ABSTRACT
A large population of genetically and antigenically diverse influenza A viruses (IAVs) are circulating among the swine population, playing an important role in influenza ecology. Swine IAVs not only cause outbreaks among swine but also can be transmitted to humans, causing sporadic infections and even pandemic outbreaks. Antigenic characterizations of swine IAVs are key to understanding the natural history of these viruses in swine and to selecting strains for effective vaccines. However, influenza outbreaks generally spread rapidly among swine, and the conventional methods for antigenic characterization require virus propagation, a time-consuming process that can significantly reduce the effectiveness of vaccination programs. We developed and validated a rapid, sensitive, and robust method, the polyclonal serum-based proximity ligation assay (polyPLA), to identify antigenic variants of subtype H3N2 swine IAVs. This method utilizes oligonucleotide-conjugated polyclonal antibodies and quantifies antibody-antigen binding affinities by quantitative reverse transcription-PCR (RT-PCR). Results showed the assay can rapidly detect H3N2 IAVs directly from nasal wash or nasal swab samples collected from laboratory-challenged animals or during influenza surveillance at county fairs. In addition, polyPLA can accurately separate the viruses at two contemporary swine IAV antigenic clusters (H3N2 swine IAV-α and H3N2 swine IAV-ß) with a sensitivity of 84.9% and a specificity of 100.0%. The polyPLA can be routinely used in surveillance programs to detect antigenic variants of influenza viruses and to select vaccine strains for use in controlling and preventing disease in swine.
KEYWORDS: H3N2, swine influenza virus, antigenic drift, antigenic variation, approximate ligation assay, clinical sample, influenza A virus, polyPLA, serological assay, vaccine strain selection
INTRODUCTION
Influenza A virus (IAV) can infect swine, causing typical signs of respiratory disease (e.g., fever, depression, coughing, nasal discharge, sneezing, and breathing difficulty) that generally last 5 to 7 days. IAVs can be transmitted between humans and swine, both of which have N-acetylneuraminic acid-α2,6-linked galactose (NeuAcα-2,6Gal) serving as a receptor for IAVs in their upper respiratory tracks (1). In addition to NeuAcα-2,6Gal, swine also have N-acetylneuraminic acid-α2,3-linked galactose (NeuAcα-2,3Gal) serving as a receptor for IAVs in their upper respiratory tracks. Thus, swine have been proposed to serve as mixing vessels, generating novel IAVs by reassorting avian IAVs and swine IAVs and facilitating adaptation of avian IAVs before their spillover to the human population (2, 3). Swine IAVs cause sporadic cases of influenza among humans as well as a pandemic in human, e.g., by a swine-origin IAV, influenza A(H1N1)pdm09 (4). On the other hand, it is not uncommon for IAVs to be introduced from humans to swine (5). For subtype H3N2 specifically, at least 13 infections caused by the introduction of virus from humans to swine were identified from 1965 to 2013 (6). Thus, understanding the genetic and antigenic diversity of IAVs in swine is key to developing an effective strategy for influenza prevention and control among domestic swine and to protect public health.
In the United States, surveillance has shown that a genetically diverse population of IAVs is circulating among domestic swine, and these viruses are subtype H1 or H3 (7, 8). At least seven genetic clusters of swine subtype H1 IAVs (H1α, H1ß, H1γ1, H1γ2, H1δ1, and H1δ2) and A(H1N1)pdm09 are circulating in the swine population, and the viruses in these H1 genetic clusters are antigenically distinct (9). A recent study suggested the proportion for the number of swine subtype H3N2 IAVs over the total number of swine IAVs recovered from domestic swine has increased, and these viruses are widely spread across the United States (10). Molecular characterization suggested that the hemagglutinin (HA) genes of contemporary swine subtype H3 IAVs belong genetically to cluster IV, and these viruses can be antigenically separated into two groups, H3N2 swine IAV-α and H3N2 swine IAV-ß (abbreviated as H3α and H3ß) (11).
Vaccination has been routinely utilized in the United States to prevent virus transmission at swine farm operations (12) and swine exhibits at agricultural fairs (13). Swine vaccines, which are available as licensed commercial products, are typically produced from culture-derived virions incubated in crude allantoic fluid from special pathogen-free chicken eggs. After incubation, the virus is chemically inactivated and formulated into a mineral oil emulsion vaccine (14). Unlike the process for producing vaccines for humans, this process for producing swine vaccines avoids costly purification steps for enrichment of the surface glycoproteins HA and neuraminidase (NA) (15); however, potential booster vaccinations are necessary to achieve and maintain protective levels of systemic HA-inhibiting antibodies (16).
Production of an effective vaccine requires an antigenic match between circulating strains and the vaccine strain, but that is not a trivial task. IAV transmission occurs rapidly among swine populations, especially on farms with medium- or large-scale operations; thus, vaccine types must be determined quickly, and vaccines must be rapidly produced and administered (within days of an outbreak) to effectively mitigate the spread of disease. However, the conventional methods for antigenic characterization (e.g., hemagglutination inhibition [HI]) require virus isolation, which usually takes at least 3 days to accomplish. Thus, because of the antigenic diversity of swine IAVs, conventional methods cannot be used to determine immunologic reactions between circulating viruses and available vaccines. Therefore, many vaccines for swine IAVs in the United States have been produced locally and autogenously without efficacy testing (17).
It is not uncommon for vaccines that are antigenically inaccurate for circulating viruses to lead to vaccine-induced immunity, causing antigenic drift and the silent spread of virus (18). Moreover, the generation of novel escape mutants could gradually lead to the replacement of circulating virus strains (19, 20), further complicating the antigenic and genetic evolution of swine IAVs. The occurrence of such antigenic drift events in swine is well documented (7, 21, 22). To develop an effective and robust swine IAV vaccination program, a rapid and robust method for antigenic characterization in swine is needed. Ideally, the assay would not require virus isolation but would be sensitive enough to directly perform antigenic characterization using clinical samples.
A polyclonal serum-based proximity ligation assay (polyPLA) was developed recently to measure antibody-antigen binding affinities without using red blood cells (RBCs) (23). The polyPLA attaches probes to antibody and then quantifies the binding affinities between antibody and antigen by quantitative reverse transcription-PCR (qRT-PCR). This method was highly sensitive and shown to effectively detect antigenic variants for human seasonal influenza subtype H3N2 virus in human nasopharyngeal swab samples, thus avoiding the need for virus isolation (23). Our goal was to develop a specific polyPLA method to quantify the antibody-antigen interaction for swine H3 IAVs. This method would be useful for vaccine strain selection for swine IAVs.
RESULTS
Comparison of HI assay and polyPLA in antigenic characterization of subtype H3 swine IAVs.
To assess the effectiveness of the polyPLA, we compared the antigenic data derived from the HI assay and the polyPLA. The seven H3N2 swine IAVs used for testing in our study had cross-reaction titers ranging from <1:10 to 1:1,600 (Table 1). HI-based antigenic cartography showed that the seven isolates were separated into two antigenic clusters: two isolates from 2009 were in cluster H3α, and the five other isolates were in cluster H3ß (Fig. 1A). The results from polyPLA indicated that the titers for these seven testing isolates ranged from 1.71 to 16.47 polyPLA units. In support of the HI cartography-derived data, polyPLA-based cartography also showed that these seven isolates were grouped in two antigenic clusters (Fig. 1A). The average distances between clusters were 5.14 U (±0.15 standard deviation) and 6.27 U (±1.22 standard deviations) in HI and polyPLA cartography, respectively. Correlation association analyses through linear regression showed that the titers between these two types of data had a coefficient (R2) of 0.82 (P < 0.0001) (Fig. 1C) and that the fold increments in HI titers and polyPLA values had an R2 coefficient of 0.85 (P < 0.0001) (Fig. 1D). Similar to HI assay results, polyPLA results suggested that subtype H3N2 swine IAVs did not react with the negative-control H1N1 virus and polyclonal antibodies.
TABLE 1.
Antigenic characterization of H3N2 swine influenza viruses using HI assay and polyPLA
| Virus | Antigenic cluster | Result with ferret antiserum and virus isolatea: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 09SW96 |
10SW215 |
11SW347 |
CA/04 |
||||||
| HI | polyPLA (SD) | HI | polyPLA (SD) | HI | polyPLA (SD) | HI | polyPLA (SD) | ||
| A/swine/Ohio/09SW64/2009 (H3N2) | H3α | 1,600 | 10.37 (0.10) | 40 | 6.14 (0.69) | <10 | 5.20 (0.36) | <10 | NA |
| A/swine/Ohio/09SW96/2009 (H3N2) | H3α | 1,280 | 11.06 (0.72) | 40 | 4.53 (0.71) | <10 | 1.71 (0.52) | <10 | NA |
| A/swine/Ohio/10SW130/2010 (H3N2) | H3ß | 40 | 6.62 (0.86) | 640 | 11.87 (0.33) | 640 | 10.62 (0.28) | <10 | NA |
| A/swine/Ohio/10SW156/2010 (H3N2) | H3ß | 20 | 4.12 (0.37) | 1,280 | 14.85 (0.36) | 640 | 9.52 (0.56) | <10 | NA |
| A/swine/Ohio/10SW215/2010 (H3N2) | H3ß | 40 | 4.01 (1.32) | 1,280 | 16.47 (0.11) | 640 | 11.64 (0.10) | <10 | NA |
| A/swine/Ohio/11SW208/2011 (H3N2) | H3ß | 40 | 7.24 (0.65) | 320 | 11.59 (0.46) | 1,024 | 12.26 (0.28) | <10 | NA |
| A/swine/Ohio/11SW347/2011 (H3N2) | H3ß | 20 | 4.99 (0.14) | 320 | 8.15 (0.44) | 1,280 | 12.63 (0.39) | <10 | NA |
| A/California/04/2009 (H1N1)b | H1pdm09 | 10 | 6.66 (0.95) | <10 | 4.83 (0.51) | <10 | NA | 1,280 | 9.70 (0.61) |
The viruses were collected from pigs at agricultural fairs in Ohio, 2009 to 2011 (11). Homologous titers are in boldface. 09SW96, A/swine/Ohio/09SW96/2009 (H3N2); 10SW215, A/swine/Ohio/10SW215/2010 (H3N2); 11SW347, A/swine/Ohio/11SW347/2011 (H3N2); CA/04, A/California/04/2009 (H1N1). Each HI experiment was repeated two times, and each HI value reported is an average number from three independent experiments. Values for polyPLA represent averages from three replicate experiments.
A/California/04/2009 (H1N1) was used as a negative control.
FIG 1.
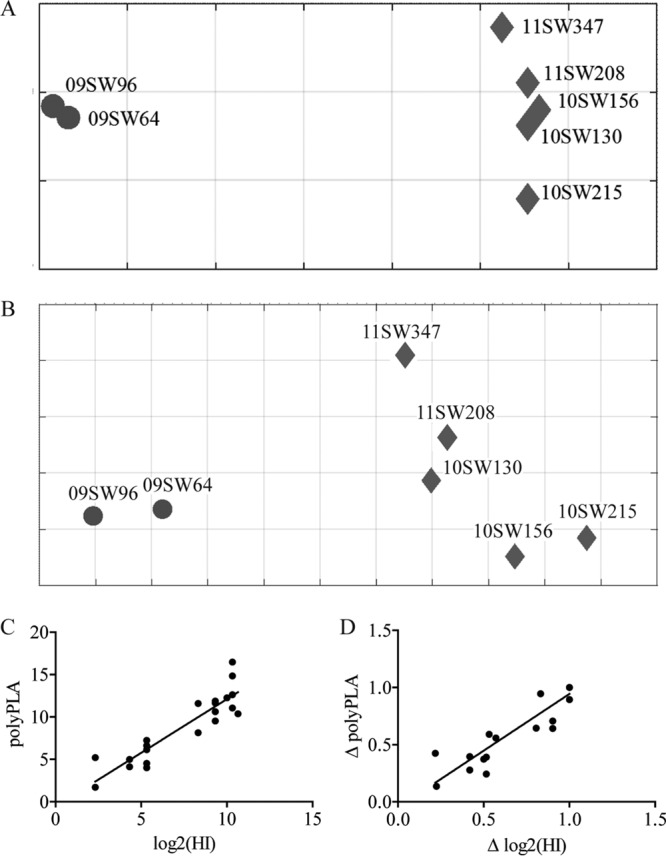
Comparison of antigenic characterization of H3N2 swine IAVs using hemagglutination inhibition (HI) assays and polyclonal serum-based proximity ligation assay (polyPLA). (A) Antigenic map derived from HI data. (B) Antigenic map derived from polyPLA data. (C) Correlation of HI titers and polyPLA values. polyPLA values can be predicted from HI titers by the following formula: polyPLA values = 1.27 × log2(HI titers) − 0.56 (R2 = 0.82). (D) Correlation of the fold increments in HI titers and those in polyPLA values. Fold increment in polyPLA values can be predicted from fold increment in HI titers by the following formula: ΔpolyPLA values = 0.10 × Δlog2(HI titers) − 0.05 (R2 = 0.85). A total of 7 representative H3N2 swine influenza A viruses (IAVs) were selected to represent antigenic clusters H3 swine IAV-α and H3 swine IAV-ß (abbreviated as H3α and H3ß) (Table 1). The homologous ferret antisera for these viruses were used to perform the HI assay and polyPLA. The HI assays were performed using 0.5% red blood cells. Antigenic maps were constructed using AntigenMap (http://sysbio.cvm.msstate.edu/AntigenMap) (33, 34). Viral isolates are 09SW64, A/swine/Ohio/09SW64/2009 (H3α); 09SW96, A/swine/Ohio/09SW96/2009 (H3α); 10SW130, A/swine/Ohio/10SW130/2010 (H3ß); 10SW156, A/swine/Ohio/10SW156/2010 (H3ß); 10SW215, A/swine/Ohio/10SW215/2010 (H3ß); 11SW208, A/swine/Ohio/11SW208/2011 (H3ß); 11SW347, A/swine/Ohio/11SW347/2011 (H3ß).
Detection of H3N2 swine IAVs in clinical samples from feral swine.
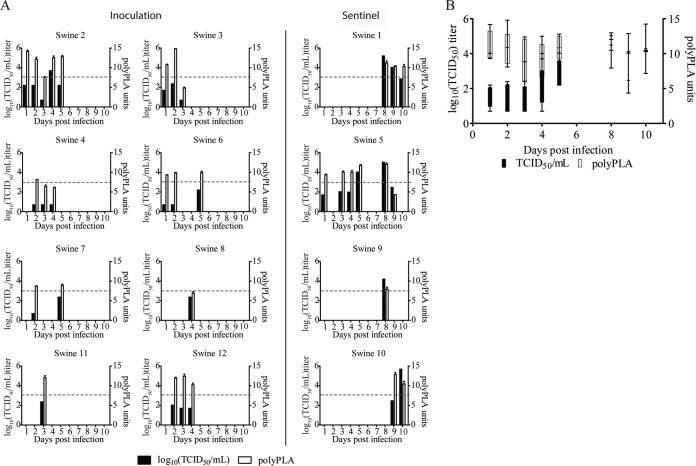
To determine whether polyPLA is sensitive enough to identify H3N2 swine IAVs in clinical samples, we used 42 nasal swab and nasal wash samples from 8 feral swine infected with A/swine/Texas/A01104013/2013(H3N2) (belonging to antigenic clade H3ß) and 4 sentinel feral swine with virus titers up to 5.00 × 105 50% tissue culture infectious doses (TCID50)/ml. Of the 42 samples, 34 were IAV positive (change in cycle threshold [ΔCT] of ≥3.00) using NP monoclonal antibodies. Further analyses using polyclonal H3ß antibodies showed that polyPLA can detect H3N2 swine IAV virus titers of <1,000 TCID50/ml (Fig. 2A); this finding is similar to that for human IAVs, which can detect virus titers of <1,000 TCID50/ml, as previously published (23). Furthermore, in the animal experiments, polyPLA could detect viral shedding from 1 to 10 days after virus challenge, and titers ranged from 4.35 to 14.83 polyPLA units (Fig. 2B; see also Table S1 in the supplemental material).
FIG 2.
Comparison of sensitivity of cell culture-based viral titration and polyclonal serum-based proximity ligation assay (polyPLA) in detecting influenza A viruses (IAVs) in nasal wash and nasal swab samples collected from feral swine infected with A/swine/Texas/A01104013/2012(H3N2). (A) Variations of TCID50 and polyPLA titers in 12 swine. The horizontal dashed line indicates 1,000 TCID50/ml. (B) Average number of days after virus challenge that virus could be detected by TCID50 and polyPLA. The whiskers of the box-and-whisker plots denote the smaller value to the larger value, while the box extends to the 25th and 75th percentiles, with the median in the middle. The infecting virus belongs to swine influenza A virus (IAV) antigenic cluster H3N2 swine IAV-ß. Swine 1 to 8 were inoculated nasally with virus; swine 9 to 12 were sentinel swine housed in the same room. Among a total of 120 samples collected from these 12 swine, 42 samples with detectable TCID50 were used in this study (see Table S1 in the supplemental material) (29).
Application of polyPLA in detecting H3N2 swine IAV antigenic variants from clinical samples collected from swine at agricultural fairs.
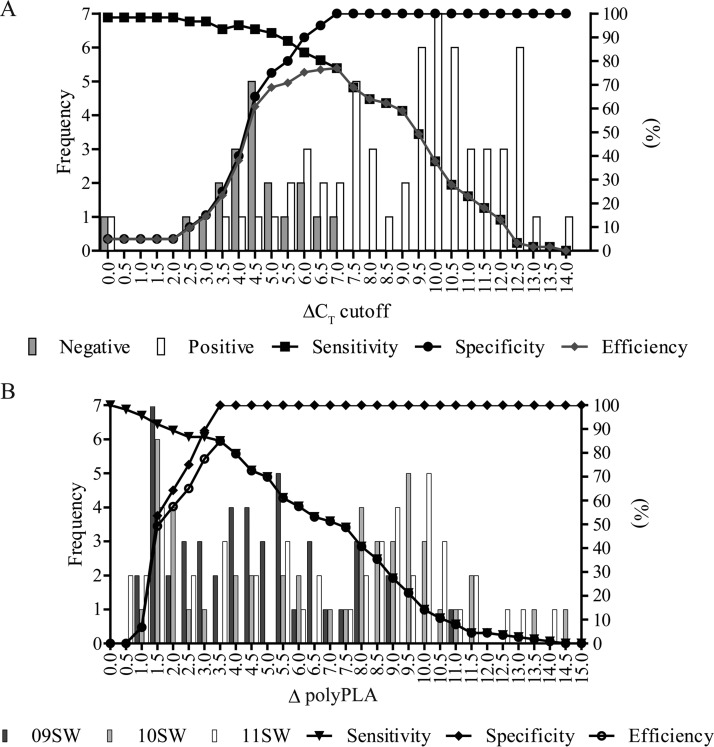
To measure specificity of polyPLA in the clinical setting, we used the assay on 81 samples taken from swine at agricultural fairs. The frequency distribution of ΔCT values for NP monoclonal antibodies for the 61 IAV-positive and 20 IAV-negative clinical samples from domestic swine showed that the greatest efficiency (77.0%) was observed at a ΔCT cutoff of 7.0 (Fig. 3A). Thus, the optimum combination for detecting IAVs in clinical samples is an assay sensitivity of 77.0% (95% confidence interval [CI], 64.5%, 86.8%) and specificity of 100.0% (95% CI, 83.2%, 100.0%). Accuracy of the polyPLA was measured by the receiver operating characteristic area under the curve, which was 0.90, an excellent test for separating IAV-positive from IAV-negative clinical samples (Fig. S1). There was 82.7% overall agreement between qRT-PCR and polyPLA, with a kappa of 62.4% (95% CI, 45.8%, 79.0%), which suggests a good strength of agreement. The typical ΔCT cutoff of 3.0 showed that the polyPLA had high sensitivity (96.7%; 95% CI, 88.7%, 99.6%) but low specificity (15.0%; 95% CI, 3.2%, 37.9%), and 59 true-positive and 17 false-positive samples were detected. At the higher ΔCT cutoff of 7.0, false positives were eliminated and 47 true-positive samples were detected.
FIG 3.
Optimization of the polyclonal serum-based proximity ligation assay (polyPLA) in detecting antigenic variants in clinical samples from swine infected with IAV. (A) Distribution of IAV-positive samples (white bars, n = 61) versus IAV-negative samples (gray bars, n = 20) obtained using NP monoclonal antibody and various ΔCT values. (B) Distribution of H3 swine IAV-α versus H3 swine IAV-ß samples at various ΔpolyPLA values. 09SW, A/swine/Ohio/09SW96/2009(H3N2); 10SW, A/swine/Ohio/10SW215/2010(H3N2); 11SW, A/swine/Ohio/11SW347/2011(H3N2). The viruses are shown in dark gray, light gray, and white bars, respectively. Data analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) with 95% CIs.
To distinguish between antigenic group H3α and antigenic group H3ß viruses, we calculated the frequency distribution of ΔpolyPLA values for H3α and H3ß polyclonal antibodies for the 47 IAV-positive clinical samples from domestic swine. At the ΔpolyPLA threshold of 3.5 U, the greatest efficiency was observed at 85.0%, with a sensitivity of 85.0% (95% CI, 77.0%, 91.0%) and specificity of 100.0% (95% CI, 87.7%, 100.0%) (Fig. 3B; Table S2). Correlation association analyses through linear regression showed the fold increment titers from homologous virus isolates and fold increment in polyPLA values had an R2 coefficient of 0.88 (P < 0.0001) (Fig. S2). An 8-fold increment in HI titer was correlated with a 3.26-fold increment in polyPLA units. polyPLA was able to distinguish between the two swine IAV H3 antigenic groups with complete agreement: 10 samples were H3α positive (sensitivity 95% CI, 69.2%, 100%), and 33 were H3ß positive (sensitivity 95% CI, 89.4%, 100%); 4 were negative to both polyclonal antibodies because they were previously identified as H1 qRT-PCR positive (Tables S3 and S4).
Effectiveness of polyPLA in detecting H1 swine IAV antigenic variants.
To evaluate whether polyPLA was effective in identifying antigenic variants for subtype H1 IAVs, cross-reactivities were measured using both polyPLA and HI assays between the CA/04 polyclonal serum and a panel of H1N1 isolates, which belong to 8 antigenically distinct clades, including clades H1α, H1ß, H1γ, H1γ1, H1γ2, H1δ1, H1δ2, and A(H1N1)pdm09. Results from polyPLA showed that CA/04 polyclonal serum cross-reacted with the homologous virus CA/04 and A/swine/Iowa/8/2013(H1N1) [both to clade A(H1N1)pdm09] with the two highest polyPLA values of 13.83 and 15.87 U, respectively; this serum cross-reacted to A/swine/Nebraska/A01240348/2011(H1N1) (H1ß) with a polyPLA value of 12.02 U, to A/swine/Indiana/13TOSU1154/2013(H1N1) (H1γ) with a polyPLA value of 11.01 U, and to A/swine/Indiana/13TOSU0832/2013(H1N1) (H1γ1) with a polyPLA value of 10.22 U. The polyPLA values for the other four viruses (H1α, H1γ2, H1δ1, and H1δ2) were less than 10.00 U (Table S3). Such results were consistent with the corresponding HI titers, validating that this method is effective in antigenic characterization of H1 viruses. Furthermore, we performed polyPLA using CA/04 serum against the 47 IAV-positive clinical samples. Results showed that CA/04 serum did not cross-react with 43 H3 IAV-positive samples but did to 4 H1 IAV-positive samples to different extents. Among these 4 H1 viruses, 2 were sequenced, 1 genetically belonged to H1γ, and the other one belonged to H1δ1 (Table S3).
DISCUSSION
In this study, a polyPLA was used to detect antigenic variants of contemporary subtype H3 IAVs in swine in the United States. This assay was validated to differentiate viruses in antigenic cluster H3α from those in H3ß directly in clinical samples, such as swine nasal swab or nasal wash samples. With the optimized ΔpolyPLA value of 3.500 U, this assay can detect antigenic variants with a specificity of 100% and a sensitivity of 84.9% in samples collected during influenza surveillance. Thus, the polyPLA is specific and sensitive enough to be used for vaccine strain selection for swine IAVs. Because it does not require virus isolation, this assay shortens the time needed for antigenic characterization using conventional methods (3 to 5 days) to only a few hours after specimen collection and therefore can increase effectiveness of vaccination programs on swine farm operations and at agricultural fairs. In addition, because it is developed based on a qRT-PCR platform, polyPLA can be used for high-throughput screening and for clinical diagnosis in most laboratories.
The HI assay is used routinely in influenza antigenic characterization because of its ease of access and its capacity for medium-throughput screening. The principle of HI is based on the competition of the glycan receptors on animal RBCs and antibody against surface glycoproteins, especially hemagglutinins of IAVs. Thus, antigenic characterization results can be affected not only by changes at antibody binding sites but also by the source of the RBCs and the variation of receptor binding sites. In addition, HI requires a large amount of virus particles, so, in general, it is necessary to recover and propagate viruses using cells or chicken eggs, which can lead to unwanted adaptive mutations, especially those at the receptor binding sites of viral hemagglutinin proteins. These mutations can skew HI data and even cause loss of binding affinity to some RBCs (24, 25). In addition, because no standard RBCs are used in HI assays, the variation in the binding affinities of IAVs to different sources of RBCs make it difficult to interpret those results across HI assays using different RBCs. Unlike the HI assay, polyPLA does not use RBCs and is not affected by variations in receptor binding sites. Instead, polyPLA utilizes oligonucleotide-labeled antibodies and quantifies the binding affinities between antibody and antigen through qRT-PCR. More strikingly, this method can be applied directly in clinical samples and can minimize biases due to virus adaptation in virus isolation. Results showed that polyPLA values are similar to those in HI assays, although the scale of fold increments are different (see Fig. S2 in the supplemental material). In this study, both HI and polyPLA clearly separated swine IAVs in antigenic cluster H3α from those in antigenic cluster H3ß. Furthermore, an 8-fold increment in HI titer was approximately equal to a 3.256-fold increment in polyPLA (Fig. S2).
Compared with clinical samples from laboratory animals, samples derived from the field could be complicated with high background in qRT-PCR due to low quantities of virus analyte, inappropriately collected/handled/transported specimens, the presence of viral inhibitor, and/or the presence of proteins from other viruses or bacterial pathogens (26). Nevertheless, for vaccine strain selection in the clinical setting, it is critical to use assays with 100% specificity and relatively high sensitivity. Relatively low assay sensitivity can be overcome by using a larger number of samples in the assays; in general, the availability of multiple samples from swine herds will not be an issue, especially during an outbreak. Based on the 81 samples we tested, polyPLA has a sensitivity of 77.0% (95% CI, 55.7%, 80.1%) and specificity of 100.0% (95% CI, 83.2%, 100.0%) when the ΔCT cutoff is set at 7.0 (Fig. 3A).
The number of polyPLAs conducted in experiments can be reduced if the IAV subtype in samples is known prior to testing. Thus, the performance of polyPLA can be maximized and the cost can be reduced if the assay is coupled with subtype-specific IAV antigen assays, such as qRT-PCR. The recommended procedure for polyPLA application includes three steps: (i) determine whether the testing sample is IAV positive by using a matrix gene-based qRT-PCR (27) rapid influenza antigen detection test (28) or an influenza test strip; (ii) use qRT-PCR to determine the subtype of IAV in the sample; and (iii) perform antigenic characterization by using subtype-specific polyPLA. As with the conventional methods for antigenic characterization, polyPLA can be used with a panel of reference sera to quantify antigenic diversity among the viruses; thus, polyPLA would be useful for antigenic characterization of IAVs and, potentially, other pathogens.
MATERIALS AND METHODS
Viruses and serum samples.
Seven contemporary (2009 to 2011) swine H3N2 IAV isolates and their homologous ferret serum samples were chosen to represent the swine IAV antigenic groups H3α and H3ß (Table 1); antigenic characterization of these isolates is described elsewhere (11). The strain of A/California/04/2009(H1N1) (abbreviated as CA/04) [A(H1N1)pdm09] and homologous ferret serum were used as negative controls. NP monoclonal antibody was obtained from BEI Resources (Manassas, VA, USA).
Clinical samples.
A total of 120 nasal wash and nasal swab samples were collected for 10 consecutive days postinfection (dpi) from 8 feral swine infected with A/swine/Texas/A01104013/2012 (H3N2) and 4 sentinel feral swine. The details for experimental designs and sample collections were available from a prior publication (29). Among these samples, 42 were tested with a detectable TCID50, and these samples were used in this study to test the sensitivity and specificity of the proposed polyPLA method.
We also tested 81 nasal swab samples that had been collected from swine at the pig exhibits at agricultural fairs in Ohio during 2009 to 2013; 61 of the samples were positive for IAV using matrix gene-based quantitative RT-PCR (qRT-PCR) (11, 30), and 20 of the samples were negative for IAV. A power analysis (OpenEpi, version 3) suggested that a sample size of 20 gave 95% probability to detect ±10% with expected specificity (31). Of those 61 IAV-positive samples, 50 were subtype H3 and 11 were subtype H1.
HA and HI assays.
HI was performed as previously described (32). In brief, receptor-destroying enzyme (RDE; Denka Seiken Co., Ltd., Tokyo, Japan) was incubated overnight at a 1:3 (vol/vol) ratio with ferret antisera. After incubation, the mixture was heat inactivated at 56°C for 30 min and then diluted 1:10 with 1× phosphate-buffered saline (PBS; pH 7.4). The treated ferret antiserum was then 1:2 serially diluted in 96-well v-bottom plates with 1× PBS, reacted with 4 hemagglutinin (HA) units of virus, and then incubated for 30 min at 37°C, after which 0.5% turkey RBCs were added to each well and incubated for 30 min at 37°C. Each experiment was repeated two times, and each HI value reported is an average number from three independent experiments; the highest dilution in which virus binding to the RBCs was blocked was expressed as the reciprocal HI titer.
polyPLA.
IgG was purified from polyclonal serum and monoclonal antibody and labeled separately with 5′ and 3′ TaqMan Prox-Oligos (Thermo Fisher Scientific, Waltham, MA, USA) for use in a proximity ligation assay, as described elsewhere (23). In brief, 5′- and 3′-labeled IgG was diluted (1:10) in assay probe dilution buffer, and 2 μl was added to 2 μl of viruses or 1× PBS (nonprotein control [NPC]) and incubated at 37°C for 1 h. The 96 μl of ligation mixture (0.1 μl of diluted [1:500] ligase, 5 μl of 20× ligation reaction buffer, and 90.9 μl of distilled H2O) was added to each incubation product, incubated at 37°C for 10 min, and then put on ice. Diluted protease was then added to the ligation products and incubated at 37°C for 10 min and at 95°C for 5 min and then put on ice. Lastly, 4.5 μl of protease products was added to 5 μl of TaqMan protein assay fast master mix (2×) (Thermo Fisher Scientific, Waltham, MA, USA) and 0.5 μl 20× universal PCR assay mix (Thermo Fisher Scientific, Waltham, MA, USA), and quantitative RT-PCR was performed with the following steps: 95°C for 20 s, 40 cycles at 95°C for 1 s, and 60°C for 20 s. The threshold was set at 0.2, and the change in the cycle threshold (ΔCT) was calculated by subtracting the average sample CT value from the average NPC CT value. Quantitative RT-PCR was performed on each sample in triplicate.
Antigenic cartography.
The antigenic maps of H3N2 swine IAVs were constructed using AntigenMap (http://sysbio.cvm.msstate.edu/AntigenMap) and data derived from the HI assay or the polyPLA (33, 34). The data entry with an HI titer of <1:10 or a ΔCT of <3.00 was determined as a low reactor for the data from HI or the polyPLA, respectively.
Data analyses.
To make the antigenic properties across the testing antigens (viruses) comparable, we calculated the polyPLA units between virus and antibody as previously described (23): polyPLA = a × (polyΔCT − monoΔCT) + b, where polyΔCT = ΔCT values for polyclonal antiserum, monoΔCT = ΔCT values for monoclonal antibody against NP, a = 1.00, and b = 10.00, to eliminate negative numbers. A monoΔCT cutoff of <3.00 has been used traditionally to determine if virus loads are too low for analyses.
Linear regression analyses were performed using HI titers of the 7 swine IAV isolates (2 to antigenic clade H3α and 5 to H3ß) versus their homologous antisera and polyPLA values of these 7 swine IAV isolates versus 3 polyclonal antibodies (1 to H3α and 2 to H3ß). The 81 nasal swab samples were assessed for monoΔCT and ΔpolyPLA cutoffs by the frequency procedure using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) to determine sensitivity and specificity for detection of IAVs and antigenic variants with confidence intervals at 95%, receiver operator characteristic area under the curve, and linear regression. The mathematical product of sensitivity times specificity, termed efficiency, was calculated and graphed for each polyPLA value to provide the probability of correct classification for unknown sample status (35). Kappa analyses and all descriptive graphs were created using GraphPad Prism, version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Hailiang Sun for technical support and Fred Cunningham for providing clinical samples from a feral swine challenge study.
This study was partially supported by the National Institutes of Health (grant 1R01AI116744-01). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02049-16.
REFERENCES
- 1.Rogers GN, Paulson JC. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 2.Scholtissek C. 1990. Pigs as “mixing vessels” for the creation of new pandemic influenza A viruses. Med Principles Pract 2:65–71. [Google Scholar]
- 3.Scholtissek C, Burger H, Kistner O, Shortridge KF. 1985. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 147:287–294. doi: 10.1016/0042-6822(85)90131-X. [DOI] [PubMed] [Google Scholar]
- 4.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson MI, Vincent AL. 2015. Reverse zoonosis of influenza to swine: new perspectives on the human-animal interface. Trends Microbiol 23:142–153. doi: 10.1016/j.tim.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson MI, Wentworth DE, Culhane MR, Vincent AL, Viboud C, LaPointe MP, Lin X, Holmes EC, Detmer SE. 2014. Introductions and evolution of human-origin seasonal influenza a viruses in multinational swine populations. J Virol 88:10110–10119. doi: 10.1128/JVI.01080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. 2008. Swine influenza viruses a North American perspective. Adv Virus Res 72:127–154. doi: 10.1016/S0065-3527(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan BS, DeBeauchamp J, Stigger-Rosser E, Franks J, Crumpton JC, Turner J, Darnell D, Jeevan T, Kayali G, Harding A, Webby RJ, Lowe JF. 2015. Influenza virus surveillance in coordinated swine production systems, United States. Emerg Infect Dis 21:1834–1836. doi: 10.3201/eid2110.140633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorusso A, Vincent AL, Harland ML, Alt D, Bayles DO, Swenson SL, Gramer MR, Russell CA, Smith DJ, Lager KM, Lewis NS. 2011. Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. J Gen Virol 92:919–930. doi: 10.1099/vir.0.027557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson TK, Nelson MI, Kitikoon P, Swenson SL, Korslund JA, Vincent AL. 2013. Population dynamics of cocirculating swine influenza A viruses in the United States from 2009 to 2012. Influenza Other Respir Viruses 7(Suppl 4):S42–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Gomez J, Bowman AS, Ye J, Long LP, Nelson SW, Yang J, Martin B, Jia K, Nolting JM, Cunningham F, Cardona C, Zhang J, Yoon KJ, Slemons RD, Wan XF. 2013. Antigenic characterization of H3N2 influenza A viruses from Ohio agricultural fairs. J Virol 87:7655–7667. doi: 10.1128/JVI.00804-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.USDA. 2012. Part II: reference of swine health and health management in the united states, 2012. USDA, Fort Collins, CO. [Google Scholar]
- 13.Bowman AS, Workman JD, Nolting JM, Nelson SW, Slemons RD. 2014. Exploration of risk factors contributing to the presence of influenza A virus in swine at agricultural fairs. Emerg Microbes Infect 3:e5. doi: 10.1038/emi.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Reeth K, Ma W. 2013. Swine influenza virus vaccines: to change or not to change-that's the question. Curr Top Microbiol Immunol 370:173–200. [DOI] [PubMed] [Google Scholar]
- 15.Bruschke CJ, Pittman M, Laddomada A. 2009. International regulations and standards for avian influenza, including the vaccine standards of the World Organisation for Animal Health. Rev Sci Tech 28:379–389. [DOI] [PubMed] [Google Scholar]
- 16.Rudolf M, Poppel M, Frohlich A, Breithaupt A, Teifke J, Blohm U, Mettenleiter T, Beer M, Harder T. 2010. Longitudinal 2 years field study of conventional vaccination against highly pathogenic avian influenza H5N1 in layer hens. Vaccine 28:6832–6840. doi: 10.1016/j.vaccine.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 17.USDA-APHIS. 2009. Autogenous biologics, 9 CFR 1. USDA, Washington, DC. [Google Scholar]
- 18.Beato MS, Xu Y, Long LP, Capua I, Wan XF. 2014. Antigenic and genetic evolution of low-pathogenicity avian influenza viruses of subtype H7N3 following heterologous vaccination. Clin Vaccine Immunol 21:603–612. doi: 10.1128/CVI.00647-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grund C, Abdelwhab el SM, Arafa AS, Ziller M, Hassan MK, Aly MM, Hafez HM, Harder TC, Beer M. 2011. Highly pathogenic avian influenza virus H5N1 from Egypt escapes vaccine-induced immunity but confers clinical protection against a heterologous clade 2.2.1 Egyptian isolate. Vaccine 29:5567–5573. doi: 10.1016/j.vaccine.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Connie Leung YH, Luk G, Sia SF, Wu YO, Ho CK, Chow KC, Tang SC, Guan Y, Malik Peiris JS. 2013. Experimental challenge of chicken vaccinated with commercially available H5 vaccines reveals loss of protection to some highly pathogenic avian influenza H5N1 strains circulating in Hong Kong/China. Vaccine 31:3536–3542. doi: 10.1016/j.vaccine.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 21.Vijaykrishna D, Smith GJ, Pybus OG, Zhu H, Bhatt S, Poon LL, Riley S, Bahl J, Ma SK, Cheung CL, Perera RA, Chen H, Shortridge KF, Webby RJ, Webster RG, Guan Y, Peiris JS. 2011. Long-term evolution and transmission dynamics of swine influenza A virus. Nature 473:519–522. doi: 10.1038/nature10004. [DOI] [PubMed] [Google Scholar]
- 22.Harder TC, Grosse Beilage E, Lange E, Meiners C, Dohring S, Pesch S, Noe T, Grund C, Beer M, Starick E. 2013. Expanded cocirculation of stable subtypes, emerging lineages, and new sporadic reassortants of porcine influenza viruses in swine populations in northwest Germany. J Virol 87:10460–10476. doi: 10.1128/JVI.00381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin BE, Jia K, Sun H, Ye J, Hall C, Ware D, Wan XF. 2015. Detection of influenza antigenic variants directly from clinical samples using polyclonal antibody based proximity ligation assays. Virology 476:151–158. doi: 10.1016/j.virol.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medeiros R, Escriou N, Naffakh N, Manuguerra JC, van der Werf S. 2001. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology 289:74–85. doi: 10.1006/viro.2001.1121. [DOI] [PubMed] [Google Scholar]
- 25.Nobusawa E, Ishihara H, Morishita T, Sato K, Nakajima K. 2000. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology 278:587–596. doi: 10.1006/viro.2000.0679. [DOI] [PubMed] [Google Scholar]
- 26.Petric M, Comanor L, Petti CA. 2006. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis 194(Suppl 2):S98–S110. doi: 10.1086/507554. [DOI] [PubMed] [Google Scholar]
- 27.WHO. 2015. WHO information for molecular diagnosis of influenza virus in humans–update. Annex 2.E and Annex 2.D. WHO, Geneva, Switzerland. [Google Scholar]
- 28.Anonymous. 1999. Rapid diagnostic tests for influenza. Med Lett Drugs Ther 41:121–122. [PubMed] [Google Scholar]
- 29.Sun H, Cunningham FL, Harris J, Xu Y, Long LP, Hanson-Dorr K, Baroch JA, Fioranelli P, Lutman MW, Li T, Pedersen K, Schmit BS, Cooley J, Lin X, Jarman RG, DeLiberto TJ, Wan XF. 2015. Dynamics of virus shedding and antibody responses in influenza A virus-infected feral swine. J Gen Virol 96:2569–2578. doi: 10.1099/jgv.0.000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. 2011. WHO information for molecular diagnosis of influenza virus in humans. WHO, Geneva, Switzerland. [Google Scholar]
- 31.Sullivan KM, Dean A, Soe MM. 2009. OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep 124:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. 2011. Manual for the laboratory diagnosis and virological surveillance of influenza. WHO Global Influenza Surveillance Network, Geneva, Switzerland. [Google Scholar]
- 33.Barnett JL, Yang J, Cai Z, Zhang T, Wan XF. 2012. AntigenMap 3D: an online antigenic cartography resource. Bioinformatics 28:1292–1293. doi: 10.1093/bioinformatics/bts105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Z, Zhang T, Wan XF. 2010. A computational framework for influenza antigenic cartography. PLoS Comput Biol 6:e1000949. doi: 10.1371/journal.pcbi.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hancock D, Holler S. 1995. Optimizing cutpoints of diagnostic tests. Popul Med Newsl 8:1–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.