ABSTRACT
The prevalence of fungi in the respiratory tracts of cystic fibrosis (CF) patients has risen. However, fungal surveillance is not routinely performed in most clinical centers in the United States, which may lead to an underestimation of the true prevalence of the problem. We conducted a prospective study comparing the rates of detection for clinically important fungi (CIF), defined as Aspergillus, Scedosporium, and Trichosporon species and Exophiala dermatitidis, in CF sputa using standard bacterial and selective fungal culture media, including Sabouraud dextrose agar with gentamicin (SDA), inhibitory mold agar (IMA), and brain heart infusion (BHI) agar with chloramphenicol and gentamicin. We described the prevalence of these fungi in an adult CF population. A total of 487 CF respiratory samples were collected from 211 unique participants. CIF were detected in 184 (37.8%) samples. Only 26.1% of CIF-positive samples were detected in bacterial culture medium, whereas greater rates of detection for fungi were found in IMA (65.8%; P < 0.001), in SDA (at 30°C, 64.7%; P = 0.005), and in BHI agar (63.0%; P = 0.001). The prevalences of Aspergillus and Scedosporium species were 40.8% and 5.2%, respectively, which are greater than the nationally reported prevalence numbers of 20.4% and 1.9%. Selective fungal culture media and longer incubation periods yielded higher rates of detection for CIF in CF sputum samples compared with that detected in bacterial culture medium, resulting in an underdetection of fungi by bacterial culture alone. The prevalence of fungi in CF may be better estimated by using selective fungal culture media, and this may translate to important clinical decisions.
KEYWORDS: fungi, cystic fibrosis, fungal culture, inhibitory mold agar, brain heart infusion agar, Sabouraud dextrose agar, Aspergillus, Scedosporium, Trichosporon, Exophiala
INTRODUCTION
Cystic fibrosis (CF) is the most common deadly autosomal recessive disease in Caucasians, affecting approximately 70,000 individuals worldwide. CF is a multiorgan disease that results from the dysfunction of the cystic fibrosis transmembrane conductance regulator gene in the epithelial cells of the sinopulmonary, pancreatic, hepatobiliary, intestinal, skin, and reproductive systems (1). Recurrent infections and bronchiectasis of the lower respiratory tract lead to respiratory failure, the leading cause of death in CF. However, a greater understanding of chronic infections and antimicrobial treatments have transformed the life expectancies of individuals with CF to greater than 40 years of age. The vast majority of antimicrobial therapies in CF are directed toward bacteria, including Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia complex.
The presence of fungi isolated from CF respiratory secretions has significantly risen. The prevalence of Aspergillus species, the most common filamentous fungi in the CF airway, has increased from 6% to 12% over the past 2 decades (2, 3). In addition, Scedosporium, Trichosporon, and Exophiala species have been increasingly detected in CF sputa (4–11). Furthermore, there is increasing evidence that the chronic presence of Aspergillus, Scedosporium, Trichosporon, or Exophiala species in CF individuals is associated with pulmonary morbidity, including respiratory symptoms and pulmonary exacerbations, and even death in the post-lung transplant population (5–9, 11, 12). The reported prevalences in CF centers have ranged from 0 to 36% and 0 to 9% for Aspergillus and Scedosporium, respectively (3, 13, 14). The variability observed may be due to differences in the clinical practice of fungal surveillance and to a lack of standardized laboratory practices for detecting fungi in CF respiratory specimens (15, 16). The presence of rapidly growing Gram-negative organisms, such as Pseudomonas aeruginosa, can interfere with the isolation of potentially clinically significant fungi in standard culture media (5). Despite this, on the basis of pilot survey data, 80% of microbiology laboratories do not routinely perform mycological examinations with selective fungal media in CF respiratory samples, unless they are requested by clinicians (Hong, unpublished data). As a result, only molds and yeasts isolated in bacterial culture medium are reported. Some clinicians order targeted fungal respiratory culture when the suspicion for atypical infection is high, but it is unclear how often this occurs. Our survey data also demonstrated that approximately 57% (95% confidence interval, 18 to 90) of clinicians in the United States report regular monitoring of fungi in CF patients. However, given the small sample size and populations limited to large academic centers, this is likely an overestimation of fungal surveillance in U.S. CF centers.
Previous data suggest that nonselective conventional culture media are insufficient for accurately diagnosing fungal infections, and more selective fungal culture media are associated with an increased identification of fungi in CF sputa (17–21). However, a direct comparison of the rates of detection for fungi by standard bacterial culture medium (based on the infection control guidelines by the Cystic Fibrosis Foundation [CFF]) and by selective fungal media have yet to be performed in a United States CF cohort (22). Accurate diagnoses of fungal infections in the CF population may have significant clinical implications, particularly for sick individuals and among candidates for lung transplantation.
The objective of our study was to evaluate the performances of bacterial and selective fungal culture media in the detection of clinically important fungi (CIF) seen in the CF respiratory tract. We hypothesized that selective fungal culture media would demonstrate greater rates of detection of fungi, and thereby, more accurately describe the prevalences of Aspergillus, Scedosporium, Trichosporon, and Exophiala species in an adult CF population.
RESULTS
A total of 501 independent respiratory samples were collected, and 487 were analyzed. Ten samples were inoculated in only two of the three culture media in the selective fungal research protocol and were omitted from analysis due to deviations from the protocol. Four samples were inoculated on expired culture medium and were excluded from the analysis. The study included 211 unique subjects with a mean of 2.28 samples per subject during the 12-month period. Filamentous fungal or yeast species were detected in 223 of 487 (45.8%) samples. Sixty specimens (12.3%) were polymicrobial (with two or more fungi). CIF were isolated from a total of 184 (37.8%) samples, with Aspergillus species being the most common.
Cohort characteristics.
We compared the baseline demographic and clinical characteristics of individuals from whom CIF were isolated during the study period with those of individuals from whom they were not (Table 1). The demographic characteristics were similar in both groups. Disease characteristics representing the severity of disease, including F508 genotype, pancreatic insufficiency, and pulmonary function defined as the predicted percent of forced expiratory volume in 1 s (FEV1), were similar in both groups. However, chronic nonmacrolide oral antibiotic and inhaled anti-pseudomonal inhaled antibiotic exposures were greater in the CIF-positive group compared to those in the CIF-negative group (oral antibiotic, 27.7% versus 13.6%, respectively, P = 0.01; inhaled antibiotic, 81.2% versus 69.1%, respectively, P = 0.05).
TABLE 1.
Baseline characteristics of CIF-positive and CIF-negative patientsa
| Characteristic | CIF positive (n = 101) | CIF negative (n = 110) | P value |
|---|---|---|---|
| Mean age (yr) | 33.2 | 32.4 | 0.60 |
| Female sex (no. [%]) | 49 (48.5) | 58 (52.7) | 0.50 |
| F508del/F508del (no. [%]) | 50 (49.5) | 63 (57.3) | 0.15 |
| FEV1 (% predicted [mean ± SD]) | 62.3 ± 23.3 | 66.1 ± 23.2 | 0.23 |
| Pancreatic insufficiency (no. [%]) | 88 (87.1) | 93 (84.6) | 0.70 |
| Pseudomonas colonization (no. [%]) | 89 (88.1) | 88 (80.0) | 0.14 |
| Treatment (no. [%]) | |||
| Inhaled antibioticb | 82 (81.2) | 76 (69.1) | 0.05 |
| Chronic oral antibioticc | 28 (27.7) | 15 (13.6) | 0.01 |
| Azithromycin | 71 (70.3) | 76 (69.1) | 0.93 |
| Antifungald | 7 (6.9) | 7 (6.4) | 0.88 |
CIF, clinically important fungi defined as Aspergillus, Scedosporium, Trichosporon, and Exophiala species.
Tobramycin powder/solution, aztreonam, colistin, or ceftazidime.
Sulfamethoxazole/trimethoprim, minocycline, doxycycline, or linezolid.
Itraconazole, voriconazole, or posaconazole.
Prevalence of clinically important fungi.
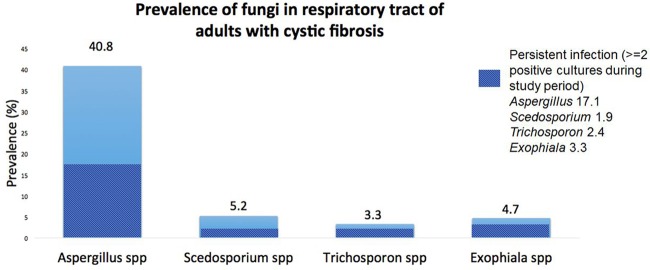
CIF were isolated from samples from 101 (47.9%) individuals during the study period. The prevalences of each of the organisms are summarized in Fig. 1. The prevalences of Aspergillus and persistent Aspergillus were 40.8% and 17.1%, respectively, with Aspergillus fumigatus as the predominant species. The prevalences of Scedosporium, Exophiala, and Trichosporon species were lower at 5.2%, 4.7%, and 3.3%, respectively. Of note, other filamentous fungal species were detected in the study. Rasamsonia species, an emerging pathogen seen in the CF airway (23), were found in samples from 2 (<1%) individuals in the cohort. Paecilomyces variotii (4.3%), Penicillium (11.2%), and other uncommon filamentous fungi, including Rhizopus, Libertella, Rhinocladiella, Cercospora, and Basidiomycete species, were also seen.
FIG 1.
Prevalences of clinically important fungi in samples from adults with cystic fibrosis.
Performance of selective fungal media for detection of Aspergillus, Scedosporium, Trichosporon, and Exophiala.
A total of 184 samples were found to have CIF isolates. The performances of each of the culture media are summarized in Table 2. Among these, CIF were detected in bacterial cultures from 48 (26.1%) samples. CIF were detected at 37°C with Sabouraud dextrose agar with gentamicin (SDA), a selective fungal culture medium, in 103 (56.0%) samples, which was statistically significantly greater that the rate of detection in bacterial culture medium (26.1%, P < 0.001). When comparing the performances of each of the selective fungal media to the rates of detection of CIF utilizing bacterial medium (26.1%), we found that each of the fungal media had significantly higher rates of detection; in SDA at 30°C, CIF were detected in 119 samples (64.7%, P = 0.005), in inhibitory mold agar (IMA), CIF were detected in 121 samples (65.8%, P < 0.001), and in brain heart infusion (BHI) agar, CIF were detected in 116 samples (63.0%, P = 0.001).
TABLE 2.
Detection of CIF in bacterial culture and selective fungal culture mediaa
| Mediumb | No. of samples with fungi detected (%) | P value |
|---|---|---|
| Bacterial culture | 48 (26.1) | reference |
| SDA at 37°C | 103 (56.0) | <0.001 |
| SDA at 30°C | 119 (64.7) | 0.005 |
| IMA | 121 (65.8) | <0.001 |
| BHI agar | 116 (63.0) | 0.001 |
| Combination of IMA, SDA at 30°C, and BHI agar | 156 (84.8) | <0.001 |
CIF, clinically important fungi defined as Aspergillus, Scedosporium, Trichosporon, and Exophiala species.
SDA, Sabouraud with gentamicin; IMA, inhibitory mold agar; BHI, brain heart infusion.
We further analyzed the performances of selective fungal media for detecting each of the CIF: Aspergillus, Scedosporium, Trichosporon, and Exophiala (see Table S1 in the supplemental material). A total of 144 samples were positive for Aspergillus species, with Aspergillus fumigatus isolated from 134 (93%). A. terreus, A. niger, A. flavus, and A. nidulans were also observed. The rate of detection of Aspergillus by bacterial culture medium was 30.6%. In comparison, the rates of detection were greater with each of the selective fungal culture media: SDA at 37°C (54.9%, P < 0.001), SDA at 30°C (50.0%, P < 0.001), IMA (50.0%, P = 0.001), and BHI agar (47.9%, P = 0.003). Twenty-five of the 144 Aspergillus-positive samples were identified by bacterial culture medium only. Among the 20 samples positive for Scedosporium apiospermum (n = 17) and S. prolificans (n = 3), bacterial culture medium detected three of the 20 (15%), whereas the rates for detecting Scedosporium using selective fungal media were greater. Of the 17 Trichosporon-positive samples, only a single specimen (5.9%) was detected by bacterial culture. SDA at 30°C and 37°C, IMA, and BHI agar performed well in comparison to bacterial medium. Finally, we failed to detect any Exophiala-positive specimens after incubating bacterial cultures for 3 days. On the contrary, IMA had a high rate of detection (95.7%), whereas the rates of detection with SDA at 30°C and 37°C and BHI agar were slightly lower.
Mean time-to-detection of Aspergillus, Scedosporium, Trichosporon, and Exophiala.
The mean times-to-isolation of CIF on bacterial culture medium and SDA at 37°C for a maximum incubation period of 3 days were 2.35 days and 2.21 days, respectively (Table 3). For SDA at 30°C, IMA, and BHI agar incubated for 14 days, the mean times-to-isolation of CIF were 3.49, 3.85, and 3.43 days, respectively, and did not exceed 4 days for each of the media. Upon examining each of the CIF, we found that Aspergillus grew in 3.48 days on IMA, Scedosporium in 3.61 days, Trichosporon in 2.75 days, and Exophiala in 5.87 days (see Table S2). Mean times-to-isolation for each of the CIF were similar to those on BHI agar and SDA at 30°C.
TABLE 3.
Time-to-detection of CIF in bacterial culture and selective fungal culture mediaa
| Mediumb | Time-to-detection (days) (mean ± SD) | P valuec |
|---|---|---|
| Bacterial culture | 2.35 ± 1.12 | reference |
| SDA at 37°C | 2.21 ± 0.51 | 0.48 |
| SDA at 30°C | 3.49 ± 1.76 | 0.002 |
| IMA | 3.85 ± 2.18 | 0.003 |
| BHI agar | 3.43 ± 1.89 | 0.003 |
CIF, clinically important fungi defined as Aspergillus, Scedosporium, Trichosporon, and Exophiala species.
SDA, Sabouraud with gentamicin; IMA, inhibitory mold agar; BHI, brain heart infusion.
Based on pair-wise comparisons.
DISCUSSION
Our study is the first to examine and compare the rates for detecting fungi in the CF airways of individuals in a North American CF cohort utilizing various culture media. The results of our study suggest CIF in CF patients vulnerable to fungal isolation in the respiratory tract are underdetected when using bacterial culture medium alone for fungal surveillance. Furthermore, when combinations of selective fungal media are used, the prevalence of CIF is notably greater than the known prevalence reported in the CFF registry (3).
The prevalence of CIF in our cohort was greater than 40%. Only one-quarter of the clinically significant fungal organisms were successfully detected in standard bacterial culture medium. Selective fungal culture media and prolonged incubation periods provided statistically significantly better rates of detection of CIF than did bacterial culture. Aspergillus species were best detected when utilizing SDA at an incubation temperature of 37°C. Aspergillus was isolated from one-third of the positive samples by bacterial culture. For other clinically significant fungi, bacterial culture performed poorly compared with the selective fungal media. Fifteen percent of the Scedosporium isolates were detected on bacterial culture media. Similarly, the rates of detection of Trichosporon and Exophiala from bacterial culture media were 5% and 0%, respectively. Among each of the CIF, the greatest rates of detection were found using a combination of IMA, SDA, and BHI agar, which may support a multimodal approach for selective fungal culturing. Although statistical comparisons between SDA, IMA, and BHI agar were not performed, with the exception of Exophiala dermatitidis, the rates of detection of each of the selective fungal media for Aspergillus, Scedosporium, and Trichosporon species appeared similar. Among the Exophiala-positive samples, a higher rate of detection occurred on IMA than on SDA or BHI agar. These data suggest that CF centers without formal mycological examinations involving selective fungal media may have unidentified cases of fungal respiratory infection or colonization in CF patients, particularly for Scedosporium, Trichosporon, and Exophiala species.
The prevalences of Aspergillus (40.8%) and Scedosporium (5.2%) were greater in our study than in the national registry data of CFF-accredited centers in the United States (43). The prevalences of Aspergillus and Scedosporium species in adults with CF has been reported as 20.4% and 1.9%, respectively (24). The prevalence numbers from our study are comparable to those from studies performed utilizing selective fungal media, such as SDA, but are not as high as those experimenting with Scedosporium-selective media (SceSel+) containing benomyl and dichloran, which have been reported to be as high as 10 to 16% (15, 17, 18, 20, 25). The prevalences of Trichosporon and Exophiala are not well described in the CFF registry. Epidemiologic data have demonstrated the prevalence of Trichosporon to be an estimated 2.1% in an American cohort (4, 11). The prevalence of Exophiala in the United States has not been described.
A number of previous studies have investigated new culturing methods and selective media for fungal species in the CF population. Nagano et al. conducted experiments to develop novel selective media for isolating yeasts and filamentous fungi and determined that SDA plus antibiotics (including co-trimethoxazole, chloramphenicol, ceftazidime, and colistin) and self-formulated Medium B+ performed with high sensitivity (96%) and specificity (82%) for the detection of fungi in CF sputa (26). Several groups have demonstrated the superior performance of SceSel+ agar containing benomyl for isolating Scedosporium species (18, 25, 27). A multicenter study in Europe and Australia comparing the performances of semiselective fungal media in 469 samples of CF sputum found yeast potato dextrose agar, SceSel+ agar, and B+ medium to be among the most sensitive for detecting fungi, including Aspergillus, Scedosporium, and Exophiala species (28). One limitation of these studies is that those selective media are not commercially available, which may prevent clinical laboratories from implementing and further standardizing laboratory protocols for fungal isolation. Despite these data and the known variability of fungal prevalence numbers secondary to nonuniform laboratory protocols, Europe and the United States lack a standardized approach for mycological examination (15, 16). In contrast to previous studies, we directly compared rates of detection between bacterial culture medium and commonly available selective fungal media and demonstrated the suboptimal performance of bacterium-specific culture medium to detect clinically significant molds and yeasts in CF sputum samples. Our findings suggest that, in the adult CF population vulnerable to respiratory fungal infection and colonization, clinicians should not solely rely on bacterial culture to make accurate fungal diagnoses.
The duration of time from inoculation to the detection of a fungal isolate was studied to determine an optimal incubation period to maximize the detection of fungi. The mean time-to-isolation of CIF utilizing selective fungal culture media at 30°C was approximately 3.5 days, which is comparable to results from previous studies (18). Upon examining the mean time-to-detection for each fungal species group during the 2-week research protocol, the observed times were notably different between fungal species. Trichosporon was isolated after a short period of incubation, whereas Exophiala dermatitidis was recovered at a mean time approaching 6 days on IMA (see Table S2). These data suggest that an incubation period of 7 days may be sufficient for detecting the majority of fungi in CF sputa.
Currently, the standard of care protocol for analyzing CF respiratory specimens in clinical microbiology laboratories involves conventional culture techniques. Although there is evidence that culture-independent tests, such as molecular sequencing, improve the sensitivity for diagnosis, these approaches are not yet universal or widely accessible to a vast majority of clinical laboratories (29, 30). In addition, the clinical relevance of the presence of DNA from organisms has yet to be demonstrated.
Evidence suggests there may be a subset of CF patients who are at greater risk of developing fungal infections and colonizations of clinical significance. The use of inhaled antibiotics, anti-staphylococcal oral antibiotics, macrolide therapy, and inhaled corticosteroids have been associated with filamentous fungal isolation, predominantly Aspergillus (4, 5, 31–36). In our cohort, individuals from whom CIF were isolated during the study period had greater usages of chronic anti-methicillin-resistant Staphylococcus aureus (MRSA) oral antibiotics and chronic inhaled anti-pseudomonal antibiotics at baseline than those from whom CIF was not isolated. These associations may further guide clinicians in assessing the presence of fungi, such as Aspergillus, Scedosporium, Trichosporon, or Exophiala species, by utilizing more accurate selective fungal culture in a subpopulation of patients that may be more vulnerable to developing these infections.
There are limitations to note in our study. First, the study population was limited to adults who were able to expectorate sputum, affecting the generalizability to the entire CF population. However, several studies indicate that fungi are more prevalent in older adults than in children with CF (4, 5, 37, 38). On the basis of CFF registry data, the mean age for acquiring a persistent isolation of Aspergillus was 17.3 years (Hong, unpublished data). Second, the incubation period for bacterial culture medium could not be extended beyond a 3-day duration. As a result, the comparison data between bacterial culture medium and selective fungal media should be interpreted cautiously. Nevertheless, one could infer that the overall standard lab practice of bacterial culture for a 3-day incubation period has a lower rate of detection for CIF compared with that from a selective fungal media protocol involving a longer incubation period. Furthermore, we were unable to determine true test characteristics, including sensitivity and specificity, due to the lack of a gold standard test for a true fungal diagnosis. Although several diagnostic tests have proved to have better performances in diagnosing certain fungal species in cystic fibrosis, such as sputum galactomannan and molecular testing, these are not routinely performed in the clinical laboratory setting (30, 39). Finally, there may be unmeasured confounders that may affect the likelihood of fungal isolation on bacterial culture, such as acute oral or intravenous antibacterial therapy for treating pulmonary exacerbation at the time of the specimen collection. However, the association between intravenous (i.v.) antibiotic use and Aspergillus isolation has been controversial (35, 40).
In conclusion, the clinical impact of certain filamentous fungi and yeast in CF remains debatable despite increasing data suggesting respiratory morbidity. However, accurate diagnoses are necessary to truly understand the clinical outcomes associated with fungal disease. Our study suggests the current practices of some CF clinicians and clinical microbiology laboratories may be limiting the fungal diagnoses if selective fungal culture methods are not utilized. These data suggest that a combination of selective fungal culture media, including IMA, SDA, and BHI agar, incubated for approximately 7 days would result in a greater isolation of CIF in a CF population. Further study is needed to guide the development of a standardized approach to detect fungi in the CF airway.
MATERIALS AND METHODS
Study design and participants.
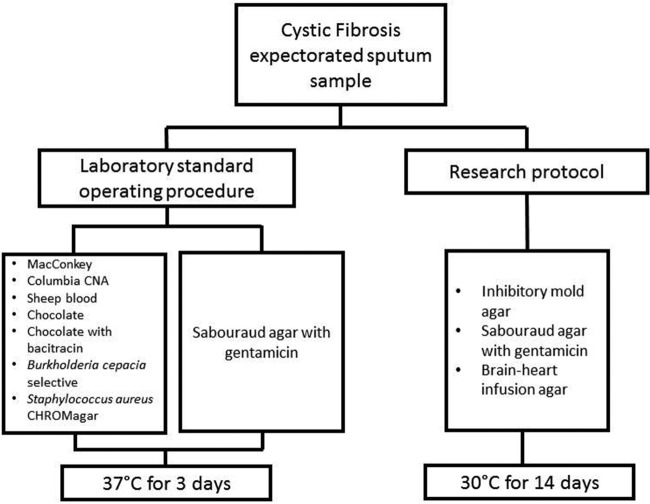
This was a 12-month prospective study involving the collection and analysis of expectorated sputa from adult CF patients at the Johns Hopkins Hospital in Baltimore, Maryland, USA. Consecutive expectorated sputum specimens were collected from patients in the outpatient clinic, regardless of clinical status, and underwent analysis by the Johns Hopkins clinical microbiology laboratory. Eligibility criteria included subjects with a baseline age of 18 years or older and with the ability to expectorate sputum. A minimum sputum sample volume of 0.5 ml was collected. Liquefaction, sonication, or other specimen modification procedures were not performed. All plates were inoculated with equal volumes of sputum specimens. Each specimen underwent two protocols simultaneously: (i) a standard laboratory protocol involving inoculating on the bacterial culture medium recommended by the CFF (MacConkey, Columbia CNA, sheep blood, chocolate with and without bacitracin, Burkholderia cepacia selective, or Staphylococcus aureus CHROMagar, [Remel, Lenexa, KS]) (22, 41) plus SDA (Remel, Lenexa, KS) and incubating for 3 days at 37°C and (ii) a selective fungal research protocol involving inoculating on IMA (Hardy Diagnostics, Santa Maria, California), SDA, and BHI agar with gentamicin and chloramphenicol (Becton Dickinson, Sparks, Maryland) and incubating for 14 days in 30°C (Fig. 2). The cultures were evaluated daily for the presence of fungal isolates. When we detected a fungal isolate, it was identified by colony characterization and morphological examination via microscopy, and if necessary, by DNA sequencing procedures (sequence analysis of 26S to 28S rDNA and internal transcribed regions using the Applied Biosystems 3500 genetic analyzer) (42). We collected the clinical and demographic information of the subjects. The primary outcome was the rate of detection of CIF, defined as Aspergillus, Scedosporium, Trichosporon, and Exophiala species. The selection of these organisms was based on published literature associated with increased respiratory symptoms, pulmonary exacerbations, and post-lung transplant complications (5–7, 10–13, 31). All filamentous fungi that were isolated were recorded. The secondary outcome was the mean time-to-detection, defined as the number of days from inoculation of the specimen to the isolation of fungi.
FIG 2.
Flow diagram depicting the study design.
Statistical analysis.
The detection rate of each culture medium was determined by the proportion of positive culture diagnoses for the respective medium over the total number of positive culture diagnoses. A positive culture was defined by the isolation of CIF in one or more of the culture media (bacterial culture media, SDA, IMA, or BHI agar). Prevalence was calculated based on the percentages of fungi detected in an individual subject in the cohort. Persistent isolation of fungi was defined as two or more occurrences of the same fungi during the study period. A descriptive analysis of the baseline cohort was performed using calculations of means and standard deviations for continuous variables and proportions for categorical variables. Demographic and clinical variables used in the analysis included: age, sex, F508del genotype, pancreatic insufficiency, FEV1 percent predicted, Pseudomonas aeruginosa colonization, and the use of inhaled anti-pseudomonal antibiotics (tobramycin powder/solution, aztreonam, colistin, and ceftazidime), nonmacrolide oral antibiotics (defined as anti-staphylococcal oral antibiotics, including sulfamethoxazole/trimethoprim, minocycline, doxycycline, and linezolid), azithromycin, and antifungals (itraconazole, voriconazole, and posaconazole). The rates of detection of fungi were compared between standard bacterial culture medium and selective fungal media (IMA, SDA, and BHI agar) using chi-square and Fishers exact tests. We used two sample t tests and Wilcoxon rank-sum tests to assess differences in the mean time-to-detection in the bacterial versus selective fungal media.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff members working in the Medical Mycology Laboratory at the Johns Hopkins Hospital for their technical support for this work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02182-16.
REFERENCES
- 1.Boyle MP. 2007. Adult cystic fibrosis. JAMA 298:1787–1793. doi: 10.1001/jama.298.15.1787. [DOI] [PubMed] [Google Scholar]
- 2.Nagano YMB, Millar BC, Johnson E, Goldsmith CE, Elborn JS, Rendall J, Moore JE. 2007. Fungal infections in patients with cystic fibrosis. Rev Med Microbiol 18:11–16. doi: 10.1097/MRM.0b013e3282e1c70a. [DOI] [Google Scholar]
- 3.Cystic Fibrosis Foundation. 2013. Cystic Fibrosis Foundation annual report 2012. Bethesda, MD. https://www.cff.org/About-Us/Assets/2012-Annual-Report/
- 4.Sudfeld CR, Dasenbrook EC, Merz WG, Carroll KC, Boyle MP. 2010. Prevalence and risk factors for recovery of filamentous fungi in individuals with cystic fibrosis. J Cyst Fibros 9:110–116. doi: 10.1016/j.jcf.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pihet M, Carrere J, Cimon B, Chabasse D, Delhaes L, Symoens F, Bouchara JP. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis–a review. Med Mycol 47:387–397. doi: 10.1080/13693780802609604. [DOI] [PubMed] [Google Scholar]
- 6.Kondori N, Lindblad A, Welinder-Olsson C, Wenneras C, Gilljam M. 2014. Development of IgG antibodies to Exophiala dermatitidis is associated with inflammatory responses in patients with cystic fibrosis. J Cyst Fibros 13:391–399. doi: 10.1016/j.jcf.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Kroner C, Kappler M, Grimmelt AC, Laniado G, Wurstl B, Griese M. 2013. The basidiomycetous yeast Trichosporon may cause severe lung exacerbation in cystic fibrosis patients - clinical analysis of Trichosporon positive patients in a Munich cohort. BMC Pulm Med 13:61. doi: 10.1186/1471-2466-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolfe NE, Haddad TJ, Wills TS. 2013. Management of Scedosporium apiospermum in a pre- and post-lung transplant patient with cystic fibrosis. Med Mycol Case Rep 2:37–39. doi: 10.1016/j.mmcr.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blyth CC, Middleton PG, Harun A, Sorrell TC, Meyer W, Chen SC. 2010. Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: identification of novel risk factors? Med Mycol 48 Suppl 1:S37–S44. doi: 10.3109/13693786.2010.500627. [DOI] [PubMed] [Google Scholar]
- 10.Hirschi S, Letscher-Bru V, Pottecher J, Lannes B, Jeung MY, Degot T, Santelmo N, Sabou AM, Herbrecht R, Kessler R. 2012. Disseminated Trichosporon mycotoxinivorans, Aspergillus fumigatus, and Scedosporium apiospermum coinfection after lung and liver transplantation in a cystic fibrosis patient. J Clin Microbiol 50:4168–4170. doi: 10.1128/JCM.01928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esther CR Jr, Plongla R, Kerr A, Lin FC, Gilligan P. 2016. Clinical outcomes in cystic fibrosis patients with Trichosporon respiratory infection. J Cyst Fibros 15:e45–e49. doi: 10.1016/j.jcf.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Amin R, Dupuis A, Aaron SD, Ratjen F. 2010. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 137:171–176. doi: 10.1378/chest.09-1103. [DOI] [PubMed] [Google Scholar]
- 13.Cimon B, Carrere J, Vinatier JF, Chazalette JP, Chabasse D, Bouchara JP. 2000. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 19:53–56. doi: 10.1007/s100960050011. [DOI] [PubMed] [Google Scholar]
- 14.Ziesing S, Suerbaum S, Sedlacek L. 2016. Fungal epidemiology and diversity in cystic fibrosis patients over a 5-year period in a national reference center. Med Mycol 54:781–786. doi: 10.1093/mmy/myw035. [DOI] [PubMed] [Google Scholar]
- 15.Borman AM, Palmer MD, Delhaes L, Carrere J, Favennec L, Ranque S, Gangneux JP, Horre R, Bouchara JP. 2010. Lack of standardization in the procedures for mycological examination of sputum samples from CF patients: a possible cause for variations in the prevalence of filamentous fungi. Med Mycol 48 Suppl 1:S88–S97. doi: 10.3109/13693786.2010.511287. [DOI] [PubMed] [Google Scholar]
- 16.Liu JC, Modha DE, Gaillard EA. 2013. What is the clinical significance of filamentous fungi positive sputum cultures in patients with cystic fibrosis? J Cyst Fibros 12:187–193. doi: 10.1016/j.jcf.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Horre R, Marklein G, Siekmeier R, Nidermajer S, Reiffert SM. 2009. Selective isolation of Pseudallescheria and Scedosporium species from respiratory tract specimens of cystic fibrosis patients. Respiration 77:320–324. [DOI] [PubMed] [Google Scholar]
- 18.Blyth CC, Harun A, Middleton PG, Sleiman S, Lee O, Sorrell TC, Meyer W, Chen SC. 2010. Detection of occult Scedosporium species in respiratory tract specimens from patients with cystic fibrosis by use of selective media. J Clin Microbiol 48:314–316. doi: 10.1128/JCM.01470-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagano Y, Elborn JS, Millar BC, Walker JM, Goldsmith CE, Rendall J, Moore JE. 2010. Comparison of techniques to examine the diversity of fungi in adult patients with cystic fibrosis. Med Mycol 48:166–176. doi: 10.3109/13693780903127506. [DOI] [PubMed] [Google Scholar]
- 20.Masoud-Landgraf L, Badura A, Eber E, Feierl G, Marth E, Buzina W. 2014. Modified culture method detects a high diversity of fungal species in cystic fibrosis patients. Med Mycol 52:179–186. doi: 10.3109/13693786.2013.792438. [DOI] [PubMed] [Google Scholar]
- 21.Horre R, Schaal KP, Siekmeier R, Sterzik B, de Hoog GS, Schnitzler N. 2004. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration 71:360–366. doi: 10.1159/000079640. [DOI] [PubMed] [Google Scholar]
- 22.Saiman L, Siegel JD, LiPuma JJ, Brown RF, Bryson EA, Chambers MJ, Downer VS, Fliege J, Hazle LA, Jain M, Marshall BC, O'Malley C, Pattee SR, Potter-Bynoe G, Reid S, Robinson KA, Sabadosa KA, Schmidt HJ, Tullis E, Webber J, Weber DJ, Cystic Fibrosis Foundation, Society for Healthcare Epidemiology of America . 2014. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect Control Hosp Epidemiol 35 Suppl 1:S1–S67. doi: 10.1086/676882. [DOI] [PubMed] [Google Scholar]
- 23.Houbraken J, Giraud S, Meijer M, Bertout S, Frisvad JC, Meis JF, Bouchara JP, Samson RA. 2013. Taxonomy and antifungal susceptibility of clinically important Rasamsonia species. J Clin Microbiol 51:22–30. doi: 10.1128/JCM.02147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cystic Fibrosis Foundation. 2012. Patient registry annual data report 2011. Bethesda, MD. [Google Scholar]
- 25.Sedlacek L, Graf B, Schwarz C, Albert F, Peter S, Wurstl B, Wagner S, Klotz M, Becker A, Haase G, Laniado G, Kahl B, Suerbaum S, Seibold M, Tintelnot K. 2015. Prevalence of Scedosporium species and Lomentospora prolificans in patients with cystic fibrosis in a multicenter trial by use of a selective medium. J Cyst Fibros 14:237–241. doi: 10.1016/j.jcf.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Nagano Y, Millar BC, Goldsmith CE, Walker JM, Elborn JS, Rendall J, Moore JE. 2008. Development of selective media for the isolation of yeasts and filamentous fungi from the sputum of adult patients with cystic fibrosis (CF). J Cyst Fibros 7:566–572. doi: 10.1016/j.jcf.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Horre R, Marklein G. 2009. Isolation and clinical significance of Pseudallescheria and Scedosporium species. Med Mycol 47:415–421. doi: 10.1080/13693780902801259. [DOI] [PubMed] [Google Scholar]
- 28.Touati KFC, Cornet M, Botterel F, Dannaoui E, Morio F, Lepape P, Grenouillet F, Favennec L, Le Gal S, Nevez F, Borman A, Saegeman V, Lagrou K, Gomez E, Caro-Luis M, Canton R, Campana S, Buzina W, Chen S, Meyer W, Roilides E, Simitsopoulou M, Manso E, Cariani L, Biffi A, Fiscarelli E, Riccioti G, Sendid B, Pihet M, Bouchara JP, Delhaes L. 2014. Evaluation of the risk of fungal colonization/infection in patients with cystic fibrosis: an international prospective study comparing the performance of media for mycological culturing MucoFong International Project (MFIP). J Mycol Med 24:e112. doi: 10.1016/j.mycmed.2014.06.008. [DOI] [Google Scholar]
- 29.Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. 2014. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev 27:490–526. doi: 10.1128/CMR.00091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxter CG, Dunn G, Jones AM, Webb K, Gore R, Richardson MD, Denning DW. 2013. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J Allergy Clin Immunol 132:560–566.e510. doi: 10.1016/j.jaci.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Noni M, Katelari A, Dimopoulos G, Doudounakis SE, Tzoumaka-Bakoula C, Spoulou V. 2015. Aspergillus fumigatus chronic colonization and lung function decline in cystic fibrosis may have a two-way relationship. Eur J Clin Microbiol Infect Dis 34:2235–2241. doi: 10.1007/s10096-015-2474-y. [DOI] [PubMed] [Google Scholar]
- 32.Burns JL, Van Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, Montgomery AB, Albers GM, Ramsey BW, Smith AL. 1999. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 179:1190–1196. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 33.Bargon J, Dauletbaev N, Kohler B, Wolf M, Posselt HG, Wagner TO. 1999. Prophylactic antibiotic therapy is associated with an increased prevalence of Aspergillus colonization in adult cystic fibrosis patients. Respir Med 93:835–838. doi: 10.1016/S0954-6111(99)90270-6. [DOI] [PubMed] [Google Scholar]
- 34.Noni M, Katelari A, Dimopoulos G, Kourlaba G, Spoulou V, Alexandrou-Athanassoulis H, Doudounakis SE, Tzoumaka-Bakoula C. 2014. Inhaled corticosteroids and Aspergillus fumigatus isolation in cystic fibrosis. Med Mycol 52:715–722. doi: 10.1093/mmy/myu038. [DOI] [PubMed] [Google Scholar]
- 35.Jubin V, Ranque S, Stremler Le Bel N, Sarles J, Dubus JC. 2010. Risk factors for Aspergillus colonization and allergic bronchopulmonary aspergillosis in children with cystic fibrosis. Pediatr Pulmonol 45:764–771. doi: 10.1002/ppul.21240. [DOI] [PubMed] [Google Scholar]
- 36.Ritz N, Ammann RA, Casaulta Aebischer C, Schoeni-Affolter F, Schoeni MH. 2005. Risk factors for allergic bronchopulmonary aspergillosis and sensitisation to Aspergillus fumigatus in patients with cystic fibrosis. Eur J Pediatr 164:577–582. doi: 10.1007/s00431-005-1701-4. [DOI] [PubMed] [Google Scholar]
- 37.de Vrankrijker AM, van der Ent CK, van Berkhout FT, Stellato RK, Willems RJ, Bonten MJ, Wolfs TF. 2011. Aspergillus fumigatus colonization in cystic fibrosis: implications for lung function? Clin Microbiol Infect 17:1381–1386. doi: 10.1111/j.1469-0691.2010.03429.x. [DOI] [PubMed] [Google Scholar]
- 38.Milla CE, Wielinski CL, Regelmann WE. 1996. Clinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr Pulmonol 21:6–10. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Prattes J, Flick H, Pruller F, Koidl C, Raggam RB, Palfner M, Eigl S, Buzina W, Zollner-Schwetz I, Thornton CR, Krause R, Hoenigl M. 2014. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med 190:922–929. doi: 10.1164/rccm.201407-1275OC. [DOI] [PubMed] [Google Scholar]
- 40.Baxter CG, Rautemaa R, Jones AM, Webb AK, Bull M, Mahenthiralingam E, Denning DW. 2013. Intravenous antibiotics reduce the presence of Aspergillus in adult cystic fibrosis sputum. Thorax 68:652–657. doi: 10.1136/thoraxjnl-2012-202412. [DOI] [PubMed] [Google Scholar]
- 41.Saiman L, Siegel J, Cystic Fibrosis Foundation Consensus Conference on Infection Control Participants . 2003. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control 31:S1–62. [PubMed] [Google Scholar]
- 42.Kwiatkowski NP, Babiker WM, Merz WG, Carroll KC, Zhang SX. 2012. Evaluation of Nucleic acid sequencing of the D1/D2 region of the large subunit of the 28S rDNA and the internal transcribed spacer region using SmartGene IDNS [corrected] software for identification of filamentous fungi in a clinical laboratory. J Mol Diagn 14:393–401. doi: 10.1016/j.jmoldx.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Cystic Fibrosis Foundation. 2014. Patient registry annual data report to the center directors 2013. Bethesda, MD. https://www.cff.org/2013_CFF_Annual_Data_Report_to_the_Center_Directors.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.