Abstract
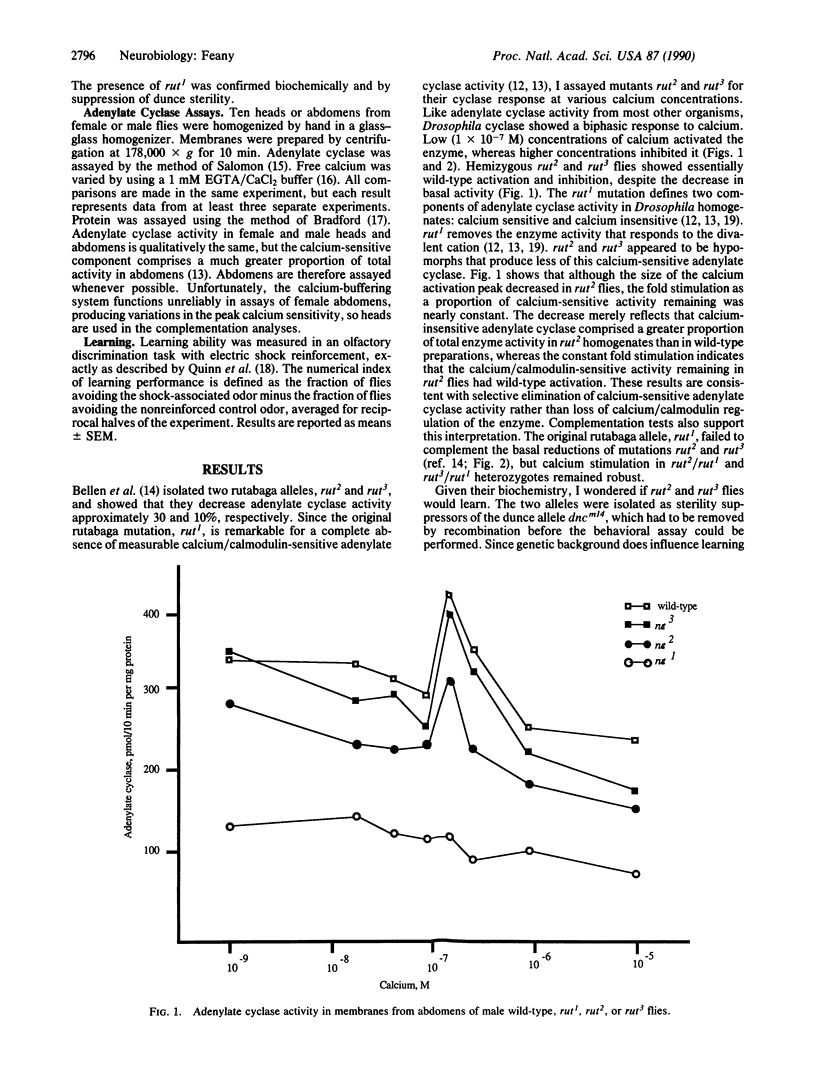
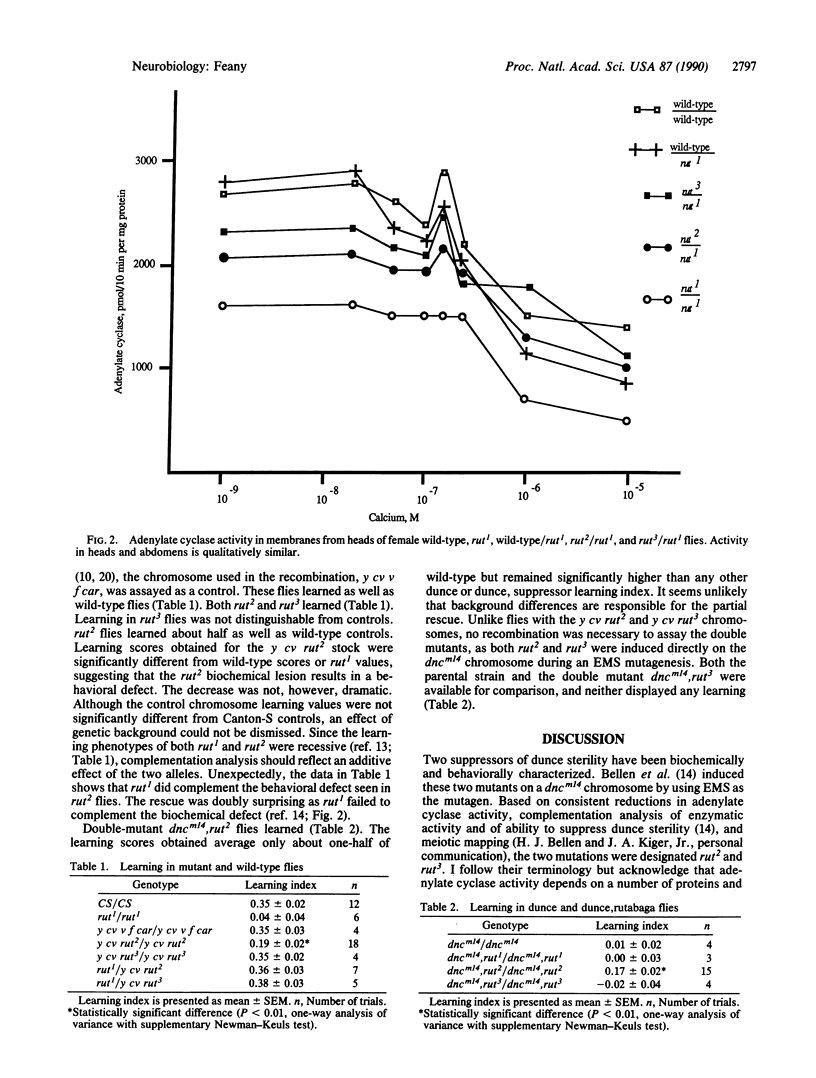
rutabaga1 (rut1), a Drosophila learning mutant, has adenylate cyclase (EC 4.6.1.1) with reduced basal activity and the absence of calcium/calmodulin-stimulated activity. A second learning mutant, dunce, is defective in cyclic AMP degradation due to decreased or absent phosphodiesterase activity. These opposing biochemical defects allow rut1 to partially suppress the female sterility caused by elevated cyclic AMP levels in dunce flies. Selection of mutations that suppress dunce sterility has led to the isolation of two rutabaga alleles. The alleles (rut2 and rut3) decrease basal adenylate cyclase activity [Bellen, H. J., Gregory, B. K., Olsson, C. L. & Kiger, J. A. (1987) Dev. Biol. 121, 432-444] but, unlike the original rutabaga mutation, leave the calcium/calmodulin-stimulated activity intact. Behaviorally, the two alleles also differ from rut1. One of the mutations partially rescues the dunce learning defect, and flies bearing both alleles learn. Calcium responsiveness may thus be the crucial component of adenylate cyclase activity required for associative learning.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellen H. J., Gregory B. K., Olsson C. L., Kiger J. A., Jr Two Drosophila learning mutants, dunce and rutabaga, provide evidence of a maternal role for cAMP on embryogenesis. Dev Biol. 1987 Jun;121(2):432–444. doi: 10.1016/0012-1606(87)90180-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byers D., Davis R. L., Kiger J. A., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981 Jan 1;289(5793):79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Chen C. N., Denome S., Davis R. L. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Kiger J. A., Jr Dunce mutants of Drosophila melanogaster: mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981 Jul;90(1):101–107. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Jan Y. N., Byers D., Quinn W. G., Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976 May;73(5):1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Mutations affect storage and use of memory differentially in Drosophila. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5445–5448. doi: 10.1073/pnas.80.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984 Jun 15;47(2):119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., Schwartz J. H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Kauvar L. M. Defective cyclic adenosine 3':5'-monophosphate phosphodiesterase in the Drosophila memory mutant dunce. J Neurosci. 1982 Oct;2(10):1347–1358. doi: 10.1523/JNEUROSCI.02-10-01347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S. Genetic dissection of Drosophila adenylate cyclase. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5992–5996. doi: 10.1073/pnas.82.17.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984 May;37(1):205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Tempel B. L. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983 May 5;303(5912):67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A., Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974 Mar;71(3):708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Tempel B. L., Livingstone M. S., Quinn W. G. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T., Quinn W. G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985 Sep;157(2):263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]



