Abstract
The release of nucleotides into extracellular space is triggered by insults like wounding and ultraviolet radiation, resulting in stimulatory or inhibitory signals via plasma membrane nucleotide receptors. As similar insults are known to activate hyaluronan synthesis we explored the possibility that extracellular UTP or its breakdown products UDP and UMP act as mediators for hyaluronan synthase (HAS) activation in human epidermal keratinocytes. UTP increased hyaluronan both in the pericellular matrix and in the culture medium of HaCaT cells. 10–100 μm UTP strongly up-regulated HAS2 expression, although the other hyaluronan synthases (HAS1, HAS3) and hyaluronidases (HYAL1, HYAL2) were not affected. The HAS2 response was rapid and transient, with the maximum stimulation at 1.5 h. UDP exerted a similar effect, but higher concentrations were required for the response, and UMP showed no stimulation at all. Specific siRNAs against the UTP receptor P2Y2, and inhibitors of UDP receptors P2Y6 and P2Y14, indicated that the response to UTP was mediated mainly through P2Y2 and to a lesser extent via UDP receptors. UTP increased the phosphorylation of p38, ERK, CREB, and Ser-727 of STAT3 and induced nuclear translocation of pCaMKII. Inhibitors of PKC, p38, ERK, CaMKII, STAT3, and CREB partially blocked the activation of HAS2 expression, confirming the involvement of these pathways in the UTP-induced HAS2 response. The present data reveal a selective up-regulation of HAS2 expression by extracellular UTP, which is likely to contribute to the previously reported rapid activation of hyaluronan metabolism in response to tissue trauma or ultraviolet radiation.
Keywords: cell signaling, hyaluronan, keratinocyte, nucleotide, purinergic receptor, P2Y, UTP, extracellular nucleotide, hyaluronan synthesis
Introduction
Cells subjected to mechanical pressure, osmotic shock, bacterial infection, chemical irritation, UV radiation, and tissue wounding rapidly release nucleotides like UTP, UDP, ATP, and ADP to the extracellular space. The nucleotide release acts as an alarm signal initiating cellular defense mechanisms (1–9). This is particularly important in the epidermis, which forms the protective outer barrier of the body (1, 4). Extracellular nucleotides have also been recognized as important mediators of other physiological signals such as pain sensation (2, 10), keratinocyte proliferation, migration, and apoptosis, and activation of inflammatory cells (11–13). Besides via cellular lysis, nucleotides can also be released by exocytosis, or by diffusion through different plasma membrane channels (14–17). In the extracellular milieu UTP and ATP are rapidly degraded to corresponding diphosphates and monophosphates (4). As nucleotides are released at relatively high concentrations and metabolized rapidly, they are ideal signaling molecules in tissues (18).
Extracellular nucleotides signal via specific ligand-gated or G protein-coupled receptor families (P2X and P2Y, respectively). These receptors are expressed in keratinocytes in a differentiation-dependent manner, members of the P2Y2 family are mainly found in the basal cell compartment, representing the proliferative cell population (reviewed in Ref. 13). UTP signals via P2Y2 and P2Y4, and UDP via P2Y6 and P2Y14 (reviewed in Ref. 19). All of the UTP/UDP receptors are expressed in keratinocytes, although the levels of P2Y4 and P2Y14 are probably low (4, 7, 11, 20). The P2Y2, P2Y4, and P2Y6 subtypes are coupled to Gq/G11 proteins and activate phospholipase C, thus inducing inositol 1,4,5-trisphosphate-mediated Ca2+ release from the ER, and activation of PKC, whereas the P2Y14 receptors inhibit adenylyl cyclase activity via Gi/o proteins (reviewed in Ref. 19).
Hyaluronan is a linear, high molecular mass polysaccharide composed of glucuronic acid and N-acetylglucosamine. The hyaluronan chain is built at the inner surface of the plasma membrane by hyaluronan synthase (HAS) enzymes, which also form a pore for hyaluronan transport into the extracellular space (21). The newly synthesized hyaluronan chain remains associated with the pericellular matrix, either bound to the synthase, or plasma membrane receptors like CD44, but is later released into the extracellular matrix, where it is often associated with aggregating proteoglycans or other extracellular membrane molecules like IαI or TSG6 (reviewed in Ref. 22). There are three hyaluronan synthase enzymes in mammals (HAS1–3). The distribution of the isoenzymes in tissues and the regulation of their expression as well as their synthetic activity differ (23) allowing context-dependent regulation of hyaluronan synthesis.
Enhanced hyaluronan metabolism occurs with tissue injury and inflammation (reviewed in Refs. 24–26), situations that are also associated with extracellular release of nucleotides. However, practically nothing is known about the possible influence of these signaling molecules on hyaluronan synthesis. In gingival fibroblasts (27) and smooth muscle cells (28), HAS1 expression is up-regulated by adenosine, a breakdown product of ATP, leading to the formation of hyaluronan-rich pericellular matrices. In human keratinocytes the sugar nucleotide UDP-glucose stimulates HAS2 expression and hyaluronan synthesis (20).
In skin epidermis hyaluronan content is rapidly increased after tissue wounding (29), exposure to chemical irritants (30), and exposure to ultraviolet B radiation (UVB) (31). Elevations of HAS2 and HAS3 mRNA are seen after skin wounding (29, 32), and UVB radiation also induces HAS1 (31). The mechanisms of HAS up-regulation after trauma remain unresolved at the moment, although activation of the EGF family growth factors by an insult may at least partly explain the up-regulation of HAS2 and HAS3 (32, 33).
The present work explores the hypothesis that the extracellular nucleotide UTP and its breakdown products UDP and UMP contribute to the rapid HAS expression and hyaluronan accumulation after various skin traumas. We establish for the first time that extracellular UTP causes a pulse of hyaluronan synthesis via a strong, specific and rapid up-regulation of HAS2 expression, mediated by the activation of p38, ERK, STAT3, CaMKII and CREB, whereas the expressions of HAS1, HAS3, HYAL1, and HYAL2 are unaffected. High concentrations of UDP reproduce the effect, whereas UMP had no significant influence on HAS2 expression.
Results
Extracellular UTP Enhances Hyaluronan Production
The influence of UTP on hyaluronan metabolism of human keratinocytes was studied by treating HaCaT cells with 100 μm UTP and analyzing hyaluronan staining of the cultures and the amount of hyaluronan secreted in the growth medium. The staining intensity was clearly higher in the UTP-treated cultures compared with the untreated cultures already after a 2-h exposure (Fig. 1, A and B). Confocal imaging confirmed the accumulation of hyaluronan (Fig. 1, C and D) and showed its localization close to the plasma membranes as seen in the side views of the cell layers (Fig. 1, E and F). The histochemical stainings indicated an early activation of its synthesis. However, the amount of hyaluronan released into the culture medium was just slightly increased after a 4-h incubation, the more substantial increase requiring a 6-h incubation with UTP (Fig. 1, G and H). At that time point the amount of hyaluronan in the culture medium was increased by 24 and 46% in the cultures treated with 10 and 100 μm UTP, respectively (Fig. 1, G and H).
FIGURE 1.
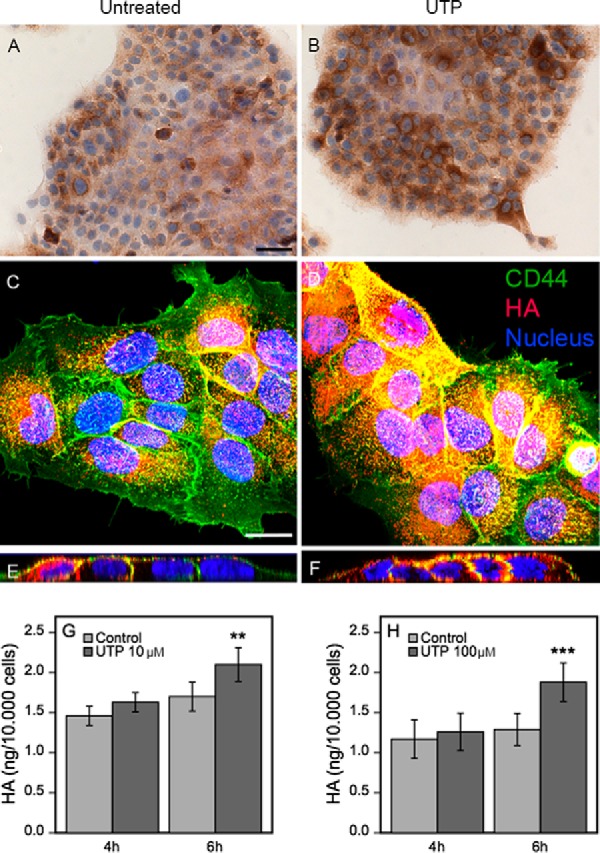
UTP addition in keratinocyte cultures rapidly increases pericellular hyaluronan, and induces a subsequent release of hyaluronan into the medium. HaCaT cultures were left untreated (A, C, and E) or treated with 100 μm UTP for 2 (B) and 4 h (D and F) and stained for hyaluronan using bHABC. In A and B, DAB was used as a chromogen (brown color). In C–F, the cultures were stained for hyaluronan using bHABC and TR-streptavidin (red), and for CD44 with a FITC-labeled secondary antibody (green). The nuclei were visualized with DAPI (blue). C and D are compressed stacks of the confocal images, E and F are side views cut through such stacks. The magnification bar for the bright field images is 50 μm, and for confocal images 20 μm. Culture media collected from HaCaT cells treated with 10 μm for 4 and 6 h (n = 3 for both) (G) and 100 μm (H) UTP for 4 and 6 h (n = 4 and n = 9, respectively) were analyzed for hyaluronan secretion. The data represent mean ± S.E. Mixed model ANOVA was used to calculate the significance of the difference to untreated cultures (**, p < 0.01; ***, p < 0.001).
UTP and UDP Markedly Up-regulate HAS2 Expression
To explore the cause of the increased hyaluronan secretion induced by UTP we first analyzed the possible influence of UTP on the level of the hyaluronan precursor sugars, UDP-GlcNAc and UDP-GlcUA, known to control the rate of hyaluronan synthesis (34–40). No significant changes in their levels were, however, observed in the UTP-treated cells compared with untreated cultures (Fig. 2A), excluding their contribution to hyaluronan accumulation. Similarly, addition of UTP to the culture medium did not influence the amount of intracellular UTP (Fig. 2B).
FIGURE 2.
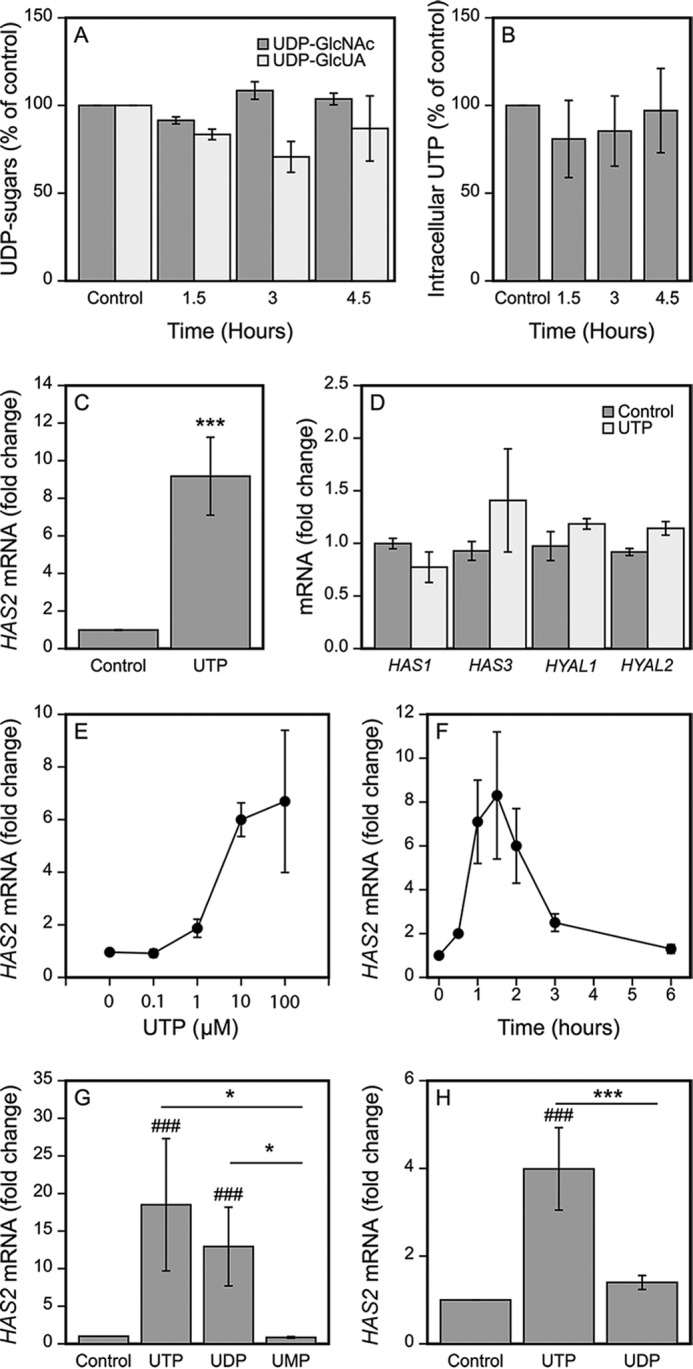
UTP strongly up-regulates HAS2 expression. HaCaT cells were incubated for the indicated times with 100 μm UTP, the amounts of the intracellular UDP-sugar precursors of hyaluronan were measured (A) as well as the intracellular UTP (B). In panels C and D, HaCaT cells were incubated for 2 h with 100 μm UTP and the levels of HAS2 mRNA (C, n = 15) and other hyaluronan-related genes (D, HAS3, n = 4; others n = 3) were analyzed by qRT-PCR. E, HaCaT cells were treated with 0.1–100 μm UTP for 2 h (n = 3). F, 100 μm UTP was added to the cultures and the samples were collected after different incubation times for HAS2 mRNA assays (n = 3). G, HaCaT cells were treated for 2 h with 100 μm UTP, UDP, and UMP prior to qRT-PCR analysis (n = 3). H, HaCaT cells were treated for 2 h with 10 μm UTP and UDP prior to mRNA analysis (n = 3). Statistical significances of the differences between the groups were tested using (in C and D) one group t test (***, p < 0.001). In G and H, mixed model ANOVA was used for comparisons between the different treatments (indicated by *, p < 0.05) and comparisons of treatments to controls (set to 1) using pnorm (indicated by ###, p < 0.001). For the UDP-sugars (A) and concentration and time series (E and F) the non-parametric Friedman test was used due to unequal variances between the groups. Both the effects of the UTP concentration (E) and of the incubation time (F) were statistically significant (χ2 = 11.46, Friedman test p = 0.022 and χ2 = 16.7, Friedman test p = 0.01, respectively). No significance was found in UDP-GlcNAc and UDP-GlcUA (A) (χ2 = 4.667, p = 0.097, and χ2 = 5.0, p = 0.172, respectively) between the untreated and UTP-treated cultures.
We then screened the expression levels of the hyaluronan-related genes by qRT-PCR at the 2-h time point (Fig. 2, C and D). HAS2 mRNA levels in the HaCaT cultures subjected to 100 μm UTP were markedly elevated, with a mean 9.2-fold increase (range 4–35-fold, n = 15) (Fig. 2C). UTP up-regulated HAS3 expression in some of the experiments, but the fold-change was more modest than for HAS2, and not statistically significant (Fig. 2D). The mRNA levels of HAS1, HYAL1, and HYAL2 were not influenced by UTP (Fig. 2D).
Different doses of UTP applied into the culture medium showed that the maximum response to UTP was obtained at about 10 μm, whereas a 1 μm concentration induced just about 2-fold stimulation in HAS2 expression (Fig. 2E). The concentration of UTP needed to stimulate HAS2 expression exceeded that present under basal conditions (nanomolar range), but in stimulated keratinocytes UTP is released at micromolar concentrations (41). Under pathological conditions the concentration of ATP can reach even at 700 μm in the tumor microenvironment (42). Under these conditions the concentration of UTP is also high, as it is released at a 1:3- 1:5 ratio to ATP in several cell types both under basal and mechanically stimulated conditions (41).
The level of HAS2 mRNA started to rise already at 30 min after adding UTP, reaching its maximum at 1.5 h (Fig. 2F). Three hours after introduction of UTP the HAS2 mRNA rise had largely faded, and completely disappeared at the end of the 6-h follow-up (Fig. 2F).
100 μm UDP exhibited a comparable stimulatory effect on HAS2 expression as 100 μm UTP (Fig. 2G). However, the effect of 10 μm UDP was markedly smaller compared with 10 μm UTP (Fig. 2H). In contrast to UTP and UDP, 100 μm of the monophosphate UMP tended to down-regulate HAS2 expression although the response did not reach statistical significance (0.6-fold) (Fig. 2G).
Induction of HAS2 Expression by UTP Involves the Purinergic P2Y2 Receptor
UTP is known to signal via the G-protein-associated receptors P2Y2 and P2Y4, whereas UDP utilizes P2Y6 and P2Y14 (43). Although it has been reported that all of these receptors are expressed in human keratinocytes, the expression levels of P2Y4 and P2Y14 seem to be low (4, 7, 11, 44).
To reveal the role of P2Y2, we suppressed its expression by specific siRNAs. The efficiency of the siRNA silencing of P2Y2 mRNA was 59% (Fig. 3A). The reduction of P2Y2 did not influence HAS2 expression under the basal culture conditions, but it suppressed the UTP-induced HAS2 up-regulation in all experiments performed, the mean reduction being 62% (Fig. 3B). As UTP may experience a rapid extracellular degradation to UDP in the culture medium, we explored the possible involvement of the UDP receptors in the UTP response. At first we silenced the P2Y6 receptor with specific siRNAs. Although the efficiency of the silencing was 59% (mean of 5 independent experiments), its influence on the UTP-induced rise in HAS2 was variable, and statistically not significant (data not shown). To further explore the role of the P2Y6 receptor we utilized a selective antagonist of P2Y6, MRS2578. At a concentration of 20 μm this inhibitor was able to reduce the UDP-induced HAS2 up-regulation by 34% (p = 0.084, Fig. 3C). It also tended to slightly (13%) reduce the UTP-induced HAS2 response, however, these effects did not reach statistical significance (Fig. 3D). The other UDP receptor, P2Y14, is coupled with the Gi protein, which is inhibited by pertussis toxin (PTX) (45). We have previously shown that PTX treatment is effective in HaCaT cells in blocking a UDP-Glc-induced HAS2 induction without causing toxic effects (20). Here, we found that PTX pretreatment reduced the UDP-induced HAS2 up-regulation by 64% (Fig. 3E), but was unable to consistently block the UTP-induced up-regulation of HAS2 (Fig. 3F). These data suggest that the UTP-induced HAS2 response is conveyed mainly through the UTP receptors.
FIGURE 3.
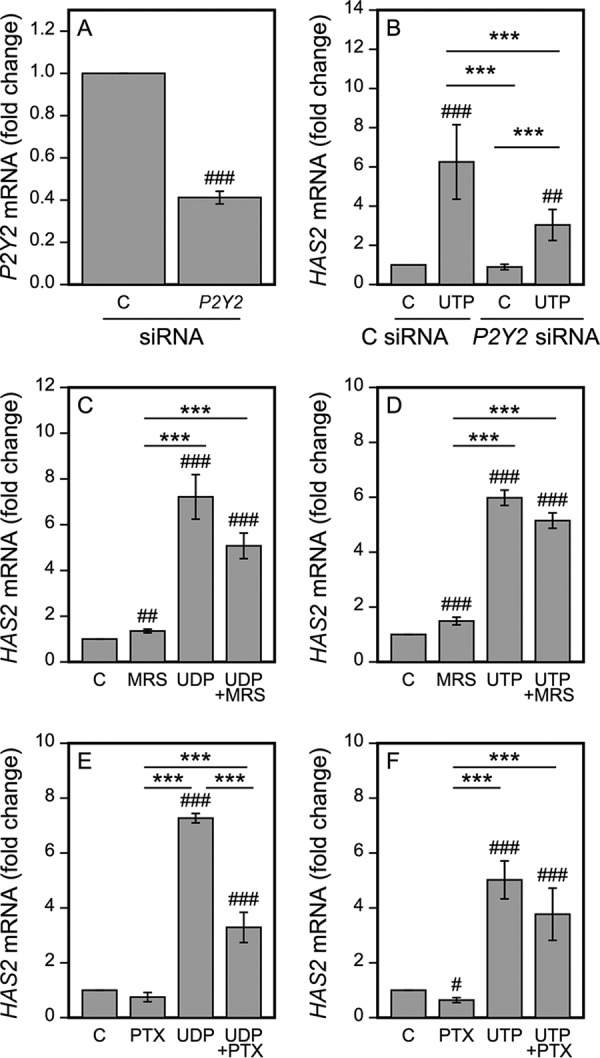
The P2Y receptors and signaling pathways involved in the UTP-induced HAS2 up-regulation. HaCaT cells were transfected with control and P2Y2-specific siRNAs (A and B). A, 2 days after the transfection the samples were collected to test for the efficiency of the siRNAs (n = 4), or B, subjected to 100 μm UTP for 2 h prior to collecting the samples for HAS2 qRT-PCR (n = 5). C and D, cells were subjected to MRS2578 (a selective antagonist of P2Y6, 20 μm) for 30 min, and E and F, PTX (100 ng/ml) for 17 h prior to the addition of 100 μm UDP (C and E) and UTP (D and F) for 2 h before mRNA assays. Statistical significances of the differences between the groups were tested using one group t test in A (##, p < 0.01). Mixed model ANOVA was used for comparisons between the different treatments (indicated by **, p < 0.01; ***, p < 0.001) and comparisons of treatments to controls (set to 1) was tested by pnorm (indicated by #, p < 0.05; ##, p < 0.01; ###, p < 0.001) in B–F.
UTP Rapidly Activates p38, ERK, STAT3, CaMKII, and CREB
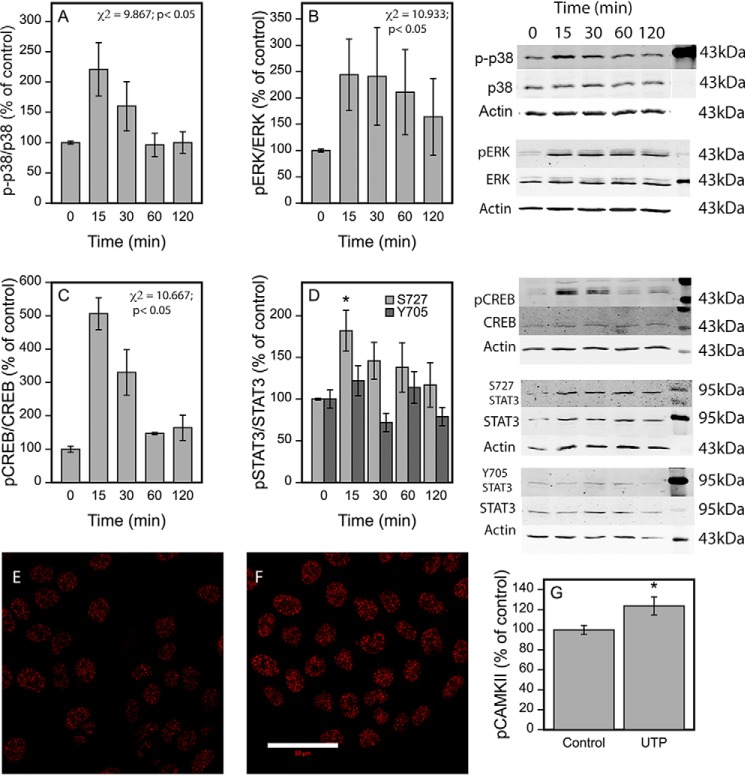
The UTP signal mediated by the Gq/11 protein coupled to P2Y2, P2Y4, and P2Y6 involves activation of calcium-related pathways, eventually leading to phosphorylation of MAP kinases, calmodulin, and the associated kinases such as CaMKII (19), STATs (46), and CREB (47). To study whether these pathways are also involved in the HAS2 response we performed Western blotting experiments on the corresponding phosphoproteins. Although UTP did not influence the amount of total protein of these signaling molecules, it caused a clear and rapid activation of phospho-p38 (Fig. 4A), pERK (Fig. 4B), and pCREB (Fig. 4C). A tendency for enhanced phosphorylation by UTP was also found at Ser-727 of STAT3 with a similar temporal pattern as in p38, ERK, and CREB (Fig. 4D), whereas there was no influence on the Tyr-705 phosphorylation of STAT3 (Fig. 4D).
FIGURE 4.
UTP induces rapid phosphorylations of p38, CREB, and Ser-727 of STAT3. HaCaT cells were subjected to 100 μm UTP for 15 min to 2 h before collecting the samples for Western blotting using antibodies against phosphorylated signaling proteins. For normalization the blots were reprobed for the total forms of signaling proteins and actin after a brief (10 min) stripping with 0.2 m NaOH. Representative blots out of 3 (p-p38, p38α (A); pERK, ERK (B); pCREB, CREB (C); pSTAT3-S727, pSTAT3-Y705, and total STAT3 (D)) experiments with the corresponding actins are shown, whereas mean ± S.E. of the quantifications of the phosphorylated forms per corresponding total proteins from all the experiments are presented as bar graphs. Statistical significances were tested using a non-parametric Friedman test because of unequal variances, indicating the overall significance values indicated in the figures. For STAT3-Y705 and -S727 the overall influence did not reach statistical significance. For Ser-727 of STAT3, allowing the use of a parametric test, pairwise comparisons of individual time points to the zero time point utilizing mixed model ANOVA and pnorm function were also performed, suggesting a significant influence at the 15-min time point (indicated by *, p < 0.05) (D). E and F, immunostainings for pCaMKII of the untreated (E) and the UTP-treated (100 μm) (F) cultures. The treatment time was 30 min. Mean ± S.E. of 5 experiments are shown in the chart (G). Statistical significance between the groups was tested using mixed model ANOVA (indicated by *, p < 0.05) (G).
Staining with the pCaMKII antibody was positive under the basal culture conditions of HaCaT cells, localizing mainly in the nucleus (Fig. 4E). After a 30-min treatment with UTP the intensity of nuclear staining was further increased (Fig. 4, F and G), indicating CaMKII activation.
The UTP-induced HAS2 Up-regulation Is Reduced by the Inhibition of PKC, p38, ERK, CaMKII, STAT3, and CREB
To obtain further support for the involvement of the above UTP-activated signaling pathways in the HAS2 response, we pretreated the HaCaT cultures with inhibitors of the corresponding proteins prior to UTP exposure. Treatment with the p38 inhibitor BIRB796 reduced the UTP-induced HAS2 up-regulation by 52%, whereas it had no influence on the basal level of HAS2 (Fig. 5A). Similarly, the MEK/ERK inhibitor PD98059 cut the UTP-induced HAS2 expression by 48%. However, it also significantly reduced the basal expression of HAS2 by 30% (Fig. 5A). As MAPKs can be activated by PKC, we treated the HaCaT cells with the PKC inhibitor BIM (Fig. 5B). It also reduced the UTP-induced HAS2 expression by 48% (Fig. 5B).
FIGURE 5.
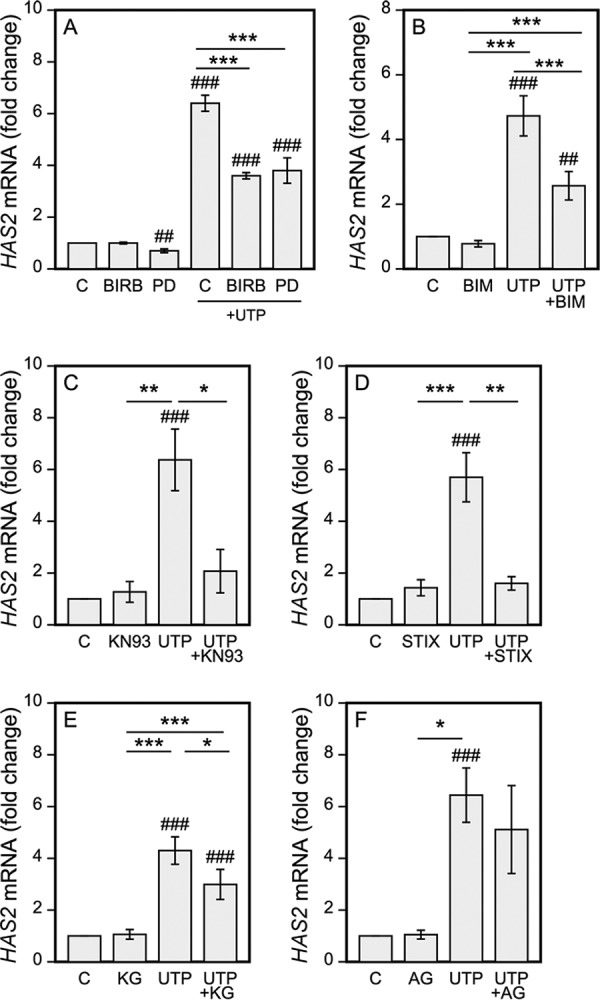
Inhibition of p38, CaMKII, STAT3, and CREB reduce the UTP-induced HAS2 up-regulation. HaCaT cells were subjected to: A, the MEK kinase inhibitor PD98059 (PD, 0.5 μm) and the p38 inhibitor BIRB796 (BIRB, 2 μm); B, the PKC inhibitor (BIM, 10 μm); C, the CaMKII inhibitor KN93 (KN93, 25 μm); D, the STAT3 IX inhibitor (STIX, 50 μm); E, the CREB inhibitor KG501 (KG, 25 μm); and F, the JAK2/EGFR inhibitor AG490 (AG, 30 μm). Preincubations with the inhibitors were 0.5 h for ERK, p38, CREB, and PKC, and 2 h for CaMKII and STAT3 prior to the addition of 100 μm UTP for 2 h. Mean ± S.E. are shown. The number of experiments is 3 for ERK and p38, 6 for BIM, 4 for CREB, 6 for CaMKII, 5 for STAT3, and 4 for JAK2/EGFR. Statistical significances of the differences between the groups were tested using mixed model ANOVA (indicated by *, **, and ***) and comparisons of the treatments to the controls (set to 1, indicated by ## and ###) using pnorm. *, p < 0.05; ** and ##, p < 0.01; *** and ###, p < 0.001.
Inhibition of CaMKII signaling with KN93 suppressed the UTP-induced HAS2 rise by 80% without influencing the basal expression level (Fig. 5C). Likewise, the STAT3 inhibitor IX (CPD188) exerted an 87% cut in the UTP-induced response without influencing the basal HAS2 expression (Fig. 5D). KG501 (naphthol-AS-E-phosphate), which blocks the binding of CPB to CREB and thereby prevents pCREB activity, reduced the HAS2 mRNA induction due to UTP in all the experiments performed (39% inhibition) (Fig. 5E). Its analog, naphthol-AS-BI-phosphate (48), caused a 31% reduction in the UTP-induced HAS2 stimulation (p < 0.001, n = 4, data not shown).
As G-protein signaling is also known to activate EGFR (reviewed by Ref. 49) and JAK2 (20, 50) we pretreated the UTP-exposed cultures with AG490, which inhibits both EGFR and JAK2. However, here it failed to block the UTP-induced HAS2 response (Fig. 5F), indicating that the rapid HAS2 response after UTP is independent of EGFR and JAK2.
Discussion
The present study shows for the first time that the extracellular uridine nucleotides UTP and UDP strongly up-regulate HAS2 expression, leading to the accumulation of hyaluronan in the pericellular matrix and later in the culture medium. As these nucleotides are often released after tissue trauma, the present findings provide a novel signaling mechanism to explain the rapid increase in hyaluronan synthesis detected after various insults (29–32). It also links hyaluronan to the defense mechanisms activated by danger signals such as extracellular nucleotides. The UTP-induced increase in HAS2 activation is transient, however, indicating that a more sustained response observed after wounding and UVR (29, 31, 32) requires subsequent activation of other signaling routes, such as growth factors or cytokines (33, 51, 52).
UTP Selectively Activates HAS2 in Keratinocytes
Although HAS2 underwent a marked increase, the effect of UTP on HAS3 was slight and inconsistent, and completely non-existent on HAS1. The absence of influence on HYAL1 and HYAL2 and the unchanged levels of the UDP-sugar substrates also support the idea that the accumulation of hyaluronan was specifically due to HAS2 up-regulation. A similar HAS2-specific response of keratinocytes was previously found for the growth factors EGF and KGF (33, 51, 52), whereas HAS2 and HAS3 contribute to keratinocyte hyaluronan production at approximately equal proportions under basal culture conditions (31). In addition to direct transcriptional regulation of HAS2, the effect of UTP could arise from the induction of HAS2-AS1, which in turn can regulate HAS2 expression via chromatin remodeling (53).
Although the UTP-induced HAS2 induction was rapid and transient, it was associated with a significant increase in the amount of hyaluronan in the pericellular matrix and in the culture medium, indicating that the elevated mRNA levels were reflected in HAS2 protein activity. The possible importance of producing hyaluronan specifically by HAS2 is not known, but it has been found that the appearance of the cell surface coat produced by overexpressed HAS2 is slightly different from that made by HAS3 (54). Although the increase in hyaluronan followed the increase in HAS2 mRNA, it is possible that post-translational modifications such as phosphorylation, ubiquitination, or O-GlcNAcylation of HAS2 were involved in the UTP-induced hyaluronan synthesis (55, 56, 39). However, the involvement of O-GlcNAcylation is less likely, because UTP did not influence the level of UDP-GlcNAc, an important substrate for O-GlcNAcylation.
The increase of hyaluronan surrounding the cells was detectable within 2 to 4 h, whereas reaching a significant change in the released hyaluronan required 6 h, probably due to the relatively long half-life (8 h) of pericellular hyaluronan in keratinocytes (57). Treatment of mesenchymal cells with a viral mimetic, or subjecting them to ER stress or hyperglycemia leads to the formation of cable-like structures of hyaluronan that attract inflammatory cells (26). Although not as prominent as in mesenchymal cells, keratinocytes can also form monocyte adhesive hyaluronan cables in response to inflammatory cytokines (58). UTP appears to differ from the above mentioned conditions in this respect, as no signs of cable formation were observed.
Receptors Involved in the UTP-induced HAS2 Response
UTP signals via the P2Y2 and P2Y4 receptors, however, the expression level of P2Y4 appears to be very low in keratinocytes (4, 7, 11), suggesting that it has a minor role in the UTP-induced responses in these cells. On the other hand, a P2Y2-specific siRNA reduced the UTP-induced HAS2 response by 62%, which considering only a partial (59%) silencing of the mRNA expression, indicates that the P2Y2 receptor mediates a major proportion of the effect.
As UTP released into the extracellular space is actively metabolized to UDP also by the HaCaT cells (4) and UDP enhanced HAS2 mRNA expression, it was possible that a part of the HAS2 induction after UTP release might occur via the P2Y6 and P2Y14 receptors for UDP (Fig. 6). Indeed, under our experimental conditions, inhibitors of P2Y6 and P2Y14, which reduced the UDP-induced HAS2 response, caused a slight, although nonsignificant down-regulation of the UTP response. The higher concentration of UDP required to achieve a stimulation comparable with that by UTP, together with the modest effect of the UDP-receptor inhibitors on UTP-induced HAS2 response, suggest that UTP degradation to UDP is not necessary for HAS2 up-regulation, although it may contribute to the response. In contrast to UTP and UDP, the corresponding monophosphate UMP failed to induce HAS2 expression. This might be explained by the fact that no known receptor for this nucleotide exists. The finding also suggests that no significant reversal of extracellular UMP to UDP or UTP occurs in the HaCaT cell cultures.
FIGURE 6.
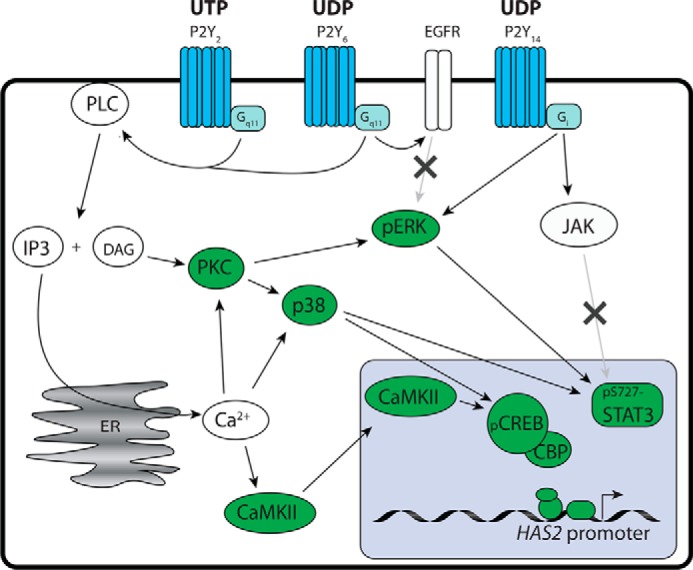
Schematic representation of the signaling pathways involved in the UTP-induced HAS2 up-regulation. Extracellular UTP and its breakdown product UDP activate the P2Y receptors that increase HAS2 expression via the indicated signaling steps. Those steps positively verified in the current study are marked green, whereas the pathways excluded are indicated by an “x.”
The UTP-induced Activation of HAS2 Expression Is Mediated via Pathways Dependent on CaMKII and PKC
As P2Y2, P2Y4, and P2Y6 are associated with the same G-protein, Gq/11, it is likely that their downstream signaling is similar, involving intracellular Ca2+ release and phospholipase C. MAP kinases, calmodulin, and related kinases as well as the transcription factors STAT3 and CREB are known to be activated by these receptors, reviewed in Ref. 13 and outlined in Fig. 6. UDP, on the other hand, via its association with P2Y14 and its inhibitory partner Gi, can suppress cAMP formation thereby reducing CREB activity. However, P2Y14 can also activate STAT3 via ERK and JAK2 (20, 59). In general, our findings on the activation of MAPK, CaMKII, STAT3, and CREB in HaCaT cells by UTP stimulation are in line with previous reports. The experiments applying specific inhibitors of these signaling molecules showed markedly reduced HAS2 responses to UTP, indicating involvement of PKC, p38, ERK, CaMKII, STAT3, and CREB. However, the true role of pERK in the HAS2 response remains uncertain due to the strong effect of the inhibitor on the basal HAS2 expression level. In any case, co-activation of both ERK and p38 signaling has been demonstrated in HaCaT cells after nucleotide exposure (60). The inability of the JAK2/EGFR inhibitor AG490 to block the UTP-dependent HAS2 response rules out EGFR activation after extracellular nucleotide exposure (61).
Although CREB is a target of several of the indicated upstream signaling pathways, STAT3 is regulated by MAP kinases and JAK2 (62). p38 and JNK have been associated with the Ser-727 phosphorylation of STAT3, whereas JAK2 is associated with Tyr-705 phosphorylation (reviewed in Ref. 63). UTP induced STAT3 phosphorylation in Ser-727, but not in Tyr-705, the former correlating temporally with p38 activation. These findings are in line with the inhibitor experiments indicating the involvement of p38 but not JAK2 in HAS2 mRNA induction. Although the specific significance of Ser-727 phosphorylation is unsettled, it is needed for full transcriptional activation of STAT-regulated gene expression, for example, by recruiting co-activators to the promoter (63, 64). Although Tyr-705 phosphorylation has been classically regarded as necessary for STAT3 activity, recent findings suggest that STAT3 may also influence gene expression independently of its phosphorylation status (reviewed Ref. 63).
Blocking CREB binding to CBP, a necessary cofactor for CREB activity (65), and inhibition of STAT3 activity both reduced the UTP-induced HAS2 up-regulation. Both of these transcription factors have active binding sites on the HAS2 promoter and regulate HAS2 expression in keratinocytes (66, 67). The lower efficiency of CREB blocking may be due to a lower potency of the inhibitors used, or a weaker activity of CREB on the HAS2 promoter. CREB alone, even with CBP, has been suggested to be insufficient to activate transcription (65). However, as both STAT3 and CREB inhibitors influenced UTP-induced HAS2 expression, it is also possible that their effect is cooperative as shown with some other promoters (68, 69).
The Interplay between UTP and Hyaluronan
The rapid nature of the HAS2 response suggests that it may be a part of the cellular defense reactions induced by UTP signaling. Indeed, the UTP-induced HAS2 expression occurred with a similar temporal pattern as the influence of UTP on the expression of the proinflammatory cytokines IL-6 and IL-8 in HaCaT cells (7, 70). The simultaneous response rules out the possibility of the cytokines controlling HAS2 expression or vice versa; rather, it is likely that the early HAS2 and cytokine responses are coordinated. Later on, breakdown of hyaluronan to low molecular mass fragments can potentiate IL-6 signaling, resulting in further IL-6 production (71), whereas intact hyaluronan dampens the inflammatory reaction by counteracting the IL-6 signaling (72–74). Apart from regulating cytokine release, hyaluronan itself can protect cells from apoptosis by scavenging reactive oxygen species. This has been shown in cultured corneal epithelial cells and keratinocytes treated with either UV radiation or toxic substances (75, 76). Furthermore, Wang and co-workers (77) showed that high levels of hyaluronan and Has2 expression were associated with resistance to UVB radiation and serum starvation in mouse fibroblasts. Thus, tissue stress that liberates UTP is followed by a rapid build-up of a hyaluronan matrix, which might protect the cells from further damage in case the harmful insult continues.
HAS2 activation and hyaluronan may also have signaling functions in their own right. Via its cell surface receptor CD44, hyaluronan has been shown to modulate the signaling of several growth factor receptors like EGFR and PDGFR, as reviewed in Ref. 78, often potentiating the effects of the native ligands. Interestingly, in keratinocytes and fibroblasts, synergism was found in the motogenic response between extracellular nucleotides and EGF, TGFα, PDGF, and TGFβ (79). Further studies are needed to check whether hyaluronan is involved in this cooperation.
UTP stimulates the migration of endothelial cells, arterial smooth muscle cells, corneal epithelial cells, prostate cancer cells, and schwannoma cells (80–83), whereas in keratinocytes it inhibits cell spreading and motility (84, 85). The influence of hyaluronan on cell motility is obviously complex. It has been shown to stimulate cell migration and in turn its removal inhibits migration (reviewed in Ref. 86). However, hyaluronan can also inhibit cell motility, especially when present in excessive amounts such as during artificial overexpression induced by HAS transfection (87, 88). The physiological response of the cells to hyaluronan depends on its molecular mass (24, 89). UTP induced mainly HAS2, which is known to synthesize high molecular mass hyaluronan (87, 90). Whether hyaluronan breakdown was activated by UTP was not investigated here. However, we did not observe any increase in the intracellular hyaluronan, which is often associated with hyaluronan breakdown (31, 91). Moreover, the expression of hyaluronidases was not changed. However, because the inhibition of cell motility occurs already within 15 to 30 min after the UTP exposure (84, 85), it unlikely relates to hyaluronan synthesis, which takes place later.
Coordination and Convergence of Signaling in Keratinocyte Hyaluronan Responses Triggered by Injury and Environmental Stress
Interestingly, the UTP-induced signaling pathways regulating HAS2 expression partially overlap with those occurring after UVB exposure, an insult known to cause nucleotide release in keratinocytes (9). Although UVB induces a multitude of signaling events, in rat epidermal keratinocytes (REK) the induction of Has2 and Has3 appears to depend mainly on p38 and CaMKII (31). However, in REK cells UVB also activates the expression of other hyaluronan-related genes, such as Has1, Hyal1, and Hyal2 (31), which did not respond to UTP. Furthermore, the UVB-induced activation of p38 and Has2 expression in rat keratinocytes is long-lasting (36 h), suggesting that for a more sustained response other signaling pathways need to be activated. One of the UVB-activated cascades originates from HB-EGF/EGFR (92), shown to regulate Has2 and Has3 expression in REK cultures (32, 93). Although the intracellular signaling mediators triggered by nucleotides and EGFR activation are partially different, they show convergence, suggesting that they can act synergistically (94).
In conclusion, nucleotides UTP and UDP, but not UMP, released into the extracellular space activate HAS2 mRNA expression in human keratinocytes. The expression of the other hyaluronan synthases or hyaluronidases are not significantly influenced by UTP. The signaling pathway for UTP involves the purinergic P2Y2 receptor, and to a smaller extent the UDP receptors P2Y6 and P2Y14, and their downstream cascades recruiting PKC, and the MAP kinases p38 and ERK, CaMKII, STAT3, and CREB (Fig. 6). The effect of UTP on HAS2 expression is strong and, although transient, causes a significant increase in hyaluronan accumulation in the pericellular matrix and culture medium. This rapid induction of a hyaluronan coat may be a good way to provide an instant response to a potentially damaging signal, whereas making sure a stronger, more sustained response is only activated if required by the circumstances.
Experimental Procedures
Cell Culture
HaCaT cells, a human spontaneously immortalized epidermal keratinocyte cell line developed by Boukamp et al. (95), were used in the present study. They were cultured in DMEM with low glucose (D5921, Sigma) containing 10% FBS (HyClone, Logan, UT), 2 mm l-glutamine (Euroclone, Milan, Italy), 50 units/ml of penicillin, and 50 μg/ml of streptomycin (Euroclone). For microscopy the cells were plated and cultured on 8-well chamber slides (Nunc Nalgene, Napperville, IL), and for biochemical experiments on 12- or 6-well plates (Greiner Bio-One, Kremsmünster, Austria). UTP, UDP, and UMP (Sigma) were dissolved in H2O as stock solutions and kept at −20 °C until used.
Signaling Inhibitors
A 2-h pretreatment before the addition of nucleotides was used with the CaMKII inhibitor KN93 (25 μm, Calbiochem, Merck Millipore, Darmstadt, Germany), JAK2 inhibitor AG490 (30 μm, Sigma), STAT3 inhibitor IX (50 μm, Calbiochem), and a 30-min pretreatment with the MEK inhibitor PD98059 (0.5 μm, Calbiochem), p38 inhibitor BIRB796 (2 μm, Axon Medchem BV, Groningen, Netherlands), CREB inhibitors (KG501, Sigma, and naphthol AS-BI-phosphate, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, both at 25 μm concentration), PKC inhibitor BIM (bisindolylmaleimide, 10 μm, Calbiochem), and the P2Y6 inhibitor MRS2578 (20 μm, Tocris Biosciences, Southampton, UK), whereas with PTX (100 ng/ml, Sigma) a 17-h preincubation was used. KN93, PTX, BIM, and naphthol AS-BI-phosphate were dissolved in water, AG490 in ethanol, and the STAT3 inhibitor IX, BIRB796, KG501, and MRS2578 in DMSO. Equal amounts of these solvents were added to the control cultures.
siRNA Treatments
The control siRNAs were obtained from Eurogentec (Liege, Belgium), P2Y2-targeted siRNAs were from Thermo Fisher (Waltham, MA), and P2Y6-targeted siRNAs from Origene (Rockville, MD). Subconfluent cultures were transfected with 50 nm siRNA using RNAiMAX (Invitrogen) according to the manufacturer's instructions. The transfection medium was replaced with ordinary culture medium after 4 h. The cells were grown for 2 days before treatment with the nucleotides. The efficacy of the knockdown was confirmed by qRT-PCR.
RNA Extraction and qRT-PCR
Total RNA was extracted with the TRI Reagent (TR118, Molecular Research Center Inc., Cincinnati, OH) and cDNA synthesis was performed using the Verso cDNA kit (Thermo Fisher). The samples were analyzed on an MX3000P thermal cycler (Stratagene, La Jolla, CA), using the Fast Start universal SYBR Green Master Mix (ROX) (Roche Applied Science, Basel, Switzerland). The cycling conditions were as follows: preincubation for 10 min at 95 °C followed by 40 cycles of 15 s denaturation at 95 °C, 1 min annealing at a primer-specific temperature, and 1 min elongation at 72 °C. Gene-specific amplification was confirmed by a melt curve analysis. The specific primers for the genes analyzed and the annealing temperatures are shown in Table 1. Fold-inductions were calculated using the formula 2−(ΔΔCt), where ΔΔCt is ΔCt(sample) − ΔCt(non-treated replicate), ΔCt is Ct(gene of interest) − Ct(ARP0) and Ct is the cycle at which the threshold is crossed.
TABLE 1.
Primer sequences for qRT-PCR of the human genes
| Gene name | Primer sequence (5′to 3′) | Tm |
|---|---|---|
| °C | ||
| ARP0 | Forword, AGATGCAGCAGATCCGCAT | 59 |
| Reverse, GTGGTGATACCTAAAGCCTG | ||
| HAS1 | Forward, CAAGATTCTTCAGTCTGGAC | 59 |
| Reverse, TAAGAACGAGGAGAAAGCAG | ||
| HAS2 | Forward, CAGAATCCAAACAGACAGTTC | 59 |
| Reverse, TAAGGTGTTGTGTGTGACTG | ||
| HAS3 | Forward, CTTAAGGGTTGCTTGCTTGC | 59 |
| Reverse, GTTCGTGGGAGATGAAGGAA | ||
| HYAL1 | Forward, GCCCTCTATCCCAGCATCTA | 59 |
| Reverse, CTCACCCAGAGCACCACTC | ||
| HYAL2 | Forward, CCTCTGGGGCTTCTACCTCT | 59 |
| Reverse, CTGAACACGGAAGCTCACAA | ||
| P2Y2 | Forward, CCTGAGAGGAGAAGCGCAG | 59 |
| Reverse, GAACTCTGCGGGAAACAGGA |
Western Blotting
Protein extraction and SDS-PAGE were performed as described before (20). Briefly, RIPA (PBS, pH 7.4, with 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/ml of phenylmethylsulfonyl fluoride, 10 μg/ml of sodium orthovanadate, and 1% phosphatase inhibitor mixture 2, and 0.5% protease inhibitor mixture (Sigma)) lysis buffer was used for protein extraction. 7.5–20 μg of protein was loaded per lane (based on Bradford assay), resolved by 10% SDS-PAGE, followed by transfer onto a nitrocellulose membrane (Amersham BiosciencesTM ProtranTM 0.45-μm NC, GE Healthcare Life Sciences, Little Chalfont, UK) with a Fastblot B43 semidry blotter (Biometra, GmbH, Göttingen, Germany). The membranes were washed with TBS containing 0.1% Tween, blocked with 1–5% BSA/TBS, and incubated with the primary antibodies: total STAT3, p38α, ERK1/2, CREB, and phospho-STAT3 (Tyr-705), phospho-STAT3 (Ser-727), phospho-CREB (Ser-133), phospho-p38 (all from Cell Signaling Technologies, Danvers, MA, diluted 1:1000), phospho-ERK (Santa Cruz Biotechnology Inc., CA, diluted 1:200), and followed by DyLight 680 or 800 anti-rabbit or anti-mouse secondary antibodies, 1:4000 (Pierce). Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) was used to image and quantitate the bands, normalizing the phosphospecific band intensities to the nonphosphorylated total proteins.
Hyaluronan ELSA
For hyaluronan assays HaCaT cells were treated with the nucleotide sugars (100 μm) for 4 or 6 h. The media were collected for hyaluronan assays and cells were released with trypsin and counted for normalization. The enzyme-linked sorbent assay (ELSA) of hyaluronan was performed as described earlier (96) using a probe complex containing the aggrecan G1 domain and link protein (HABC, prepared as described in Ref. 97). ELISA plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with HABC, followed by 1-h sequential incubations with hyaluronan standards (1–50 ng/ml) and appropriately diluted samples, then by biotinylated HABC (bHABC) and horseradish peroxidase-streptavidin (Vector Laboratories, Burlingame, CA). A 3,3′,5,5′-tetramethylbenzidine substrate was used for color development (0.01% 3,3′,5,5′-tetramethylbenzidine, Sigma) in 0.005% H2O2, 0.1 m sodium acetate, and 1.5 mm citric acid buffer, pH 5.0. The reaction was stopped with 2 m H2SO4, and the absorbance read at 450 nm.
Hyaluronan and Immunostainings
The stainings were performed as described previously (57). Cells fixed in 2% paraformaldehyde were permeabilized and blocked with 1% BSA in 0.1 m phosphate buffer, pH 7.0, containing 0.1% Triton X-100, followed by overnight incubation at 4 °C with bHABC (3 μg/ml). The binding of bHABC was detected using the ABC complex (1:200, Vectastain Kit, Vector Laboratories) and 0.05% diaminobenzidine (DAB, Sigma) containing 0.03% hydrogen peroxide. The nuclei were counterstained with Mayer's hematoxylin. The stained cultures were viewed and imaged with a Zeiss Axio Imager.M2 microscope (Carl Zeiss Microimaging GmbH, Jena, Germany).
For dual stainings the anti-CD44 antibody Hermes 3 (a generous gift of professor Sirpa Jalkanen, University of Turku) at a 1:100 final dilution was added to the bHABC solution. Fluorescently tagged anti-mouse IgG (1:200) and Texas Red streptavidin (1:1000) (both from Vector Laboratories) were used as the secondary reagents. Nuclei were stained with DAPI (1 μg/ml, Sigma). The cells were viewed and imaged with confocal microscopy using a ×40 NA 1.3 oil objective on a Zeiss Axio Observer inverted microscope, equipped with a Zeiss LSM 700 confocal module. The images were processed using ZEN 2009 software (Carl Zeiss), and Adobe Photoshop Elements 9 software (Adobe Systems Inc., San Jose, CA).
For the imaging of pCaMKII the cells were fixed in 4% paraformaldehyde for 20 min on ice, permeabilized with cold methanol for 10 min, followed by blocking with 1% BSA in Tris containing 0.1% Tween for 10 min at room temperature. Overnight incubation with a pCaMKII antibody (Promega, Madison, WI), diluted 1:500 in the blocking buffer, was followed by a 1-h incubation with a Texas Red-labeled anti-rabbit IgG antibody (1:500, Vector Laboratories).
UDP-Sugar Determination
For UDP-sugar determinations cells were treated with UTP (100 μm) for 1.5, 3, and 4.5 h after which the intracellular contents were extracted and UDP-GlcUA, UDP-GlcNAc, and nucleotides measured as described previously (54, 98). Briefly, the cells were washed twice with ice-cold PBS and scraped into PBS, followed by sonication and centrifugation (6000 × g for 20 min, all steps on ice or at 4 °C). A small aliquot was collected for total protein estimation with a Pierce BCA kit (Thermo Fisher) and the rest of the sample was further purified with Superclean Envi-Carb SPE cartridges (Sigma). The eluted samples were evaporated and dissolved in water for HPLC on a CarpoPacTM PA1 column (Dionex, Thermo Fisher Scientific) as described before (98). Peak areas were normalized to the protein contents.
Statistical Methods
The results were analyzed with PASW Statistics 18 (SPSS Inc., Chicago, IL). Log transformation was used if the data were not normally distributed or the variances were unequal. Mixed model ANOVA and pairwise comparisons between the treatments were performed using the estimated marginal means (LSD) correcting for multiple comparisons. For comparisons between control (set as 1) and different treatments the cumulative distribution function (pnorm) calculated in R for Apple, version 3.2.3 (The R Project for Statistical Computing (99)), was used, correcting for multiple comparisons. For comparisons of a single group, normalized to a control group, the single group t test was used.
Author Contributions
T. J. conceived and designed the study, analyzed the results, and contributed to paper writing and prepared all figures. R. H. T. designed and coordinated the study, analyzed results, and wrote the paper. R. K. provided technical expertise for all experiments. G. B. took part in study conception. L. R., S. O., and S. P. S. performed and analyzed part of the experiments, and critically revised the manuscript. M. I. T. took part in the study conception and critically revised the manuscript. All authors approved the manuscript.
Acknowledgment
We thank Dr. Jarmo T. Laitinen for critical review of the manuscript.
This work was supported by grants from the Sigrid Juselius Foundation (to R. H. T., M. I. T., and S. P. S.), the Special Government Funding of Kuopio University Hospital (to M. I. T.), The Spearhead Funds of the University of Eastern Finland/Cancer Center of Eastern Finland (to M. I. T. and R. H. T.), and the Cancer Foundation of Northern Savo (to L. R.). The authors declare that they have no conflicts of interest with the contents of this article.
- P2Y
- G protein-coupled purinergic receptor
- qRT
- quantitative RT
- HAS
- hyaluronan synthase
- HYAL
- hyaluronidase
- UDP-GlcNAc
- UDP-N-acetylglucosamine
- UDP-GlcUA
- UDP-glucuronic acid
- UDP-Glc
- UDP-glucose
- PTX
- pertussis toxin
- CREB
- cAMP response element-binding protein
- CBP
- CREB-binding protein
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- p38
- p38 mitogen-activated protein kinase
- HaCaT
- spontaneously transformed aneuploid immortal keratinocyte cell line from human skin
- REK
- rat epidermal keratinocytes
- ER
- endoplasmic reticulum
- BIM
- bisindolylmaleimide
- EGFR
- epidermal growth factor receptor
- ELSA
- enzyme-linked sorbent assay
- DAB
- diaminobenzidine
- ANOVA
- analysis of variance.
References
- 1. Inoue K., Hosoi J., and Denda M. (2007) Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J. Invest. Dermatol. 127, 362–371 [DOI] [PubMed] [Google Scholar]
- 2. Pastore S., Mascia F., Gulinelli S., Forchap S., Dattilo C., Adinolfi E., Girolomoni G., Di Virgilio F., and Ferrari D. (2007) Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J. Invest. Dermatol. 127, 660–667 [DOI] [PubMed] [Google Scholar]
- 3. Holzer A. M., and Granstein R. D. (2004) Role of extracellular adenosine triphosphate in human skin. J. Cutan. Med. Surg. 8, 90–96 [DOI] [PubMed] [Google Scholar]
- 4. Ho C. L., Yang C. Y., Lin W. J., and Lin C. H. (2013) Ecto-nucleoside triphosphate diphosphohydrolase 2 modulates local ATP-induced calcium signaling in human HaCaT keratinocytes. PLoS ONE 8, e57666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azorin N., Raoux M., Rodat-Despoix L., Merrot T., Delmas P., and Crest M. (2011) ATP signalling is crucial for the response of human keratinocytes to mechanical stimulation by hypo-osmotic shock. Exp. Dermatol. 20, 401–407 [DOI] [PubMed] [Google Scholar]
- 6. Tsutsumi M., Inoue K., Denda S., Ikeyama K., Goto M., and Denda M. (2009) Mechanical-stimulation-evoked calcium waves in proliferating and differentiated human keratinocytes. Cell Tissue Res. 338, 99–106 [DOI] [PubMed] [Google Scholar]
- 7. Yoshida H., Kobayashi D., Ohkubo S., and Nakahata N. (2006) ATP stimulates interleukin-6 production via P2Y receptors in human HaCaT keratinocytes. Eur. J. Pharmacol. 540, 1–9 [DOI] [PubMed] [Google Scholar]
- 8. Boarder M. R., and Hourani S. M. (1998) The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 19, 99–107 [DOI] [PubMed] [Google Scholar]
- 9. Takai E., Tsukimoto M., Harada H., and Kojima S. (2011) Involvement of P2Y6 receptor in p38 MAPK-mediated COX-2 expression in response to UVB irradiation of human keratinocytes. Radiat. Res. 175, 358–366 [DOI] [PubMed] [Google Scholar]
- 10. Mandadi S., Sokabe T., Shibasaki K., Katanosaka K., Mizuno A., Moqrich A., Patapoutian A., Fukumi-Tominaga T., Mizumura K., and Tominaga M. (2009) TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 458, 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burrell H. E., Bowler W. B., Gallagher J. A., and Sharpe G. R. (2003) Human keratinocytes express multiple P2Y-receptors: evidence for functional P2Y1, P2Y2, and P2Y4 receptors. J. Invest. Dermatol. 120, 440–447 [DOI] [PubMed] [Google Scholar]
- 12. Greig A. V., Linge C., Cambrey A., and Burnstock G. (2003) Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J. Invest. Dermatol. 121, 1145–1149 [DOI] [PubMed] [Google Scholar]
- 13. Burnstock G., and Verkhratsky A. (2010) Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell. Death Dis. 1, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Filippini A., Taffs R. E., and Sitkovsky M. V. (1990) Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc. Natl. Acad. Sci. U.S.A. 87, 8267–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Q., Mak D., Devidas S., Schwiebert E. M., Bragin A., Zhang Y., Skach W. R., Guggino W. B., Foskett J. K., and Engelhardt J. F. (1998) Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J. Cell Biol. 143, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixon C. J., Bowler W. B., Littlewood-Evans A., Dillon J. P., Bilbe G., Sharpe G. R., and Gallagher J. A. (1999) Regulation of epidermal homeostasis through P2Y2 receptors. Br. J. Pharmacol. 127, 1680–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burrell H. E., Wlodarski B., Foster B. J., Buckley K. A., Sharpe G. R., Quayle J. M., Simpson A. W., and Gallagher J. A. (2005) Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J. Biol. Chem. 280, 29667–29676 [DOI] [PubMed] [Google Scholar]
- 18. Pellegatti P., Falzoni S., Pinton P., Rizzuto R., and Di Virgilio F. (2005) A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol. Biol. Cell 16, 3659–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glaser T., Resende R. R., and Ulrich H. (2013) Implications of purinergic receptor-mediated intracellular calcium transients in neural differentiation. Cell. Commun. Signal. 11, 12–811X-11–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jokela T. A., Kärnä R., Makkonen K. M., Laitinen J. T., Tammi R. H., and Tammi M. I. (2014) Extracellular UDP-glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) Tyr705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J. Biol. Chem. 289, 18569–18581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weigel P. H., Hascall V. C., and Tammi M. (1997) Hyaluronan synthases. J. Biol. Chem. 272, 13997–14000 [DOI] [PubMed] [Google Scholar]
- 22. Wang A., de la Motte C., Lauer M., and Hascall V. (2011) Hyaluronan matrices in pathobiological processes. FEBS J. 278, 1412–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tammi R. H., Passi A. G., Rilla K., Karousou E., Vigetti D., Makkonen K., and Tammi M. I. (2011) Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 278, 1419–1428 [DOI] [PubMed] [Google Scholar]
- 24. Oikari S., Jokela T. A., Tammi R. H., and Tammi M. I. (2012) in Extracellular Matrix: Pathobiology and Signaling (Karamanos N., ed) pp. 39–65, DeGruyter, Berlin [Google Scholar]
- 25. Lee-Sayer S. S., Dong Y., Arif A. A., Olsson M., Brown K. L., and Johnson P. (2015) The where, when, how, and why of hyaluronan binding by immune cells. Front. Immunol. 6, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrey A. C., and de la Motte C. A. (2014) Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 5, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murakami S., Hashikawa T., Saho T., Takedachi M., Nozaki T., Shimabukuro Y., and Okada H. (2001) Adenosine regulates the IL-1 β-induced cellular functions of human gingival fibroblasts. Int. Immunol. 13, 1533–1540 [DOI] [PubMed] [Google Scholar]
- 28. Grandoch M., Hoffmann J., Röck K., Wenzel F., Oberhuber A., Schelzig H., and Fischer J. W. (2013) Novel effects of adenosine receptors on pericellular hyaluronan matrix: implications for human smooth muscle cell phenotype and interactions with monocytes during atherosclerosis. Basic Res. Cardiol. 108, 340. [DOI] [PubMed] [Google Scholar]
- 29. Tammi R., Pasonen-Seppänen S., Kolehmainen E., and Tammi M. (2005) Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J. Invest. Dermatol. 124, 898–905 [DOI] [PubMed] [Google Scholar]
- 30. Maytin E. V., Chung H. H., and Seetharaman V. M. (2004) Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am. J. Pathol. 165, 1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rauhala L., Hämäläinen L., Salonen P., Bart G., Tammi M., Pasonen-Seppänen S., and Tammi R. (2013) Low dose ultraviolet B irradiation increases hyaluronan synthesis in epidermal keratinocytes via sequential induction of hyaluronan synthases Has1–3 mediated by p38 and Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling. J. Biol. Chem. 288, 17999–18012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monslow J., Sato N., Mack J. A., and Maytin E. V. (2009) Wounding-induced synthesis of hyaluronic acid in organotypic epidermal cultures requires the release of heparin-binding egf and activation of the EGFR. J. Invest. Dermatol. 129, 2046–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pasonen-Seppänen S., Karvinen S., Törrönen K., Hyttinen J. M., Jokela T., Lammi M. J., Tammi M. I., and Tammi R. (2003) EGF up-regulates, whereas TGF-β down-regulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J. Invest. Dermatol. 120, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 34. Jokela T. A., Jauhiainen M., Auriola S., Kauhanen M., Tiihonen R., Tammi M. I., and Tammi R. H. (2008) Mannose inhibits hyaluronan synthesis by down-regulation of the cellular pool of UDP-N-acetylhexosamines. J. Biol. Chem. 283, 7666–7673 [DOI] [PubMed] [Google Scholar]
- 35. Kultti A., Pasonen-Seppänen S., Jauhiainen M., Rilla K. J., Kärnä R., Pyöriä E., Tammi R. H., and Tammi M. I. (2009) 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and down-regulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 315, 1914–1923 [DOI] [PubMed] [Google Scholar]
- 36. Oikari S., Makkonen K., Deen A. J., Tyni I., Kärnä R., Tammi R. H., and Tammi M. I. (2016) Hexosamine biosynthesis in keratinocytes: roles of GFAT and GNPDA enzymes in the maintenance of UDP-GlcNAc content and hyaluronan synthesis. Glycobiology 26, 710–722 [DOI] [PubMed] [Google Scholar]
- 37. Deen A. J., Arasu U. T., Pasonen-Seppänen S., Hassinen A., Takabe P., Wojciechowski S., Kärnä R., Rilla K., Kellokumpu S., Tammi R., Tammi M., and Oikari S. (2016) UDP-sugar substrates of HAS3 regulate its O-GlcNAcylation, intracellular traffic, extracellular shedding and correlate with melanoma progression. Cell Mol. Life Sci. 73, 3183–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vigetti D., Ori M., Viola M., Genasetti A., Karousou E., Rizzi M., Pallotti F., Nardi I., Hascall V. C., De Luca G., and Passi A. (2006) Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian xenopus laevis and its involvement in hyaluronan synthesis. J. Biol. Chem. 281, 8254–8263 [DOI] [PubMed] [Google Scholar]
- 39. Vigetti D., Deleonibus S., Moretto P., Karousou E., Viola M., Bartolini B., Hascall V. C., Tammi M., De Luca G., and Passi A. (2012) Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J. Biol. Chem. 287, 35544–35555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vigetti D., Karousou E., Viola M., Deleonibus S., De Luca G., and Passi A. (2014) Hyaluronan: biosynthesis and signaling. Biochim. Biophys. Acta 1840, 2452–2459 [DOI] [PubMed] [Google Scholar]
- 41. Gendaszewska-Darmach E., and Kucharska M. (2011) Nucleotide receptors as targets in the pharmacological enhancement of dermal wound healing. Purinergic Signal. 7, 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Falzoni S., Donvito G., and Di Virgilio F. (2013) Detecting adenosine triphosphate in the pericellular space. Interface Focus 3, 20120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burnstock G. (2012) Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays 34, 218–225 [DOI] [PubMed] [Google Scholar]
- 44. Nagakura C., Negishi Y., Tsukimoto M., Itou S., Kondo T., Takeda K., and Kojima S. (2014) Involvement of P2Y11 receptor in silica nanoparticles 30-induced IL-6 production by human keratinocytes. Toxicology 322, 61–68 [DOI] [PubMed] [Google Scholar]
- 45. Kopf G. S., and Woolkalis M. J. (1991) ADP-ribosylation of G proteins with pertussis toxin. Methods Enzymol. 195, 257–266 [DOI] [PubMed] [Google Scholar]
- 46. Washburn K. B., and Neary J. T. (2006) P2 purinergic receptors signal to STAT3 in astrocytes: difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience. 142, 411–423 [DOI] [PubMed] [Google Scholar]
- 47. Ichiki T. (2006) Role of cAMP response element binding protein in cardiovascular remodeling: good, bad, or both? Arterioscler. Thromb. Vasc. Biol. 26, 449–455 [DOI] [PubMed] [Google Scholar]
- 48. Lee J. W., Park H. S., Park S. A., Ryu S. H., Meng W., Jürgensmeier J. M., Kurie J. M., Hong W. K., Boyer J. L., Herbst R. S., and Koo J. S. (2015) A novel small-molecule inhibitor targeting CREB-CBP complex possesses anti-cancer effects along with cell cycle regulation, autophagy suppression and endoplasmic reticulum stress. PLoS ONE 10, e0122628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Z. (2016) Transactivation of epidermal growth factor receptor by G protein-coupled receptors: recent progress, challenges and future research. Int. J. Mol. Sci. 17, 10.3390/ijms17010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng S. E., Lee I. T., Lin C. C., Wu W. L., Hsiao L. D., and Yang C. M. (2013) ATP mediates NADPH oxidase/ROS generation and COX-2/PGE2 expression in A549 cells: role of P2 receptor-dependent STAT3 activation. PLoS ONE 8, e54125. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Pienimaki J. P., Rilla K., Fulop C., Sironen R. K., Karvinen S., Pasonen S., Lammi M. J., Tammi R., Hascall V. C., and Tammi M. I. (2001) Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J. Biol. Chem. 276, 20428–20435 [DOI] [PubMed] [Google Scholar]
- 52. Karvinen S., Pasonen-Seppänen S., Hyttinen J. M., Pienimäki J. P., Törrönen K., Jokela T. A., Tammi M. I., and Tammi R. (2003) Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J. Biol. Chem. 278, 49495–49504 [DOI] [PubMed] [Google Scholar]
- 53. Vigetti D., Deleonibus S., Moretto P., Bowen T., Fischer J. W., Grandoch M., Oberhuber A., Love D. C., Hanover J. A., Cinquetti R., Karousou E., Viola M., D'Angelo M. L., Hascall V. C., De Luca G., and Passi A. (2014) Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J. Biol. Chem. 289, 28816–28826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rilla K., Oikari S., Jokela T. A., Hyttinen J. M., Kärnä R., Tammi R. H., and Tammi M. I. (2013) Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J. Biol. Chem. 288, 5973–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vigetti D., Genasetti A., Karousou E., Viola M., Clerici M., Bartolini B., Moretto P., De Luca G., Hascall V. C., and Passi A. (2009) Modulation of hyaluronan synthase activity in cellular membrane fractions. J. Biol. Chem. 284, 30684–30694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karousou E., Kamiryo M., Skandalis S. S., Ruusala A., Asteriou T., Passi A., Yamashita H., Hellman U., Heldin C. H., and Heldin P. (2010) The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J. Biol. Chem. 285, 23647–23654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tammi R., MacCallum D., Hascall V. C., Pienimäki J. P., Hyttinen M., and Tammi M. (1998) Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J. Biol. Chem. 273, 28878–28888 [DOI] [PubMed] [Google Scholar]
- 58. Jokela T. A., Lindgren A., Rilla K., Maytin E., Hascall V. C., Tammi R. H., and Tammi M. I. (2008) Induction of hyaluronan cables and monocyte adherence in epidermal keratinocytes. Connect. Tissue Res. 49, 115–119 [DOI] [PubMed] [Google Scholar]
- 59. Fricks I. P., Carter R. L., Lazarowski E. R., and Harden T. K. (2009) Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. J. Pharmacol. Exp. Ther. 330, 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giltaire S., Lambert S., and Poumay Y. (2011) HB-EGF synthesis and release induced by cholesterol depletion of human epidermal keratinocytes is controlled by extracellular ATP and involves both p38 and ERK1/2 signaling pathways. J. Cell. Physiol. 226, 1651–1659 [DOI] [PubMed] [Google Scholar]
- 61. Li W. H., Qiu Y., Zhang H. Q., Tian X. X., and Fang W. G. (2015) P2Y2 receptor and EGFR cooperate to promote prostate cancer cell invasion via ERK1/2 pathway. PLoS ONE 10, e0133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bromberg J., and Chen X. (2001) STAT proteins: signal tranducers and activators of transcription. Methods Enzymol. 333, 138–151 [DOI] [PubMed] [Google Scholar]
- 63. Qi Q. R., and Yang Z. M. (2014) Regulation and function of signal transducer and activator of transcription 3. World J. Biol. Chem. 5, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schuringa J. J., Schepers H., Vellenga E., and Kruijer W. (2001) Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett. 495, 71–76 [DOI] [PubMed] [Google Scholar]
- 65. Zhang X., Odom D. T., Koo S. H., Conkright M. D., Canettieri G., Best J., Chen H., Jenner R., Herbolsheimer E., Jacobsen E., Kadam S., Ecker J. R., Emerson B., Hogenesch J. B., Unterman T., Young R. A., and Montminy M. (2005) Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. U.S.A. 102, 4459–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Makkonen K. M., Pasonen-Seppänen S., Törrönen K., Tammi M. I., and Carlberg C. (2009) Regulation of the hyaluronan synthase 2 gene by convergence in cyclic AMP response element-binding protein and retinoid acid receptor signaling. J. Biol. Chem. 284, 18270–18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saavalainen K., Pasonen-Seppänen S., Dunlop T. W., Tammi R., Tammi M. I., and Carlberg C. (2005) The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J. Biol. Chem. 280, 14636–14644 [DOI] [PubMed] [Google Scholar]
- 68. Le Goff W., Zheng P., Brubaker G., and Smith J. D. (2006) Identification of the cAMP-responsive enhancer of the murine ABCA1 gene: requirement for CREB1 and STAT3/4 elements. Arterioscler. Thromb. Vasc. Biol. 26, 527–533 [DOI] [PubMed] [Google Scholar]
- 69. Ichiba M., Nakajima K., Yamanaka Y., Kiuchi N., and Hirano T. (1998) Autoregulation of the Stat3 gene through cooperation with a cAMP-responsive element-binding protein. J. Biol. Chem. 273, 6132–6138 [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi D., Ohkubo S., and Nakahata N. (2006) Contribution of extracellular signal-regulated kinase to UTP-induced interleukin-6 biosynthesis in HaCaT keratinocytes. J. Pharmacol. Sci. 102, 368–376 [DOI] [PubMed] [Google Scholar]
- 71. Vistejnova L., Safrankova B., Nesporova K., Slavkovsky R., Hermannova M., Hosek P., Velebny V., and Kubala L. (2014) Low molecular weight hyaluronan mediated CD44 dependent induction of IL-6 and chemokines in human dermal fibroblasts potentiates innate immune response. Cytokine 70, 97–103 [DOI] [PubMed] [Google Scholar]
- 72. Austin J. W., Gilchrist C., and Fehlings M. G. (2012) High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J. Neurochem. 122, 344–355 [DOI] [PubMed] [Google Scholar]
- 73. Wood M. W., Breitschwerdt E. B., and Gookin J. L. (2011) Autocrine effects of interleukin-6 mediate acute-phase proinflammatory and tissue-reparative transcriptional responses of canine bladder mucosa. Infect. Immun. 79, 708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rooney P., Srivastava A., Watson L., Quinlan L. R., and Pandit A. (2015) Hyaluronic acid decreases IL-6 and IL-8 secretion and permeability in an inflammatory model of interstitial cystitis. Acta Biomater. 19, 66–75 [DOI] [PubMed] [Google Scholar]
- 75. Ye J., Wu H., Wu Y., Wang C., Zhang H., Shi X., and Yang J. (2012) High molecular weight hyaluronan decreases oxidative DNA damage induced by EDTA in human corneal epithelial cells. Eye (Lond.) 26, 1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pauloin T., Dutot M., Joly F., Warnet J. M., and Rat P. (2009) High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol. Vis. 15, 577–583 [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Y., Lauer M. E., Anand S., Mack J. A., and Maytin E. V. (2014) Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J. Biol. Chem. 289, 32253–32265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Misra S., Hascall V. C., Markwald R. R., and Ghatak S. (2015) Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 6, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang D. J., Huang N. N., and Heppel L. A. (1990) Extracellular ATP shows synergistic enhancement of DNA synthesis when combined with agents that are active in wound healing or as neurotransmitters. Biochem. Biophys. Res. Commun. 166, 251–258 [DOI] [PubMed] [Google Scholar]
- 80. Kaczmarek E., Erb L., Koziak K., Jarzyna R., Wink M. R., Guckelberger O., Blusztajn J. K., Trinkaus-Randall V., Weisman G. A., and Robson S. C. (2005) Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb. Haemost. 93, 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pillois X., Chaulet H., Belloc I., Dupuch F., Desgranges C., and Gadeau A. P. (2002) Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ. Res. 90, 678–681 [DOI] [PubMed] [Google Scholar]
- 82. Li W. H., Qiu Y., Zhang H. Q., Liu Y., You J. F., Tian X. X., and Fang W. G. (2013) P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br. J. Cancer 109, 1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lamarca A., Gella A., Martiañez T., Segura M., Figueiro-Silva J., Grijota-Martinez C., Trullas R., and Casals N. (2014) Uridine 5′-triphosphate promotes in vitro Schwannoma cell migration through matrix metalloproteinase-2 activation. PLoS ONE 9, e98998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Taboubi S., Milanini J., Delamarre E., Parat F., Garrouste F., Pommier G., Takasaki J., Hubaud J. C., Kovacic H., and Lehmann M. (2007) Gα(q/11)-coupled P2Y2 nucleotide receptor inhibits human keratinocyte spreading and migration. FASEB J. 21, 4047–4058 [DOI] [PubMed] [Google Scholar]
- 85. Faure E., Garrouste F., Parat F., Monferran S., Leloup L., Pommier G., Kovacic H., and Lehmann M. (2012) P2Y2 receptor inhibits EGF-induced MAPK pathway to stabilise keratinocyte hemidesmosomes. J. Cell Sci. 125, 4264–4277 [DOI] [PubMed] [Google Scholar]
- 86. Evanko S. P., Tammi M. I., Tammi R. H., and Wight T. N. (2007) Hyaluronan-dependent pericellular matrix. Adv. Drug Deliv. Rev. 59, 1351–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brinck J., and Heldin P. (1999) Expression of recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces cell migration and cell surface CD44. Exp. Cell Res. 252, 342–351 [DOI] [PubMed] [Google Scholar]
- 88. Takabe P., Bart G., Ropponen A., Rilla K., Tammi M., Tammi R., and Pasonen-Seppänen S. (2015) Hyaluronan synthase 3 (HAS3) overexpression down-regulates MV3 melanoma cell proliferation, migration and adhesion. Exp. Cell Res. 337, 1–15 [DOI] [PubMed] [Google Scholar]
- 89. Ruppert S. M., Hawn T. R., Arrigoni A., Wight T. N., and Bollyky P. L. (2014) Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 58, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., and Kimata K. (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 274, 25085–25092 [DOI] [PubMed] [Google Scholar]
- 91. Tammi R., Rilla K., Pienimaki J. P., MacCallum D. K., Hogg M., Luukkonen M., Hascall V. C., and Tammi M. (2001) Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 276, 35111–35122 [DOI] [PubMed] [Google Scholar]
- 92. Seo M., and Juhnn Y. S. (2010) Gq protein mediates UVB-induced cyclooxygenase-2 expression by stimulating HB-EGF secretion from HaCaT human keratinocytes. Biochem. Biophys. Res. Commun. 393, 190–195 [DOI] [PubMed] [Google Scholar]
- 93. Pasonen-Seppänen S. M., Maytin E. V., Törrönen K. J., Hyttinen J. M., Hascall V. C., MacCallum D. K., Kultti A. H., Jokela T. A., Tammi M. I., and Tammi R. H. (2008) All-trans retinoic acid-induced hyaluronan production and hyperplasia are partly mediated by EGFR signaling in epidermal keratinocytes. J. Invest. Dermatol. 128, 797–807 [DOI] [PubMed] [Google Scholar]
- 94. Grimm I., Messemer N., Stanke M., Gachet C., and Zimmermann H. (2009) Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J. Cell Sci. 122, 2524–2533 [DOI] [PubMed] [Google Scholar]
- 95. Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., and Fusenig N. E. (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hiltunen E. L., Anttila M., Kultti A., Ropponen K., Penttinen J., Yliskoski M., Kuronen A. T., Juhola M., Tammi R., Tammi M., and Kosma V. M. (2002) Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res. 62, 6410–6413 [PubMed] [Google Scholar]
- 97. Tammi R., Ågren, Tuhkanen U. M. A. L., and Tammi M. (1994) Hyaluronan metabolism in skin. Prog. Histochem. Cytochem. 29, 1–81 [DOI] [PubMed] [Google Scholar]
- 98. Oikari S., Venäläinen T., and Tammi M. (2014) Borate-aided anion exchange high-performance liquid chromatography of uridine diphosphate-sugars in brain, heart, adipose and liver tissues. J. Chromatogr. A 1323, 82–86 [DOI] [PubMed] [Google Scholar]
- 99. RCore Team, R. (2015) R: a language and environment for statistical computing: R foundation for statistical computing. Vienna, Austria [Google Scholar]