ABSTRACT
Fecal microbiota transplantation is an efficacious and inexpensive therapy for recurrent Clostridium difficile infection, yet its safety is thought to depend on appropriate fecal donor screening. FDA guidance for regulation of this procedure is in flux, but screening and manufacture of fecal material from asymptomatic donors present many challenges to clinical laboratories. This minireview summarizes FDA regulatory changes, principles of donor selection, and recommended laboratory screening practices for fecal microbiota transplantation.
KEYWORDS: Clostridium difficile, FMT, fecal microbiota transplantation, gut microbial therapeutics, microbiome, stool donor screening
INTRODUCTION
The intestinal microbiota is composed of a diverse array of microorganisms that play a vital role in metabolism, immune function, and gut homeostasis (1, 2). Dysbiosis, or abnormal alterations in intestinal microbiotal composition or diversity, has been implicated in a range of diseases, including Clostridium difficile infection (CDI), inflammatory bowel disease (IBD), and irritable bowel syndrome (IBS). Recently, there has been rapid expansion in interest for microbiotal enrichment as a treatment for disease or means to improve health, chiefly through transplantation of screened and processed fecal material from a healthy donor to a patient. However, regulation of this process is in transition, and there is variation in donor screening practices, which can present challenges to microbiology laboratories.
Prior to the ability to culture for C. difficile, fecal microbiota transplantation (FMT) was occasionally used in humans to treat enterocolitis that was presumed to be due to Staphylococcus species (3). FMT may have therapeutic utility for other conditions linked with changes in gut microbiota, such as obesity, diabetes, atherosclerosis, colon cancer, and nonalcoholic fatty liver disease (4). However, FMT is presently best understood as a safe and highly effective therapy for recurrent Clostridium difficile infection (RCDI), which is commonly defined as 3 or more episodes of mild to moderate CDI or 2 episodes resulting in hospitalization (5).
Over the last decade, the prevalence, severity and mortality of CDI and RCDI have been increasing (6). The annual cost of CDI in the United States has been estimated at $4.8 billion to acute-care centers in 2008 U.S. dollars, and the epidemiologic burden of disease in the United States in 2011 was estimated to include 434,000 infections and 29,000 deaths in 2011 (6, 7). A subsequent analysis of RCDI costs from 2013 suggested a CDI-associated cost of $34,104 per patient and a national estimate of $2.8 billion in 2013 dollars for RCDI alone (8). FMT has emerged as a highly effective and inexpensive therapeutic option for RCDI, with mean cure rates of around 90% (9–13). Given the numerous and potentially unrecognized functions of gut microbiota, the risk of transmission of diseases from a donor to recipient mandate the optimization of donor selection and screening for potentially transmissible infections and other conditions associated with gut microbiotal function. Here, we review the background of the U.S. Food and Drug Administration (FDA) regulation of FMT as well as FMT donor selection and laboratory screening recommendations.
STOOL BANKS AND FOOD AND DRUG ADMINISTRATION REGULATION OF FECAL TRANSPLANTATION
In May 2013, the FDA announced that FMT would be regulated as an investigational new drug (IND) due to its use for the treatment of disease and the absence of large randomized control studies supporting its efficacy and safety (4, 14). Subsequently, the U.S. Center for Biologics Evaluation and Research within the FDA classified human feces as a biologic agent and drug (15). This decision was met with concerns from physicians and patients that requiring an IND would impede access to this therapy (16). Later, in July 2013, the FDA amended its statement to instead exercise enforcement discretion. This decision allowed physicians to provide FMT to patients specifically for RCDI unresponsive to other treatments without an IND application, provided patients gave informed consent (17). The FDA maintains that the enforcement discretion policy does not apply to other uses of FMT, including research or treatment of conditions other than RCDI (14). In the same month, the presidents of the Infectious Diseases Society of America (IDSA), American Society for Gastrointestinal Endoscopy (ASGE), North American Society for Pediatric Gastroenterology (NASPG), Hepatology and Nutrition (NASPGHAN), American Gastroenterological Association (AGA), and American College of Gastroenterology (ACG) issued consensus guidance for FMT donor health and stool screening in an open letter to the FDA (18). This joint society consensus guidance built on prior recommendations from the FMT workgroup (5).
The FDA and 2013 joint professional society consensus recommendations have suggested that fecal donors should be known to the recipient or treating physician (14, 18). However, since that time, use of banked stool for FMT (a case in which stool donors are never known to the patient) has become increasingly common in research and clinical practice settings. The logistical benefits of stool banking have led some institutions to organize internal programs for donor recruitment and screening and stool testing and inventory. Yet, the barriers to the development of internal banking programs are not trivial, since the process requires significant resources, administrative buy-in, and coordination among clinical and laboratory areas. Perhaps due to these barriers, stool from donors unknown to patients or physicians is often sourced through independent stool banks operating separately from the point of care. While one such independent stool bank, OpenBiome (www.openbiome.org), operates as a nonprofit, a number of for-profit enterprises have also been founded which are developing microbiome-based therapeutics. Since 2013, the FDA has periodically issued draft guidance suggesting that it is considering whether or not independent stool banks should be required to produce an IND application for all indications, including RCDI, which could have significant impacts on patient access to FMT.
In March 2014, the FDA issued draft guidance for public comment recommending that stool donors be known to either the patient or treating physician and requiring stool banking organizations to obtain IND approval from the FDA when distributing universal donor material for clinical or research purposes (4, 16). However, this draft guidance was not issued as a rule. More recently, in March 2016, the FDA issued updated draft guidance raising additional concerns about independent stool banks. The FDA noted that manufacturing of FMT product by a stool bank (defined “as an establishment that collects, prepares, and stores FMT product for distribution to other establishments, health care providers, or other entities for use in patient therapy or clinical research”) presents specific safety concerns by using a limited number of donors of stool administered for multiple recipients (19). Notably, hospital laboratories were specifically excluded from this definition of stool banking organizations and would still not be required to sponsor an IND application for CDI not responsive to standard therapies. The draft guidance would allow an independent stool bank sponsoring an IND application to waive certain IND regulations for investigators. Requirements may be waived through the application in the original IND or included in an amendment and must contain reason for waiving the requirement or alternative actions satisfying the requirement. Requirements may also be waived with FDA approval if such a waiver poses no risks or if compliance cannot be achieved (20). The FDA is currently seeking comments on which regulations can be waived, specifically in IND review of FMT therapies (21). It is not clear if this draft guidance in this form might shift the relative regulatory burden from independent stool banks to internal hospital stool banks. However, if this led to an increase in sourcing stool for FMT from internal banks, many centers would not be prepared for this change. Ideally, internal stool bank best practices in laboratory testing would be collected, studied, and disseminated to facilitate process improvement while further guidance from the FDA is pending.
Ultimately, many have suggested that due to the inherent variability of stool, the FDA may choose to regulate the processes of screening and manufacturing material for FMT rather than the contents of the material itself in a way that may be analogous to the regulation of whole blood or cord blood donations (22, 23). However, this has yet to be determined.
PRINCIPLES OF DONOR SELECTION
To date, there have been no trials evaluating outcomes of fecal transplantation as related to donor characteristics. Thus, current criteria for selection and screening of fecal donors are primarily a product of expert opinion. While there are safety concerns related to exposing multiple FMT recipients to stool from a more limited group of donors (for example, if a donor was asymptomatically shedding infectious norovirus and this was transmitted to a pool of recipients), we are not aware of any published reports of such an outcome. As noted previously in the earlier section on FDA regulation, regulatory considerations are in flux, and laboratories conducting internal donor selection and screening should check for the latest guidance (http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm).
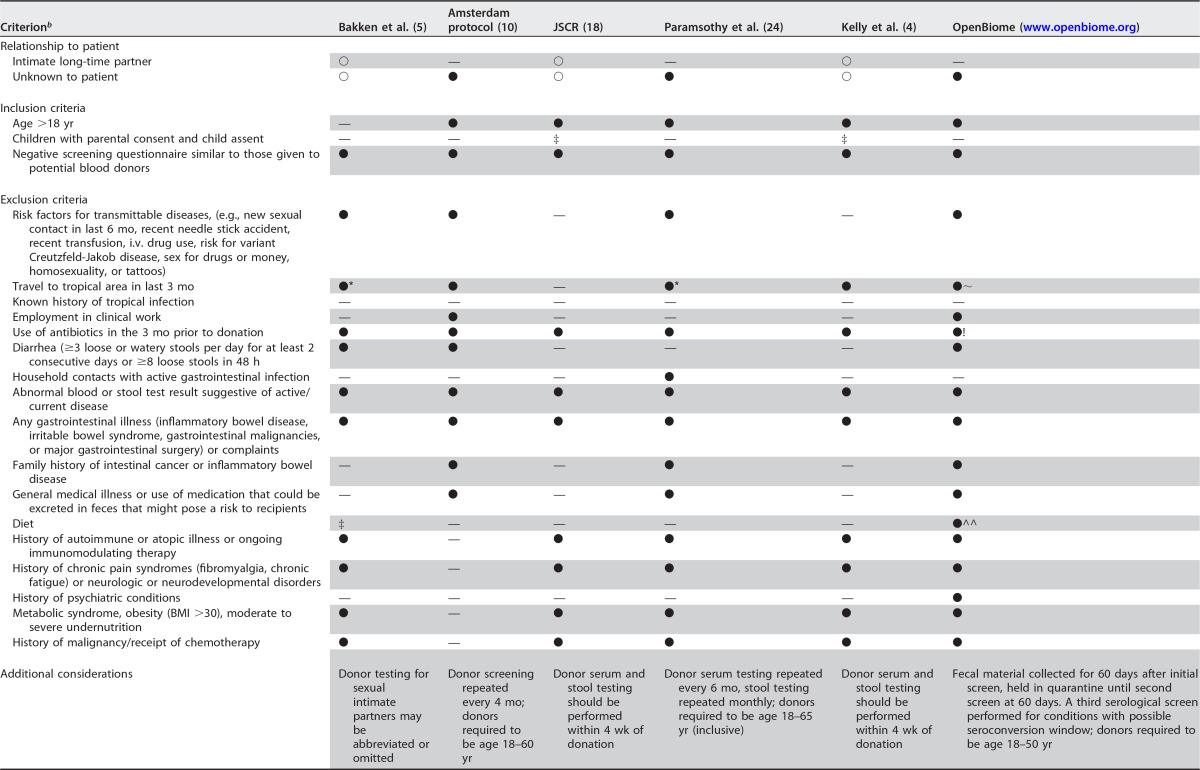
In determining initial eligibility, all reviewed recommendations require that potential donors undergo initial screening using a questionnaire analogous to those provided to blood donors. Donor responses to these questionnaires should have no responses indicating a risk factor or illness that could potentially be transmitted by FMT. Many protocols also require follow-up questionnaires or other means of assessment at or near the time of donation to screen for interval change in inclusion or exclusion criteria (10, 24). Donors with active symptoms of infection or risk factors that suggest a risk of infection between the period of screening and collection of feces should also be excluded (10). Other microbiome-specific exclusion criteria related to conditions that may influence the gut microbiome have been suggested in prior screening recommendations, which include conditions, like neurological or metabolic disorders, malignancy, and recent antibiotic use (18). These and other fecal donor inclusion and exclusion criteria are summarized in Table 1.
TABLE 1.
Recommended fecal donor selection criteriaa
JSCR, Joint Society Consensus Recommendations to the FDA for fecal donor selection and screening; ○, options equally reasonable; ●, recommended or routine practice; —, method or recommendation not noted in article or not applicable; ‡, could be considered on case-by-case basis as suggested by exposure status of donor and immune status of recipient; *, donors were excluded if they had traveled within the prior 6 months to areas endemic for diarrheal illness; ∼, donors excluded if any history of travel to regions with high risk of acquiring infectious pathogens endemic to the area; !, no date of last antibiotic use specified; ^^, dietary screening not further specified.
i.v., intravenous; BMI, body mass index.
RECOMMENDED DONOR AND DONATED STOOL TESTING
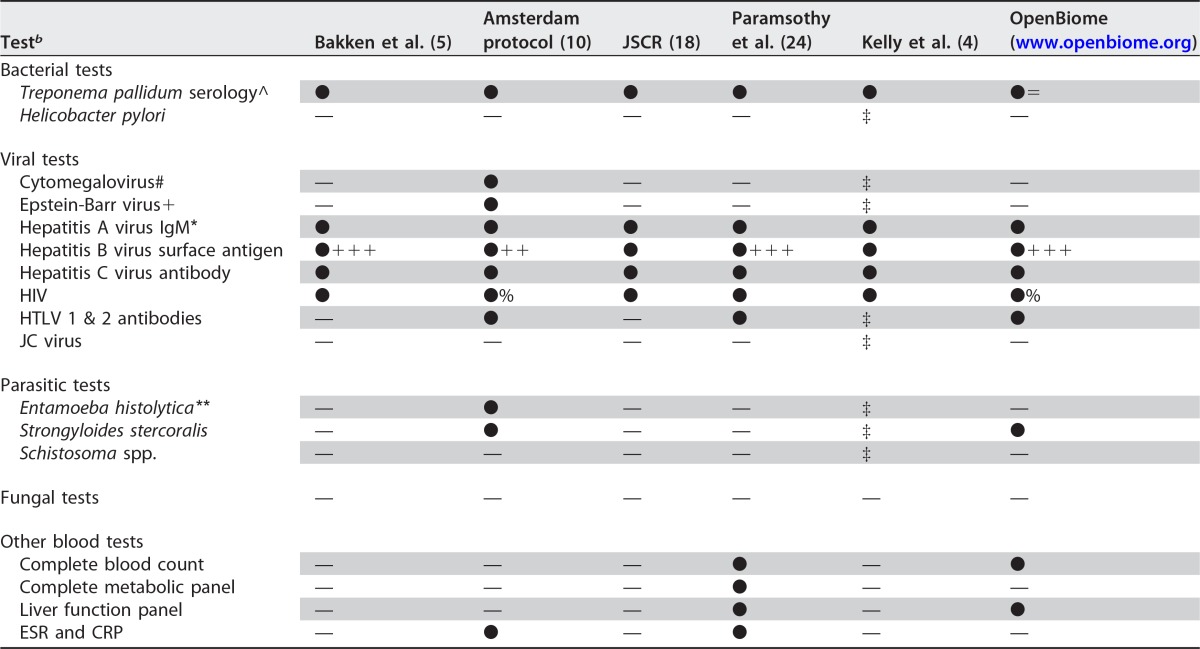
After donor selection and laboratory screening, prior studies have reported that surprisingly few donors are found to be suitable by current recommendations. In reports from OpenBiome, published results of donor screening in a trial of FMT for IBD, and another trial of FMT for RCDI, 3%, 10%, and 32% of potential donors, respectively, ultimately met criteria for donation (10, 24, 25; www.openbiome.org). Potential donors may be excluded for multiple reasons, but in these studies, high rates of donor candidates were asymptomatic carriers of potential pathogens, underscoring the importance of laboratory testing of all potential donors (10, 24, 25). Of donors excluded based on the results of stool or blood testing, common reasons for exclusion included detection of Dientamoeba fragilis, Blastocystis hominis, Clostridium difficile, and Rotavirus in stool (10, 24, 25). Positive serological screens were infrequent in these studies, with one exclusion for apparent Strongyloides exposure, another exclusion for an indeterminate hepatitis C virus (HCV) antibody screen with abnormal transaminase levels, and no positive serological screens reported in the series reported by OpenBiome (10, 24, 25).
Some screening practices have been built upon the donor screening protocol described in the Amsterdam protocol (10, 24). However, such practices are periodically updated to reflect improved understanding of asymptomatic carriage of potential pathogens and conditions related to the gut microbiome. For example, independent stool banks have expanded their screening practices to test for asymptomatic carriage of viral pathogens, like rotavirus (25). Also, as increasing data become available on FMT in immunocompromised patients, a broader range of opportunistic pathogens has been considered when screening immunocompetent stool donors who may be asymptomatic carriers. For FMT in immunocompromised patients, it may be important to consider screening of donors for potential viral opportunistic infections (e.g., JC or BK virus) with quantitative PCR or with a serological screen followed by quantitative PCR. While outcomes in RCDI are increasingly well described with randomized controlled trials and a recent meta-analysis, long-term safety data are lacking (26). An FMT national registry has recently been spearheaded by the AGA to assess the short-term and long-term (up to 10 years of patient-reported follow up) safety of FMT, with secondary objectives of evaluating the efficacy of FMT practice in the United States (C. Kelly, Warren Alpert Medical School of Brown University, personal communication). Further research is needed to clarify the relative merits of selecting known or unknown donors as well as inclusion and exclusion criteria. The 2011 FMT Workgroup Recommendations, Amsterdam protocol, 2013 society consensus recommendations, donor screening protocol from an IBD FMT clinical trial, 2015 AGA recommendations, and OpenBiome routine screening practices as of September 2016 are summarized in Tables 1 to 3.
TABLE 3.
Recommended stool testing for potential fecal donorsa
JSCR, Joint Society Consensus Recommendations to the FDA for fecal donor selection and screening; —, recommendation not noted in article; ●, recommended or routinely performed test; ○, options equally reasonable; ‡, test should be considered on a case-by-case basis as suggested by exposure status of donor and immune status of recipient; =, H. pylori antigen testing recommended for upper routes of FMT.
EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; MRSA, methicillin-resistant Staphylococcus aureus; vancomycin-resistant enterococci.
JSCR, Joint Society Consensus Recommendations to the FDA for fecal donor selection and screening.
After initial donor selection with screening questionnaires with or without physical examination, potential donors should undergo a battery of serologic and stool tests to rule out potentially transmissible infectious diseases. There is general agreement between published joint society consensus recommendations and published research protocols; however, the frequency or timing of donor testing is less well defined. The frequency of recommended testing ranges from once within 4 weeks of stool donation to as long as 74 days prior to donation, with blood and stool screening tests at the beginning and end of a 60-day collection period, followed by a third serological screen to account for potential late exposure and delayed seroconversion (18; www.openbiome.org). Another discrepancy in donor blood testing recommendations is the recommendation to screen for Strongyloides and Schistosoma, which was recommended by Kelly et al. and the Joint Society Consensus Recommendations if exposure to these pathogens is suggested by travel history (4, 18). If thought to be indicated, a serological test for these pathogens is likely to be sufficient, and donors with positive screens should likely be excluded. The recommended serologic and stool tests for potential fecal donors are listed in Tables 2 and 3, respectively.
TABLE 2.
Recommended blood testing for potential fecal donorsa
JSCR, Joint Society Consensus Recommendations to the FDA for fecal donor selection and screening; ^, Treponema pallidum testing with rapid plasma reagin or Treponema pallidum particle agglutination assay; ●, recommended or routinely performed test; =, T. pallidum screen is performed as cascade with reflex to rapid plasma reagin (RPR); —, recommendation not noted in article; ‡, test should be considered on a case-by-case basis as suggested by exposure status of donor and immune status of recipient; #, cytomegalovirus IgG and IgM; +, Epstein-Barr virus viral capsid antigen (VCA) IgM, VCA IgG, VCA, and anti-Epstein Barr nuclear antigen (EBNA); *, total hepatitis A antibodies, and if positive, also hepatitis A IgM; +++, hepatitis B surface antigen and core antibody IgM/IgG; ++, hepatitis B surface antigen and surface antibody; %, HIV-1 p24 antigen test also included; **, Entamoeba histolytica serum latex agglutination and dipstick.
HTLV, human T-cell lymphotrophic virus; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
In the United States, if providers are considering FMT for indications other than RCDI, an IND must be submitted to the FDA, as outlined by others (16). Depending on the planned study indication, review by the FDA may include recommendations for additional blood or stool testing of donors specific to that study.
DONOR STOOL SCREENING AND THE CLINICAL MICROBIOLOGY LABORATORY
As FMT became more frequently utilized during this past decade, requests to perform donor stool screening presented clinical laboratories with an array of considerations. Perhaps the most perplexing concern was about the validity of testing formed stool from healthy donors for the presence of C. difficile. The reference method C. difficile toxigenic culture has become largely obsolete due to barriers of cost and longer turnaround times, and many laboratories now rely on commercial antigen detection assays that target C. difficile common antigen and toxin, and/or molecular assays that detect the C. difficile tcdA or tcdB gene. In the United States, these commercial assays are FDA cleared, with on-label indications restricted to unformed stool from diarrheal symptomatic patients suspected of having CDI. Indeed, the analytical sensitivity for detection of the assays' target(s) was determined in this specific patient population. There are not any on-label claims for the performance or utility of these commercial C. difficile assays for testing stool from an asymptomatic healthy donor for FMT donation. Clinical laboratories often perform tests for off-label indications with appropriate disclaimers in the result reports. However, depending on their location and accrediting agency, clinical laboratories stand to be in violation of regulatory mandates unless specific test validation is performed to support such off-label use on formed stool. This kind of test validation is beyond the resources of many hospital and commercial laboratories, so most laboratories are likely using commercial C. difficile assays off-label to screen for asymptomatic carriage. It may provide some reassurance that independent stool banks, like OpenBiome, claim to screen donor stool for C. difficile using standardized processes. However, it should be acknowledged that these screening tests are likely still performed off-label, and the performance characteristics may be different than those reported for unformed stool.
Our group currently uses an off-label molecular assay for screening asymptomatic donors for toxigenic C. difficile. Commercial nucleic acid testing has previously been shown to have a higher negative predictive value in testing symptomatic patients than that with a glutamate dehydrogenase (GDH) or multistep testing algorithm (27). However, a recent study of detection of C. difficile in asymptomatic patients suggested a very similar negative predictive value of GDH compared to a commercial nucleic acid amplification test (NAAT) (99.3% versus 99.9%, respectively) (28). Most clinical laboratories that will be screening donor stool are likely committed to one of these testing strategies for symptomatic patients, and these data suggest that GDH or NAAT is likely to be appropriate.
Similarly, donor stool testing with multiplex PCR for viral or other parasitic pathogens has not been validated for this indication, and the general approach for validating these tests has been on loose or watery stool. However, given the reported exclusion of donors based on viral testing of stool donor fecal samples, further study in this area may serve to expedite the preparation of fecal material for FMT (24, 25). There are no data to support routine testing of potential donors for fungal pathogens.
As others have recommended a range of tests from what could be considered a minimum battery to a number of tests that may be more relevant in light of donor exposures or recipient characteristics (e.g., immunocompromise or comorbid conditions associated with changes in the microbiome), it remains important for clinicians and clinical laboratorians to remain in dialogue about the most appropriate diagnostics as new data become available. Well-designed clinical trials to evaluate associations between donor characteristics and clinical outcomes, as well as iterative planning and review of current practices, will be important to ensure patient safety, accessibility to FMT, and cost-effectiveness of testing.
FUTURE DIRECTIONS
Many questions about optimal donor selection and screening laboratory tests remain, while FDA regulation remains in flux. It is unclear if frequency of donor laboratory screening should be static, applying rigid testing to all samples equally, or dynamic to adjust for seasonal variation in diarrheal diseases, like rotavirus (29). Further, learning health systems, which utilize big data in real time, might be able to anticipate need for FMT supplies based on local rates of RCDI within a particular health system. Adjustments in the frequency of donor testing prior to using a particular donor's stool can have significant impacts on cost, although the relative risks of such models require further study (29).
Routine practice for donor selection and laboratory testing, as well as stool testing, will need to be reevaluated on a regular basis as the roles of human gut microbiota are further clarified. Future study may support use of NAATs, 16S ribosomal gene, or shotgun genome sequencing of bacterial, archaea, viral, and fungal reads to screen donors. Host genome expression profiles may provide additional information about ideal donor selection. Still, other techniques may more simply and cost-effectively identify source donors with keystone microbiotal populations. Current research examining high-level extracts or cultured whole microbiota of donor material may illuminate certain therapeutic components of FMT and target its use either for specific disorders or more general application (30).
The advent of FMT therapy in response to the CDI epidemic highlights the limitations of antimicrobial therapy, as well as the yet-to-be discovered complexities of the human microbiome. Ongoing systematic appraisal of the evolving body of scientific FMT research is needed to inform regulatory oversight and guide the development of evidence-based best clinical practices.
ACKNOWLEDGMENTS
This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Ley RE, Peterson DA, Gordon JI. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman B, Silen W, Bascom GS, Kauvar AJ. 1958. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44:854–859. [PubMed] [Google Scholar]
- 4.Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. 2015. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA, Russell G, Surawicz C, Fecal Microbiota Transplantation Workgroup . 2011. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubberke ER, Olsen MA. 2012. Burden of clostridium difficile on the healthcare system. Clin Infect Dis 55:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues R, Barber GE, Ananthakrishnan AN. 2017. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol 38:196–202. doi: 10.1017/ice.2016.246. [DOI] [PubMed] [Google Scholar]
- 9.Cammarota G, Ianiro G, Gasbarrini A. 2014. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 48:693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 10.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 11.Kassam Z, Lee CH, Yuan Y, Hunt RH. 2013. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 12.Waye A, Atkins K, Kao D. 2016. Cost averted with timely fecal microbiota transplantation in the management of recurrent Clostridium difficile infection in Alberta, Canada. J Clin Gastroenterol 50:747–753. doi: 10.1097/MCG.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 13.Merlo G, Graves N, Brain D, Connelly LB. 2016. Economic evaluation of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection in Australia. J Gastroenterol Hepatol 31:1927–1932. doi: 10.1111/jgh.13402. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. 2013. Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM361393.pdf. [Google Scholar]
- 15.Moore T, Rodriguez A, Bakken JS. 2014. Fecal microbiota transplantation: a practical update for the infectious disease specialist. Clin Infect Dis 58:541–545. doi: 10.1093/cid/cit950. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CR, Kunde SS, Khoruts A. 2014. Guidance on preparing an investigational new drug application for fecal microbiota transplantation studies. Clin Gastroenterol Hepatol 12:283–288. doi: 10.1016/j.cgh.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. 2013. Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm387023.htm. [Google Scholar]
- 18.Relman D, Vender RJ, Rustgi AK, Wang KK, Bousvaros A. 2013. Current consensus guidance on donor screening and stool testing for FMT. American Gastroenterological Association, Bethesda, MD: https://www.gastro.org/research/Joint_Society_FMT_Guidance.pdf. [Google Scholar]
- 19.FDA. 2013. Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 20.Code of Federal Regulations. 2016. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 312. Subpart A. Section 312.10 Waivers. 21 CFR 312.10 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm. [Google Scholar]
- 21.Brennan Z. 2016. Fecal transplants to treat C. difficile: FDA seeks comment on what IND requirements to waive. Regulatory Affairs Professionals Society, Rockville, MD: http://raps.org/Regulatory-Focus/News/2016/02/29/24428/Fecal-Transplants-to-Treat-C-difficile-FDA-Seeks-Comment-on-What-IND-Requirements-to-Waive/. [Google Scholar]
- 22.Sachs RE, Edelstein CA. 2015. Ensuring the safe and effective FDA regulation of fecal microbiota transplantation. J Law Biosci 2:396–415. doi: 10.1093/jlb/lsv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelstein CA, Kassam Z, Daw J, Smith MB, Kelly CR. 2015. The regulation of fecal microbiota for transplantation: an international perspective for policy and public health. Clin Res Regul Aff 32:101–109. [Google Scholar]
- 24.Paramsothy S, Borody TJ, Lin E, Finlayson S, Walsh AJ, Samuel D, van den Bogaerde J, Leong RWL, Connor S, Ng W, Mitchell HM, Kaakoush N, Kamm MA. 2015. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis 21:1600–1606. doi: 10.1097/MIB.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 25.Burns LJ, Dubois N, Smith MB, Mendolia GM, Burgess J, Edelstein C, Noh A, Alm E, Kassam Z. 2015. 499 donor recruitment and eligibility for fecal microbiota transplantation: results from an international public stool bank. Gastroenterology 148(Suppl 1):S-96–S-97. [Google Scholar]
- 26.Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, Yan F, Cao H, Wang B. 2016. Systematic review: adverse events of fecal microbiota transplantation. PLoS One 11:e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novak-Weekley SM, Marlowe EM, Miller JM, Cumpio J, Nomura JH, Vance PH, Weissfeld A. 2010. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol 48:889–893. doi: 10.1128/JCM.01801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terveer EM, Crobach MJT, Sanders IMJG, Vos MC, Verduin CM, Kuijper EJ. Detection of Clostridium difficile in feces of asymptomatic patients admitted to the hospital. J Clin Microbiol, in press. doi: 10.1128/JCM.01858-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kazerouni A, Burgess J, Burns LJ, Wein LM. 2015. Optimal screening and donor management in a public stool bank. Microbiome 3:75. doi: 10.1186/s40168-015-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borody TJ, Brandt LJ, Paramsothy S. 2014. Therapeutic faecal microbiota transplantation: current status and future developments. Curr Opin Gastroenterol 30:97–105. doi: 10.1097/MOG.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]