Abstract
Inflammation plays a significant role in the development of obesity-related complications, but the molecular events that initiate and propagate such inflammation remain unclear. Here, we report that mice fed a high-fat diet (HFD) for as little as 1–3 days show increased differentiation of myeloid progenitors into neutrophils and monocytes but reduced B lymphocyte production in the bone marrow. Levels of neutrophil elastase (NE) and the nuclear factors CCAAT/enhancer-binding protein α (C/EBPα) and growth factor-independent 1 (GFI-1) are elevated in hematopoietic stem and progenitor cells from HFD-fed mice, but mice lacking either NE or C/EBPα are resistant to HFD-induced myelopoiesis. NE deletion increases expression of the inhibitory isoform of p30 C/EBPα, impairs the transcriptional activity of p42 C/EBPα, and reduces expression of the C/EBPα target gene GFI-1 in hematopoietic stem and progenitor cells, suggesting a mechanism by which NE regulates myelopoiesis. Furthermore, NE deletion prevents HFD-induced vascular leakage. Thus, HFD feeding rapidly activates bone marrow myelopoiesis through the NE-dependent C/EBPα-GFI-1 pathway preceding vascular damage and systemic inflammation.
Keywords: bone marrow, inflammation, metabolic disease, neutrophil, obesity, neutrophil elastase, myelopoiesis, vascular leakage, systemic inflammation
Introduction
Obesity is a major contributing factor to the development of insulin resistance, type II diabetes, and cardiovascular diseases. Extensive investigation has demonstrated the relationship between obesity and increased systemic inflammation (1–4). However, the molecular events that initiate and propagate obesity-related inflammation remain unknown. Neutrophils are the most abundant leukocytes that are critical for innate immunity and acute inflammation (5). In response to an inflammatory insult, circulating neutrophils adhere to the vasculature and undergo transendothelial migration to the site of inflammation. Furthermore, activated neutrophils can interact with and activate other inflammatory cells, such as macrophages and lymphocytes. Neutrophils thus play a pivotal role in initiating inflammation-associated tissue damage. Emerging evidence suggests that neutrophils contribute to obesity-related inflammation. Obese humans have elevated neutrophil counts (6, 7). Our group and others have shown that short-term consumption of a high-fat diet (HFD)5 is sufficient to increase neutrophil infiltration into white adipose tissue, and mice that lack neutrophil elastase (NE) are resistant to HFD-induced leukocyte infiltration in adipose tissues and subsequent insulin resistance (8–10). However, little is known of the mechanisms of neutrophil activation and NE involvement in inflammation at the very early stages of HFD-induced obesity.
Neutrophil homeostasis is highly regulated and involves dynamic changes in the production of neutrophils in the bone marrow (BM). BM hematopoietic stem cells (HSCs) can differentiate into cells of myeloid and lymphoid lineages. During differentiation, differential expression of transcription factors plays a major role in regulating myelopoiesis and lymphopoiesis. In this study, we investigated the mechanisms underlying HFD-induced increases in neutrophil infiltration by examining the effects of an HFD on BM hematopoiesis and the involvement of NE and hematopoiesis-related transcription factors in this process. We found that HFD feeding selectively increases emergency myelopoiesis and subsequent systemic inflammation through activation of the C/EBPα-GFI-1 pathway. This study also suggests that NE regulates C/EBPα-GFI-1 signaling and diet-induced myelopoiesis.
Results and Discussion
Myelopoiesis Is Rapidly Increased by Short-term HFD Feeding
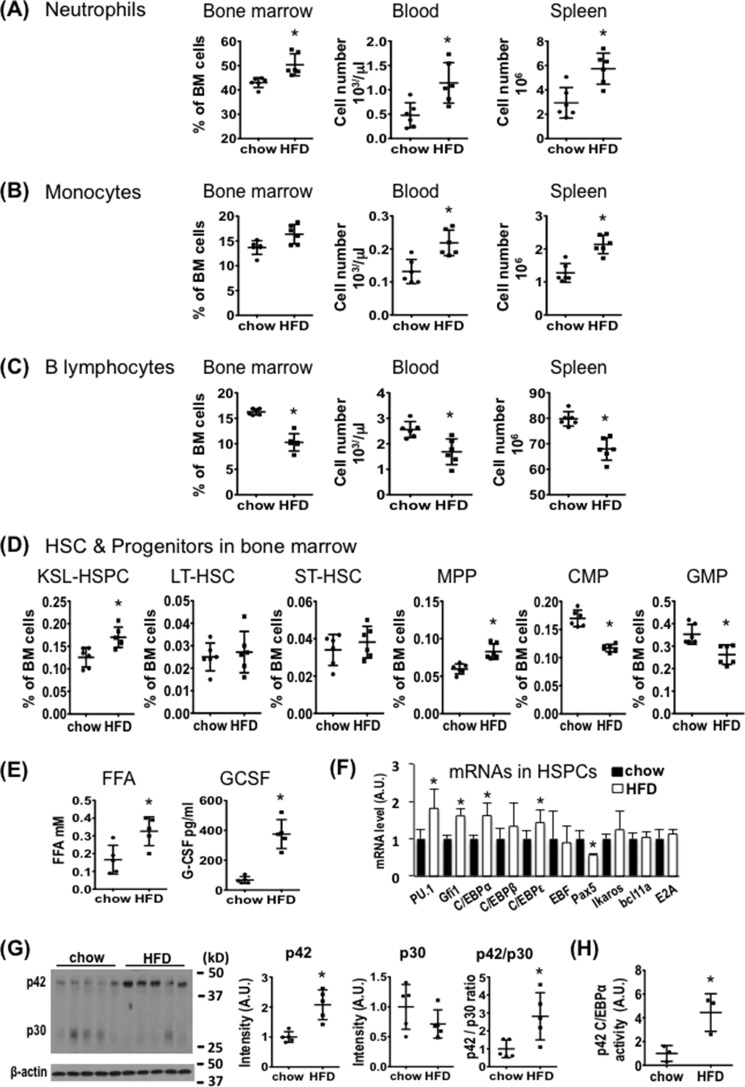
To determine whether HFD-induced leukocyte infiltration in adipose tissues is due to an increase in neutrophil production, we fed WT C57BL/6J mice with a normal chow diet (NCD) or an HFD (60 kcal% with lard fat, Research Diets, Inc., product number D12492) for 3 days, and then measured the frequency and absolute number of neutrophils, monocytes, and B-lymphocytes in the BM, blood, and spleen by flow cytometry (supplemental Fig. 1). Mice fed the HFD had significantly more neutrophils and, to a lesser extent, monocytes, but fewer B-lymphocytes in the BM, blood, and spleen than mice fed an NCD (Fig. 1, A–C, supplemental Fig. 2). Consistent with the increase in myeloid cells, production of Kit+Sca-1+Lin− (KSL) hematopoietic stem and multipotent progenitor cells (KSL-HSPCs) was also higher in the BM of HFD-fed as compared with NCD-fed mice (Fig. 1D, supplemental Fig. 2). Further analyses of the subset populations of KSL-HSPCs revealed that short-term HFD feeding rapidly increased multipotent progenitors (MPPs), whereas no significant change was observed with long-term and short-term hematopoietic stem cells (LT-HSCs and ST-HSCs, Fig. 1D). Interestingly, however, common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs) were decreased by short-term exposure to an HFD.
FIGURE 1.
Short-term HFD feeding differentially affects hematopoiesis. A–D, C57BL/6J mice (10-week-old males) were fed normal chow or an HFD (60 kcal% fat) for 3 days. BM, spleen, and blood samples were analyzed by FACS. Graphs show the frequency (%) or absolute number of neutrophils (CD11b+Ly-6G+) (A), monocytes (CD11b+Ly-6Chigh) (B), B-lymphocytes (CD19+) (C), and HSC/progenitors including KSL-HSPCs (c-Kit+Sca-1+Lin−), LT-HSCs (Lin−Sca1+cKit+CD135−CD34−), ST-HSCs (Lin−Sca1+cKit+CD135−CD34+), MPPs (Lin−Sca1+cKit+CD135+CD34+), CMPs (Lin−Sca1−c-Kit+CD34+FcγRII/IIIinterm), and GMPs (c-Kit+Lin−Sca1−CD34+FcγRII/IIIhigh) (D). A–D, n = 6 mice per group. E, plasma FFA and G-CSF levels. n = 5 per group. F, qRT-PCR analysis of hematopoietic transcription factors in purified BM HSPCs. n = 6 mice per group. A.U., arbitrary units. G, immunoblotting of p42 and p30 C/EBPα in BM cells. n = 5 per group. H, HSPCs (5 × 105 cells) enriched from two mice were pooled for a ChIP assay with p42 C/EBPα antibody followed by quantitative PCR analysis of the promoter region of the Gfi1 gene (sense, 5′-GGGACTTAGTTTCTGAAGC-3′; antisense, 5′-GTTTGCCTAGGGACTATGTG-3′, from −259 to −58). Mouse IgG was used as negative control for immunoprecipitation, and lysate for each sample was used as input control for qRT-PCR. n = 3 per group. A–H, *, p < 0.05, normal chow versus HFD. Experiments were repeated at least two times for A–G and one time for H. All data are expressed as mean ± S.D.
We further performed HFD studies with shorter and longer feeding periods. The changes in neutrophils, BM KSL-HSPCs, LT-HSCs, MPPs, CMPs, and GMPs in mice fed the HFD for 7 days were similar to those in mice fed the same diet for 3 days (supplemental Fig. 3, A–C). HFD feeding for 7 days was also sufficient to increase both Cd11b+Ly6G+ neutrophil and CD45+F4/80+CD11c+ M1-type macrophage infiltration in stromal vascular fractions from adipose tissue (supplemental Fig. 3D). HFD feeding for 24 h increased neutrophil production with concomitant reduction of myeloid progenitors CMPs and GMPs. However, no significant change was observed with BM KSL-HSPCs and MPPs in 1-day HFD-fed mice. This observation suggests that differentiation of myeloid progenitors into neutrophils occurs earlier than enhanced HSC function and MPP production in HFD-fed mice.
We also measured plasma levels of FFAs and G-CSF. As shown in Fig. 1E, plasma FFAs and G-CSF were significantly increased in the mice fed the HFD for 3 days. G-CSF is essential for the amplification and terminal differentiation of neutrophil progenitors and precursors, whereas lipolysis that increases FFAs may be involved in the release of BM neutrophils (11, 12). Thus, our data support the notion that buildup plasma levels of G-CSF and FFAs are associated with HFD-induced neutrophil production. In addition, we observed more blood neutrophils, but not BM neutrophils, in mice fed an HFD (60% kcal) made of coconut oil (medium-chain saturated fat) for 3 days in comparison with chow diet-fed mice (supplemental Fig. 4). Interestingly, plasma levels of FFAs, but not G-CSF, were increased in coconut oil-fed mice. Further studies are needed to evaluate the roles of G-CSF and different species of FFAs in diet-induced neutrophil production and functions.
HFD Feeding Selectively Regulates the Expression of Transcription Factors and Rapidly Activates C/EBPα Transcriptional Activity in HSPCs
To investigate how HFD feeding increases myelopoiesis and concomitantly suppresses lymphopoiesis, isolated BM HSPCs were used to examine gene expression of several hematopoietic transcription factors. PU.1 is known to be required for HSC differentiation to the myeloid lineage, and C/EBPα/ϵ and GFI-1 are necessary for terminal differentiation of myeloid cells (13, 14). We found that HFD feeding for 3 days significantly increased PU.1, C/EBPα, C/EBPϵ, and GFI-1 mRNA levels in HSPCs (Fig. 1F). In contrast, expression of Pax5, which is required for lymphocyte differentiation (15), was significantly reduced in HSPCs after HFD feeding, providing an explanation for the decreased production of B-lymphocytes. These data suggest that short-term HFD feeding selectively stimulates HSC differentiation toward the myeloid lineage and suppresses lymphopoiesis in the BM by differentially regulating transcription factor expression. Consistent with the results of the qRT-PCR analysis, we found that expression of the full-length active form of p42 C/EBPα protein was increased in BM cells from HFD-fed mice, whereas there was a tendency of reduced expression of p30 C/EBPα, which acts as a dominant-negative inhibitor) (16). Hence, HFD feeding significantly increased the ratio of p42 over p30 C/EBPα (Fig. 1G).
C/EBPα promotes GFI-1 transcription in BM progenitors and neutrophils by binding to a C/EBP consensus recognition site in the GFI-1 promoter region (17). To determine whether HFD feeding affects C/EBPα transcriptional activity in BM HSPCs, we performed ChIP assays using an antibody that specifically recognizes the transcriptionally active p42 C/EBPα. Indeed, HFD feeding for 3 days significantly increased C/EBPα binding to the GFI-1 promoter in WT HSPCs (Fig. 1H). Because both C/EBPα and GFI-1 are involved in the late stages of granulocyte differentiation (14), our data suggest that HFD feeding rapidly stimulates myeloid cell differentiation by activating the C/EBPα-GFI-1 pathway. To evaluate whether this pathway is necessary for HFD-induced myelopoiesis, conditional depletion of C/EBPα in BM hematopoietic cells was introduced by injection of poly(I:C) to Mx1-Cre+/CEBPαF/F and control Mx1-Cre−/CEBPαF/F mice (18). Efficient transgene deletion was confirmed by measuring C/EBPα protein in BM cells from Mx1-Cre+/CEBPαF/F (supplemental Fig. 5A). Notably, a dramatic loss of neutrophils and monocytes from the BM and blood of Mx1-Cre+/CEBPαF/F mice as compared with control mice (supplemental Fig. 5, B and C), both fed with HFD for 3 days, was observed. However, the frequency and number of KSL-HSPCs, CMPs, and GMPs were markedly increased in the BM of HFD-fed Mx1-Cre+/CEBPαF/F mice (supplemental Fig. 5D). These data suggest that C/EBPα is required for HFD-induced myeloid cell differentiation.
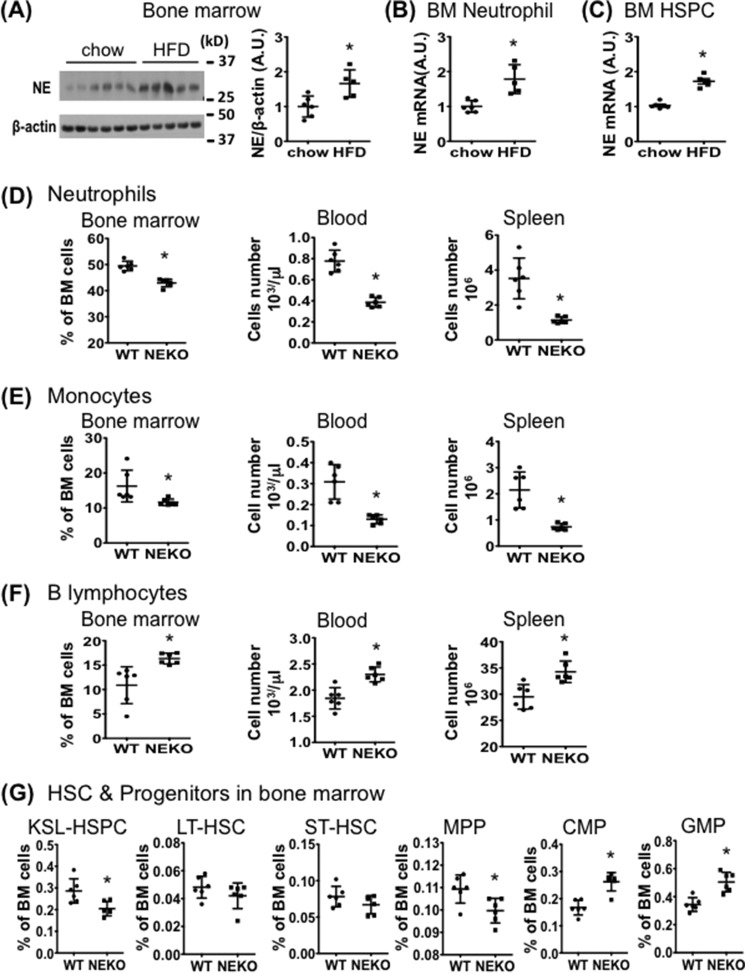
Neutrophil Elastase Deficiency Reduces HFD-induced Myelopoiesis
HFD feeding of WT mice significantly increased NE protein levels in BM (Fig. 2A) and NE mRNA levels in BM neutrophils and HSPCs (Fig. 2, B and C). We asked whether HFD-induced myelopoiesis might also be affected by deletion of NE. Notably, NE KO mice fed an HFD had a significantly lower number of neutrophils and monocytes, but more B-lymphocytes, in the BM, blood, and spleen as compared with HFD-fed WT mice (Fig. 2, D–F, supplemental Fig. 6, A–C). Interestingly, BM KSL-HSPCs and MPPs, but not ST-HSCs and LT-HSCs, were significantly reduced in HFD-fed NE KO mice (Fig. 2G, supplemental Fig. 6D). However, both GMPs and CMPs were significantly increased (Fig. 2G). Thus, HFD-fed NE KO mice and HFD-fed WT mice displayed opposing myelopoietic and lymphopoietic cell profiles. NE KO mice fed an HFD for 4 weeks have similar immune cell profiles to the mice fed for 3 days (supplemental Fig. 7). Of note, in comparison with WT controls, NE KO mice had lower basal levels of KSL-HSPCs, neutrophils, and monocytes accompanied with higher basal levels of B-lymphocytes, CMPs, and GMPs in the BM under the feeding condition with a normal chow diet (supplemental Fig. 8).
FIGURE 2.
NE expression in BM cells is enhanced by short-term HFD feeding and is required for HFD-induced myelopoiesis. A–C, C57BL/6J mice (9-week-old males) were fed normal chow or an HFD for 3 days before BM cells were isolated. A, immunoblotting of NE protein in BM cells. B and C, qRT-PCR analysis of NE mRNA in BM neutrophils (B) and HSPCs (C). A–C, normal chow diet n = 6; HFD n = 5. *, p < 0.05, normal chow versus HFD. A.U., arbitrary units. D–G, NE KO mice and WT controls (10-week-old males) were fed an HFD for 3 days before FACS analyses of cells in BM, spleen, and blood samples. Graphs show the frequency (%) or absolute numbers of neutrophils (D), monocytes (E), B-lymphocytes (F), and BM HSPCs (G). *, p < 0.05, WT versus NE KO mice. n = 6 for each group. Experiments were repeated at least two times for A–G. All data are expressed as mean ± S.D.
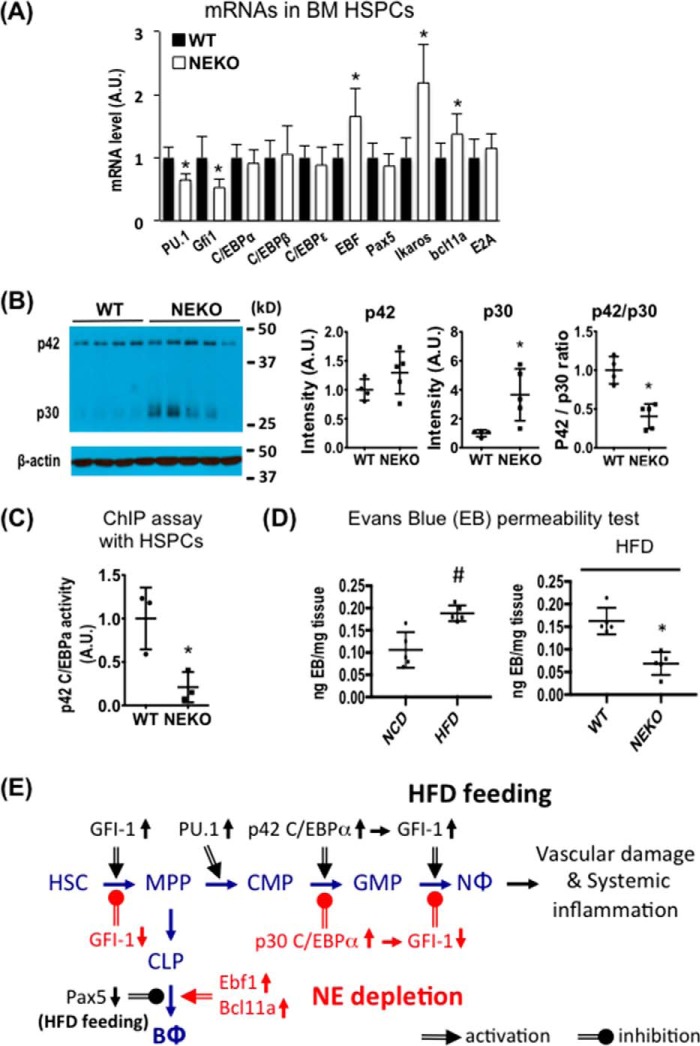
HFD-induced Changes in C/EBPα Isoform Expression and Transcriptional Activity Are Reversed by Deletion of Neutrophil Elastase
We observed that PU.1 and GFI-1, but not C/EBPα/ϵ, mRNA levels were lower in HSPCs from NE KO mice than WT mice (Fig. 3A). In contrast, expression of several transcription factors involved in B-lymphocyte differentiation, such as EBF1, Ikaros, and Bcl11a, was higher in HSPCs from HFD-fed NE KO than HFD-fed WT mice. We also examined the effects of NE deletion on expression of the two C/EBPα protein isoforms in BM cells from HFD-fed mice. As shown in Fig. 3B, BM cells from HFD-fed NE KO mice expressed significantly more of the dominant-negative p30 C/EBPα isoform than HFD-fed WT mice, shifting the ratio of the transcriptionally active and inhibitory C/EBPα isoforms (Fig. 3B). Thus, deletion of NE reversed the HFD-induced change in the C/EBPα isoform ratio observed in WT mice.
FIGURE 3.
NE is required for HFD-induced activation of the C/EBPα-GFI-1 pathway and vascular leakage. A–C, WT or NE KO mice (10-week-old males) were fed an HFD for 3 days before isolation of BM cells. A, qRT-PCR analysis of hematopoietic transcription factors in BM HSPCs. n = 6 per group. A.U., arbitrary units. B, immunoblotting of p42 and p30 C/EBPα in BM cells. WT n = 4, NE KO n = 5. C, HSPCs from HFD-fed WT and NE KO mice were used for a ChIP assay with p42 C/EBPα antibody followed by qRT-PCR analysis of the promoter region of the Gfi1 gene. n = 3 per group. A–C, *, p < 0.05, WT mice versus NE KO mice. D, WT and NE KO mice (8-week-old males) were fed with either an HFD or chow for 4 weeks and then injected with Evans Blue (EB, i.v.) for 2 h before being perfused with PBS. Evans Blue leakage into epididymal fat was quantified as described in the supplemental Experimental Procedures. #, p < 0.05, chow versus HFD for WT mice, n = 4; *, p < 0.05, WT versus NE KO mice. WT n = 4, NE KO n = 5. Experiments were repeated at least one time for A–D. E, illustration of the BM hematopoietic pathway showing the transcription factors involved in HFD-induced changes in myelopoiesis and lymphopoiesis (black letters and arrows) and the corresponding effects of deletion of NE (red letters and arrows). NΦ, neutrophils; BΦ, B lymphocytes. All data are expressed as mean ± S.D.
Short-term HFD feeding increased the expression of GFI-1 mRNA in BM HSPCs in WT mice (Fig. 1F). This change was abolished by deletion of NE (Fig. 3A). Thus, we further performed a ChIP assay to determine whether NE deletion affects C/EBPα transcriptional activity in BM HSPCs from mice fed an HFD for 3 days. Indeed, deletion of NE significantly reduced p42 C/EBPα binding to the GFI-1 promoter in HSPCs (Fig. 3C). These data suggest that loss of NE suppresses HFD-induced myelopoiesis, possibly through inhibiting C/EBPα activity and GFI-1 expression.
Deletion of NE Prevents HFD-induced Vascular Leakage
We further performed a functional test to evaluate whether HFD feeding and depletion of NE affect vascular permeability. As shown in Fig. 3D (left panel), an HFD feeding for 4 weeks increased Evan blue leakage into adipose tissue in C57BL/6J mice. Interestingly, NE KO mice were fully protected from HFD-induced vascular damage in adipose tissue (Fig. 3D, right panel).
HFD Feeding Selectively Activates Transcription Factors That Favor Myelopoiesis at the Expense of Lymphopoiesis in Bone Marrow
In this study, we showed that HFD feeding rapidly increases neutrophil numbers and concomitantly decreases B-lymphocyte numbers in the BM, spleen, and blood. At the molecular level, short-term HFD feeding increases expression of the myelopoietic transcription factors C/EBPα, PU.1, and GFI-1 and decreases expression of the key lymphopoietic transcription factor Pax5 in BM HSPCs, suggesting a mechanism for the differential effects of short-term HFD feeding on myelopoiesis and lymphopoiesis. It is interesting to note that HFD feeding increases the expression of the transcriptionally active p42 C/EBPα. This is consistent with the finding that both p42 C/EBPα transcriptional activity and expression of its downstream target GFI-1 are increased by HFD feeding. We also observed a significant increase in MPPs in the BM of HFD-fed mice, whereas myeloid progenitors, particularly GMPs, were decreased. Previous studies have suggested that C/EBPα is involved in the conversion of CMPs to GMPs and terminally differentiated cells (18), whereas GFI-1 is required for the commitment of GMPs to neutrophils (14). This is consistent with our data showing that C/EBPα deletion significantly increased myeloid progenitors and reduced mature neutrophils and monocytes in HFD-fed mice. Thus, short-term HFD-induced decrease of GMPs and CMPs could be the result of increased expression and activity of C/EBPα and GFI-1. HFD feeding also increases levels of BM MPPs. This could be the result of enhanced HSC differentiation into MPPs. It was previously reported that GFI-1 regulates HSC function or HSC differentiation (19, 20). Hence, our data support the notion that activation of the C/EBPα-GFI-1 pathway at the early stage of HFD feeding leads to: 1) enhanced HSC function and 2) rapid differentiation of the myeloid progenitors to matured myeloid cells at the expense of lymphopoiesis in the BM. Our data elucidate a signaling pathway that is involved in HFD-induced myelopoiesis in the BM (21, 22).
NE Is Required for HFD-induced Activation of the C/EBPα-GFI-1 Pathway and Myelopoiesis
We also found that short-term HFD feeding increases NE mRNA levels in enriched BM HSPCs. Interestingly, NE deletion prevents the HFD-induced changes of immune cell profiles observed in WT mice. The regulatory effects of NE on hematopoiesis during HFD feeding are consistent with, and may explain the resistance of NE KO mice to HFD-induced vascular leakage, leukocyte infiltration, and inflammation of adipose tissues, as well as the development of insulin resistance (8, 9). We also explored the molecular mechanisms by which NE regulates BM myelopoiesis. One mechanistic finding was that NE ablation does not affect the expression of C/EBPα mRNA and its active p42 protein isoform but enhances the expression of the dominant-negative p30 C/EBPα isoform, which lacks the N-terminal 117-amino acid sequence containing two transactivation domains (16, 23). Although the molecular basis for this selective effect is unclear, it is possible that the loss of NE favors expression of the short isoform of C/EBPα. Nevertheless, it is clear that NE deletion decreases the transcriptional activity of C/EBPα and subsequent expression of the downstream target gene GFI-1 required for commitment of late stage granulocytosis (Fig. 3A).
Collectively, the data presented here have uncovered a signaling pathway through which HFD feeding regulates myelopoiesis and neutrophil production (Fig. 3E). Our study provides evidence that HFD feeding rapidly induces neutrophil production in BM preceding vascular damage and systemic inflammation. Given that NE deletion prevents neutrophil production and subsequent systemic inflammation and metabolic dysfunction (8, 9), it is possible that neutrophils and NE are involved in initiating inflammatory damage at the early stages of obesity. Further understanding of molecular events leading to the activation of neutrophils and NE in diet-induced obesity may help to develop strategies for the prevention and treatment of obesity-related vascular damage, inflammation, and diseases.
Experimental Procedures
All methods are described in detail in the supplemental Experimental Procedures, including experimental animals; isolation of mouse bone marrow cells, splenocytes, and peripheral blood leukocytes; FACS analysis; real-time quantitative RT-PCR; PCR primers; immunoblotting analysis; ChIP and C/EBPα transcriptional activity assay; measurement of plasma FFAs and G-CSF; and Evans Blue vascular permeability test.
Statistical Analysis
All quantitative results are expressed as means ± S.D. Differences between two groups were evaluated using an unpaired Student's t test with two-tailed distribution. A p value <0.05 was considered statistically significant.
Study Approval
All mouse work was performed according to Boston University Institutional Animal Care and Use Committee (IACUC) guidelines.
Author Contributions
Z. Y. J., J. Y. H., and Q. L. Z. designed experiments. J. Y. H., Q. L. Z., Y. S., C. H. H., A. G. S., Z. L., K. Z., and Z. Y. J. performed animal experiments. J. Y. H. and Q. L. Z. performed FACS analysis and ChIP assay. J. Y. H. performed immunoblotting analysis and qRT-PCR. Q. L. Z., M. W. M., and R. R. J. performed the permeability test. J. Y. Z. and D. G. T. maintained the Mx1-Cre+/CEBPαF/F mice. Z. Y. J. wrote the manuscript with help from J. Y. H. and Q. L. Z.
Supplementary Material
Acknowledgments
We thank Dr. Anna Belkina (Boston University), and Dr. Carey Lumeng (University of Michigan) for flow cytometry technique assistance. We also thank Dr. Steve Farmer (Boston University School of Medicine (BUSM)) for scientific suggestions.
This work was supported by American Diabetes Association Grant 1-16-IBS-146 and National Institutes of Health Grant R01DK094025 (to Z. Y. J.), a Boston University Clinical & Translational Science Institute (CTSI) Pilot Grant 1UL1TR001430 (to Q. L. Z.), a grant from the National Research Foundation Singapore and the Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award (to D. G. T.), and visiting scholarships from the China Scholarship Council (to C. H., Y. S., and Z. L.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Experimental Procedures and supplemental Figs. 1–8.
- HFD
- high-fat diet
- BM
- bone marrow
- C/EBPα
- CCAAT/enhancer-binding protein α
- CMP
- common myeloid progenitors
- GFI-1
- growth factor-independent 1
- GMP
- granulocyte-monocyte progenitors
- HSC
- hematopoietic stem cell
- HSPC
- hematopoietic stem and progenitor cell
- LT-HSC
- long-term hematopoietic stem cells
- ST-HSC
- long-term hematopoietic stem cells
- MPP
- multipotent progenitor cell
- NCD
- normal chow diet
- NE
- neutrophil elastase
- qRT-PCR
- quantitative RT.
References
- 1. Hotamisligil G. S., Shargill N. S., and Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 2. Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z. W., Karin M., and Shoelson S. E. (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293, 1673–1677 [DOI] [PubMed] [Google Scholar]
- 3. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., and Ferrante A. W. Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., and Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mantovani A., Cassatella M. A., Costantini C., and Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 [DOI] [PubMed] [Google Scholar]
- 6. Xu X., Su S., Wang X., Barnes V., De Miguel C., Ownby D., Pollock J., Snieder H., Chen W., and Wang X. (2015) Obesity is associated with more activated neutrophils in African American male youth. Int. J. Obes. (Lond.) 39, 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trellakis S., Rydleuskaya A., Fischer C., Canbay A., Tagay S., Scherag A., Bruderek K., Schuler P. J., and Brandau S. (2012) Low adiponectin, high levels of apoptosis and increased peripheral blood neutrophil activity in healthy obese subjects. Obes. Facts 5, 305–318 [DOI] [PubMed] [Google Scholar]
- 8. Mansuy-Aubert V., Zhou Q. L., Xie X., Gong Z., Huang J. Y., Khan A. R., Aubert G., Candelaria K., Thomas S., Shin D. J., Booth S., Baig S. M., Bilal A., Hwang D., Zhang H., et al. (2013) Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 17, 534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talukdar S., Oh D. Y., Bandyopadhyay G., Li D., Xu J., McNelis J., Lu M., Li P., Yan Q., Zhu Y., Ofrecio J., Lin M., Brenner M. B., and Olefsky J. M. (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elgazar-Carmon V., Rudich A., Hadad N., and Levy R. (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 11. Manz M. G., and Boettcher S. (2014) Emergency granulopoiesis. Nat. Rev. Immunol. 14, 302–314 [DOI] [PubMed] [Google Scholar]
- 12. Roth Flach R. J., Matevossian A., Akie T. E., Negrin K. A., Paul M. T., and Czech M. P. (2013) β3-Adrenergic receptor stimulation induces E-selectin-mediated adipose tissue inflammation. J. Biol. Chem. 288, 2882–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenbauer F., and Tenen D. G. (2007) Transcription factors in myeloid development: balancing differentiation with transformation. Nat. Rev. Immunol. 7, 105–117 [DOI] [PubMed] [Google Scholar]
- 14. Hock H., Hamblen M. J., Rooke H. M., Traver D., Bronson R. T., Cameron S., and Orkin S. H. (2003) Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 18, 109–120 [DOI] [PubMed] [Google Scholar]
- 15. Cobaleda C., Schebesta A., Delogu A., and Busslinger M. (2007) Pax5: the guardian of B cell identity and function. Nat. Immunol. 8, 463–470 [DOI] [PubMed] [Google Scholar]
- 16. Lin F. T., MacDougald O. A., Diehl A. M., and Lane M. D. (1993) A 30-kDa alternative translation product of the CCAAT/enhancer binding protein α message: transcriptional activator lacking antimitotic activity. Proc. Natl. Acad. Sci. U.S.A. 90, 9606–9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lidonnici M. R., Audia A., Soliera A. R., Prisco M., Ferrari-Amorotti G., Waldron T., Donato N., Zhang Y., Martinez R. V., Holyoake T. L., and Calabretta B. (2010) Expression of the transcriptional repressor Gfi-1 is regulated by C/EBPα and is involved in its proliferation and colony formation-inhibitory effects in p210BCR/ABL-expressing cells. Cancer Res. 70, 7949–7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang P., Iwasaki-Arai J., Iwasaki H., Fenyus M. L., Dayaram T., Owens B. M., Shigematsu H., Levantini E., Huettner C. S., Lekstrom-Himes J. A., Akashi K., and Tenen D. G. (2004) Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBPα. Immunity 21, 853–863 [DOI] [PubMed] [Google Scholar]
- 19. Zeng H., Yücel R., Kosan C., Klein-Hitpass L., and Möröy T. (2004) Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J. 23, 4116–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hock H., Hamblen M. J., Rooke H. M., Schindler J. W., Saleque S., Fujiwara Y., and Orkin S. H. (2004) Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431, 1002–1007 [DOI] [PubMed] [Google Scholar]
- 21. Singer K., DelProposto J., Morris D. L., Zamarron B., Mergian T., Maley N., Cho K. W., Geletka L., Subbaiah P., Muir L., Martinez-Santibanez G., and Lumeng C. N. (2014) Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol. Metab. 3, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagareddy P. R., Kraakman M., Masters S. L., Stirzaker R. A., Gorman D. J., Grant R. W., Dragoljevic D., Hong E. S., Abdel-Latif A., Smyth S. S., Choi S. H., Korner J., Bornfeldt K. E., Fisher E. A., Dixit V. D., et al. (2014) Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19, 821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirstetter P., Schuster M. B., Bereshchenko O., Moore S., Dvinge H., Kurz E., Theilgaard-Mönch K., Månsson R., Pedersen T. A., Pabst T., Schrock E., Porse B. T., Jacobsen S. E., Bertone P., Tenen D. G., and Nerlov C. (2008) Modeling of C/EBPα mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell 13, 299–310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.