ABSTRACT
BK virus (BKV)-associated diseases in transplant recipients are an emerging issue. However, identification of the various BK virus subtypes/subgroups is a long and delicate process on the basis of currently available data. Therefore, we wanted to define a simple and effective one-step strategy for characterizing all BK virus strains from the VP1 gene sequence. Based on the analysis of 199 available complete DNA VP1 sequences, phylogenetic trees, alignments, and isolated polymorphisms were used to define an effective strategy for distinguishing the 12 different BK virus subtypes/subgroups. Based on the 12 subtypes identified from the 199 complete BKV VP1 sequences (1,089 bp), 60 mutations that can be used to differentiate these various subtypes/subgroups were identified. Some genomic areas were more variable and comprised mutational hot spots. From a subregion of only 100 bp in the VP1 region (1977 through 2076), we therefore constructed an algorithm that enabled rapid determination of all BKV subtypes/subgroups with 99% agreement (197/199) relative to the complete VP1 sequence. We called this domain of the BK viral genome the BK typing and grouping region (BKTGR). Finally, we validated our viral subtype identification process in a population of 100 transplant recipients with 100% efficiency. The new simpler method of BKV subtyping/subgrouping reported here constitutes a useful tool for future studies that will help us to more clearly understand the impact of BKV subtypes/subgroups on diagnosis, infection, and BK virus-associated diseases.
KEYWORDS: BK virus, BK virus strains, subtyping, transplantation
INTRODUCTION
First described in 1971, the polyomavirus BK virus (BKV) has become increasingly prevalent since the introduction of more potent immunosuppressive agents. In immunocompromised patients, human BK polyomavirus has been linked to two major complications in transplant recipients, polyomavirus-associated nephropathy (PyVAN) (in 1 to 10% of kidney transplant recipients) (1) and polyomavirus-associated hemorrhagic cystitis (PyVHC) (in 5 to 15% of allogeneic hematopoietic stem cell transplant [HSCT] recipients) (2). A notable feature of BKV is the remarkable diversity of its genomic sequences. Genetic heterogeneity in the region of the VP1 gene has been used to classify BKV into four major subtypes: I, II, III, and IV. Jin developed the first scheme for BKV subtyping based on the sequence and type-specific restriction of endonuclease sites of a 327-bp variable region of the gene coding for the major capsid protein VP1 (3) (nucleotides 1630 to 1956, according to Dunlop numbering). Subtype I is the most common subtype worldwide (80%), followed by subtype IV (15%) (4). Subtype II and III viruses have been found to be sister groups and are rarely detected. Based on DNA sequence variations, four subgroups of subtype I have been identified (Ia, Ib-1, Ib-2, and Ic), and six subgroups of subtype IV have been identified (IVa-1, IVa-2, IVb-1, IVb-2, IVc-1, and IVc-2) (5, 6). The distribution of BKV subtypes and subgroups in human populations has been studied in many countries (7–9). BKV subtype I subgroup distributions vary across countries; subgroup Ia is highly prevalent in Africa, subgroup Ib-1 is highly prevalent in southeast Asia, subgroup Ib-2 is highly prevalent in Europe, and subgroup Ic is highly prevalent in northeast Asia (10, 11). As with subtype I, each subtype IV subgroup has a specific geographic distribution (6). A better knowledge of viral subtypes and subgroups is needed for quality assurance of diagnostic assays performed in molecular biology laboratories and also is important for tracing infection trails in epidemiologic investigations. Furthermore, whether any differences exist between the various BKV subgroups/subtypes and their causative roles in the development of clinical syndromes and nephropathy remains largely unknown.
Moreover, in order to detect all viral subtypes and subgroups, various regions of the viral genome must be amplified, and alignments must be performed, which makes determination of these 12 subgroups and subtypes a long and tedious process. An obvious need exists for clinical virologists to easily identify viral subtypes associated with emerging BK virus diseases.
Therefore, the aim of this study was to develop a simple and rapid method for the determination of all 12 BK virus subtypes/subgroups. In this study, we found that amplification of a 100-bp region in VP1 is sufficient for resolving this problem, and we called this region the BK typing and grouping region (BKTGR).
RESULTS
Phylogeny of BKV VP1 gene.
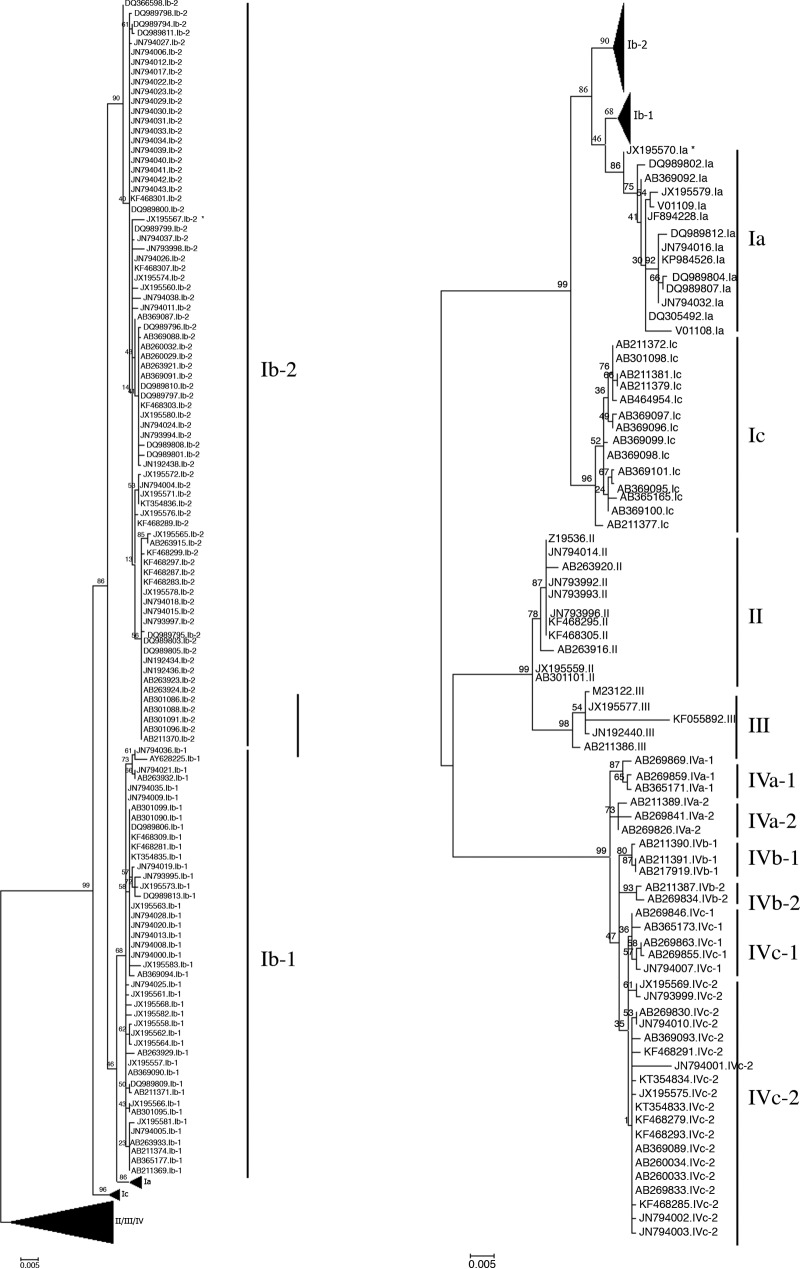
All 199 complete BKV VP1 gene sequences retrieved from GenBank were aligned. A phylogenetic tree was then constructed in order to cluster all 199 strains according to their BKV subtype and subgroup (Fig. 1), which resulted in 14 Ia subgroup strains, 44 Ib-1 subgroup strains, 76 Ib-2 subgroup strains, 14 subgroup Ic strains, 11 subtype II strains, 5 subtype III strains, 3 subgroup IVa-1 strains, 3 subgroup IVa-2 strains, 3 subgroup IVb-1 strains, 2 subgroup IVb-2 strains, 5 subgroup IVc-1 strains, and 19 subgroup IVc-2 strains. These data reflect the BKV genotype distribution observed in the European population, which suggests that this cohort of reference strains is relevant.
FIG 1.
Phylogenetic tree constructed by the maximum likelihood method using 199 complete BKV VP1 gene sequences available in GenBank. The asterisk indicates the strain for which subtyping/subgrouping showed discordant results according to the BKTGR scheme.
Distribution of polymorphisms in the BKV VP1 gene.
To detect the single nucleotide polymorphisms (SNPs) and enable subclassification of these BKV subtype and subgroup isolates, the complete VP1 gene sequences of the 199 reference strains were aligned from position 1564 to position 2652 (representing 1,089 bp). The locations of all subtype-informative SNPs are shown in Table 1; only mutations found at a position number that uniquely identified BKV subtypes and subgroups are listed. Note that 60 mutations throughout this region of 1,089 bp differentiated between these various subtypes and subgroups. The entire BKV VP1 region contains 12 specific informative SNPs (positions 1761, 1766 through 1768, 1170, 1857, 1926, 1989, 1998, 2076, 2181, 2277, 2280, and 2391), that is, when the mutation was found for only one subtype/subgroup. For example, at position 1761, the C nucleotide is found for only the IVc-2 subgroup. Some regions are more variable than others and comprise mutational hot spots. We also observed that more than one-third of these discriminative mutations (21/60) were concentrated in the region between positions 1962 and 2154.
TABLE 1.
Informative single nucleotide polymorphisms for BKV subtypes/subgroups on VP1 genomic positions (Dunlop-based numbering)
| VP1 position | SNP for BKV subtype or subgroup (no. of sequences): |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ia (14) | Ib-1 (44) | Ib-2 (76) | Ic (14) | II (11) | III (5) | IVa-1 (3) | IVa-2 (3) | IVb-1 (3) | IVb-2 (2) | IVc-1 (5) | IVc-2 (19) | |
| 1760 | T | T | T | T | A | A | A | A | A | A | A | A |
| 1761 | T | T | T | T | T | T | T | T | T | T | T | C |
| 1766, 1767, 1768 | TAA | TAA | TAA | TAA | TAA | AGC | TAA | TAA | TAA | TAA | TAA | TAA |
| 1770 | G | G | G | G | G | C | A | A | A | A | A | A |
| 1787 | A | A | A | A | C | C | C | C | C | C | C | C |
| 1824 | C | C | C | C | T | T | C | C | C | C | C | C |
| 1848 | C | C | C | C | A | A | A | A | A | A | A | A |
| 1857 | T | T | T | T | T | C | T | T | T | T | T | T |
| 1858 | T | T | T | T | C | C | T | T | T | T | T | T |
| 1860 | A | A | A | G | A | A | A | A | G | G | G | G |
| 1869 | C | C | C | C | C | C | T | T | T | T | T | T |
| 1912 | C | C | C | C | A | A | A | A | A | A | A | A |
| 1926 | T | T | T | T | T | T | T | T | T | G | T | T |
| 1938 | C | C | C | C | C | C | T | T | T | T | T | T |
| 1962 | A | A | A | A | C | C | A | A | A | A | A | A |
| 1965 | A | A | A | A | A | A | G | G | G | G | G | G |
| 1971 | G | G | G | A | T | T | A | A | A | A | A | A |
| 1977 | G | G | G | G | G | G | G | G | G | G | A | A |
| 1978 | C | C | C | C | A | A | A | A | A | A | A | A |
| 1989 | A | T | T | G | C | T | C | C | C | C | C | C |
| 1992 | A | A | A | G | A | A | A | A | A | A | A | A |
| 1998 | T | T | T | T | G or C | C | T | T | T | T | T | T |
| 2007 | T | T | T | T | T | T | C | C | C | C | C | C |
| 2013 | C | C | C | C | C | C | T | T | T | T | T | T |
| 2028 | T | T | T | T | G | G | G | G | G | G | G | G |
| 2034 | A | A | A | A | A | A | G | G | G | G | G | G |
| 2058 | G | G | G | G | G | G | G | C | A | A | A | A |
| 2067 | T | T | T | T | T | T | C | C | C | C | C | C |
| 2076 | A | C | A | A | A | A | A | A | A | A | A | A |
| 2086 | G | G | G | G | C | C | G | G | G | G | G | G |
| 2088 | T | T | A | A | A | A | A | A | A | A | A | A |
| 2100 | C | C | C | T | C | C | C | C | C | C | C | C |
| 2112 | A | A | A | A | T | T | C | C | C | C | C | C |
| 2142 | C | C | C | C | T | T | C | C | C | C | C | C |
| 2154 | T | T | T | T | T | T | C | C | C | C | C | C |
| 2181 | T | T | T | T | T | T | T | T | T | G | T | T |
| 2184 | G | G | G | G | G | G | A | A | A | A | A | A |
| 2191 | G | G | A | A | A | A | A | A | A | A | A | A |
| 2235 | T | T | T | T | T | T | A | A | A | A | A | A |
| 2237 | T | T | T | T | A | A | A | A | A | A | A | A |
| 2259 | C | C | C | C | C | T | C | C | C | C | C | C |
| 2274 | G | G | G | G | T | T | A | A | A | A | A | A |
| 2277 | C | C | C | C | C | C | C | C | T | C | C | C |
| 2280 | C | C | C | C | C | C | T | C | C | C | C | C |
| 2301 | A | A | A | A | G | G | G | G | G | G | G | G |
| 2316 | G | G | G | G | G | G | G | G | G | A | G | G |
| 2370 | C | C | C | C | G | G | A | A | A | A | A | A |
| 2391 | A | A | A | A | G | G | A | A | A | A | A | A |
| 2406 | A | A | A | A | A | A | G | G | G | G | G | G |
| 2413 | G | G | G | G | G | G | C | C | C | C | C | C |
| 2451 | A | A | A | A | G | G | A | A | A | A | A | A |
| 2457 | T | T | T | T | T | T | C | C | C | C | C | C |
| 2510 | G | G | G | G | A | A | G | G | G | G | G | G |
| 2541 | A | A | A | A | A | A | G | G | G | G | G | G |
| 2544 | C | C | C | C | T | T | T | T | T | T | T | T |
| 2550 | A | A | A | A | T | T | G | G | G | G | G | G |
| 2581 | A | A | A | A | C | C | C | C | A | C | C | C |
| 2583 | A | A | A | A | G | G | G | G | G | G | G | G |
| 2621 | A | A | A | A | G | G | G | G | G | G | G | G |
| 2625 | G | A | A | A | A | A | G | G | G | G | G | G |
Identification of BKTGR and the typing algorithm.
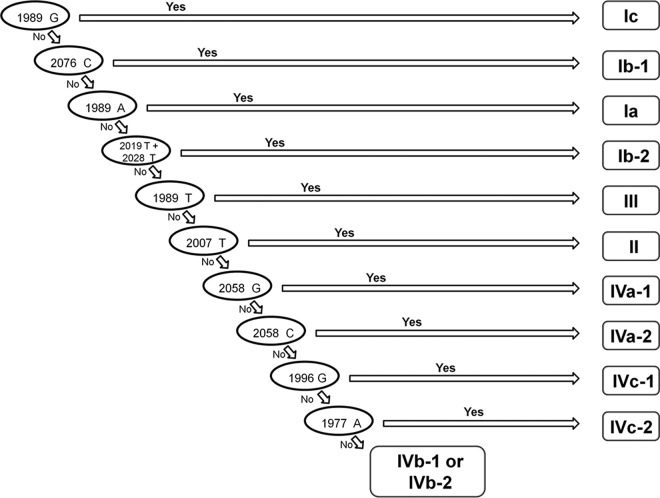
After detailed analysis of these mutational hot spots, we deduced an algorithm in order to determine the 12 BKV subgroups and subtypes (Fig. 2) from a minimum sequence. BKV subgrouping or subtyping, therefore, can be based on a region comprising only 100 bp of the VP1 gene (1977 through 2076), according to the presence or absence of specific mutations in this part of the viral genome. This region of the viral genome was therefore called the BK virus typing and grouping region (BKTGR). In summary, a sequence is manually analyzed for each informative SNP, step by step, according to the algorithm presented in Fig. 2: if, for the first informative SNP mentioned (1989G), the answer is yes, then subgroup Ic is determined. If the answer is no, then we have to look at the following position (2076C), and so on. Only the two subgroups IVb-1 and IVb-2 cannot be differentiated by using this algorithm. Such strains are then classified as the IVb subgroup. In order to confirm the robustness of the proposed algorithm, the 199 reference sequences studied were then aligned for the region between positions 1977 and 2076. We blindly tested our subtyping/subgrouping algorithm on these 199 sequences step by step in our decision-making process to assess agreement with respect to the phylogenetic tree shown in Fig. 1. Only two of the 199 strains studied (about 1%) presented a discordant determination of viral subtype between the subtyping/subgrouping that was based on the complete VP1 gene sequence and the one that was based on our 100-bp BKTGR.
FIG 2.
BKV subtyping/subgrouping algorithm based on the mutations detected in the BKTGR region of VP1.
An isolate from an Ib-2 strain (GenBank accession number JX195567) was misclassified as subgroup Ib-1, and an isolate from an Ia strain (JX195570) was misclassified as subtype III. The 100-bp sequences of these strains were submitted to the NCBI BLAST tool and were also misclassified as subgroup Ib-1. Thus, we observed the complete VP1 gene sequences (nucleotides 1564 to 2652) to understand these failures. The isolate from an Ib-2 strain (JX195567) has Ib-2-specific SNPs except at positions 2019 and 2076, which are specific to the Ib-1 subgroup. Since these two positions are included in the BKTGR, BLAST, alignment analysis, and the algorithm classified JX195567 as subgroup Ib-1, whereas the same tools used on the complete VP1 gene classified this strain as subgroup Ib-2. The isolate from an Ia strain (JX195570) has SNPs of subgroups Ia and Ib-1. The region from nucleotides 1698 to 2074, which includes a part of the BKTGR, best match the sequence of subgroup Ib-1, whereas the other part of the VP1 gene best matches subgroup Ia. Thus, the different methods used to determine the subtype according to this 100-bp region gave discordant results. In the case of our algorithm, this strain was classified as subtype III, because of the process of elimination. First, it took into account the position 2076 to determine the subgroup Ib-1, but at this position, the sequence was similar to that of subgroup Ia, eliminating the hypothesis of it being subgroup Ib-1. Second, it took into account position 1989 to determine subgroup Ia, but at this position, the sequence was similar to that of subgroup Ib-1, which had already been eliminated during the preceding steps and induced the steps that followed and the misclassification. Such sequences might be the result of recombination or mixed-infection events, which have already been noticed (12). The misclassification of the 2 isolates (JX195567 and JX195570) by the algorithm on the BKTGR were due to the sequences themselves rather than to a lack of accuracy of the algorithm. Nevertheless, it is important to note the inability of our algorithm to type such events.
Validation of the BKV subtyping algorithm and BKV subtype distribution among a population of French kidney transplant recipients.
In order to validate our BKV subtyping algorithm, BKV subtypes were identified in a population of 100 renal transplant recipients presenting with BKV viral replication. Subtypes were first determined according to the VP1 and large T antigen (LTA) partial gene-sequencing method (nucleotides 1564 to 2215 and 3021 to 3715). Sequences obtained were then used to blindly determine the subtypes according to the 100-bp BKTGR scheme, since it just needs the sequence from nucleotides 1977 to 2076. A concordance of 100% was obtained using these two methods. The main patient characteristics, BKV viral load and subtype distribution, are listed in Table 2. On the basis of these results, we designed new degenerate consensus primers that target only this 100-bp region. These primers provide a simple and reliable way to amplify and sequence the BKTGR of the 12 different subtypes/subgroups of the BKV. The 100 BKV DNA-positive samples, representatives of 5 different subtypes/subgroups (Ia, Ib-1, Ib-2, II, and IVc-2), with viral loads ranging from 4.41 to 9.46 log10 copies/ml were all successfully amplified and sequenced using this technique.
TABLE 2.
Clinical characteristics of renal transplant recipients
| Characteristic | Value |
|---|---|
| Age (median ± SD) (yr)a | 48.4 ± 15.9 |
| Sex (no. of males/females) | 66:34 |
| Urinary BKV viral load (mean ± SD) (log10 copies/ml) | 7.08 ± 1.7 |
| BKV genotype/subgroup | |
| Ia | 4 |
| Ib-1 | 22 |
| Ib-2 | 60 |
| Ic | 0 |
| II | 3 |
| III | 0 |
| IVa-1 | 0 |
| IVa-2 | 0 |
| IVb-1 | 0 |
| IVb-2 | 0 |
| IVc-1 | 0 |
| IVc-2 | 11 |
SD, standard deviation.
DISCUSSION
In this study, we identified a 100-bp region of the VP1 gene that can be used to simply identify the various BKV subtypes/subgroups. We called this region the BK typing and grouping region (BKTGR). We also developed consensus primers that enable sequencing of the different subtypes on this part of the BKV VP1 gene. We then tested the proposed algorithm in a population of kidney transplant recipients, which allowed us to establish the distribution of BKV subtypes/subgroups in Amiens, France. This distribution has been established in many countries but was still unknown in France. The analysis of 100 sequences derived from specimens of renal transplant recipients in France revealed the predominant Ib-2 subgroup. Further studies are needed to complete these preliminary epidemiological data. It was not possible to validate the primers we designed in this study on subtype III, or on subgroups Ic and IV (except IV-c2), due to the lack of clinical samples. Nevertheless, the algorithm was validated for this subtype and these subgroups using the reference sequences panel. Moreover, these two primers are located on regions presenting low variability between the different subtypes/subgroups. The sequence alignment of the reference strains on the complete VP1 gene shows that, from nucleotides 1920 to 1941 (position of the BKV-S1920 primer), the subtype III and subgroup Ic sequences are identical to those of subgroup Ia and subtype II. Concerning genotype IV, all subgroups share the same sequence as that of subgroup IVc-2 in this region, except IVb-2, which presents only one nucleotide mismatch. From nucleotides 2138 to 2159 (position of the BKV-A2159 primer), the subgroup Ic sequences are the same as those of subgroups Ia, Ib-1, and Ib-2, the subtype III sequences are identical to those of subtype II, and all the subtype IV subgroups share the same sequences as those of subgroup IVc-2. These observations suggest that the two primers, BKV-S1920 and BKV-A2159, can amplify and sequence all 12 subtypes/subgroups of the BKV.
Historically, BKV subtyping and subgrouping were based mainly on the phylogenetic analysis of some partial gene sequences. However, these methods are slower and, in some cases, less discriminating. For example, the distinction between major subtypes and subgroups is not discriminating enough when the analysis is based on the 327-bp region used in some BKV-typing studies. DNA sequencing followed by construction of a phylogenetic tree or BLAST analysis is an efficient process but requires a long time to set up, since it involves the retrieval and correct alignment of numerous sequences of reference in order to cover all possibilities. Appropriate selection of certain specific SNPs in BKV typing can enable determination of an appropriate region that presents enough variability to discriminate all subtypes and subgroups. This selection might make the BKV typing faster and simpler to perform, both of which are useful in a routine diagnostic laboratory. Our analysis reveals that mutations in VP1 can be used to distinguish all subtypes/subgroups. Luo et al. described the main polymorphisms over the entire coding portion of the BKV genome but did not propose a simple subtyping/subgrouping strategy (13). It is unknown why 1 to 10% of kidney transplant recipients develop symptomatic diseases, but the intensity and nature of immunosuppression probably play a major role, and the role of viral factors, particularly subtypes/subgroups, also seems obvious. Simpler BKV subtyping/subgrouping would therefore enable a better understanding of their impact on diagnosis, infection, and BK virus-associated disease (14).
Among the 60 subtype-informative SNPs listed in Table 1, 15 (25%) are nonsynonymous mutations (data not shown) and are not specifically located on the BC, DE, or HI loop as described by other authors in the study of VP1 quasi-species (12). However, only mutations that occur at a given position for all subtypes are indicated here.
The algorithm we have described in this study presents, on the whole, some limitations. First, it is unable to discriminate between the two subgroups of IVb. Subgroups IVb-1 and IVb-2 are uncommon and concern only 5 of the 199 (2.4%) sequences analyzed. However, 4 positions in the BKV VP1 gene outside the BKTGR can be used to differentiate these two subtypes if necessary, that is, positions 1926, 2181, 2316, and 2581 (see Table 1). Second, the discordant results our algorithm gave for two reference strains reveals its inability to subtype putative mixed-genotype samples or recombination events that need an analysis based on a larger region or on more than one region simultaneously (19). Compared with other typing methods, the BK virus typing and grouping region on which our algorithm is based is, thus, accurate enough to discriminate among the different subtypes and subgroups, but it is too short to type recombinant strains or mixed-genotype samples.
Other teams have described BK virus strain classification strategies based on various alternative options. For example, Gard et al. described a real-time PCR technique, but this technique did not determine subgroups (15). Matsuda et al. developed a BKV-genotyping tool based on high-resolution melting analysis (16), but it was limited by its difficulty in distinguishing strains with the same polymorphisms. Finally, other complete VP1 sequences are available in GenBank, and it will be interesting to see whether our subtyping/subgrouping algorithm maintains an efficiency greater than 99%.
In conclusion, the proposed protocol for simple identification of all BKV subtypes/subgroups is easily accessible to all routine laboratories and should enable a better understanding of the impact of each virus strain on the emergence of BK virus-associated diseases.
MATERIALS AND METHODS
Recovery of public sequences.
Publicly available whole-genome sequences or complete VP1 gene sequences were retrieved from GenBank using “BK virus complete genome” or “BK virus VP1 gene complete sequence” as key words in the nucleotide search. All data were collected before 1 January 2016. We selected 199 reference strain sequences, all isolated from human samples. The accession numbers of these sequences are V01108, JF894228, JN794032, DQ305492, DQ989807, AB369092, DQ989804, DQ989802, DQ989812, V01109, JX195579, JN794016, KP984526, JX195570, AB211369, AB263929, AB211371, AB369094, AB365177, AB301099, AB301090, AB211374, AB263933, AB301095, AB263932, AY628225, AB369090, DQ989813, DQ989809, DQ989806, KF468309, KF468281, KT354835, JX195583, JX195582, JX195581, JX195573, JX195568, JX195566, JX195564, JX195563, JX195562, JX195561, JX195558, JX195557, JN794036, JN794035, JN794028, JN794025, JN794021, JN794020, JN794019, JN794013, JN794009, JN794008, JN794005, JN794000, JN793995, AB211370, AB263915, JN192438, AB260032, AB369087, AB260029, AB301096, AB301091, AB301088, AB301086, AB263924, AB263923, AB263921, JN192436, JN192434, JX195560, AB369091, AB369088, DQ989800, DQ989799, DQ989811, DQ989810, DQ989808, DQ989805, DQ989803, DQ989801, DQ989798, DQ989797, DQ989796, DQ989795, DQ989794, KF468307, KF468303, KF468301, KF468299, KF468297, KF468289, KF468287, KF468283, DQ366598, KT354836, JX195580, JX195578, JX195576, JX195574, JX195572, JX195571, JX195565, JN794043, JX195567, JN794042, JN794041, JN794040, JN794039, JN794038, JN794037, JN794034, JN794033, JN794031, JN794030, JN794029, JN794027, JN794026, JN794024, JN794023, JN794022, JN794018, JN794017, JN794015, JN794012, JN794011, JN794006, JN794004, JN793998, JN793997, JN793994, AB211372, AB301098, AB211377, AB211381, AB369101, AB365165, AB464954, AB211379, AB369100, AB369099, AB369098, AB369097, AB369096, AB369095, Z19536, JN794014, AB263916, AB263920, AB301101, KF468305, KF468295, JX195559, JN793996, JN793993, JN793992, M23122, KF055892, JN192440, AB211386, JX195577, AB269869, AB269859, AB365171, AB211389, AB269841, AB269826, AB211390, AB211391, AB217919, AB211387, AB269834, AB269846, AB269863, AB365173, AB269855, JN794007, AB269830, AB269833, AB260033, AB260034, AB369093, AB369089, KF468293, KF468291, KF468285, KF468279, KT354834, KT354833, JX195575, JX195569, JN794010, JN794003, JN794002, JN794001, and JN793999.
Phylogenetic analysis.
Analyses were performed for sequences retrieved from GenBank and for sequences obtained from patient samples. Comparative alignment was carried out using the ClustalW program of MEGA 6.0 software. A phylogenetic tree was constructed using the maximum likelihood method with the Tamara-Nei model in MEGA 6.0, and 1,000 bootstrap replications were used to assess the reliability of the tree (17).
Mapping of gene polymorphism in VP1.
The reference sequence used for numbering in this analysis was the Dunlop strain sequence (GenBank accession no. V01108; subgroup Ia), in which nucleotide position 1 is the noncoding control region (NCCR) position next to the start codon of the large T antigen. All polymorphisms found in the VP1 region were recorded for these 199 sequences. We then retained only the polymorphisms that were found for all sequences of a given subtype/subgroup.
Clinical samples.
A total of 100 BKV DNA-positive urine samples were collected from 100 different renal transplant recipients at the Amiens University Hospital. Viral DNA was extracted from 200 μl of urine by using protocol B of the NucliSENS easyMAG system (bioMérieux, France). The extracted DNA was eluted with 50 μl of elution buffer and frozen at −80°C before use for quantification and genotyping.
Quantification of BKV viral load.
The BKV viral load of each clinical sample was determined by quantitative real-time PCR. The PCR (R-gene kit [Argene, France]) assay was performed in a 25-μl volume containing 15 μl of amplification premix and 10 μl of standard or sample DNA using the following amplification profile: 1 cycle for 15 min at 95°C followed by 45 two-step cycles at 95°C for 10 s and 60°C for 40 s.
BKV genotyping.
BKV subtyping and subgrouping were based on the phylogenetic analysis of fragments within VP1 and large T antigen genes (nucleotides 1564 to 2215 and 3021 to 3715, respectively) as previously described (18). In our study, another genotyping scheme was set up, based on a shorter 100-bp segment from nucleotides 1977 to 2076 within VP1. To briefly summarize, viral DNA was first amplified using primers BKV-S1920 (5′-GGTYATTGGAATAACTAGYATGC-3′) and BKV-A2159 (5′-TCCAARTAGGCCTTATGRTCAG-3′), and Sanger sequencing of the purified amplicons was performed using the Applied Biosystems BigDye Terminator v1.1 kit according to manufacturer instructions. These primers were used individually for the generation of forward and reverse sequences which were read with the use of an Applied Biosystems 3500 genetic analyzer. The nucleotide sequences obtained were then manually reviewed and submitted to the new established algorithm.
ACKNOWLEDGMENTS
The Amiens University Medical Center provided resources for this study.
We have no conflicts of interest to declare.
REFERENCES
- 1.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 2.Dropulic LK, Jones RJ. 2008. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant 41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin L. 2001. Molecular methods for identification and genotyping of BK virus. Methods Mol Biol 165:33–48. [DOI] [PubMed] [Google Scholar]
- 4.Krumbholz A, Bininda-Emonds OR, Wutzler P, Zell R. 2008. Evolution of four BK virus subtypes. Infect Genet Evol 8:632–643. doi: 10.1016/j.meegid.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhong S, Randhawa PS, Ikegaya H, Chen Q, Zheng HY, Suzuki M, Takeuchi T, Shibuya A, Kitamura T, Yogo Y. 2009. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J Gen Virol 90:144–152. doi: 10.1099/vir.0.83611-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishimoto Y, Zheng HY, Zhong S, Ikegaya H, Chen Q, Sugimoto C, Kitamura T, Yogo Y. 2007. An Asian origin for subtype IV BK virus based on phylogenetic analysis. J Mol Evol 65:103–111. doi: 10.1007/s00239-006-0269-6. [DOI] [PubMed] [Google Scholar]
- 7.Baksh FK, Finkelstein SD, Swalsky PA, Stoner GL, Ryschkewitsch CF, Randhawa P. 2001. Molecular genotyping of BK and JC viruses in human polyomavirus-associated interstitial nephritis after renal transplantation. Am J Kidney Dis 38:354–365. doi: 10.1053/ajkd.2001.26101. [DOI] [PubMed] [Google Scholar]
- 8.Di Taranto C, Pietropaolo V, Orsi GB, Jin L, Sinibaldi L, Degener AM. 1997. Detection of BK polyomavirus genotypes in healthy and HIV-positive children. Eur J Epidemiol 13:653–657. doi: 10.1023/A:1007371320999. [DOI] [PubMed] [Google Scholar]
- 9.Takasaka T, Goya N, Tokumoto T, Tanabe K, Toma H, Ogawa Y, Hokama S, Momose A, Funyu T, Fujioka T, Omori S, Akiyama H, Chen Q, Zheng HY, Ohta N, Kitamura T, Yogo Y. 2004. Subtypes of BK virus prevalent in Japan and variation in their transcriptional control region. J Gen Virol 85:2821–2827. doi: 10.1099/vir.0.80363-0. [DOI] [PubMed] [Google Scholar]
- 10.Krumbholz A, Zell R, Egerer R, Sauerbrei A, Helming A, Gruhn B, Wutzler P. 2006. Prevalence of BK virus subtype I in Germany. J Med Virol 78:1588–1598. doi: 10.1002/jmv.20743. [DOI] [PubMed] [Google Scholar]
- 11.Ikegaya H, Saukko PJ, Tertti R, Metsarinne KP, Carr MJ, Crowley B, Sakurada K, Zheng HY, Kitamura T, Yogo Y. 2006. Identification of a genomic subgroup of BK polyomavirus spread in European populations. J Gen Virol 87:3201–3208. doi: 10.1099/vir.0.82266-0. [DOI] [PubMed] [Google Scholar]
- 12.Luo C, Hirsch HH, Kant J, Randhawa P. 2012. VP-1 quasispecies in human infection with polyomavirus BK. J Med Virol 84:152–161. doi: 10.1002/jmv.22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Bueno M, Kant J, Martinson J, Randhawa P. 2009. Genotyping schemes for polyomavirus BK, using gene-specific phylogenetic trees and single nucleotide polymorphism analysis. J Virol 83:2285–2297. doi: 10.1128/JVI.02180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konietzny R, Fischer R, Ternette N, Wright CA, Turney BW, Chakera A, Hughes D, Kessler BM, Pugh CW. 2012. Detection of BK virus in urine from renal transplant subjects by mass spectrometry. Clin Proteomics 9:4. doi: 10.1186/1559-0275-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gard L, Niesters HG, Riezebos-Brilman A. 2015. A real time genotyping PCR assay for polyomavirus BK. J Virol Methods 221:51–56. doi: 10.1016/j.jviromet.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda Y, Qazi Y, Iwaki Y. 2011. A rapid and efficient method BK polyomavirus genotyping by high-resolution melting analysis. J Med Virol 83:2128–2134. doi: 10.1002/jmv.22239. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Descamps V, Martin E, Morel V, Francois C, Helle F, Duverlie G, Castelain S, Brochot E. 2015. Comparative evaluation of three nucleic acid-based assays for BK virus quantification. J Clin Microbiol 53:3822–3827. doi: 10.1128/JCM.02116-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morel V, Fournier C, Francois C, Brochot E, Helle F, Duverlie G, Castelain S. 2011. Genetic recombination of the hepatitis C virus: clinical implications. J Viral Hepat 18:77–83. [DOI] [PubMed] [Google Scholar]