of Cav-1 mRNA in the heart and the urogenital ridge of both male and female mouse embryos, and weak expression in intersometic vessels at E9.5 (Bullejos et al., 2002). Cav-1 mRNA appeared to be expressed for the first time in lungs at E12 (Engelman et al., 1998b; Ramirez et al., 2002) and retained high expression levels until E17 (Ramirez et al., 2002). In the period between E12.5 and E16.5, during differentiation of the testis cords but before differentiation of the ovary, expression of Cav-1 mRNA dramatically increased in the ovary, but remained at low or moderate levels in the testis (Bullejos et al., 2002). Female-specific up-regulation of Cav-1 mRNA expression at the urogenital ridges suggests that Cav-1 may be identified as a candidate female-specific gene.
FIGURE 2.
A schematic showing developmental expression of murine Cav-1, Cav-2, and Cav-3 in whole mouse embryos. mRNA for both Cav-1 and Cav-2 are expressed on embryonic day (E) 7, downregulated by E11, upregulated by E15, and remain elevated until E17. Cav-3 mRNA expression, although very weak, is first visible at E10. Cav-1 protein is first detected at E10, but the levels of all three caveolin proteins are significantly upregulated by E15 and remain elevated until E17.
TABLE 2.
Tissue Distribution of Cav-1, Cav-2, and Cav-3 mRNA and Protein During Mouse Embryogenesis
| Age | mRNA | Protein | Genes |
|---|---|---|---|
| E7 | Whole mouse embryo (Engelman et al., 1998b). | Unknown | Cav-1 |
| E9.5 | Heart, Intersomitic Vessels, Urogenital ridge (Bullejos et al., 2002). | Cav-1 | |
| E10 | Unknown | Lung ECs (Jasmin et al., 2012; Ramirez et al., 2002) | Cav-1α |
| Ventricular heart chamber (Biederer et al., 2000). | No observable protein | Cav-3 | |
| E11 | Somites, Atrial heart chambers (Biederer et al., 2000). | Cav-3 | |
| E12 | Lung (Ramirez et al., 2002). | No observable protein | Cav-1 |
| Tongue, Skeletal muscles (Biederer et al., 2000). | Cav-3 | ||
| E12.5 | Testis, Ovaries (Bullejos et al., 2002). | Cav-1 | |
| E13.5 | Testis, Ovaries (Bullejos et al., 2002). | Cav-1 | |
| E15 | Kidney, Gut, Brain (Engelman et al., 1998b). | Cav-1 | |
| Myocardium (Biederer et al., 2000). | Cav-3 | ||
| E16 | Lung parenchyma (Engelman et al., 1998b; Ramirez et al., 2002). | Cav-1 & Cav-2 | |
| Lung epithelial cells (Engelman et al., 1998b; Ramirez et al., 2002). | Cav-1β & Cav-2 | ||
| Diaphragm, Tongue, Hindlimb muscle, Cardiomyocyte (Engelman et al., 1998b). | Cav-3 | ||
| E19 | Lung alveolar ECs (Jasmin et al., 2012). | Cav-1α |
Cav-1 protein showed delayed expression pattern compared to its mRNA. Engelman et al. reported that mRNA expressions of Cav-1 and Cav-2 in whole mouse embryo were first detected at E7, but they detected little to no caveolin proteins from embryos harvested on E11 using Western blot analysis. Subsequently, in accordance with their Northern blot results, the levels of all three caveolin proteins were significantly upregulated by E15 and stayed elevated on E17 (Engelman et al., 1998b). A few years later, Ramirez et al. reported the presence of Cav-1 protein in lung ECs as early as E10 using immunostaining (Ramirez et al., 2002). Later, Cav-1 protein was detected in testis and ovaries at E13.5 (Bullejos et al., 2002) and in kidney, gut, and brain at E15 (Engelman et al., 1998b). Interestingly, both Cav-1 and Cav-2 proteins were detected in lung parenchyma and lung epithelial cells at E16 (Engelman et al., 1998b; Ramirez et al., 2002). Unfortunately, information about Cav-2 mRNA or protein expression during murine embryogenesis is extremely scarce, perhaps due to structural similarities and functional redundancy between Cav-1 and Cav-2.
An early study reported that Cav-3 mRNA first became detectable on E15 and remained elevated until E17; however, no tissue-specific observation of Cav-3 mRNA expression had been described (Engelman et al., 1998b). In a more recent study, in situ hybridization of mouse embryo tissue sections with a Cav-3 specific probe revealed that Cav-3 mRNA expression, although very weak, was first visible in the myocardial wall of the ventricular heart chamber as early as E10. At E11, Cav-3 mRNA was also detected in the developing somites and atrial chambers of the heart. Stronger gene expression was observed in tongue and skeletal muscles at E12 and in the entire myocardium at E15. In summary, Cav-3 mRNA expression appears to be restricted to striated muscle, both in cardiac and skeletal muscle tissues and is induced after the gastrulation stage of mouse embryogenesis (Biederer et al., 2000).
In accordance with mRNA expression patterns, Cav-3 protein was detected exclusively in skeletal and cardiac muscle tissue, including developing diaphragm, tongue, hind limb muscles, and cardiomyocytes. Surprisingly, little or no expression of Cav-3 protein was observed in those tissues until E16 (Engelman et al., 1998b). However, currently available literature contain limited information and therefore, more research on tissue-specific expression of Cav-3 protein at earlier points during mouse embryogenesis is urgent.
Up to date, there has been no mechanistic study to explain how and why caveolin genes are regulated during embryogenesis. In the mouse, gastrulation, a stage of development in which the primary germ layers are formed and from which all the fetal tissues will develop, begins shortly after a blastula implants into uterine wall at around E6.5 (Fig. 2; Tam and Loebel, 2007). This is immediately followed by organogenesis, the development of the various organs. During gastrulation, the mouse embryo changes dramatically in size and shape and undergoes different cellular and molecular events (Tam and Behringer, 1997). For example, formation of the primitive node on the posterior side of the embryo induces the secretion of various cellular signals, such as fibroblasts growth factor (FGF), transforming growth factor beta (TGF-β; Tam and Behringer, 1997), and PDGF (Andrae et al., 2008). These growth factors and signals are thought to help cell migration and proliferation, which are the major morphogenetic force that leads to the expansion of three germ layers -the ectoderm, endoderm, and mesoderm. Interestingly, Cav-1 gene was first expressed in a whole mouse embryo at E7, at early gastrula stage, downregulated at E11, and significantly upregulated again at E15. Earlier in this review, we have described that Cav-1 plays an important role in recruiting and regulating PDGF signaling events, which take place in caveolae (Liu et al., 1996). In fact, Cav-1 has also been shown to negatively regulate TGF-β and FGF2 induced cell migration or angiogenesis in many different cell types (Razani et al., 2001b; Miyasato et al., 2011; Feng et al., 2012). Additionally, there is evidence that caveolae is involved in signal transduction by bone morphogenetic protein (BMPs), which play important roles during embryonic development (Nohe et al., 2005). BMP signaling begins by BMP binding to serine/threonine kinase receptors to initiate the Smad signaling cascade which further leads to the regulation of gene expression events associated with osteogenesis, chondrogenesis, neurogenesis, and hematopoiesis (Wang et al., 2014). Recent studies indicated that BMP receptors are flexible and dynamic on the cell surface and that these proteins complex with caveolae protein. Nohe et al. showed that Cav-1 binds to receptors for BMP2, one of the most important BMPs in bone formation. This interaction inhibits Smad2 phosphorylation and activation of the Smad signaling pathway (Nohe et al., 2005). Furthermore, Cav-1 has been shown to regulate the Cripto-1 signaling pathway, which plays a crucial role in early embryonic development (Bianco et al., 2008). Cripto-1 functions in formation of the primitive streak during gastrulation, anterior/posterior axis patterning, specification of mesoderm and endoderm, and arrangement of left/right asymmetry of developing organs (Cheng et al., 2003; Chen et al., 2006). Taken together, it is possible that Cav-1 gene expression and protein distribution changes during embryonic development act to orchestrate other key signaling molecules that are directly involved in germ layer cell migration and formation. For example, it is likely that caveolins are upregulated at certain stages of embryonic development as a negative feedback mechanism to suppress PDGF, TGF-β, FGF2, and/or BMP2 signaling pathways.
Caveolae and Caveolins in Human Disease
Development of caveolin-deficient murine models has provided clues that caveolae and caveolin genes play a critical role in mammalian physiology. More importantly, these animal models show that even if caveolin proteins are not essential for life, their absence or alterations still effect pathogenesis of a number of human diseases, including cancer, pulmonary disease, neurodegeneration, aging, muscular dystrophy, cardiomyopathy, atherosclerosis, and others (Table 3).
TABLE 3.
Phenotypes of Caveolin KO Mice and Associated Human Disease
| Gene | Phenotype/abnormalities in KO mice | Associated human disease phenotypes |
|---|---|---|
| Cav-1 |
|
|
| Cav-2 |
|
|
| Cav-3 |
|
|
CANCER
The role of caveolae in cancer remains controversial with either beneficial or detrimental effects. Clinically, the genes for Cav-1 and Cav-2 are localized in close proximity to a region commonly deleted in a variety of human cancer, including breast, kidney, lung, pancreas, neck, ovary, colon, and stomach cancers (Cohen et al., 2004a; Williams and Lisanti, 2004, 2005). In case of breast cancer, several human cancer cell lines display decreased Cav-1 and caveolae expression levels compared to benign mammary epithelial cells. Interestingly, re-expression of Cav-1 in these cells was sufficient to decrease cancer cell proliferation and even prevented them from forming colonies in vitro (Engelman et al., 1998a; Lee et al., 2002). Importantly, sequence analysis of Cav-1 in patients revealed sporadic mutations in up to 16% of the cases examined, and patients harboring Cav-1 mutation have been reported to have an 82% chance of cancer recurrence (Hayashi et al., 2001; Jasmin et al., 2012). In addition, Cav-1 and/or Cav-2 have been reported to be down-regulated in oral squamous cell and thyroid carcinomas (Aldred et al., 2003; Han et al., 2004). Recently, several studies have reported that changes in stromal Cav-1 expression closely correlate with tumor progression and growth. For example, the loss of stromal Cav-1 could promote tumor growth through up-regulation of collagen type VI and other ECM components in the tumor/stromal microenvironment (Trimmer et al., 2011). On the other hand, increased stromal Cav-1 expression is associated with reduced tumor size, grade, metastasis, and improved survival (Sotgia et al., 2012; Martins et al., 2013).
Generation of Cav-1 KO animal models has significantly advanced understanding of the functional roles of Cav-1 in cancer. Several studies reported that although Cav-1 KO mice did not spontaneously develop tumors, they were more susceptible to chemically induced skin tumors and transgenically induced mammary dysplasia (Williams and Lisanti, 2004; Jasmin et al., 2012). These studies further suggested that the loss of Cav-1 alone appeared insufficient to induce tumor cell growth and transformation in vivo; however, low or down-regulation of Cav-1 expression could potentiate the development of cancer when combined with a transforming agent, such as a carcinogen (Cohen et al., 2004a; Williams and Lisanti, 2004, 2005; Jasmin et al., 2012). These observations from clinical, biochemical, and molecular studies suggest that in particular, Cav-1 may be a tumor suppressor.
Cav-1 has also been reported to be consistently upregulated in bladder, esophagus, and prostate carcinomas (Li et al., 2001; Tahir et al., 2001; Williams and Lisanti, 2005; Jasmin et al., 2012). It has been suggested that during the early stages of tumorigenesis, Cav-1 may act as a tumor suppressor by inhibiting tumor cell cycle, cell growth, and cell proliferation. Some evidence shows, however, that Cav-1 may also act as an oncogene and induce more advanced cancer phases, including metastasis (Li et al., 2001; Tahir et al., 2001; Williams and Lisanti, 2005). In the case of prostate cancer, Cav-1 is present in the serum of patients, and circulating Cav-1 levels correlates with the extent of disease, suggesting that elevated Cav-1 may promote prostate cancer progression (Jasmin et al., 2012). In addition, mouse and human prostate tumor cells secrete Cav-1 in vitro, stimulated by androgen. More recently, Diaz et al. laid out the mechanism behind Cav-1 and its effect on metastatic colon and melanoma cancer cells migration. They proposed that elevated Cav-1 prevents inactivation of Rab5, a small GTPase that promotes Rac1 and cancer cell migration, and increased Cav-1-Rab5-Rac1 signaling axis led to enhanced tumor cell migration and invasion (Lobos-Gonzalez et al., 2013; Diaz et al., 2014).
Interestingly, caveolin gene expressions could be both downregulated and upregulated in thyroid carcinoma, depending on the stage of the cancer. The most common subtypes of thyroid carcinoma are papillary carcinoma and follicular carcinoma (LiVolsi and Asa, 1994). Cav-1 overexpression was observed as an early event in the progression of papillary carcinoma (Ito et al., 2002), whereas both Cav-1 and Cav-2 were significantly down-regulated in sporadic follicular carcinoma (Aldred et al., 2003). In addition to thyroid cancer, Cav-1 expression levels compared with normal tissue varied, unchanged or inconclusive in several different types of cancer, including colon, kidney, lung, brain, and pancreas (Williams and Lisanti, 2005). Taken together, these observations strongly suggest that Cav-1 may truly possess both tumor suppressor and oncogenic characteristics as its expression levels are downregulated, unchanged, or upregulated, depending on the tumor cell type or stage of the disease. Future research on identifying factors that determine caveolin gene expression level at each stage or type of cancer will greatly advance current understanding of tumorigenesis.
PULMONARY DISEASE
Compared with other tissues, the lung demonstrates one of the highest levels of Cav-1 and Cav-2 expression, compared to the “detectable” level of expression in ECs and alveolar Type I epithelial cells in the adult lung (Newman et al., 1999; Jasmin et al., 2012). The importance of Cav-1 and Cav-2 in normal lung function was dramatically demonstrated through generation of Cav-1 and Cav-2 deficient mice.
Cav-1 KO mice suffered from pulmonary hypertension as well as a hyper-proliferative morphological phenotype which is characterized by constricted alveolar spaces, thickening of the alveolar wall, fibrosis, and endothelial hyper-cellularity, which resulted in severe physical limitations (Drab et al., 2001; Razani et al., 2002a,b). Moreover, pulmonary abnormalities in Cav-1 KO mice contributed to increased exercise intolerance, as manifested by the early onset of exhaustion in those mice during a swimming test (Cohen et al., 2004a). Previously, Kasper et al. reported that loss of Cav-1 expression in type I pneumocytes increased the severity of pulmonary fibrosis in rats after irradiation-induced lung injury (Kasper et al., 1998). It has been shown that caveolae regulate lung fibrosis by trafficking TGF-β receptor and control cell signaling process via Cav-1. Caveolae endocytosis can facilitate ubiquitination and degradation of TGF-β receptor, thus turning off TGF-β signaling pathways. Therefore, when Cav-1 is depleted or deficient, TGF-β activity would be uninhibited and would lead to further perturbation in fibrosis upon lung injury (Jasmin et al., 2012).
Interestingly, many defects observed in Cav-1 deficient mice are also found in Cav-2 KO mice. Cav-2 deficient mice are characterized with histologically abnormal lungs that are indistinguishable from that of Cav-1 deficient mice (Razani et al., 2002b; Cohen et al., 2004a). These results were unexpected since Cav-2 is co-expressed with Cav-1 in most cell types, including lung. These findings suggest that Cav-2 may play an important role in normal lung function independent of Cav-1, but further studies need to be done to confirm this hypothesis.
NEURODEGENERATION AND AGING
The importance of caveolins in neurodegenerative disease and aging has previously been reported by various groups. These studies demonstrated that brains from Cav-1 KO mice developed central nervous system pathology similar to Alzheimer’s disease (AD) and other forms of dementia, such as altered N-methyl-D-asparatate receptor activation and downstream signaling, motor and behavioral abnormalities, increased ischemic cerebral injury, impaired spatial memory, and cholinergic function (Trushina et al., 2006; Jasmin et al., 2007; Gioiosa et al., 2008; Head et al., 2008). In addition, other groups reported distinctive neurological phenotypes, including reduction in brain size and behavior changes that progressed with age (Trushina et al., 2006; Head et al., 2010) from Cav-1 KO mice.
More recently, Head and colleagues also demonstrated that the loss of Cav-1 accelerated neurodegeneration and aging in mice (Willmann et al., 2006; Head et al., 2010). In mice, one of the reasons for age-related synapse degradation is the age-related decline in the expression of the cholesterol-binding protein Cav-1. Cav-1 organizes synaptic signaling components of the neurotransmitter and neurotrophic receptor signaling pathways within membrane/lipid rafts (MLR), which are the discrete regions of the plasmalemma enriched in cholesterol, glycosphingolipids, and sphingomyelin, essential for the development and stabilization of synapses. The authors demonstrated that the amount of synaptic signaling molecules and Cav-1 in the hippocampal MLR of Cav-1 KO mice was dramatically decreased compared to that of wild-type counterparts and more importantly, the degree of severity increased with age. Also, young Cav-1 KO mice showed signs of premature neuronal aging and exhibited pathological signs indicative of AD, including significantly elevated levels of amyloid-β (Aβ), astrogloiosis and P-Tau in the hippocampus (Head et al., 2010). Interestingly, neuron targeted re-expression of Cav-1 in neurons derived from Cav-1 KO mice in vitro decreased Aβ expression, suggesting that loss of Cav-1 protein is associated with premature neurodegenerative phenotype.
Furthermore, Cav-1has recently been identified as a substrate of parkin, one of the major genes found to be mutated in Parkinson’s disease (PD). Cha et al. had reported that loss of parkin function promoted disruption of the ubiquitination and degradation of Cav-1, resulting in accumulation of Cav-1 protein in cells (Cha et al., 2015). The elevated Cav-1 protein further led to altered total cholesterol level and membrane fluidity in parkin KO cells, causing dysregulation of lipid rafts-dependent endocytosis. The authors suggested that dysfunction of proteins in lipid rafts, including Cav-1, may be a causal factor of PD. In conclusion, the above studies highlight the importance of Cav-1 in neurodegenerative pathology and its implications in AD and PD. Taken together, these studies suggest that Cav-1 may be a novel therapeutic target for AD or PD.
MUSCULAR DYSTROPHY
Cav-3, a muscle specific form of caveolin protein, is the principal structural protein of caveolae membrane domains in striated muscle cells (Tang et al., 1996). Cav-3 is expressed in most muscle tissue types, including diaphragm, heart, skeletal muscle, and smooth muscle. Its gene expression increases dramatically during the differentiation and fusion of myoblasts (Galbiati et al., 2001b). Since its identification as the main caveolae protein expressed in skeletal and cardiac myocytes, extensive research has now implicated Cav-3 in skeletal myopathies. Beginning in 1998, Cav-3 mutations were identified in patients with autosomal dominant limb-girdle muscular dystrophy (LGMD) and were associated with severe deficiencies in Cav-3 expression in skeletal muscle fibers (Minetti et al., 1998). Cav-3 deficient mice were first generated in the early 2000s by two groups (Galbiati et al., 1999; Hagiwara et al., 2000), Indeed, Cav-3KO mice lacked sarcolemmal caveolae in skeletal and cardiac muscle cells, and demonstrated histologically abnormal skeletal muscle with necrotic muscle fibers and centralized nuclei, which recapitulate the mild muscular dystrophy phenotype described in patients with Type IC-LGMD. Mechanically, Cav-3 interacts with the dystrophin-glycoprotein complex (DGC) at the sarcolemma and Cav-3 binds specifically to β-dystroglycan. Therefore, Cav-3 KO mice completely lack caveolae and functional Cav-3 protein, resulting in the exclusion of the DGC from lipid raft microdomains (Galbiati et al., 2001a). In addition to muscle degeneration, abnormalities in the organization of T-tubule systems with dilated and longitudinally oriented T-tubules were identified (Galbiati et al., 1999; Hagiwara et al., 2000; Galbiati et al., 2001b). More recently, additional mutations in Cav-3 have been identified among patients with other myopathies, including rippling muscle disease, distal myopathy, and idiopathic hyperCKemia (Carbone et al., 2000; Betz et al., 2001; Kubisch et al., 2003; Woodman et al., 2004b).
CARDIOMYOPATHY
Both Cav-1 and Cav-3 KO mice suffer from heart pathology, including cardiac hypertrophy, cardiomyopathy, and reduced fractional shortening (Woodman et al., 2002; Cohen et al., 2003; Hayashi et al., 2004). Histological examination of cardiac tissue sections demonstrated perivascular fibrosis, myocyte hypertrophy, and cellular infiltration (Woodman et al., 2002). Cav-3 is the chief caveolin family member expressed in cardiac myocytes (Galbiati et al., 2001a) and Cav-1 is expressed mostly in non-muscle cell types in the heart, including cardiac fibroblasts and ECs (Cohen et al., 2003). Hayashi et al. (2004) recently reported that Cav-3 mutation was detected among patients with familial hypertrophic cardiomyopathy and dilated cardiomyopathy. The mutation occurred at Thr63Ser, which was also involved in other mutations causing LGMD.
In order to create a truly caveolae-deficient mouse, Park and colleagues created Cav-1/Cav-3 double knockout (dKO) mice, which were viable and fertile, despite the fact that they lacked morphologically identifiable caveolae in both muscle and non-muscle cells (Park et al., 2002). As expected, these dKO mice exhibited lung, fat and skeletal muscle defects similar to their single KO counterparts, suggesting that combined loss of caveolin proteins (Cav-1 and Cav-3) did not produce any alterations in the phenotypes previously identified in single KO animals. However, functional, molecular, and histological analysis revealed that these mice developed more severe cardiomyopathy (Park et al., 2002). Although the mechanism behind the increased severity of cardiomyopathy upon combined deletion of caveolin genes still needs to be deciphered, this study emphasizes the importance of both caveolin isoforms for normal cardiac structure and function.
UROGENITAL DISEASES
Cav-1 deficient mice exhibited urogenital organ disorders that are similar to the lower urinary tract dysfunction (LUTD) observed in elderly adult male humans. Interestingly, these pathological changes were detected in the bladders of male Cav-1 KO mice only, but not Cav-2 or Cav-3 KO mice (Williams and Lisanti, 2004; Woodman et al., 2004a; Le Lay and Kurzchalia, 2005). This observation is unexpected since the bladder is composed primarily of smooth muscle cells that express all three caveolin proteins. Only loss of the Cav-1, not Cav-2 or Cav-3, resulted in the absence of morphologically identifiable caveolae in the bladder of male mice, highlighting again that Cav-1 is essential and necessary for caveoliogenesis in smooth muscle cells (Woodman et al., 2004a). Cav-1 KO mice demonstrate significant fluid accumulation in the prostate, seminal vesicles, and kidneys, which eventually results in marked dilation and even necrosis of these organs. Moreover, loss of Cav-1 in male mice show increases in bladder weight, smooth muscle layer thickness, and bladder pressure (Woodman et al., 2004a). Additionally, Cao et al. demonstrated that disruption of Cav-1 gene resulted in urolithiasis, presumably through the loss of renal calcium reabsorption and subsequent hypercalciuria (Cao et al., 2003). The above studies indicate that Cav-1 is an important contributor to normal urogenital function and development.
ATHEROSCLEROSIS
In the 1950s, caveolae were first identified as plasmalemmal vesicles that play an important role in the transport of macromolecules from the lumen of blood vessels to peripheral tissues (Bruns and Palade, 1968a,b). However, until recently, very little work had been done to demonstrate the role of caveolae in the development of atherosclerosis. Atherosclerosis is a disease of the blood vessel characterized by the lesions formed by the initial buildup of cholesterol-enriched lipoproteins followed by the deposition of smooth muscle cells and macrophages (Nordestgaard, 1996). As presented earlier in this review, Cav-1 and caveolae are highly expressed in ECs, macrophages, and smooth muscle cells (Williams and Lisanti, 2004). Given the relative cholesterol enrichment of caveolae compared to the rest of the plasma membrane, several studies have suggested that dysregulation of Cav-1 and/or caveolae might be associated with vascular dysfunction. Utilizing Cav-1 deficient mice has proven that loss of Cav-1 resulted in profound vascular dysfunction with defects in endothelium-dependent contractile responses, mediated primarily through a loss of inhibition of eNOS activity in mice (Drab et al., 2001; Razani et al., 2001a; Williams and Lisanti, 2004). In addition, Drab et al. also demonstrated that Cav-1 KO mice displayed thickening of lung alveolar septa, mainly caused by an uncontrolled proliferation of ECs and fibrosis (Drab et al., 2001). To directly examine the in vivo role of Cav-1 and caveolae in atherosclerosis, Frank et al. interbred Cav-1 KO mice with atherosclerosis-prone ApoE−/− mice, and found that Cav-1 loss significantly increased plasma cholesterol and triglyceride levels with elevated atherosclerotic lesions in the ApoE−/− background (Frank et al., 2004), clearly demonstrating the involvement of Cav-1 in the development of vascular disease.
LIPODYSTROPHY
As stated in the beginning of this review, the functional roles of Cav-1 and caveolae in lipid and cholesterol homeostasis of mammalian physiology have been recognized. Cholesterol is a major component of caveolae, and depletion of cellular cholesterol significantly reduces the number of invaginated caveolae. In addition, Cav-1 can directly bind to cholesterol and Cav-1 has been postulated to have a role in homeostasis of not only cellular cholesterol but also fatty acids and triglycerides. The highest levels of Cav-1 and caveolae are detected in adipocytes where Cav-1 expression levels are induced about 10–25-fold during differentiation of pre-adipocytes into mature adipocytes, suggesting that Cav-1 may be associated with body fat production and metabolism (Scherer et al., 1994). Utilizing Cav-1 KO mice, several research groups have reported that loss in Cav-1, but not Cav-2, resulted in dramatic reduction in white adipose tissue deposition and a lean phenotype (Razani et al., 2002a; Cohen et al., 2004b; Kim et al., 2008). Additionally, these mice were resistant to diet-induced obesity and displayed several metabolic disorders similar to humans, including elevated levels of serum free fatty acids, triglycerides, and very-low-density lipoproteins/chylomicrons (Cohen et al., 2004b). These phenotypic characteristics parallel many of those associated with type II diabetes in humans, suggesting the crucial role of Cav-1 in normal lipid metabolism. Lastly, Cav-1 KO mice displayed an impaired lipolytic activity and perturbations in the architecture of the lipid droplet cortex (Cohen et al., 2004b), suggesting that Cav-1deficiency results in abnormal lipid droplet biogenesis and metabolism.
CAVEOLINS IN BIRTH DEFECTS
Descriptions of caveolins and caveolae involvement in congenital birth defects are very limited as their importance is only beginning to be recognized; however, there is an increasing body of evidence that Cav-1 also plays a central role in birth defects of lung and brain.
Lung
Hofmann et al. recently investigated the role of Cav-1 in the nitrofen-induced congenital diaphragmatic hernia (CDH). A diaphragmatic hernia is a birth defect present in the diaphragm, where a hole in the underdeveloped diaphragm muscle causes some of the organs that are normally found in the abdomen to move up into the chest cavity. In the fetus, the lungs are developing at the same time as the diaphragm and with heart, lungs, and abdominal organs all taking up space in the chest cavity, the lungs do not develop properly. The underdevelopment of the lungs is termed pulmonary hypoplasia (Keijzer and Puri, 2010; Keijzer et al., 2010). It had been reported that the high morbidity and mortality of newborn infants diagnosed with CDH is generally due to severe pulmonary hypoplasia and persistent pulmonary hypertension (PH) (Keijzer and Puri, 2010). Cav-1 has been implicated to play an important role in maintaining EC homeostasis in lungs by regulating eNOS activity (Drab et al., 2001; Jasmin et al., 2004). Hofmann et al. reported imbalance of Cav-1 and eNOS expression in the mouse and the rat CDH models. Pregnant mice and rats were exposed to nitrofen during Day 9 (D9) of gestation. Nitrofen exposure resulted in a high rate of CDH with associated PH and pulmonary vasculature abnormalities in fetuses sacrificed on D21, strikingly similar to the human situation (Hofmann et al., 2014). They observed that pulmonary Cav-1 gene expression and protein levels were significantly downregulated, whereas eNOS expression levels were dramatically upregulated in these CDH animal models. These results suggest that chronic activation of eNOS induced by downregulation or loss of Cav-1 may be closely linked to the pathogenesis of PH in the nitrofen-induced CDH.
Brain
More recently, Yang et al. reported that increased expressions of Cav-1 may contribute to Fragile X syndrome (FXS) (Yang et al., 2015). FXS is an inherited birth defect that causes mental retardation in humans and results from expansion of CGG repeats in the FMR1 gene, which leads to the loss of fragile X mental retardation protein (Feng et al., 1995; O’Donnell and Warren, 2002). It has been previously shown that the interaction between estrogen receptor (ER) and lipid raft caveolae is critical for the estrogen signaling pathways and that impaired ER-caveolae coupling could contribute to the mental retardation of FXS. In the study, Yang et al. used FMR1 KO mouse as the model of FXS and found increased Cav-1 expression in the cultured neurons and prefrontal cortex derived from the KO animals. This increase led to high coupling of ERα-Cav-1 and further contributed to the impairment of ER signaling in neurons derived from FMR1 KO mice (Yang et al., 2015). While there is currently no cure for Fragile X syndrome, various therapies may help the child develop. Results from this study first demonstrated that increased coupling of ERα-Cav-1may elucidate a critical abnormal mechanism of FXS and provided insights into the cellular mechanisms underlying FXS, which will greatly help developing therapeutics in the future.
Concluding Remarks
The study of caveolae and their marker proteins, the caveolins, has been improving, yet still remains a challenging endeavor. Caveolae have been involved in numerous cellular functions, including membrane trafficking, mechanosensing, ECM remodeling, cell migration, cell signaling, lipid metabolism, and tissue regeneration. To date, studies using caveolin-deficient mouse models dramatically show that caveolae and caveolins are implicated in various types of pathogenesis of human diseases (Fig. 3). Despite the fact that currently existing caveolin KO mice, whether it is a single gene or double gene (Cav-1 and Cav-3) KO, are viable and fertile (Park et al., 2002), there has been an increasing body of evidence that Cav-1 is also implicated in birth defects of lung and brain (Hofmann et al., 2014; Yang et al., 2015). Most interestingly, evidence for caveolae/Cav-1 functions in stem cell-mediated tissue repair/regeneration and wound healing is plentiful (see review by Baker and Tuan, 2013); however, more sophisticated approaches to delineate the role of caveolae/caveolin in stem cell biology are needed in the future. Now, with the availability of caveolin-deficient mice, biochemical, cell culture, gene editing, 3D organotypic culture model systems, and other non-gene related approaches can finally be intermeshed to provide a complete picture of caveolar and caveolin functions both in vivo and in stem cell model systems. For example, the clustered regularly interspaced short palindromic sequences (CRISPR)/Cas based technique may be extremely useful in creating caveolin-KO cell lines. As opposed to siRNA knockdowns or downregulation, the CRISPR/Cas system directly edits the organism’s genome which offers a permanent knockdown solution (Makarova et al., 2011), ideal for generating a Cav-1/Cav-2/Cav-3 deficient or domain-mutated cell line. This would better enable researchers to carry out in vitro experiments to further elucidate the role of caveolins in regulating signal transduction and lipid raft aggregation as well as stem cell proliferation, differentiation, substrate-driven tissue specification, and homing and mobilization. Investigating the effect of membrane cholesterol, caveolae, and caveolins in modulating stem cell substrate-rigidity responses in 3D will also greatly benefit our understanding of how stem cells need to be manipulated in some areas of regenerative medicine. Lastly, the effect of nongene related perturbations in cholesterol/caveolae/caveolins homeostasis on cell membrane properties and adhesive characteristics bear further investigation, research that will undoubtedly reveal new mechanisms of caveolae and caveolin function. These additional studies will shed light on understanding the mechanisms of action of the ubiquitous plasma membrane microstructure of caveolae and associated caveolins on cellular responses and functions that are related to human health and diseases, embryonic development, and birth defects.
FIGURE 3.
Functional involvement of caveolae and caveolin-1 in cellular activities and developmental and disease mechanisms. See text for details. CSD: caveolin scaffolding domain. Figures adapted from www.bioon.com, www.dementiablog.org, www.bionews-tx.com, www.readtiger.com, www.columbiasurgery.org, www.bio.cmu.edu, www.mesotheliomaclinic.org, and Baker and Yuan (2013).
Acknowledgments
Supported by a grant from the NIH (1R01 EB019430).
References
- Aldred MA, Ginn-Pease ME, Morrison CD, et al. Caveolin-1 and caveolin-2, together with three bone morphogenetic protein-related genes, may encode novel tumor suppressors down-regulated in sporadic follicular thyroid carcinogenesis. Cancer Res. 2003;63:2864–2871. [PubMed] [Google Scholar]
- Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asterholm IW, Mundy DI, Weng J, et al. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012;15:171–185. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitt J, Trigatti B, Rigotti A, et al. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- Baker N, Sohn J, Tuan RS. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res Ther. 2015;6:238. doi: 10.1186/s13287-015-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, Tuan RS. The less-often-traveled surface of stem cells: caveolin-1 and caveolae in stem cells, tissue repair and regeneration. Stem Cell Res Ther. 2013;4:90. doi: 10.1186/scrt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, Zhang G, You Y, Tuan RS. Caveolin-1 regulates proliferation and osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2012;113:3773–3787. doi: 10.1002/jcb.24252. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron. 2004;44:905–908. doi: 10.1016/j.neuron.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Betz RC, Schoser BG, Kasper D, et al. Mutations in CAV3 cause mechanical hyperirritability of skeletal muscle in rippling muscle disease. Nat Genet. 2001;28:218–219. doi: 10.1038/90050. [DOI] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Mancino M, et al. Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells. Am J Pathol. 2008;172:345–357. doi: 10.2353/ajpath.2008.070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer CH, Ries SJ, Moser M, et al. The basic helix-loop-helix transcription factors myogenin and Id2 mediate specific induction of caveolin-3 gene expression during embryonic development. J Biol Chem. 2000;275:26245–26251. doi: 10.1074/jbc.M001430200. [DOI] [PubMed] [Google Scholar]
- Bilderback TR, Gazula VR, Lisanti MP, Dobrowsky RT. Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways. J Biol Chem. 1999;274:257–263. doi: 10.1074/jbc.274.1.257. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Mari M, Herms A, et al. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr Biol. 2011;21:681–686. doi: 10.1016/j.cub.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968a;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968b;37:277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Bowles J, Koopman P. Extensive vascularization of developing mouse ovaries revealed by caveolin-1 expression. Dev Dyn. 2002;225:95–99. doi: 10.1002/dvdy.10128. [DOI] [PubMed] [Google Scholar]
- Cao G, Yang G, Timme TL, et al. Disruption of the caveolin-1 gene impairs renal calcium reabsorption and leads to hypercalciuria and urolithiasis. Am J Pathol. 2003;162:1241–1248. doi: 10.1016/S0002-9440(10)63920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology. 2000;54:1373–1376. doi: 10.1212/wnl.54.6.1373. [DOI] [PubMed] [Google Scholar]
- Cha SH, Choi YR, Heo CH, et al. Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: implications for Parkinson’s disease. Mol Neurodegener. 2015;10:63. doi: 10.1186/s13024-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, et al. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development. 2006;133:319–329. doi: 10.1242/dev.02210. [DOI] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Bennett JT, et al. EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1. Genes Dev. 2003;17:31–36. doi: 10.1101/gad.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004a;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Park DS, Woodman SE, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Razani B, Schubert W, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004b;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- Czarny M, Schnitzer JE. Neutral sphingomyelinase inhibitor scyphostatin prevents and ceramide mimics mechanotransduction in vascular endothelium. Am J Physiol Heart Circ Physiol. 2004;287:H1344–1352. doi: 10.1152/ajpheart.00222.2004. [DOI] [PubMed] [Google Scholar]
- Das M, Cui J, Das DK. Generation of survival signal by differential interaction of p38MAPKalpha and p38MAPKbeta with caveolin-1 and caveolin-3 in the adapted heart. J Mol Cell Cardiol. 2007;42:206–213. doi: 10.1016/j.yjmcc.2006.08.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Mendoza P, Ortiz R, et al. Rab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasion. J Cell Sci. 2014;127:2401–2406. doi: 10.1242/jcs.141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Franzini-Armstrong C. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1975;250:513–539. doi: 10.1113/jphysiol.1975.sp011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Lee RJ, Karnezis A, et al. Reciprocal regulation of neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for human breast cancer. J Biol Chem. 1998a;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Galbiati F, Lisanti MP. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, −2, and −3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31) FEBS Lett. 1998b;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- Everson WV, Smart EJ. Influence of caveolin, cholesterol, and lipoproteins on nitric oxide synthase: implications for vascular disease. Trends Cardiovasc Med. 2001;11:246–250. doi: 10.1016/s1050-1738(01)00119-0. [DOI] [PubMed] [Google Scholar]
- Feng L, Liao WX, Luo Q, et al. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. J Cell Physiol. 2012;227:2480–2491. doi: 10.1002/jcp.22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Zhang F, Lokey LK, et al. Translational suppression by trinucleotide repeat expansion at FMR1. Science. 1995;268:731–734. doi: 10.1126/science.7732383. [DOI] [PubMed] [Google Scholar]
- Fernandez MA, Albor C, Ingelmo-Torres M, et al. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, et al. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Fu Y, Hoang A, Escher G, et al. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem. 2004;279:14140–14146. doi: 10.1074/jbc.M311061200. [DOI] [PubMed] [Google Scholar]
- Fujigaki Y, Sakakima M, Sun Y, et al. Immunohistochemical study on caveolin-1alpha in regenerating process of tubular cells in gentamicin-induced acute tubular injury in rats. Virchows Arch. 2007;450:671–681. doi: 10.1007/s00428-007-0417-4. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113(Pt 19):3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamada Y. Review: extracellular matrix regulates tooth morphogenesis. Connect Tissue Res. 2005;46:220–226. doi: 10.1080/03008200500344017. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Engelman JA, Volonte D, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001a;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Caveolae and caveolin-3 in muscular dystrophy. Trends Mol Med. 2001b;7:435–441. doi: 10.1016/s1471-4914(01)02105-0. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Liu J, et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell. 2001c;12:2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Minetti C, et al. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the golgi complex. J Biol Chem. 1999;274:25632–25641. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986;102:1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L, Raggi C, Ricceri L, et al. Altered emotionality, spatial memory and cholinergic function in caveolin-1 knock-out mice. Behav Brain Res. 2008;188:255–262. doi: 10.1016/j.bbr.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- Goetz JG, Minguet S, Navarro-Lerida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf GA, Connell PM, van der Westhuyzen DR, Smart EJ. The class B, type I scavenger receptor promotes the selective uptake of high density lipoprotein cholesterol ethers into caveolae. J Biol Chem. 1999;274:12043–12048. doi: 10.1074/jbc.274.17.12043. [DOI] [PubMed] [Google Scholar]
- Grande-Garcia A, Echarri A, de Rooij J, et al. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffoni C, Spisni E, Santi S, et al. Knockdown of caveolin-1 by antisense oligonucleotides impairs angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2000;276:756–761. doi: 10.1006/bbrc.2000.3484. [DOI] [PubMed] [Google Scholar]
- Hagiwara Y, Sasaoka T, Araishi K, et al. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–3054. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- Han SE, Park KH, Lee G, et al. Mutation and aberrant expression of Caveolin-1 in human oral squamous cell carcinomas and oral cancer cell lines. Int J Oncol. 2004;24:435–440. [PubMed] [Google Scholar]
- Hayashi K, Matsuda S, Machida K, et al. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- Hayashi T, Arimura T, Ueda K, et al. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2004;313:178–184. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Tsutsumi YM, et al. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. Faseb J. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- Head BP, Peart JN, Panneerselvam M, et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 2010;5:e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A, Gosemann JH, Takahashi T, et al. Imbalance of caveolin-1 and eNOS expression in the pulmonary vasculature of experimental diaphragmatic hernia. Birth Defects Res B Dev Reprod Toxicol. 2014;101:341–346. doi: 10.1002/bdrb.21117. [DOI] [PubMed] [Google Scholar]
- Huang CS, Zhou J, Feng AK, et al. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem. 1999;274:36707–36714. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yoshida H, Nakano K, et al. Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid. Br J Cancer. 2002;86:912–916. doi: 10.1038/sj.bjc.6600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin J-Fo, Frank PG, Lisanti MP. Caveolins and caveolae: roles in signaling and disease mechanisms. New York Austin, Tex: Springer Science+Business Media; Landes Bioscience; 2012. p. 184. [Google Scholar]
- Jasmin JF, Malhotra S, Singh Dhallu M, et al. Caveolin-1 deficiency increases cerebral ischemic injury. Circ Res. 2007;100:721–729. doi: 10.1161/01.RES.0000260180.42709.29. [DOI] [PubMed] [Google Scholar]
- Jasmin JF, Mercier I, Hnasko R, et al. Lung remodeling and pulmonary hypertension after myocardial infarction: pathogenic role of reduced caveolin expression. Cardiovasc Res. 2004;63:747–755. doi: 10.1016/j.cardiores.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Kasper M, Reimann T, Hempel U, et al. Loss of caveolin expression in type I pneumocytes as an indicator of subcellular alterations during lung fibrogenesis. Histochem Cell Biol. 1998;109:41–48. doi: 10.1007/s004180050200. [DOI] [PubMed] [Google Scholar]
- Keijzer R, Puri P. Congenital diaphragmatic hernia. Semin Pediatr Surg. 2010;19:180–185. doi: 10.1053/j.sempedsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Keijzer R, van de Ven C, Vlot J, et al. Thoracoscopic repair in congenital diaphragmatic hernia: patching is safe and reduces the recurrence rate. J Pediatr Surg. 2010;45:953–957. doi: 10.1016/j.jpedsurg.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Kim CA, Delepine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- Kimmel AR, Firtel RA. Breaking symmetries: regulation of Dictyostelium development through chemoattractant and morphogen signal-response. Curr Opin Genet Dev. 2004;14:540–549. doi: 10.1016/j.gde.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kozera L, White E, Calaghan S. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One. 2009;4:e8312. doi: 10.1371/journal.pone.0008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schoser BG, von During M, et al. Homozygous mutations in caveolin-3 cause a severe form of rippling muscle disease. Ann Neurol. 2003;53:512–520. doi: 10.1002/ana.10501. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- Le Lay S, Kurzchalia TV. Getting rid of caveolins: phenotypes of caveolin-deficient animals. Biochim Biophys Acta. 2005;1746:322–333. doi: 10.1016/j.bbamcr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Lee H, Park DS, Razani B, et al. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (−/−) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002;161:1357–1369. doi: 10.1016/S0002-9440(10)64412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, et al. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Li L, Yang G, Ebara S, et al. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61:4386–4392. [PubMed] [Google Scholar]
- Li S, Song KS, Koh SS, et al. Baculovirus-based expression of mammalian caveolin in Sf21 insect cells. A model system for the biochemical and morphological study of caveolae biogenesis. J Biol Chem. 1996;271:28647–28654. doi: 10.1074/jbc.271.45.28647. [DOI] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- LiVolsi VA, Asa SL. The demise of follicular carcinoma of the thyroid gland. Thyroid. 1994;4:233–236. doi: 10.1089/thy.1994.4.233. [DOI] [PubMed] [Google Scholar]
- Lobos-Gonzalez L, Aguilar L, Diaz J, et al. E-cadherin determines Caveolin-1 tumor suppression or metastasis enhancing function in melanoma cells. Pigment Cell Melanoma Res. 2013;26:555–570. doi: 10.1111/pcmr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza-Coll MA, Perera S, Shi W, Filmus J. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene. 2005;24:1727–1737. doi: 10.1038/sj.onc.1208379. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Haft DH, Barrangou R, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan CL, Iglesias PA, Long Y, Devreotes PN. Chemoattractant signaling in dictyostelium discoideum. Annu Rev Cell Dev Biol. 2004;20:223–253. doi: 10.1146/annurev.cellbio.20.011303.132633. [DOI] [PubMed] [Google Scholar]
- Martins D, Beca FF, Sousa B, et al. Loss of caveolin-1 and gain of MCT4 expression in the tumor stroma: key events in the progression from an in situ to an invasive breast carcinoma. Cell Cycle. 2013;12:2684–2690. doi: 10.4161/cc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci USA. 2002;99:1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T, Simard JR, Wharton J, et al. Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry. 2006;45:2882–2893. doi: 10.1021/bi051999b. [DOI] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Hawkins BJ, et al. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta. 2008;1783:1866–1875. doi: 10.1016/j.bbamcr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, Sotgia F, Bruno C, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet. 1998;18:365–368. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- Miyasato SK, Loeffler J, Shohet R, et al. Caveolin-1 modulates TGF-beta1 signaling in cardiac remodeling. Matrix Biol. 2011;30:318–329. doi: 10.1016/j.matbio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier S, Parton RG, Vogel F, et al. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy DI, Machleidt T, Ying YS, et al. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- Navarro A, Anand-Apte B, Parat MO. A role for caveolae in cell migration. Faseb J. 2004;18:1801–1811. doi: 10.1096/fj.04-2516rev. [DOI] [PubMed] [Google Scholar]
- Newman GR, Campbell L, von Ruhland C, et al. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res. 1999;295:111–120. doi: 10.1007/s004410051217. [DOI] [PubMed] [Google Scholar]
- Nohe A, Keating E, Underhill TM, et al. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci. 2005;118:643–650. doi: 10.1242/jcs.01402. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG. The vascular endothelial barrier–selective retention of lipoproteins. Curr Opin Lipidol. 1996;7:269–273. doi: 10.1097/00041433-199610000-00002. [DOI] [PubMed] [Google Scholar]
- Norman LL, Oetama RJ, Dembo M, et al. Modification of Cellular Cholesterol Content Affects Traction Force, Adhesion and Cell Spreading. Cell Mol Bioeng. 2010;3:151–162. doi: 10.1007/s12195-010-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. Find structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- Park DS, Woodman SE, Schubert W, et al. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119:787–796. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner KT, Kanse SM, May AE. Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol. 2000;12:621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Pollack L, Millien G, et al. The alpha-isoform of caveolin-1 is a marker of vasculogenesis in early lung development. J Histochem Cytochem. 2002;50:33–42. doi: 10.1177/002215540205000104. [DOI] [PubMed] [Google Scholar]
- Ramprasad OG, Srinivas G, Rao KS, et al. Changes in cholesterol levels in the plasma membrane modulate cell signaling and regulate cell adhesion and migration on fibronectin. Cell Motil Cytoskeleton. 2007;64:199–216. doi: 10.1002/cm.20176. [DOI] [PubMed] [Google Scholar]
- Razani B, Combs TP, Wang XB, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002a;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001a;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Razani B, Lisanti MP. Caveolin-deficient mice: insights into caveolar function human disease. J Clin Invest. 2001;108:1553–1561. doi: 10.1172/JCI14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Wang XB, Engelman JA, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002b;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002c;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001b;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lewis RY, Volonte D, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lisanti MP, Baldini G, et al. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994;127:1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, et al. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Tang Z, Chun M, et al. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Razani B, et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–48622. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Severs NJ. Caveolae: static inpocketings of the plasma membrane, dynamic vesicles or plain artifact? J Cell Sci. 1988;90:341–348. doi: 10.1242/jcs.90.3.341. [DOI] [PubMed] [Google Scholar]
- Shatz M, Liscovitch M. Caveolin-1: a tumor-promoting role in human cancer. Int J Radiat Biol. 2008;84:177–189. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- Simionescu N, Siminoescu M, Palade GE. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975;64:586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Koster D, Ruez R, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Graf GA, McNiven MA, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- Song KS, Scherer PE, Tang Z, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Martinez-Outschoorn UE, Howell A, et al. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Woodman SE, Bonuccelli G, et al. Phenotypic behavior of caveolin-3 R26Q, a mutant associated with hyperCKemia, distal myopathy, and rippling muscle disease. Am J Physiol Cell Physiol. 2003;285:C1150–1160. doi: 10.1152/ajpcell.00166.2003. [DOI] [PubMed] [Google Scholar]
- Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir SA, Yang G, Ebara S, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–3885. [PubMed] [Google Scholar]
- Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Torres VA, Tapia JC, Rodriguez DA, et al. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol Cell Biol. 2007;27:7703–7717. doi: 10.1128/MCB.01991-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VA, Tapia JC, Rodriguez DA, et al. Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J Cell Sci. 2006;119:1812–1823. doi: 10.1242/jcs.02894. [DOI] [PubMed] [Google Scholar]
- Trimmer C, Sotgia F, Whitaker-Menezes D, et al. Caveolin-1 and mitochondrial SOD2 (MnSOD) function as tumor suppressors in the stromal microenvironment: a new genetically tractable model for human cancer associated fibroblasts. Cancer Biol Ther. 2011;11:383–394. doi: 10.4161/cbt.11.4.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, Du Charme J, Parisi J, McMurray CT. Neurological abnormalities in caveolin-1 knock out mice. Behav Brain Res. 2006;172:24–32. doi: 10.1016/j.bbr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Smart EJ. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J Biol Chem. 2000;275:25595–25599. doi: 10.1074/jbc.M003401200. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipo-protein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte D, Liu Y, Galbiati F. The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. Faseb J. 2005;19:237–239. doi: 10.1096/fj.04-2215fje. [DOI] [PubMed] [Google Scholar]
- Wang RN, Green J, Wang Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yang X, Liu Q, et al. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol. 1999;144:1285–1294. doi: 10.1083/jcb.144.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- Willmann R, Pun S, Stallmach L, et al. Cholesterol and lipid microdomains stabilize the postsynapse at the neuromuscular junction. Embo J. 2006;25:4050–4060. doi: 10.1038/sj.emboj.7601288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Ashton AW, Schubert W, et al. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003;162:2059–2068. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Cheung MW, Tarr M, et al. Urogenital alterations in aged male caveolin-1 knockout mice. J Urol. 2004a;171:950–957. doi: 10.1097/01.ju.0000105102.72295.b8. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Park DS, Cohen AW, et al. Caveolin-3 knockout mice develop a progressive cardiomyopathy and show hyper-activation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Sotgia F, Galbiati F, et al. Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology. 2004b;62:538–543. doi: 10.1212/wnl.62.4.538. [DOI] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Yang L, Zhang K, et al. Increased coupling of caveolin-1 and estrogen receptor alpha contributes to the fragile X syndrome. Ann Neurol. 2015;77:618–636. doi: 10.1002/ana.24358. [DOI] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Liu Y, Stan RV, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]