Abstract
Hereditary tyrosinemia type 1 (HT1) is a severe human autosomal recessive disorder caused by the deficiency of fumarylacetoacetate hydroxylase (FAH), an enzyme catalyzing the last step in the tyrosine degradation pathway. Lack of FAH causes accumulation of toxic metabolites (fumarylacetoacetate and succinylacetone) in blood and tissues, ultimately resulting in severe liver and kidney damage with onset that ranges from infancy to adolescence. This tissue damage is lethal but can be controlled by administration of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), which inhibits tyrosine catabolism upstream of the generation of fumarylacetoacetate and succinylacetone. Notably, in animals lacking FAH, transient withdrawal of NTBC can be used to induce liver damage and a concomitant regenerative response that stimulates the growth of healthy hepatocytes. Among other things, this model has raised tremendous interest for the in vivo expansion of human primary hepatocytes inside these animals and for exploring experimental gene therapy and cell-based therapies. Here, we report the generation of FAH knock-out rabbits via pronuclear stage embryo microinjection of transcription activator-like effector nucleases. FAH−/− rabbits exhibit phenotypic features of HT1 including liver and kidney abnormalities but additionally develop frequent ocular manifestations likely caused by local accumulation of tyrosine upon NTBC administration. We also show that allogeneic transplantation of wild-type rabbit primary hepatocytes into FAH−/− rabbits enables highly efficient liver repopulation and prevents liver insufficiency and death. Because of significant advantages over rodents and their ease of breeding, maintenance, and manipulation compared with larger animals including pigs, FAH−/− rabbits are an attractive alternative for modeling the consequences of HT1.
Keywords: animal model, gene knock-out, liver, stem cells, transplantation
Introduction
Animal models bearing mutations/truncations in genes causing human disease are essential to understand the mechanisms underlying those diseases and to identify new diagnostic or therapeutic procedures. In some cases, the idiosyncrasy of human disease has also made these animals valuable for unexpected applications (1). One such example are Fah−/− mice (2), which have been genetically engineered to lack fumarylacetoacetate hydrolase (FAH),3 an enzyme responsible for catalyzing the last step in the degradation of the amino acid tyrosine (3). In humans, lack of FAH causes hereditary tyrosinemia type 1 (HT1), a rare autosomal recessive condition characterized by retrograde accumulation of toxic metabolites including fumarylacetoacetate and succinylacetone in blood and tissues, which ultimately causes liver failure and renal tubular dysfunction as the most prominent manifestations (4, 5). Liver failure can develop acutely in the first few months after birth or be more progressive. Treatment of HT1 consists mainly of administration of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), which inhibits the conversion of 4-hydroxyphenylpyruvate to homogentisic acid by 4-hydroxyphenylpyruvate dioxygenase, the second step in the tyrosine degradation pathway, thus preventing the accumulation of fumarylacetoacetate and succinylacetone (6). However, if patients are diagnosed too late or do not comply with NTBC treatment, there is end stage liver disease with cirrhosis and high risk of hepatocellular carcinoma due to chronic damage. Notably, in Fah−/− mice, controlled withdrawal of NTBC can be used to cause liver damage in a desired manner, inducing the generation of a regenerative response that allows repopulation of the damaged liver by the remaining normal hepatocytes after NTBC is added back (2, 7). When Fah−/− mice are simultaneously deficient in key genes regulating the immune response (e.g. Rag2 and/or Il2rg), the same principle allows liver repopulation with transplanted heterologous primary hepatocytes (8), stem cell-derived hepatocytes (9), or transdifferentiated hepatocytes (10, 11) without immune rejection.
For a long time, genetic engineering of mammals has been mostly restricted to mice. This is because of the availability of mouse embryonic stem cells (ESCs) and the relative ease of manipulating these cells with traditional gene modification techniques. Genetically modified ESCs can then be injected into mouse blastocysts to produce chimeric animals with germ line transmission (12). Somatic cell modification and nuclear transfer have been traditionally an alternative for genetic engineering of species for which bona fide ESCs are not available, but the procedure is inefficient (13). More recently, the development of designer nuclease technologies (zinc finger nucleases, transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) (14) has expanded significantly the repertoire of species (e.g. rats, rabbits, dogs, pigs, sheep, and cattle) (15–20) amenable to routine genetic engineering and made the procedure a less time-consuming effort; bona fide rat ESCs were also isolated recently (21, 22). Accordingly, several groups have reported the generation of FAH-deficient pigs (23, 24) and rats (25, 26), which offer some advantages over Fah−/− mice.
Rabbits are widely used for animal experimentation. Their physiology is closer to humans than rodents, and they have relatively low cost maintenance and are easy to breed (27, 28). Herein, we report the generation of FAH−/− rabbits by injecting TALENs into the cytoplasm of rabbit pronuclear stage embryos. These FAH−/− rabbits have liver and kidney phenotypic features analogous to FAH-deficient rodents and pigs but also develop frequent ocular manifestations including corneal keratitis. We further show that allogeneic transplantation of wild-type rabbit primary hepatocytes achieves efficient liver repopulation and improves the liver function and survival rate of FAH−/− rabbits. These results demonstrate that genetically engineered FAH−/− rabbits are an attractive choice for modeling the consequences of HT1.
Results
Construction of TALENs and Generation of FAH Knock-out Rabbits
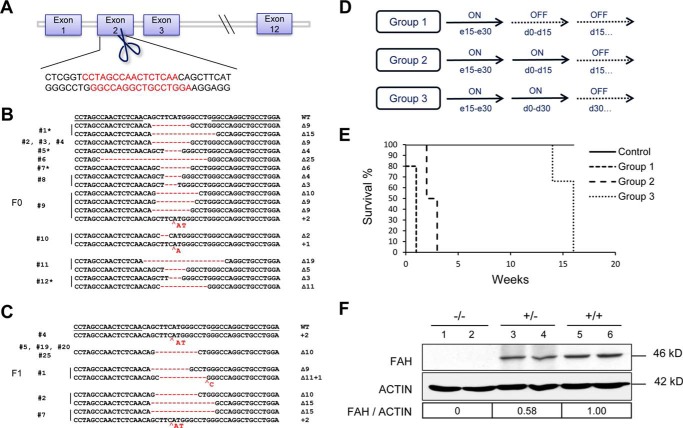
We designed a pair of TALENs targeting exon 2 of the rabbit FAH gene (Fig. 1A) and assembled them according to the Golden Gate method with the following codes: NI for adenine, NG for thymine, HD for cytosine, and NN for guanine. We injected different concentrations (10, 20, 30, 50, and 100 ng/μl) of in vitro transcribed TALEN-coding mRNAs into the cytoplasm of rabbit pronuclear stage embryos (20, 27) and then transferred the embryos into surrogate mothers. NTBC was administered to pregnant rabbits from day 15 of pregnancy to prevent intrauterine death (2). With the highest concentration of TALEN mRNAs, all foster mothers miscarried, and no rabbits were born (Table 1). The pregnancy was not affected with the other TALEN mRNA concentrations; a total of 31 rabbits were born, and the ear tissue of each animal was collected for genotyping. With the lowest concentration of TALEN mRNAs, all six newborn rabbits were negative for gene targeting. However, we detected FAH mutations using 20 and 30 ng/μl, and the targeting efficiency was 100% (four of four newborns) using 50 ng/μl (Table 1). These FAH mutant rabbits were mostly mosaic with different insertions and/or deletions (indels) within the FAH locus as shown in (Fig. 1B). Through breeding of FAH mutant founder (F0) animals, we obtained a total of nine FAH−/− first filial generation (F1) rabbits (Fig. 1C) and eight FAH+/− rabbits, which appeared healthy at birth. These animals were bred further to produce additional filial generations.
FIGURE 1.
Preparation of TALENs and generation of FAH knock-out rabbits. A, design of TALENs targeting exon 2 of rabbit FAH gene. Bases in red indicate the TALEN recognition sequences. B, Sanger sequencing of the targeted region in the FAH locus in F0 rabbits; * represents rabbits in which the wild-type FAH sequence was detected. C, Sanger sequencing of the targeted region in the FAH locus in FAH−/− F1 rabbits. D, schematic of the three groups of FAH−/− rabbits used to study the dependence on NTBC for long term survival (ON, NTBC administration; OFF, NTBC withdrawal); e means embryonic day and d means day after birth. E, survival curve for the same three groups of FAH−/− rabbits. F, Western blotting analysis confirms that FAH−/− rabbits (−/−) are negative for FAH protein expression in liver tissue lysates, whereas FAH+/− (+/−) rabbits express a reduced amount of FAH. Band intensities (FAH/Actin) were quantified using ImageJ software and are shown relative to wild-type (+/+) rabbits.
TABLE 1.
Generation of FAH knock-out rabbits using TALENs
| No. surrogates | TALEN mRNA concentration | No. embryos transferred | No. newborns (%) | No. mutant rabbits (%) |
|---|---|---|---|---|
| ng/μl | ||||
| 3 | 10 | 34 | 6 (12) | 0 |
| 3 | 20 | 39 | 12 (31) | 3 (25) |
| 2 | 30 | 32 | 9 (28) | 5 (56) |
| 3 | 50 | 37 | 4 (11) | 4 (100) |
| 4 | 100 | 40 | 0 | 0 |
To ascertain the importance of NTBC administration in preventing death of FAH−/− rabbits, we prepared 13 FAH−/− rabbits and divided them into three groups, each with different NTBC administration modes (Fig. 1D). The rabbits were closely monitored to collect blood before death and to store the tissues in good condition. In the first group, no NTBC was administered at any time after birth, and these animals died shortly (within 1 week after birth) (Fig. 1E). In the second and third groups, NTBC was continuously administered until the rabbits were 2 weeks or 1 month old, respectively. Rabbits in the second group survived no longer than 3 weeks after birth, whereas rabbits in the third group were able to live as long as 4 months after birth (Fig. 1E). Western blotting analysis of liver tissue lysates from two dead FAH−/− rabbits showed that FAH was undetectable when compared with wild-type rabbits (Fig. 1F). Likewise, heterozygous knock-out (FAH+/−) rabbits expressed half of the amount of protein of the wild type. Thus, we have generated FAH−/− rabbits that can only be maintained alive with NTBC, and we next proceeded to study whether these animals develop characteristic phenotypic features of HT1.
FAH−/− Rabbits Have Liver and Kidney Phenotypic Characteristics of HT1
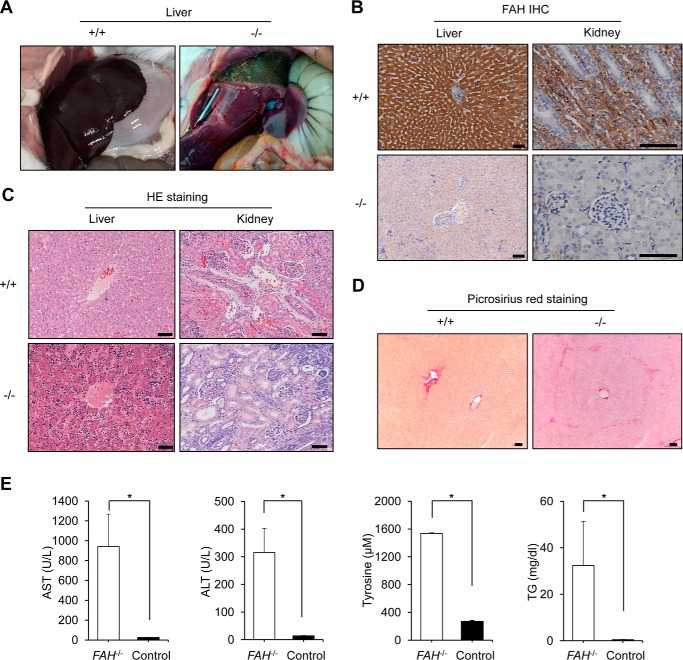
We dissected the bodies of dead FAH−/− rabbits belonging to the above mentioned groups and observed prominent liver swelling, hemorrhage, and yellow/green discoloration suggestive of both cholestasis and liver necrosis in contrast to the normal aspect of their wild-type counterparts (Fig. 2A). We also collected and sectioned the liver and kidney tissues for immunohistological analysis with FAH antibodies. As expected, FAH protein was completely absent in FAH−/− livers and kidneys, two tissues that normally express it at a high level, but displayed strong staining in wild-type rabbits (Fig. 2B). In addition, hematoxylin and eosin staining showed diffuse hepatocellular injury with dysplastic hepatocytes and tubule interstitial nephritis in FAH−/− rabbits only (Fig. 2C), both of which are hallmarks of HT1. Moreover, picrosirius red staining (29) revealed mild fibrosis in the liver of FAH−/− rabbits but not in wild type (Fig. 2D), which is in agreement with Fah−/− mice but contrasts with the development of cirrhosis in FAH-deficient rats and pigs (25, 30). In addition, we extracted serum from blood samples and analyzed the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglycerides, all of which are elevated as a consequence of liver damage (23). A significant increase of all three parameters was observed in FAH−/− rabbits maintained without NTBC compared with wild-type rabbits (Fig. 2E). We also detected that tyrosine levels had increased in FAH−/− rabbits maintained without NTBC, illustrating that, as expected, deletion of FAH blocks the tyrosine metabolic pathway (2, 5). Altogether, these results confirm that FAH−/− rabbits develop features similar to HT1 patients and rodent/pig models (5, 7, 23–26).
FIGURE 2.
FAH−/− rabbits develop progressive liver failure in the absence of NTBC. A, severe necrosis in the liver of a FAH−/− rabbit (right) belonging to group 3 of NTBC administration (as in Fig. 1D) in contrast to a healthy wild-type rabbit. B, immunohistochemistry (IHC) of liver and kidney sections of a FAH−/− rabbit belonging to group 2 of NTBC administration shows no expression of FAH in contrast to a wild-type rabbit. Scale bars, 50 μm. C, hematoxylin and eosin (HE) staining shows abnormal tissue architecture in liver and kidney sections of the same FAH−/− rabbit in B in contrast to a wild-type rabbit. In the FAH−/− rabbit, diffused hepatocellular injury with dysplastic hepatocytes and tubular epithelial injury of kidney were observed. Scale bars, 50 μm. D, picrosirius red staining shows the existence of mild interstitial fibrosis in the liver of the same FAH−/− rabbit in A but not in the wild-type rabbit. Scale bars, 50 μm. E, serum biochemical parameters indicate liver damage in FAH−/− rabbits compared with a wild-type rabbit. TG stands for triglycerides. Error bars represent S.E. (n = 3 replicate measurements). * corresponds to p < 0.01 according to Student's t test.
Ocular Manifestations in FAH Knock-out Rabbits
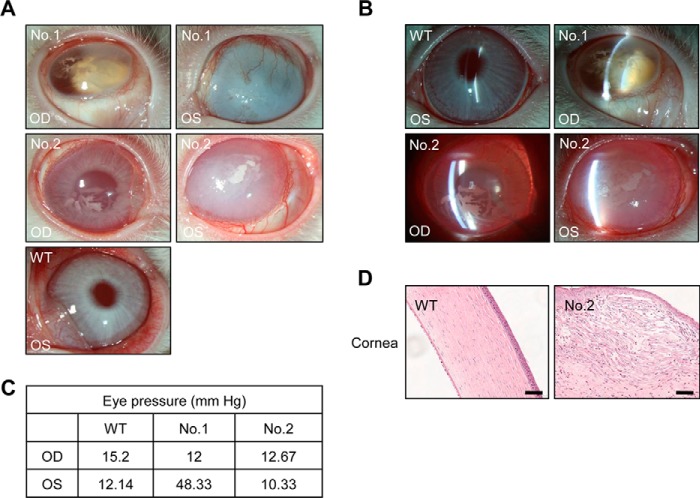
Ocular involvement is not frequent in HT1 patients, but in those rare cases corneal keratitis is the main manifestation (31, 32). This has been attributed to inflammation produced by local tyrosine deposition in the form of crystals caused by low compliance with a low protein diet and the secondary effect of using NTBC on tyrosine accumulation. Interestingly, in the course of our study, we noticed that FAH knock-out rabbits develop frequent ocular manifestations too. This problem was observed in all third filial generation FAH−/− rabbits and a small proportion of FAH+/− rabbits. Of note, although the latter were not treated with NTBC at any time after birth, it was administered to their mothers during pregnancy. We performed ophthalmological analysis of two FAH+/− rabbits compared with a wild-type rabbit. Through direct eye inspection, we discovered keratoleukoma accompanied by edema and opacity in both eyes of the two FAH+/− rabbits (Fig. 3A). Using slit lamp imaging, we also noticed deeper chamber depth and lens opacification accompanied with dilated pupil and posterior synechia of the iris in the right eye of the first FAH+/− rabbit (Fig. 3B), implying cataract and iritis. Moreover, in the same rabbit, there was a pathogenic high intraocular pressure of 48.33 mm Hg in the left eye (Fig. 3C), indicating secondary glaucoma, whereas intraocular pressure in the right eye was normal. Conversely, chamber depth and intraocular pressure were normal in the second FAH+/− rabbit (Fig. 3, B and C), and no significant cataract was found. In addition, hematoxylin and eosin staining showed edema and thickening in the corneal epithelium and stroma of both FAH+/− rabbits, and the corneal surface became irregular in particular in the left eye of the second FAH+/− rabbit (Fig. 3D). Moreover, swelling, condensed nuclei, and fragmented bodies could be seen in corneal epithelial and stroma cells in both FAH+/− rabbits, indicating necrocytosis (Fig. 3D). Therefore, there is frequent ocular involvement, mostly manifested as corneal keratitis, in FAH knock-out rabbits.
FIGURE 3.
Ocular manifestations in FAH knock-out rabbits. A, photographs of right (OD) and left (OS) eyes of two FAH+/− rabbits show corneal abnormalities; a wild-type (WT) rabbit was used as control. B, slit lamp photographs of the same three rabbits show abnormalities of chamber depth and lens opacification in FAH+/− rabbit number 1 compared with the wild type. C, measurement of intraocular pressure using a tonometer (Icare, Finland) in the same three rabbits. Only the left eye of FAH+/− rabbit number 1 shows pathogenic levels. D, hematoxylin and eosin staining shows corneal structural abnormalities (edema, thickening, cellular defects, and disorganization of the corneal epithelium and stroma) in the left eye of FAH+/− rabbit number 2 compared with the wild type. Scale bars, 50 μm.
Allogeneic Primary Hepatocyte Transplantation Rescues Liver Damage of FAH−/− Rabbits
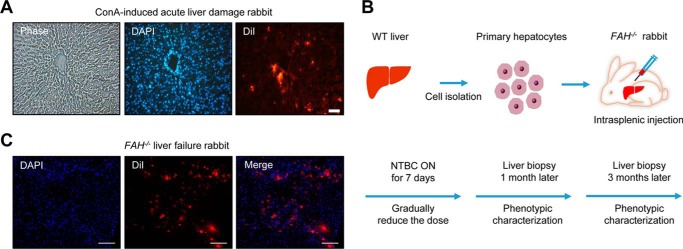
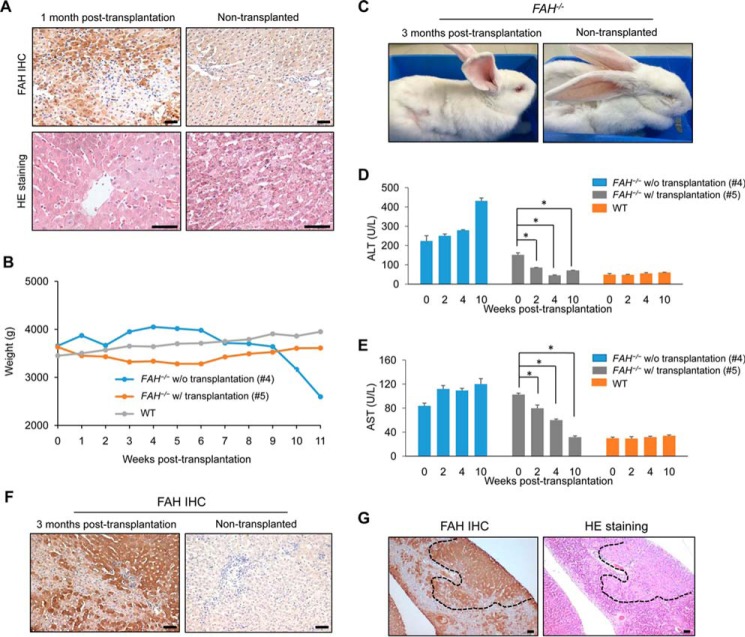
We then studied whether transplantation of FAH-competent hepatocytes could rescue the liver phenotype and prevent death of FAH−/− rabbits untreated with NTBC. First, to assess feasibility, we induced acute liver damage in wild-type rabbits with concanavalin A (33), which causes a rapid regenerative response that facilitates engraftment of transplanted cells, and 24 h later 107 rabbit primary hepatocytes extracted from a healthy wild-type rabbit were injected intrasplenically. To assist with their identification, transplanted hepatocytes were labeled with DiI, as this fluorescent compound can last as long as 1 month in vivo (34, 35). The recipient animals were immunosuppressed with cyclosporin A starting 24 h before transplantation. We observed a large number of DiI-positive cells in liver sections 3 weeks after transplantation, proving the efficacy of the approach (Fig. 4A). Next, we transplanted wild-type rabbit primary hepatocytes into two FAH−/− rabbits that had been treated with NTBC after birth. A wild-type rabbit and a non-transplanted FAH−/− rabbit treated with NTBC were used as controls. NTBC was gradually decreased in the three FAH−/− rabbits and completely withdrawn 1 week after the transplantation (Fig. 4B). One month after NTBC withdrawal, a small liver biopsy was obtained from one of the transplanted FAH−/− rabbits and the non-transplanted FAH−/− rabbit for histological analysis. Approximately 30% of liver cells in the examined liver sections of the transplanted FAH−/− rabbit were DiI-positive at this time point (Fig. 4C). Likewise, FAH immunohistochemistry confirmed significant engraftment of FAH-positive hepatocytes in the same FAH−/− transplanted rabbit, and these hepatocytes displayed normal morphology in contrast with damaged cells in non-engrafted areas (Fig. 5A). We also measured weight in these FAH−/− rabbits and the wild-type control over the post-transplantation period. We noticed that 11 weeks after NTBC withdrawal the FAH−/− rabbit that did not receive transplantation showed a 40% decrease in body weight and died shortly after, whereas the wild-type and FAH−/− rabbits receiving allogeneic hepatocyte transplantation had similar weight and appeared healthy (Fig. 5, B and C). In addition, we performed serum biochemical analysis over the post-transplantation period to see whether liver function can be recovered in FAH−/− rabbits compared with the control. Indeed, ALT and AST values of wild type and a transplanted FAH−/− rabbit were alike at week 10 post-transplantation, whereas the non-transplanted FAH−/− rabbit showed significantly higher values (Fig. 5, D and E). Interestingly, we also observed more engrafted FAH-positive cells at 3 months in the transplanted FAH−/− rabbits (Fig. 5, F and G), indicating long term stability of the engrafted cells and suggesting in vivo proliferation. These data prove the utility of FAH−/− rabbits as tools for testing experimental methodologies involving liver cell transplantation.
FIGURE 4.
Hepatocyte transplantation of FAH−/− rabbits. A, transplantation of wild-type rabbit primary hepatocytes into the liver of a wild-type rabbit treated with concanavalin A (ConA; 5.0 mg/kg, intrasplenic injection) to induce acute liver damage. Engraftment of DiI-labeled hepatocytes can be observed under the fluorescence microscope at 3 weeks post-transplantation. DAPI was used to stain nuclei. Scale bars, 50 μm. B, schematic showing the allogeneic transplantation procedure in FAH−/− rabbits. NTBC was administered for another 2 days after the first liver biopsy. C, engraftment of DiI-labeled cells observed under the fluorescence microscope in a FAH−/− rabbit without NTBC at 1 month post-transplantation. Scale bars, 50 μm.
FIGURE 5.
Wild-type primary hepatocyte transplantation rescues liver architecture and prevents death of FAH−/− rabbits. A, top panels, FAH immunohistochemistry shows wild-type hepatocyte repopulation of the liver of a FAH−/− rabbit 1 month post-transplantation compared with the control (non-transplanted FAH−/− rabbit). Lower panels, hematoxylin and eosin staining shows restoration of liver architecture in the same transplanted FAH−/− rabbit compared with the non-transplanted FAH−/− rabbit. Scale bars, 50 μm. B, body weight changes of the two FAH−/− rabbits in A (with or without transplantation) and a control wild-type rabbit. * corresponds to p < 0.01. C, photographs of a transplanted FAH−/− rabbit at 3 months post-transplantation and a non-transplanted FAH−/− rabbit. The non-transplanted FAH−/− rabbit appears weak. D and E, serum levels of ALT and AST in a wild-type rabbit and two FAH−/− rabbits (with (w/) or without (w/o) transplantation). Error bars represent S.E. (n = 3 replicate measurements). * corresponds to p < 0.01. F, FAH immunohistochemistry shows extensive wild-type hepatocyte repopulation of the liver of a FAH−/− rabbit 3 months post-transplantation compared with the non-transplanted FAH−/− rabbit. Scale bars, 50 μm. G, right panel, hematoxylin and eosin (HE) staining shows restoration of liver architecture (circled by dashed lines) in the same transplanted FAH−/− rabbit at 3 months post-transplantation. Left panel, adjacent liver section of the transplanted FAH−/− rabbit showing co-localization of areas with normal structure and positive FAH staining. Scale bars, 50 μm.
Discussion
Patients with HT1 are treated with NTBC and dietary restrictions, but these are not curative, and, besides, a number of individuals fail to respond and require liver transplantation (7). Because a shortage of donors limits organ transplantation, other therapeutic strategies (e.g. stem cell-based or gene therapy approaches) are urgently needed to treat this patient population. Appropriate preclinical animal models are required for testing these experimental therapies, and although Fah−/− mice present many phenotypic features of HT1, their physiology (e.g. inflammatory responses (36)) differs significantly from humans. Likewise, their small size and short life span pose a limitation for analytical studies and long term assessments. Aiming to solve these issues, Fah−/− rats (25, 26) and in particular pigs (23, 24) have been generated recently. However, although pigs have many advantages over rodents for HT1 disease modeling, their handling and breeding is laborious and costly.
Rabbits are excellent animals for state-of-the-art animal experimentation. They are closer phylogenetically to primates than rodents and have a longer life span (8–10 years), and their medium size, short pregnancy period (1 month versus 4 months in pigs), and relatively straightforward husbandry requirements facilitate production of large cohorts at relatively low cost (27, 28). Like pigs, rabbits also have a more diverse genetic background than rodents, a situation that is closer to that in humans. Notably, the first transgenic rabbits were generated over 3 decades ago (37, 38), but the lack of bona fide rabbit ESCs for more complex genetic engineering and the inefficiency of rabbit somatic cell nuclear transfer (39) hampered the development of the field until the arrival of highly efficient designer nuclease technologies (20, 40–42).
Our work presented here is the first description of genetically engineered FAH−/− rabbits. We used TALEN mRNA microinjection into pronuclear stage rabbit embryos (20) rather than the CRISPR/Cas9 system (40, 43) because we envisaged that for embryo injections the toxicity and off-target effects of the former are easier to control (44), although recent improvements of the CRISPR/Cas9 technique could solve this issue (45). FAH−/− rabbits display liver and kidney manifestations of the human genetic disorder but also have frequent ocular alterations (mostly corneal keratitis). Ocular involvement is rare in patients with HT1 but frequent in patients with tyrosinemia type II (46), which is produced by mutations in tyrosine transaminase, the enzyme undertaking the first step of tyrosine catabolism. As with tyrosinemia type II, corneal keratitis in HT1 patients and possibly FAH knock-out rabbits as well is caused by enhanced local accumulation of tyrosine, which is boosted by NTBC (31, 32). The frequency with which ocular manifestations happen in FAH−/− rabbits potentially makes them a useful model for studying how to prevent and treat this potential complication in HT1 patients. FAH−/− rabbits did not develop cirrhosis in our study, although it is likely that varying the NTBC administration/withdrawal routine and allowing more chronic damage would induce it. Besides being an excellent choice for modeling HT1 and studying chronic liver damage, FAH−/− rabbits could also be used for testing gene therapy approaches, which have proved successful in FAH-deficient mice and pigs but require additional studies to evaluate long term safety and efficacy (24). In addition, FAH−/− rabbits could be exceptional bioreactors for growing primary human hepatocytes for in vivo human disease modeling (e.g. hepatitis B or C), potential xenotransplantation, or for in vitro studies, but this would require producing FAH−/−RAG2−/−IL2RG−/− rabbits to avoid immune rejection. Such triple knock-out animals would be valuable too as preclinical models for experimental stem cell-based therapies including the transplantation of hepatocyte-like cells derived from induced pluripotent stem cells (11) or produced through transdifferentiation (10, 11). Given the time and cost of pursuing this approach in pigs, rabbits offer an attractive option.
In summary, we have demonstrated that FAH−/− rabbits are a promising alternative for modeling HT1 and for developing therapeutic strategies aiming to cure this disease. From a wider perspective, our work also shows that genetically engineered rabbits offer a powerful approach to recapitulating human disease.
Experimental Procedures
Animals and Ethics Statement
New Zealand White rabbits were obtained from the Laboratory Animal Centre of Southern Medical University (Guangzhou, China). All rabbit experiments were conducted under the approval of the Animal Care and Use committee of the Guangzhou Institutes of Biomedicine and Health (ID 2012040) and the Department of Science and Technology of Guangdong Province (ID SYXK 2005-0063). Animals were observed at least once daily for clinical signs and symptoms consistent with acute liver failure. To obtain FAH−/− and FAH+/− F1 rabbits, we bred F0 animals with each other, and then we bred F0 with F1, F1 with F1, and so on for producing other filial generations. For details of which rabbits were used in each experiment and their respective genotypes see supplemental Table 1. Pregnant rabbits giving birth to FAH−/− rabbits received 7.5 mg/liter NTBC/200 ml of drinking water per day beginning on day 15 of pregnancy. The same dose was used for maintenance of FAH−/− rabbits. Ophthalmological evaluation was performed in Zhongshan Ophthalmic Center, Sun Yat-sen University.
TALEN Preparation, Embryo Microinjection, and Embryo Transfer
TALENs were designed and assembled according to the golden gate assembly method (27). In vitro transcribed TALEN mRNAs were prepared using an mMESSAGE mMACHINE® T7 kit (Ambion, Austin, TX) and purified using an RNeasy Mini Elute Cleanup kit (Qiagen, Valencia, CA). The TALEN microinjection procedure was essentially as described previously (27). Briefly, 6–8-month-old female rabbits were induced to superovulate with 50 IU of FSH every 12 h for 3 days, mated with male rabbits about 72 h later, and injected with 100 IU of human chorionic gonadotropin. Female rabbits were euthanized 18 h after injection, and the oviducts were flushed with prewarmed embryo manipulation medium for collecting embryos at the pronuclear stage. Mixed in vitro transcribed TALEN mRNAs were microinjected into the cytoplasm, and then embryos were transferred to Earle's balanced salt solution medium for in vitro culture in a 5% CO2 incubator at 38.5 °C with 100% humidity. Approximately 15–30 optimal quality (as judged by microscope inspection) injected embryos were transferred into unilateral pavilions of the oviducts for each recipient mother.
Genotyping
DNA of newborn rabbits was extracted from a small piece of ear tissue using a Hipure Tissue DNA Mini kit (Magen, Beijing, China) following the manufacturer's protocol. PCR products spanning the TALEN target sites were amplified with KOD-Plus-Neo DNA polymerase (Toyobo, Tokyo, Japan) with the primers 5′-GCACTTGAGCCATCGTCCGT (FAH-F) and 5′-ACCAGCAGCAGGCAATCCCA (FAH-R) and then sequenced.
Western Blotting Assay
For Western blotting, liver tissue samples were homogenized in radioimmune precipitation assay buffer and centrifuged at 14,500 × g at 4 °C for 30 min; supernatants were then collected, and protein concentration was quantified. Lysates were subjected to 10% polyacrylamide-SDS gel electrophoresis followed by immunoblotting onto a polyvinylidene fluoride membrane (Millipore, Temecula, CA). Membranes were blocked with 5% evaporated milk in TBS-Tween 20 for 2 h and incubated overnight at room temperature with primary antibodies against FAH (ab140167, Abcam, Cambridge, UK). Membranes were then incubated with a secondary HRP-conjugated anti-rabbit antibody (sc-2004, Santa Cruz Biotechnology, Santa Cruz, CA) for 60 min at room temperature and imaged using a SuperSignalTM West Pico Chemiluminescence Substrate kit (Thermo, Rockford, IL). ACTIN antibody (sc-47778, Santa Cruz Biotechnology) was probed as a loading control.
Histology
Liver and kidney tissues were fixed with 4% neutral buffered paraformaldehyde for 48 h and processed for paraffin embedding and sectioning. For immunohistochemistry staining, antigen retrieval was performed in a 1 m citrate buffer (pH 6.0) bath for 20 min. Tissues were immunostained with anti-FAH primary antibodies (ab140167) and visualized using VECTASTAIN Elite ABC HRP kit (Vector Laboratories, Burlingame, CA). Hematoxylin and eosin staining and picrosirius red staining were performed using standard protocols.
Serum Analysis
Blood was obtained via puncture of the ear vein using heparinized tubes. Serum was separated by centrifugation at 900 × g for 20 min and stored at −80 °C prior to biochemistry and amino acid analysis. Concentrations of ALT, AST, and triglycerides were measured with a CL-8000 Hitachi 7180 automatic biochemical analyzer (Shimadzu, Kyoto, Japan). Tyrosine was quantified by liquid chromatography-mass spectrometry using the ABI 3200 Q TRAP LC-MS/MS system (Applied Biosystems, Foster City, CA).
Allogeneic Hepatocyte Transplantation
Fresh hepatocytes were isolated from wild-type rabbit livers by in situ collagenase perfusion as reported previously (47). Briefly, the liver was perfused with calcium- and magnesium-free Hanks' balanced salt solution (Thermo) supplemented with 0.5 mm EGTA for 10 min and 10 mm HEPES for 3 min. The solution was changed to Earle's balanced salt solution supplemented with 0.1 mg/ml collagenase IV (Sigma) for 10 min. The liver was then gently minced in the second solution and filtered through 150-, 75-, 50-, and 37.5-μm nylon mesh, sequentially. After centrifugation at 50 × g for 5 min, the pellet was washed with DMEM Gibco) supplemented with 4.5 g/ml glucose three times at 50 × g for 2 min. The number and viability of cells were assessed by trypan blue. 107 viable cells were then incubated in a 20 μm DiI solution (Sigma) for 5 min at 37 °C and for 15 min at 4 °C for labeling and washed in DMEM supplemented with 4.5 g/ml glucose. Eventually, 107 hepatocytes in 2.5 ml of Clonetics® hepatocyte culture medium (Lonza, Walkersville, MD) were injected into the spleen of recipient rabbits via a small flank incision. Concanavalin A and cyclosporin A were purchased from Sigma.
Author Contributions
M.A.E. and L. Lai had the idea for the study. L. Li, L. Lai, and M.A.E. designed the experiments. L. Li and M.A.E. analyzed the data. L. Li conducted most of the experiments. Q. Zhang, H. Y., Q. Zou, C. L., F. J., P. Z., Z. L., J. Y., Q. C., Y. W., P. N. N., J. F., P. H. M., W. L., S. C., D. W., T.-K. S., S. T., H.-F. T., B. Q., and X. B. contributed to the experiments or provided critical advice. M. A. E., X. B., and L. Lai provided funding. M. A. E. wrote the manuscript with help from L. Li. M. A. E. and L. Lai approved the final version of the manuscript for submission.
Supplementary Material
Acknowledgments
We thank the members in the Laboratory Animal Center of the Guangzhou Institutes of Biomedicine and Health for histological analysis and Yan Chen for technical advice.
This work was supported by National Key Research and Development Program of China Grant 2016YFA0100102; Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDA01020106; National Natural Science Foundation of China (NSFC) Grant 31401275; Guangdong Province Science and Technology Program Grants 2013B050800010, 2013B060300017, 2014030304010, 2014B030301058, 2014A030312001, 2015B020229002, and 2016B030229007; Guangzhou Science and Technology Cooperation Program Grants 2012J5100040 and 201508030027; Key Deployment Project of the Chinese Academy of Sciences Grant KSZD-EW-Z-005-003-002; Pearl River Science and Technology Nova Program of Guangzhou Grant 201610010107; Youth Innovation Promotion Association of the Chinese Academy of Sciences Grant 2015294; Wellcome Trust Senior Investigator Award 096956/Z/11/Z (to P. H. M.); and NSFC and Hong Kong Research Grants Council Joint Research Fund Grants 81261160506 and N-HKU 730/12. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Table 1.
- FAH
- fumarylacetoacetate hydroxylase
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- CRISPR
- clustered regularly interspaced short palindromic repeats
- Cas9
- CRISPR-associated protein 9
- DiI
- 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate
- ESCs
- embryonic stem cells
- F0
- founder generation
- F1
- first filial generation
- HT1
- hereditary tyrosinemia type 1
- NTBC
- 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
- TALEN
- transcription activator-like effector nuclease.
References
- 1. Nakamura K., Tanaka Y., Mitsubuchi H., and Endo F. (2007) Animal models of tyrosinemia. J. Nutr. 137, Suppl. 1, 1556S–1560S [DOI] [PubMed] [Google Scholar]
- 2. Grompe M., Lindstedt S., al-Dhalimy M., Kennaway N. G., Papaconstantinou J., Torres-Ramos C. A., Ou C. N., and Finegold M. (1995) Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 10, 453–460 [DOI] [PubMed] [Google Scholar]
- 3. Grompe M. (2001) The pathophysiology and treatment of hereditary tyrosinemia type 1. Semin. Liver Dis. 21, 563–571 [DOI] [PubMed] [Google Scholar]
- 4. Russo P., and O'Regan S. (1990) Visceral pathology of hereditary tyrosinemia type I. Am. J. Hum. Genet. 47, 317–324 [PMC free article] [PubMed] [Google Scholar]
- 5. Vogel A., van Den Berg I. E., Al-Dhalimy M., Groopman J., Ou C. N., Ryabinina O., Iordanov M. S., Finegold M., and Grompe M. (2004) Chronic liver disease in murine hereditary tyrosinemia type 1 induces resistance to cell death. Hepatology 39, 433–443 [DOI] [PubMed] [Google Scholar]
- 6. Lindstedt S., Holme E., Lock E. A., Hjalmarson O., and Strandvik B. (1992) Treatment of hereditary tyrosinemia type-I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340, 813–817 [DOI] [PubMed] [Google Scholar]
- 7. Ashorn M., Pitkänen S., Salo M. K., and Heikinheimo M. (2006) Current strategies for the treatment of hereditary tyrosinemia type I. Paediatr. Drugs 8, 47–54 [DOI] [PubMed] [Google Scholar]
- 8. Azuma H., Paulk N., Ranade A., Dorrell C., Al-Dhalimy M., Ellis E., Strom S., Kay M. A., Finegold M., and Grompe M. (2007) Robust expansion of human hepatocytes in Fah(−/−)/Rag2(−/−)/Il2rg(−/−) mice. Nat. Biotechnol. 25, 903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huch M., Dorrell C., Boj S. F., van Es J. H., Li V. S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M. J., Haft A., Vries R. G., Grompe M., and Clevers H. (2013) In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang P., He Z., Ji S., Sun H., Xiang D., Liu C., Hu Y., Wang X., and Hui L. (2011) Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 [DOI] [PubMed] [Google Scholar]
- 11. Zhu S., Rezvani M., Harbell J., Mattis A. N., Wolfe A. R., Benet L. Z., Willenbring H., and Ding S. (2014) Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 508, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Capecchi M. R. (1989) Altering the genome by homologous recombination. Science 244, 1288–1292 [DOI] [PubMed] [Google Scholar]
- 13. Lai L., Kolber-Simonds D., Park K. W., Cheong H. T., Greenstein J. L., Im G. S., Samuel M., Bonk A., Rieke A., Day B. N., Murphy C. N., Carter D. B., Hawley R. J., and Prather R. S. (2002) Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295, 1089–1092 [DOI] [PubMed] [Google Scholar]
- 14. Gaj T., Gersbach C. A., and Barbas C. F. 3rd (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carlson D. F., Lancto C. A., Zang B., Kim E. S., Walton M., Oldeschulte D., Seabury C., Sonstegard T. S., and Fahrenkrug S. C. (2016) Production of hornless dairy cattle from genome-edited cell lines. Nat. Biotechnol. 34, 479–481 [DOI] [PubMed] [Google Scholar]
- 16. Zou Q., Wang X., Liu Y., Ouyang Z., Long H., Wei S., Xin J., Zhao B., Lai S., Shen J., Ni Q., Yang H., Zhong H., Li L., Hu M., et al. (2015) Generation of gene-target dogs using CRISPR/Cas9 system. J. Mol. Cell. Biol. 7, 580–583 [DOI] [PubMed] [Google Scholar]
- 17. Yang D., Yang H., Li W., Zhao B., Ouyang Z., Liu Z., Zhao Y., Fan N., Song J., Tian J., Li F., Zhang J., Chang L., Pei D., Chen Y. E., and Lai L. (2011) Generation of PPARγ mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res. 21, 979–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geurts A. M., Cost G. J., Freyvert Y., Zeitler B., Miller J. C., Choi V. M., Jenkins S. S., Wood A., Cui X., Meng X., Vincent A., Lam S., Michalkiewicz M., Schilling R., Foeckler J., et al. (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu M., Wei C., Lian Z., Liu R., Zhu C., Wang H., Cao J., Shen Y., Zhao F., Zhang L., Mu Z., Wang Y., Wang X., Du L., and Wang C. (2016) Rosa26-targeted sheep gene knock-in via CRISPR-Cas9 system. Sci. Rep. 6, 24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song J., Zhong J., Guo X., Chen Y., Zou Q., Huang J., Li X., Zhang Q., Jiang Z., Tang C., Yang H., Liu T., Li P., Pei D., and Lai L. (2013) Generation of RAG 1- and 2-deficient rabbits by embryo microinjection of TALENs. Cell Res 23, 1059–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buehr M., Meek S., Blair K., Yang J., Ure J., Silva J., McLay R., Hall J., Ying Q. L., and Smith A. (2008) Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 [DOI] [PubMed] [Google Scholar]
- 22. Li P., Tong C., Mehrian-Shai R., Jia L., Wu N., Yan Y., Maxson R. E., Schulze E. N., Song H., Hsieh C. L., Pera M. F., and Ying Q. L. (2008) Germline competent embryonic stem cells derived from rat blastocysts. Cell 135, 1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hickey R. D., Mao S. A., Glorioso J., Lillegard J. B., Fisher J. E., Amiot B., Rinaldo P., Harding C. O., Marler R., Finegold M. J., Grompe M., and Nyberg S. L. (2014) Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res. 13, 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hickey R. D., Mao S. A., Glorioso J., Elgilani F., Amiot B., Chen H., Rinaldo P., Marler R., Jiang H., DeGrado T. R., Suksanpaisan L., O'Connor M. K., Freeman B. L., Ibrahim S. H., Peng K. W., et al. (2016) Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1. Sci. Transl. Med. 8, 349ra99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang L., Shao Y., Li L., Tian F., Cen J., Chen X., Hu D., Zhou Y., Xie W., Zheng Y., Ji Y., Liu M., Li D., and Hui L. (2016) Efficient liver repopulation of transplanted hepatocyte prevents cirrhosis in a rat model of hereditary tyrosinemia type I. Sci. Rep. 6, 31460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuijk E. W., Rasmussen S., Blokzijl F., Huch M., Gehart H., Toonen P., Begthel H., Clevers H., Geurts A. M., and Cuppen E. (2016) Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci. Rep. 6, 22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y., Fan N., Song J., Zhong J., Guo X., Tian W., Zhang Q., Cui F., Li L., Newsome P. N., Frampton J., Esteban M. A., and Lai L. (2014) Generation of knockout rabbits using transcription activator-like effector nucleases. Cell Regen. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bosze Z., and Houdebine L. M. (2006) Application of rabbits in biomedical research: a review. World Rabbit Sci. 14, 1–14 [Google Scholar]
- 29. Goodman Z. D. (2007) Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 47, 598–607 [DOI] [PubMed] [Google Scholar]
- 30. Elgilani F., Mao S. A., Glorioso J. M., Yin M., Iankov I. D., Singh A., Amiot B., Rinaldo P., Marler R. J., Ehman R. L., Grompe M., Lillegard J. B., Hickey R. D., and Nyberg S. L. (2017) Chronic phenotype characterization of a large-animal model of hereditary tyrosinemia type 1. Am. J. Pathol. 187, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schauwvlieghe P. P., Jaeken J., Kestelyn P., and Claerhout I. (2013) Confocal microscopy of corneal crystals in a patient with hereditary tyrosinemia type I, treated with NTBC. Cornea 32, 91–94 [DOI] [PubMed] [Google Scholar]
- 32. Gissen P., Preece M. A., Willshaw H. A., and McKiernan P. J. (2003) Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J. Inherit. Metab. Dis. 26, 13–16 [DOI] [PubMed] [Google Scholar]
- 33. Trautwein C., Rakemann T., Malek N. P., Plümpe J., Tiegs G., and Manns M. P. (1998) Concanavalin A-induced liver injury triggers hepatocyte proliferation. J. Clin. Investig. 101, 1960–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrari A., Hannouche D., Oudina K., Bourguignon M., Meunier A., Sedel L., and Petite H. (2001) In vivo tracking of bone marrow fibroblasts with fluorescent carbocyanine dye. J. Biomed. Mater. Res. 56, 361–367 [DOI] [PubMed] [Google Scholar]
- 35. Li Y., Song Y., Zhao L., Gaidosh G., Laties A. M., and Wen R. (2008) Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat. Protoc. 3, 1703–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., Richards D. R., McDonald-Smith G. P., Gao H., Hennessy L., Finnerty C. C., López C. M., Honari S., Moore E. E., Minei J. P., et al. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 110, 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brem G., Brenig B., Goodman H. M., Selden R. C., Graf F., Kruff B., Springman K., Hondele J., Meyer J., Winnacker E. L., and Krausslich H. (1985) Production of transgenic mice, rabbits and pigs by microinjection into pronuclei. Reprod. Domest. Anim. 20, 251–252 [Google Scholar]
- 38. Hammer R. E., Pursel V. G., Rexroad C. E. Jr., Wall R. J., Bolt D. J., Ebert K. M., Palmiter R. D., and Brinster R. L. (1985) Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315, 680–683 [DOI] [PubMed] [Google Scholar]
- 39. Tian J., Song J., Li H., Yang D., Li X., Ouyang H., and Lai L. (2012) Effect of donor cell type on nuclear remodelling in rabbit somatic cell nuclear transfer embryos. Reprod. Domest. Anim. 47, 544–552 [DOI] [PubMed] [Google Scholar]
- 40. Yuan L., Sui T., Chen M., Deng J., Huang Y., Zeng J., Lv Q., Song Y., Li Z., and Lai L. (2016) CRISPR/Cas9-mediated GJA8 knockout in rabbits recapitulates human congenital cataracts. Sci. Rep. 6, 22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flisikowska T., Thorey I. S., Offner S., Ros F., Lifke V., Zeitler B., Rottmann O., Vincent A., Zhang L., Jenkins S., Niersbach H., Kind A. J., Gregory P. D., Schnieke A. E., and Platzer J. (2011) Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PLoS One 6, e21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ji D., Zhao G., Songstad A., Cui X., and Weinstein E. J. (2015) Efficient creation of an APOE knockout rabbit. Transgenic Res. 24, 227–235 [DOI] [PubMed] [Google Scholar]
- 43. Yan Q., Zhang Q., Yang H., Zou Q., Tang C., Fan N., and Lai L. (2014) Generation of multi-gene knockout rabbits using the Cas9/gRNA system. Cell Regen. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fu Y., Foden J. A., Khayter C., Maeder M. L., Reyon D., Joung J. K., and Sander J. D. (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang D., Song J., Zhang J., Xu J., Zhu T., Wang Z., Lai L., and Chen Y. E. (2016) Identification and characterization of rabbit ROSA26 for gene knock-in and stable reporter gene expression. Sci. Rep. 6, 25161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Macsai M. S., Schwartz T. L., Hinkle D., Hummel M. B., Mulhern M. G., and Rootman D. (2001) Tyrosinemia type II: nine cases of ocular signs and symptoms. Am. J. Ophthalmol. 132, 522–527 [DOI] [PubMed] [Google Scholar]
- 47. Reese J. A., and Byard J. L. (1981) Isolation and culture of adult hepatocytes from liver biopsies. In Vitro 17, 935–940 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.